Abstract
OBJECTIVE
Sphenoorbital meningioma (SOM) is a unique skull base tumor, characterized by infiltrative involvement and hyperostosis primarily of the lesser wing of sphenoid bone, with frequent involvement of the orbital compartment. SOM often manifests with proptosis and visual impairment. Surgical technique and outcome are highly variable among studies reported in the literature. The authors present a single-surgeon experience with SOM.
METHODS
A retrospective review of a prospectively maintained institutional database was performed. A blinded imaging review by 2 study team members was completed to confirm SOM, after which chart review was carried out to capture demographics and outcomes. All statistical testing was completed using JMP Pro version 14.1.0, with significance defined as p < 0.05.
RESULTS
Forty-seven patients who underwent surgery between 2000 and 2017 were included. The median age at surgery was 47 years (range 36–70 years), 81% of patients were female, and the median follow-up was 43 months (range 0–175 months). All operations were performed via a frontotemporal craniotomy, orbitooptic osteotomy, and anterior clinoidectomy, with extensive resection of all involved bone and soft tissue. Preoperatively, proptosis was noted in 44 patients, 98% of whom improved. Twenty-eight patients (60%) had visual deficits before surgery, 21 (75%) of whom improved during follow-up. Visual field defect other than a central scotoma was the only prognostic factor for improvement in vision on multivariate analysis (p = 0.0062). Nine patients (19%) had recurrence or progression during follow-up.
CONCLUSIONS
SOM is a unique skull base tumor that needs careful planning to optimize outcome. Aggressive removal of involved bone and periorbita is crucial, and proptosis and visual field defect other than a central scotoma can improve after surgery.
Keywords: en plaque meningioma, sphenoorbital meningioma, oncology, skull base
Sphenoorbital meningioma (SOM) is a unique and uncommon subset of skull base meningioma that has significant tumor infiltration and hyperostosis involving predominantly the lesser wing of the sphenoid bone that may extend to the greater sphenoid wing, anterior clinoid, and frontal and temporal bones. These tumors usually present with insidious, painless proptosis and/or visual impairment. Historically, Cushing and Eisenhardt first classified this tumor as meningioma en plaque.21 Since that initial characterization, as with many types of intracranial meningioma, there is a great deal of heterogeneity on how these tumors are classified in the neurosurgical literature; the most common of them are sphenoid wing meningioma, en plaque meningioma, and pterional meningioma. Due to the rarity and confusing classification terms, obtaining a pure cohort is difficult, and surgical outcome is highly variable among studies in terms of visual improvement, tumor recurrence, and so on. Thus, we conducted a retrospective review of a single surgeon’s experience to investigate characteristics of a very specifically defined SOM to describe our surgical strategy and postoperative outcome, especially as it relates to change in preoperative visual deficits.
Methods
Patient Population
Following IRB approval, medical records and a prospectively maintained database at our institution were searched for terms of “meningioma,” “sphenoorbital,” “sphenoid wing,” “optic,” “cavernous sinus,” “tuberculum,” “planum,” “anterior skull base,” “anterior clinoid,” and “orbitofrontal” for patients who underwent craniotomy for tumor resection from 2000 to 2017. SOM was defined as a pathologically proven meningioma arising from predominantly the lesser wing of the sphenoid bone, with the majority of tumor being evident in hyperostosis components of the sphenoid bone on CT and/or MR images. Meningiomas originating from other locations such as clinoid meningiomas, optic nerve sheath meningiomas, planum sphenoidale meningiomas, tuberculum sellae meningiomas, and lateral sphenoid wing meningiomas were excluded. Clinical data regarding age, sex, tumor size, pre- and postoperative visual function (acuity, field, and motility), duration of symptoms prior to surgery, measured proptosis, previous treatment, WHO tumor grade, any additional treatment rendered after the index operation, and recurrence on follow-up MRI were reviewed. Clinical and radiographic outcomes were evaluated 3 months after surgery and then annually thereafter. Formal ophthalmological evaluation was performed pre- and postoperatively. In some cases in which an ophthalmologist’s evaluation was not available, detailed neurological evaluation by a neurologist or our senior attending neurosurgeon was utilized for evaluation as a general estimation of postoperative visual function. Fractionated radiation therapy or Gamma Knife radiosurgery (GKRS; Elekta AB) was selectively used for treatment of residual or recurrent tumors. Tumor histology was reviewed by a neuropathologist, and classification of the tumor type was based on WHO classification.
