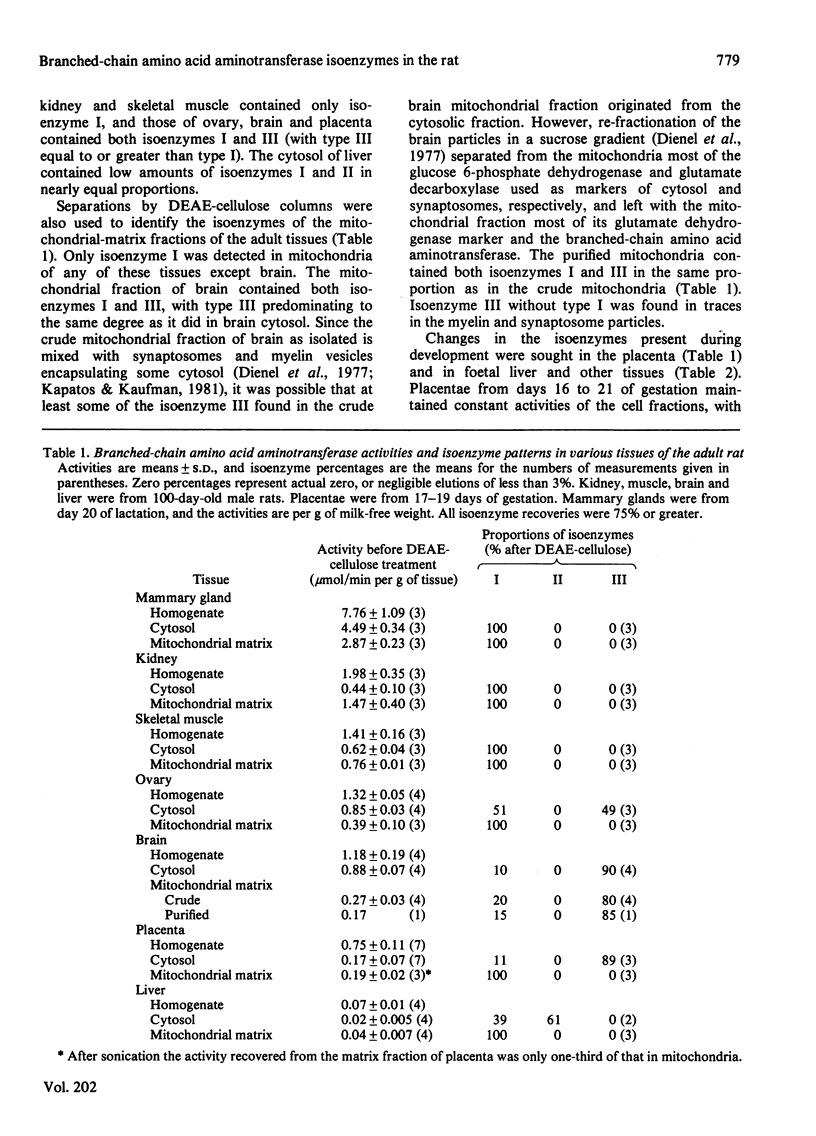
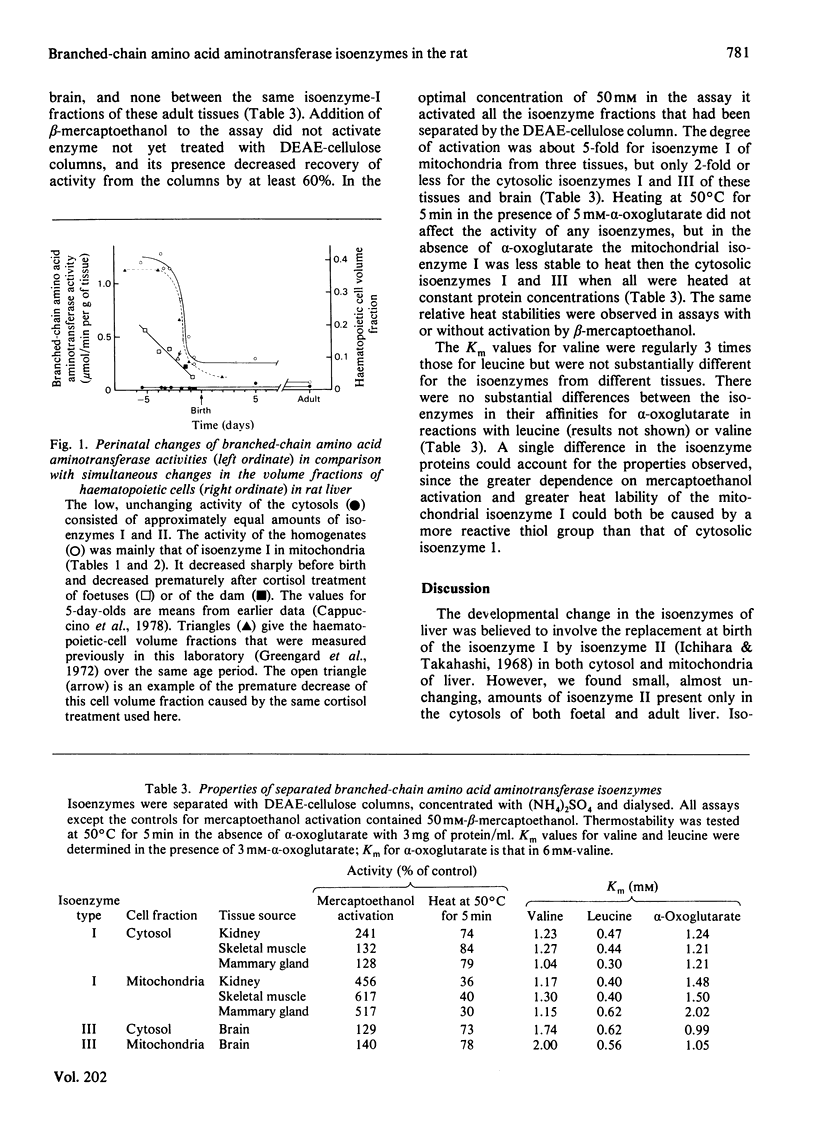
Abstract
The isoenzymic forms of branched-chain amino acid aminotransferase in mitochondria of rat tissues were compared with the better-known cytosolic forms in order to find any regular pattern of expression of these isoenzymes during development. Mitochondria of all tissues examined except brain contained only a type-I isoenzyme differing from the cytosolic type-I isoenzyme in heat stability and activation by mercaptoethanol. Foetal and adult brain mitochondria contained isoenzymes type III as well as type I. The large excess of type-I isoenzyme in foetal liver was localized in mitochondria, apparently of haematopoietic cells. The activity of this isoenzyme declined precipitously (by 80%) from day 19 of gestation at the same period and rate as does the volume fraction of haematopoietic cells that are then leaving the liver. Cortisol treatment accelerated the loss of these cells, and proportionally accelerated loss of the mitochondrial isoenzyme I. A development succession of type-I isoenzyme by the unique type II of liver parenchymal cell cytosols could not be demonstrated, since small, about equal, amounts of types I and II were always present in cytosols of foetal and adult liver. Developmental succession of isoenzymes within tissues was limited to cytosols and was demonstrated by the presence of cytosolic isoenzyme III in foetal and newborn skeletal muscle and kidney, organs which contain only isoenzyme I in the adult.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aki K., Ogawa K., Ichihara A. Transaminases of branched chain amino acids. IV. Purification and properties of two enzymes from rat liver. Biochim Biophys Acta. 1968 Jun 4;159(2):276–284. doi: 10.1016/0005-2744(68)90076-4. [DOI] [PubMed] [Google Scholar]
- Aki K., Ogawa K., Shirai A., Ichihara A. Transaminase of branched chain amino acids. 3. Purification and properties of the mitochondrial enzyme from hog heart and comparison with the supernatant enzyme. J Biochem. 1967 Nov;62(5):610–617. doi: 10.1093/oxfordjournals.jbchem.a128712. [DOI] [PubMed] [Google Scholar]
- Aki K., Yokojima A., Ichihara A. Transaminase of branched chain amino acids. VI. Purification and properties of the hog brain enzyme. J Biochem. 1969 Apr;65(4):539–544. doi: 10.1093/oxfordjournals.jbchem.a129047. [DOI] [PubMed] [Google Scholar]