Data Analysis
In visual deficit evaluation, visual acuity deficit was regarded positive if the acuity was equal to or worse than 20/30. Visual improvement was defined as either improvement in visual acuity of 10 or more (e.g., from 20/40 to 20/30 or better) or a decrease of visual field defect compared with the patient’s preoperative status. Five patients underwent previous treatment elsewhere prior to presenting to our institution (surgery in 4 patients and surgery followed by radiation treatment in 1 patient). Tumor size was measured on preoperative axial CT or MR images as the maximal anteroposterior length, from the anterior margin of the tumor adjacent to the optic nerve to the posterior margin of the tumor. Oya et al. introduced visual deficit severity criteria that addresses both visual acuity and field deficit simultaneously, which we utilized in our data analysis: normal (20/20 with no visual field deficit), mild (20/25–20/100 with or without blurriness or visual field loss), and severe (20/200 or worse) (Table 1).13 Proptosis was evaluated using a Hertel exophthalmometer, and, in patients who did not have this measurement during the ophthalmologist’s evaluation, it was measured on axial imaging studies as the difference in distance on the right/left sides between the anterior margin of the eyeball and the transverse line, binding the bilateral anterior tips of the lateral orbit, as previously described.12,18,22 We conducted 3 statistical analyses. First, we conducted univariate analysis of the entire patient cohort to determine prognostic factors contributing to normal postoperative vision (normal vision that is either maintained from before the surgery or improved from a preoperative deficit back to normal) among the following factors: age (< 49 or ≥ 50 years), sex, previous treatment (first surgery [yes/no]), ocular pain/discomfort (yes/no), proptosis (≥ 2 mm compared with the other eye), duration of symptoms until surgery (≤ 6 months or > 6 months), preoperative visual deficit severity per Oya et al.’s criteria (none or mild/severe),13 and tumor size (≥ 40 mm or < 40 mm). Then, multivariate analysis was performed to find potential favorable prognostic factors contributing to visual improvement among patients with preoperative visual deficits: age, tumor size, first surgery or not, symptom duration before surgery, visual deficit severity, visual field deficit (yes/no), ocular pain/discomfort (yes/no), proptosis, and extent of resection (subtotal resection [STR] vs gross-total resection [GTR]). To investigate potential factors determining tumor control, we performed multivariate analysis between recurrence/progression and the following factors: tumor size, any previous treatments, extent of resection, WHO grade of the tumor, and any prophylactic radiation treatments. All statistical data were computed using JMP Pro (version 14.1.0, SAS Institute Inc.); p < 0.05 was considered significant.
TABLE 1.
Visual deficit severity criteria
| Visual Acuity | Visual Field Deficit | |
|---|---|---|
| None | 20/20 | None |
| Mild | 20/25–20/100 | Blurriness &/or field defect present |
| Severe | 20/200 or worse |
According to Oya et al.13
Surgical Technique
The surgical technique for all patients with SOM was very uniform. The patient is positioned supine on the operating table in 15° of reverse-Trendelenburg with the head placed in 3-point pin fixation and rotated 30° to the contralateral side. The patient’s frontotemporal area and lateral thigh on the ipsilateral side are clipped, prepped, and draped in a sterile fashion. We use a modified Dolenc approach for exposure of the lesion.5 A standard curvilinear skin incision is made, beginning in front of the tragus of the ipsilateral ear and ending just behind the hairline in the midline, and the skin flap is turned above the superficial temporalis fascia. Interfascial dissection is performed to protect the frontalis branch of the facial nerve. To incise the temporalis muscle, we leave a cuff of 1 cm under the linea temporalis so that the temporalis muscle can be reattached for closure. An incision of the temporalis muscle is followed to the root of the zygoma, and the temporalis muscle is turned inferiorly and anteriorly. Typically, 3 burr holes are made to perform a standard frontotemporal craniotomy. A burr hole is placed in the anatomical keyhole to expose the frontal dura and periorbita, another just above the root of the zygoma, and a third burr hole at approximately the superior temporal line along the planned course of the craniotomy.20 Additional burr holes are created when the dura is very adherent to the inner table of the skull. Since the majority of the tumor is intraosseous and the bony resection is all done extradurally, it is optimal to not have any durotomies during the craniotomy. A frontotemporal craniotomy is turned in standard fashion, but this may need to be modified when the tumor is invading the calvaria in the region of the pterion or temporalis muscle. In this case, a trough is made with a cutting or diamond burr through the affected bone. When the deep temporalis muscle looks abnormal, this abnormal-looking component is sent to pathology as a specimen until negative margin is confirmed. It is rare that the full thickness of the temporalis muscle needs to be resected.
The dura is dissected from the lateral sphenoid wing, which is drilled and removed with a variety of cutting and diamond burrs. In most cases, prominent bleeding is encountered in this drilling process and can be controlled with bone wax. The lateral and superior wall of the orbit is exposed and resected with an orbital rongeur and diamond burrs. The meningoorbital band is identified and sectioned for better exposure and complete resection of the lesser sphenoid wing.6 Drilling of the lesser sphenoid wing is continued until the anterior clinoid process is hollowed out. The optic canal is identified en route, and, after unroofing of the optic canal, the anterior clinoid process is removed with a small punch. The orbital roof is removed medially until normal-appearing bone is encountered. When the ethmoid or sphenoid sinus is opened, as is often the case, it is plugged with fat or muscle tissue harvested from the lateral thigh or temporalis muscle. The bony resection is continued along the lesser wing of the sphenoid down to the middle cranial fossa, and the foramen rotundum and cranial nerve (CN) V2 are identified and skeletonized. As resection proceeds posteriorly, the foramen ovale and CN V3 are skeletonized in the same fashion. Although frameless stereotactic guidance may help guide the bony resection, we have rarely found it necessary. Usually, the skull base foramina serve as excellent anatomical landmarks to guide the resection of involved bone.
After aggressive resection of abnormal bone, the dura is widely opened in a semilunar fashion posterior to the tumor-involved sphenoid wing. Frontal and temporal dural margins are sent for frozen-section pathological analysis until negative margins are achieved. The dural incisions are then extended frontally and temporally down to the skull base. A 2-mm dural margin is maintained over the foramen ovale, foramen rotundum, the meningo-orbtial band, and the optic canal. The tumor is carefully dissected from the frontal and temporal lobes under a microscope and sent for pathologic evaluation. When the tumor invades the cavernous sinus (CS), the lateral wall of the CS is removed, but we do not continue the resection into the CS medial to the CNs. The dural incision is then extended lateral to the optic canal, and the falciform ligament is opened along the course of the lateral margin of the optic nerve to allow safe mobilization of the optic nerve for removal of tumor extending along the optic nerve and optic canal (Fig. 1). A fascia lata graft is harvested from the ipsilateral thigh through a linear incision and sewn in to replace the resected dura using a running double-armed 5-0 Prolene suture. Of course, where the falciform ligament has been opened lateral to the optic nerve, a watertight closure is not possible, and care has to be taken to not constrict the optic canal. After resection of the intradural component and dural repair, the intraorbital tumor is addressed. When there is significant volume of the intraorbital component, we work together with an ophthalmologist to resect the periorbita and intraorbital tumor. If the lacrimal gland is involved, this is resected. The orbital fat will extensively herniate into the wound once the periorbita is removed; therefore, it is optimal to reconstruct the dura before the periorbita is resected to make it easier to see the inferior dural margins above the optic canal, foramen rotundum, and foramen ovale.
FIG. 1.
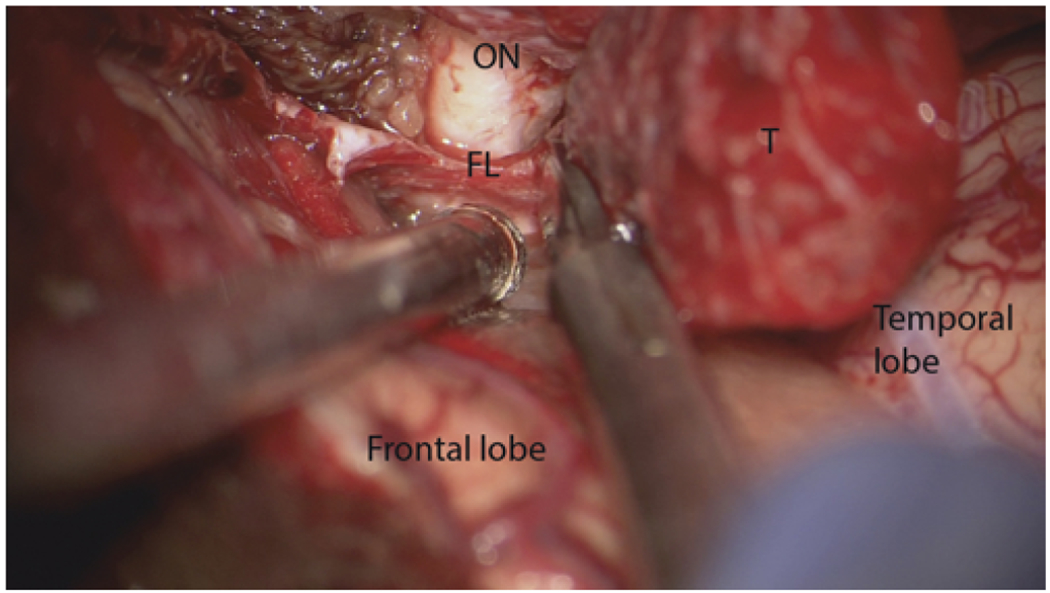
Intraoperative microscopic view in a case of right SOM. The dural incision around the tumor (T) is extended to the falciform ligament (FL) to decompress the optic nerve (ON). Figure is available in color online only.
The bone flap is replaced with titanium plates and screws, but when a significant amount of the bone flap is invaded by the tumor, titanium mesh is preferably used for reconstruction. We do not ever reconstruct the roof or lateral wall of the orbit. The temporalis muscle is re-secured to the muscle cuff, and the skin flap is closed in layers over a subgaleal drain hooked to bulb suction.
Results
Patient Demographics
Forty-seven patients (38 female and 9 male patients) underwent surgery for SOM over a period of 17 years. The median follow-up duration was 43 months (range 0–175 months). The median patient age was 47 years (range 36–70 years); 28 patients had a tumor on the right side, and 19 had one on the left side.
Patient characteristics are detailed in Table 2. The median tumor size was 30 mm (range 13–58 mm). Five patients had previous treatment (4 underwent surgery, and 1 underwent surgery followed by radiation therapy). The median duration of symptoms before surgery was 8 months (range 1–180 months). Twenty-eight patients (60%) had visual deficits preoperatively; 5 patients had visual acuity deficit only, 6 patients had visual field defect only, and 17 patients had both. According to Oya et al.’s visual deficit severity criteria (Table 1), the conditions were classified as severe (in 5 patients [18%]) and mild (in 23 patients [82%]).13 Sixteen patients (34%) complained of ocular pain or discomfort, and proptosis greater than or equal to 2 mm compared with the uninvolved eye was present in 44 patients (94%).
TABLE 2.