- Cappuccino C. C., Kadowaki H., Knox W. E. Assay of leucine aminotransferase in rat tissues and tumors. Enzyme. 1978;23(5):328–338. doi: 10.1159/000458597. [DOI] [PubMed] [Google Scholar]
- Dienel G., Ryder E., Greengard O. Distribution of mitochondrial enzymes between the perikaryal and synaptic fractions of immature and adult rat brain. Biochim Biophys Acta. 1977 Feb 28;496(2):484–494. doi: 10.1016/0304-4165(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Greengard O., Federman M., Knox W. E. Cytomorphometry of developing rat liver and its application to enzymic differentiation. J Cell Biol. 1972 Feb;52(2):261–272. doi: 10.1083/jcb.52.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara A. Isozyme patterns of branched-chain amino acid transaminase during cellular differentiation and carcinogenesis. Ann N Y Acad Sci. 1975 Aug 22;259:347–354. doi: 10.1111/j.1749-6632.1975.tb25431.x. [DOI] [PubMed] [Google Scholar]
- Ichihara A., Takahashi H. Transaminases of branched-chain amino acids. V. Activity change in developing and regenerating rat liver. Biochim Biophys Acta. 1968 Oct 8;167(2):274–279. doi: 10.1016/0005-2744(68)90206-4. [DOI] [PubMed] [Google Scholar]
- Kapatos G., Kaufman S. Peripherally administered reduced pterins do enter the brain. Science. 1981 May 22;212(4497):955–956. doi: 10.1126/science.7233193. [DOI] [PubMed] [Google Scholar]
- Knox W. E., Lister-Rosenoer L. M. Timing of gestation in rats by fetal and maternal weights. Growth. 1978 Mar;42(1):43–53. [PubMed] [Google Scholar]
- Ogawa K., Ichihara A. Isozyme patterns of branched-chain amino acid transaminase in various rat hepatomas. Cancer Res. 1972 Jun;32(6):1257–1263. [PubMed] [Google Scholar]
- Ogawa K., Yokojima A., Ichihara A. Transaminase of branched chain amino acids. VII. Comparative studies on isozymes of ascites hepatoma and various normal tissues of rat. J Biochem. 1970 Dec;68(6):901–911. doi: 10.1093/oxfordjournals.jbchem.a129429. [DOI] [PubMed] [Google Scholar]
- Roth S. L., Delotto R., Kaji A. Changes in leucine aminotransferase isozymes by viral transformation and its correlation with the isozyme changes occurring during differentiation. Cancer Res. 1977 Apr;37(4):1147–1153. [PubMed] [Google Scholar]
- Shirai A., Ichihara A. Transaminase of branched chain amino acids. 8. Further studies on regulation of isozyme activities in rat liver and kidney. J Biochem. 1971 Nov;70(5):741–748. doi: 10.1093/oxfordjournals.jbchem.a129691. [DOI] [PubMed] [Google Scholar]