Patient characteristics
| Value | |
|---|---|
| Median follow-up period, mos (range) | 43 (0–175) |
| Median age, yrs (range) | 47 (36–70) |
| Sex, F/M | 38/9 |
| Side, rt/lt | 28/19 |
| Median tumor size, mm (range) | 30 (13–58) |
| Previous treatments | |
| Surgery | 4 |
| Surgery followed by fractionated radiation therapy | 1 |
| Median preop symptom duration, mos (range) | 8 (1–180) |
| Visual deficits | 28 (60%) |
| Severe | 5 |
| Mild | 23 |
| Visual acuity deficit only | 5 |
| Visual field defect only | 6 |
| Both visual acuity & field deficit | 17 |
| Ocular pain/discomfort | 16 (34%) |
| Improved postop | 15 (94%) |
| Proptosis | 44 (94%) |
| Improved postop | 43 (98%) |
| Resection | |
| GTR | 16 (34%) |
| STR | 31 (66%) |
| Pathology | |
| WHO grade I | 43 (91%) |
| WHO grade II | 4 (9.3%) |
| Radiographic recurrence | 9 (19%) |
| Treatment | |
| Fractioned radiation | 6 |
| Surgery | 2 |
| Stereotactic radiosurgery | 1 |
| Postop visual outcome | |
| Improved | 21 (45%) |
| Stable | 26 (55%) |
| Worse | 0 (0%) |
| Complication | 4 |
| Deep venous thrombosis | 2 |
| CSF leak | 1 |
| Superficial wound infection | 1 |
| Postop CN III palsy | 11 (23%) |
| Complete | 2 |
| Partial | 9 |
| Improved | 8 |
| Postop CN V deficit | 19 (40%) |
| Hypesthesia | 13 |
| Improved in follow-up | 2 |
| Hyperesthesia | 6 |
| Improved in follow-up | 4 |
Values are presented as the number of patients unless stated otherwise.
Surgical Outcomes
Seventy-five percent of patients (21 patients) with any visual deficits (n = 28) had postoperative improvement. Ocular pain/discomfort was relieved in all except 1 patient postoperatively (15/16, 94%). Ninety-eight percent of patients with proptosis had improvement postoperatively (43/44), either resolution of proptosis or decrease of proptosis measurement of equal or more than 3 mm. GTR was achieved in 16 patients (34%), while STR was performed in the remaining 31 patients, usually with a small amount of tumor left in the orbital apex to avoid ophthalmoplegia. Ophthalmologists were involved in the tumor resection in 37 patients (79%).
Pathology of the Tumor
Pathological examination of the tumor revealed WHO grade I in 45 patients (96%) and grade II in 2 patients (4.3%). One patient with a grade II tumor had undergone surgery and adjuvant radiation therapy at another institution before presentation to our hospital. GTR was achieved; however, significant radiographic recurrence was noted at the 47-month follow-up for which additional resection was recommended. To date, the patient has not undergone this salvage surgery. In the other patient, adjuvant radiation therapy was deferred because of satisfactory GTR and a postoperative MRI finding of no residual tumor. No radiographic recurrence was noted at the last follow-up of 83 months. Thirteen patients (28%) underwent prophylactic radiation treatment after STR of the tumor (GKRS in 5 and fractionated radiation therapy in 8). This was almost always directed at residual tumor in the CS or orbital apex. Radiographic recurrence or growth of the residual tumor was identified in 9 patients (19%) at a median interval of 28 months (range 14–74 months): 6 patients underwent fractionated radiation therapy, 2 patients underwent additional surgery, and 1 patient underwent GKRS.
Complications
Nonneurological postoperative complications occurred in 4 patients (8.5%); 2 patients experienced deep venous thrombosis in the lower extremities that necessitated anticoagulation. One patient had CSF leak that resolved spontaneously, and another patient experienced a superficial wound infection that was successfully treated medically with antibiotics. Postoperative third nerve palsy occurred in 11 patients (23.4%), and 2 of the palsies were complete. One patient required strabismus surgery, and the other did not because of preoperative severely impaired vision that did not improve postoperatively. The other 9 patients had partial third nerve palsy, 8 of whom improved at a median follow-up of 3 months (range 1–25 months). Postoperative CN V dysfunction occurred in 19 patients (40%). Hypesthesia was noted in 13 patients and resolved in 2 at last follow-up. Six patients developed hyperesthesia following tumor resection, which resolved in 4.
Statistical Analysis
Having no preoperative visual deficit was the only statistically significant factor for normal postoperative vision (p < 0.0001), and age, sex, first surgery or not, ocular pain or discomfort, proptosis, duration of symptoms, and tumor size (≥ 40 mm) were not related (Table 3). Among 28 patients with preoperative visual deficits (acuity and/or field deficits), visual field defect was the only favorable prognostic factor associated with visual function improvement in multivariate analysis (p = 0.0062) (Table 4). Age, tumor size, first surgery or not, duration of symptoms, visual deficit severity, ocular pain or discomfort, preoperative proptosis, and extent of resection were not related to postoperative visual improvement. No contributing factors for tumor recurrence/progression were detected in multivariate analysis among tumor size, previous treatment, extent of resection, WHO grade of the tumor, and prophylactic radiation treatments (Table 5).
TABLE 3.
Univariate analysis of postoperative normalization of vision
| Factor | p Value |
|---|---|
| Age (<49 or ≥50 yrs) | 0.26 |
| Sex | 0.78 |
| First surgery or not | 0.057 |
| Ocular pain/discomfort (yes/no) | 0.36 |
| Proptosis (≥2 mm) | 0.80 |
| Duration of symptoms until surgery (≤6 or >6 mos) | 0.94 |
| Preop visual deficit severity (none or mild/severe) | <0.0001* |
| Tumor size (≥40 or <40 mm) | 0.78 |
Statistically significant.
TABLE 4.
Multivariate analysis of visual improvement among patients with visual deficits
| Factor | p Value |
|---|---|
| Age | 0.58 |
| Tumor size | 0.58 |
| First surgery or not | 0.29 |
| Symptom duration | 0.092 |
| Visual deficit severity (mild or severe) | 0.23 |
| Visual field defect | 0.0062* |
| Ocular pain/discomfort | 0.36 |
| Proptosis | 0.13 |
| Extent of resection (STR or GTR) | 0.91 |
Statistically significant.
TABLE 5.
Multivariate analysis of tumor control
| Factor | p Value |
|---|---|
| Tumor size | 0.78 |
| Previous treatments | 0.25 |
| Extent of resection | 0.32 |
| WHO grade of the tumor | 0.43 |
| Prophylactic radiation treatments | 0.13 |
Illustrative Case
A 50-year-old woman with a history of medically well-controlled hypertension presented with a 9-month history of left eye discomfort. Ophthalmological evaluation revealed 5 mm of left-sided proptosis with no visual acuity and field. CT scanning and MRI revealed a left 43-mm SOM displacing the left optic nerve (Fig. 2A and B). A left frontotemporal craniotomy, orbitooptic osteotomy, anterior clinoidectomy with tumor resection, and left fascia lata graft reconstruction was performed. Postoperative CT scanning demonstrated satisfactory resection of abnormal bone (Fig. 2C). The patient was discharged home on postoperative day 3 with moderate diplopia consistent with partial oculomotor nerve palsy. Pathology revealed WHO grade I meningioma. Postoperative ophthalmological evaluation demonstrated improvement in proptosis with a Hertel exophthalmometer difference from 5 mm down to 1 mm, and no change in visual acuity or field. The partial CN III palsy completely resolved at the 6-month follow-up.
FIG. 2.
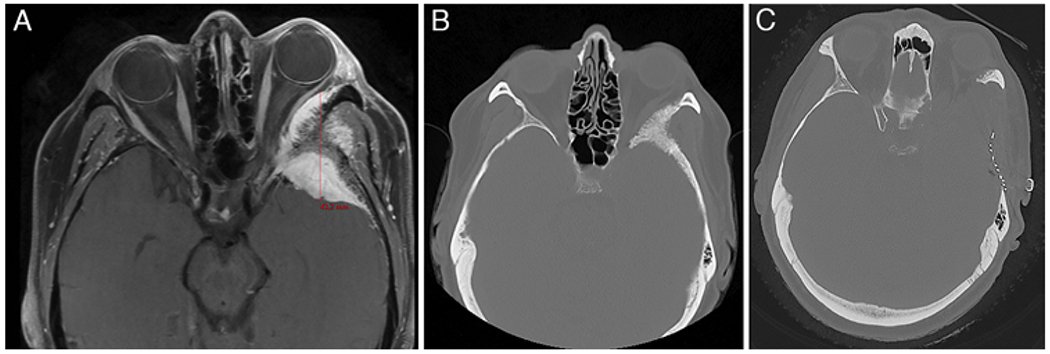
A: Gadolinium-enhanced axial T1-weighted MR image of the brain obtained preoperatively, showing a mass with peripheral dominant enhancement in the lesser wing of the sphenoid bone on the left side. The tumor size is demonstrated, from the anterior margin to the posterior margin of the tumor. B: CT scan revealing significant hyperostosis of the tumor. C: Postoperative CT scan demonstrating satisfactory resection of the hyperostotic tumor. The defect was reconstructed with titanium mesh. Figure is available in color online only.
Discussion
Sphenoorbital Meningioma
We defined SOM as a very specific subset of sphenoid wing meningioma predominantly involving the lesser wing of the sphenoid bone and causing prominent hyperostosis with secondary involvement of the greater sphenoid wing, orbit, and possibly ethmoids and central skull base as well as the frontal and temporal bones in very extensive tumors. Typically, the intradural tissue aspect of the tumor is a minor component. We excluded other originating locations such as lateral sphenoid wing meningiomas with a large intradural tumor mass without significant hyperostosis, clinoid meningiomas, optic nerve sheath meningiomas, planum sphenoidale meningiomas, tuberculum sellae meningiomas, and convexity meningiomas (Fig. 3). Multiple terminologies have existed historically referring to this specific tumor subtype, such as sphenoid wing meningioma, en plaque meningioma, hyperostosing meningioma of the sphenoid ridge, and pterional meningioma, and therefore it is difficult to compare results based on historical literature reports. Another confusing factor hindering the obtaining of a pure cohort of SOM patients besides confusing nomenclature is that many studies classify meningioma as “en plaque” and meningioma as “en masse” per Cushing’s criteria into the same category.3 A report by Pompili et al. from 1982, in which the authors reviewed a single institution’s 20-year experience with intracranial meningiomas, described a 9% incidence of SOM.14 However, they included the “en masse” subtype, which grows primarily as an intradural mass, rather than spreading over dural surfaces,18 as well as recurrent hyperostosing meningiomas of the sphenoid wing. Thus, the incidence of a pure SOM as we define it would be less. Indeed, Castellano et al. found SOM in just 15 of all 608 encountered meningiomas (2.5%).2 The exact incidence of SOM is lacking evidence, but SOM stands as an uncommon subtype of meningioma.
FIG. 3.
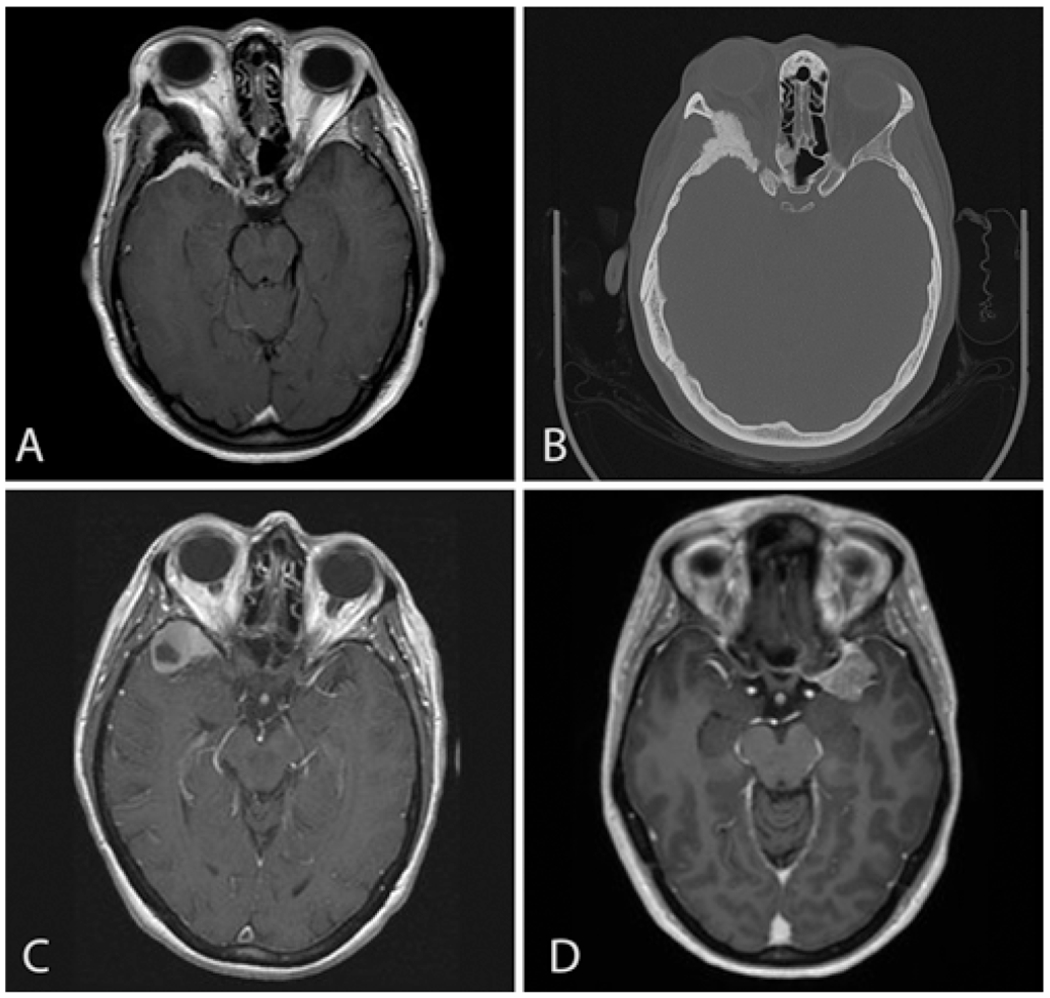
Imaging studies of SOM and other meningiomas involving the sphenoid bone. A: Gadolinium-enhanced T1-weighted MR image of the SOM, demonstrating the main focus of the tumor being evident in the right lesser wing of the sphenoid bone. There is also an intradural and intraorbital component of the tumor, compressing the right optic nerve. Proptosis is also noted. B: CT in bone window of the SOM, revealing abnormal thickening of the lesser wing of the sphenoid bone. C: Example of a lateral sphenoid wing meningioma on the right side. The main component of the tumor exists intradurally, and hypertrophy of the lesser wing of the sphenoid bone is not significant. D: Example of a medial sphenoid wing meningioma on the left side. The tumor is located near the lesser wing of the sphenoid bone; however, this is an intradural tumor without an intraosseous component.
The classic triad of SOM consists of proptosis, visual impairment, and ocular paresis.8 In our cohort, the presence of each of these preoperative symptoms was 94%, 75%, and 11%, respectively. As Shrivastava et al. pointed out, ocular paresis is typically secondary to mechanical restriction of extraocular muscles rather than true CN palsy, explaining the low occurrence in our cohort.21
Pathological analysis of SOM is very limited in the literature as well. In our series, 2 patients had WHO grade II tumors (4.3%), and the other 45 patients had grade I tumors. Mirone et al. (n = 71), Shrivastava et al. (n = 25), and Terrier et al. (n = 130) reported that 100% of SOM in their cohorts were WHO grade I.12,21,22 Only 1 patient with a WHO grade II SOM experienced recurrence in our cohort, and statistical analysis did not demonstrate significant correlation of WHO grade with recurrence, but with only 2 grade II tumors, firm conclusions cannot really be drawn. The recurrence rate of SOM is highly variable among studies, ranging from 8% to 56.3%.13,17,19,21 Potential reasons for this variability include disparate tumor inclusion criteria, a limited follow-up period, differences in surgical strategies, e.g., aggressiveness toward abnormal bone removal, and tumor characteristics (WHO grade). In our series, the recurrence rate of 19% at a median follow-up of 43 months is well within the range of prior studies.
Postoperative Visual Improvement
SOM has been reported to cause visual deficit in 30%–74% of patients, and improvement with surgery has been reported in 37%–75% of patients.7,13,15 This correlates well with our study, in which 60% of patients had visual deficits preoperatively, and improvement was achieved by surgery in 75% at last follow-up. Oya et al. concluded that severe preoperative visual deficit was the only statistically significant risk factor for unfavorable postoperative vision, while other factors such as age, duration of symptoms, extent of proptosis, ocular pain, or previous surgery were not.13 Our statistical analysis revealed similar results: patients without visual deficits before surgery are more likely to have normal vision postoperatively, and those with visual deficits are less likely to have normalized vision (p < 0.0001). Our study uniquely revealed that among 28 patients with preoperative visual deficits, visual field defect was the only favorable prognostic factor for visual improvement (p = 0.0062). As Maroon et al. previously suggested, the orbital component of the tumor typically exerts mass effect on the orbital structures rather than invades into them.11 Thus, it is likely that the visual field deficit is related to direct compression of the optic nerve by the tumor/hyperostotic bone. Therefore, any type of visual field defect other than a central scotoma may be reversible after surgery by removing the component of the mass compressing the optic nerve. A visual acuity deficit, on the other hand, may be the result of irreversible degeneration of the optic nerve by severe or prolonged compression. Indeed, the importance of decompression of the optic nerve has been emphasized in multiple studies.11,15
Postoperative Improvement of Proptosis and Cosmetic Outcomes
Proptosis is one of the most common symptoms of SOM, reported in 49%–100% of patients.18 Postoperative proptosis improvement is reported to be 50%–100%.1,4,9,10,15,18,21 This correlates with our results: 94% of patients presented with proptosis, among whom postoperative improvement was achieved in 98%. Several potential mechanisms have been suggested for proptosis, including direct progression of the tumor into the orbit, as well as contribution of venous obstruction due to tumor in the CS and the superior orbital fissure causing reduction of venous drainage from the orbit.18 Terrier et al. concluded that periorbital removal significantly improved proptosis for patients with severe preoperative proptosis greater than 6 mm.22 We agree that resection of the periorbita from the superior and lateral orbit is a critical step in the procedure. The only patient whose preoperative proptosis did not improve in our cohort did not have any unusual characteristics compared with other patients: the tumor size was 28 mm, the duration of symptoms until surgery was 36 months, and preoperative visual acuity was 20/50 with a visual field deficit. The visual deficit did not improve postoperatively in this patient. Our multivariate analysis did not demonstrate a statistically significant relationship between preoperative proptosis measurement and visual improvement (p = 0.51). It is possible that a long duration of symptoms may result in fibrosis of intraorbital soft tissues that would prevent the structures from returning to their normal positions. However, we do not have any direct evidence to support this.
We do not reconstruct the roof or lateral wall of the orbit. We do not typically remove the orbital rim, and therefore the cosmetic result has been very good. Most patients have noted some pulsatile enophthalmos for a few days after surgery, with resolution in 100% of cases by the 3-month follow-up. As SOM often extends laterally to the temporal bone and temporalis muscles, the defect of the craniotomy and bone flap is occasionally large, depending on extension of the intraosseous component of the tumor. If the defect was significantly large, we used a titanium mesh for reconstruction. The temporalis muscle is also occasionally resected; however, it is very rare that we have to resect a large volume of the muscle. These patients visit our follow-up clinic with expected atrophy in the temporal area, but so far, no patient has requested reconstruction for discomfort or cosmetic reasons.
Complications and Neurological Deficits
Postoperative oculomotor palsy and sensory trigeminal neuropathies are 2 common procedure-related cranial neuropathies. The incidence has been reported as 1.8%–14.2% for CN III palsy and 3.0%–21.0% for hypesthesia or CN V neuropathies.13 Three of our patients (6.4%) had persistent CN III palsy (complete in 2 and partial in 1), and 13 patients (28%) had permanent CN V neuropathies. The higher rate of CN V neuropathies in our cohort than that reported in the literature probably reflects our aggressive drilling strategy near the foramen rotundum and ovale. Subgaleal fluid collection and CSF leakage have been uncommonly encountered in previous studies.15,16 Ringel et al. reported subgaleal fluid collection in 6 patients in their series of 63 patients, 2 of whom required surgical revision of the dural leak, followed by implantation of a lumboperitoneal shunt.15 In a series of 66 patients by Saeed et al., 5 patients developed subgaleal fluid collection, one of whom underwent surgical dural repair and lumboperitoneal shunt.16 One patient in our series of 47 patients had transient CSF leakage from the nostril, likely related to opening the sphenoid sinus or an ethmoid air cell with drilling, which fortunately resolved spontaneously and did not require a lumbar drain or return to the operating room. Since we opened the falciform ligament to decompress the optic nerve, complete watertight dural closure is unobtainable; however, we sew the fascia lata graft or pericranial graft to the dural defect in a continuous fashion with 5-0 or 6-0 Prolene to minimize CSF leakage. Those areas without stitches are reinforced with placement of a small autologous fat graft and fibrin sealant. Indeed, the importance of a watertight dural closure except for segments in which one cannot be obtained, such as the middle fossa, orbital roof, and anterior clinoid process, as well as using a fat graft, has been emphasized in multiple previous studies.15,16
Conclusions
SOM is a rare, unique subtype of skull base meningioma that is primarily intraosseous and often manifests with proptosis and visual impairment. Aggressive removal of abnormal bone is crucial for tumor control, and proptosis improvement is expected, especially when the periorbita is removed. A visual field defect without a central scotoma is a statistically significant favorable prognostic factor for visual improvement. Long-term follow-up is necessary to truly establish a reliable recurrence rate and need for potential adjuvant treatment.
ABBREVIATIONS
- CN
cranial nerve
- CS
cavernous sinus
- GKRS
Gamma Knife radiosurgery
- GTR
gross-total resection
- SOM
sphenoorbital meningioma
- STR
subtotal resection
Footnotes
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Supplemental Information
Previous Presentations
Portions of this work were presented in abstract form at the North American Skull Base Society 29th Annual Meeting, Orlando, FL, February 15–17, 2019.
References
- 1.Bikmaz K, Mrak R, Al-Mefty O: Management of bone-invasive, hyperostotic sphenoid wing meningiomas. J Neurosurg 107:905–912, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Castellano F, Guidetti B, Olivecrona H: Pterional meningiomas en plaque. J Neurosurg 9:188–196, 1952 [DOI] [PubMed] [Google Scholar]
- 3.Cushing H: The meningiomas (dural endotheliomas): their source, and favoured seats of origin. Brain 45:282–316, 1922 [Google Scholar]
- 4.De Jesús O, Toledo MM: Surgical management of meningioma en plaque of the sphenoid ridge. Surg Neurol 55:265–269, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Dolenc V: Direct microsurgical repair of intracavernous vascular lesions. J Neurosurg 58:824–831, 1983 [DOI] [PubMed] [Google Scholar]
- 6.Froelich SC, Aziz KM, Levine NB, Theodosopoulos PV, van Loveren HR, Keller JT: Refinement of the extradural anterior clinoidectomy: surgical anatomy of the orbitotemporal periosteal fold. Neurosurgery 61 (5 Suppl 2):179–186, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Honig S, Trantakis C, Frerich B, Sterker I, Schober R, Meixensberger J: Spheno-orbital meningiomas: outcome after microsurgical treatment: a clinical review of 30 cases. Neurol Res 32:314–325, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Krayenbühl HA: Unilateral exophthalmos. Clin Neurosurg 14:45–71, 1966 [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Shi JT, An YZ, Zhang TM, Fu JD, Zhang JL, et al. : Sphenoid wing meningioma en plaque: report of 37 cases. Chin Med J (Engl) 122:2423–2427, 2009 [PubMed] [Google Scholar]
- 10.Mariniello G, Maiuri F, Strianese D, Donzelli R, Iuliano A, Tranfa F, et al. : Spheno-orbital meningiomas: surgical approaches and outcome according to the intraorbital tumor extent. Zentralbl Neurochir 69:175–181, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Maroon JC, Kennerdell JS, Vidovich DV, Abla A, Sternau L: Recurrent spheno-orbital meningioma. J Neurosurg 80:202–208, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Mirone G, Chibbaro S, Schiabello L, Tola S, George B: En plaque sphenoid wing meningiomas: recurrence factors and surgical strategy in a series of 71 patients. Neurosurgery 65 (6 Suppl):100–109, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Oya S, Sade B, Lee JH: Sphenoorbital meningioma: surgical technique and outcome. J Neurosurg 114:1241–1249, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Pompili A, Derome PJ, Visot A, Guiot G: Hyperostosing meningiomas of the sphenoid ridge—clinical features, surgical therapy, and long-term observations: review of 49 cases. Surg Neurol 17:411–416, 1982 [DOI] [PubMed] [Google Scholar]
- 15.Ringel F, Cedzich C, Schramm J: Microsurgical technique and results of a series of 63 spheno-orbital meningiomas. Neurosurgery 60 (4 Suppl 2):214–222, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Saeed P, van Furth WR, Tanck M, Freling N, van der Sprenkel JW, Stalpers LJ, et al. : Surgical treatment of sphenoorbital meningiomas. Br J Ophthalmol 95:996–1000, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Sandalcioglu IE, Gasser T, Mohr C, Stolke D, Wiedemayer H: Spheno-orbital meningiomas: interdisciplinary surgical approach, resectability and long-term results. J Craniomaxillofac Surg 33:260–266, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Scarone P, Leclerq D, Héran F, Robert G: Long-term results with exophthalmos in a surgical series of 30 sphenoorbital meningiomas. Clinical article. J Neurosurg 111:1069–1077, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Schick U, Bleyen J, Bani A, Hassler W: Management of meningiomas en plaque of the sphenoid wing. J Neurosurg 104:208–214, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Shimizu S, Tanriover N, Rhoton AL Jr, Yoshioka N, Fujii K: MacCarty keyhole and inferior orbital fissure in orbitozygomatic craniotomy. Neurosurgery 57 (1 Suppl): 152–159, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Shrivastava RK, Sen C, Costantino PD, Della Rocca R: Sphenoorbital meningiomas: surgical limitations and lessons learned in their long-term management. J Neurosurg 103:491–497, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Terrier LM, Bernard F, Fournier HD, Morandi X, Velut S, Hénaux PL, et al. : Spheno-orbital meningiomas surgery: multicenter management study for complex extensive tumors. World Neurosurg 112:e145–e156, 2018 [DOI] [PubMed] [Google Scholar]


