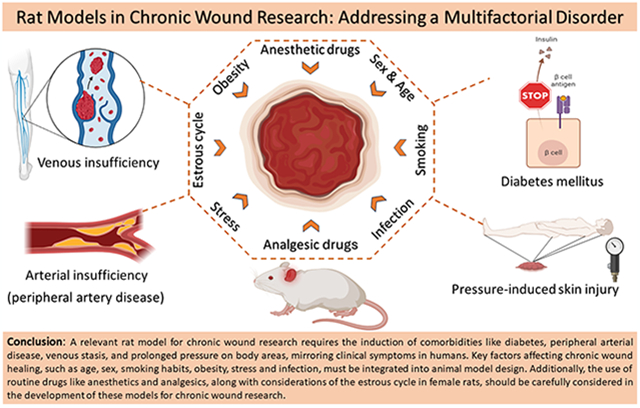
Abstract
The increasing global prevalence of chronic wounds underscores the growing importance of developing effective animal models for their study. This review offers a critical evaluation of the strengths and limitations of rat models frequently employed in chronic wound research and proposes potential improvements. It explores these models in the context of key comorbidities, including diabetes, venous and arterial insufficiency, pressure-induced blood flow obstruction, and infections. Additionally, the review examines important wound factors including age, sex, smoking, and the impact of anesthetic and analgesic drugs, acknowledging their substantial effects on research outcomes. A thorough understanding of these variables is crucial for refining animal models and can provide valuable insights for future research endeavors.
Keywords: Rat model, Chronic wound, Peripheral arterial disease, Diabetic ulcer, Pressure ulcer, Venous ulcer
Graphical Abstract
1. Introduction
Chronic skin wounds, such as peripheral arterial disease (PAD), venous, pressure, and diabetic ulcers, are estimated to affect 1–2 % of populations in developed countries [1]. These wounds not only diminish the quality of life for patients but also impose significant clinical and economic burdens on the healthcare system [2]. The prevalence of chronic wounds is on a steady rise, with a global projected increase in diabetic foot ulcers by approximately 6.6 % between 2016 and 2024 [3]. As a result, there is an increasing need for the development of various wound care techniques [4]. Numerous therapeutic approaches have been tested for healing chronic wounds in preclinical animal models [5,6], yet they frequently fail in clinical trials. In general, new drug treatments that have been effective in preclinical (animal) studies have had a failure rate of 90 % in human clinical trials [7–9]. The cause of this failure in translation is multifactorial but can be attributed in part to inadequate animal models or deficient experimental designs [10,11].
In chronic wound research, the most commonly utilized animal models are rodents, specifically Mus musculus (mice) and Rattus norvegicus (rats). Although mice have been the preferred model due to the extensive range of genetic tools available, recent advancements in genome editing technologies are increasingly making rats a comparable alternative [12]. The selection between rats and mice as models should, therefore, hinge more on their respective physiological, anatomical, and pharmacological characteristics relevant to the targeted biological study. Notably, adult rats have a weight 8–10 times greater than that of mice, rendering them significantly larger and thus easier to handle and perform surgery on. This advantage enables researchers to simulate extensive wound areas, which, in conjunction with disease modeling, is particularly beneficial for studying chronic wound types [13]. Rats also demonstrate a lower stress response to human interaction than mice, further enhancing their value as research models [12,14]. Furthermore, Rattus norvegicus has a lengthy history of more than 150 years in biological research, providing a wealth of literature that can inform and support contemporary basic and translational research efforts [15–17]. In this review article, we focus on the latest progress in experimental rat models used for the study of chronic wounds. We compare and discuss the current animal models of the four main types of chronic wounds (i.e., PAD, diabetic, venous, and pressure ulcers). Additionally, we examine significant accompanying factors such as age, sex, side effects of anesthetic and analgesic drugs, smoking, obesity, stress, infection, and wound contraction that should be considered for better translatability to humans.
Our critical evaluation is intended to assist readers in choosing the most fitting models for simulating chronic wounds of diverse etiologies, as well as in identifying models that accurately depict certain underlying diseases and are optimally adaptable for inducing wounds of these specific types.
2. Non-rodent animal models for chronic wounds
2.1. Rabbits
Rabbits have long been utilized in wound healing research, specifically using the ear excisional wound model. In this model, wounds penetrating the epidermis, dermis, and cartilage are made with a biopsy punch [18]. It has been frequently used for studies of the toxicology and pharmacology of new medicine since skin permeability in rabbits is higher than that of humans and other animals, including rodents and pigs. Moreover, rabbit skin, particularly in response to aging and drugs, closely mirrors human reactions. The extensive ear vasculature facilitates the easy creation of ischemic wounds through a minimally invasive procedure and allows for the induction of multiple wounds in the ear [19,20]. The loose skin of rabbits presents a major challenge for wound healing models. Therefore, either the rabbit ears, where the dermis is firmly attached to the subcutaneous tissue (acting as a natural splint), or an external splint are employed. In addition, rabbit models have limitations like reduced genetic tractability and a lack of species-specific reagents, especially when compared to rodent models [20].
2.2. Pigs
Pig and human skin have remarkable anatomical and physiological similarities including the dermal-epidermal thickness ratio which is 10: 1 to 13: 1 in the pigs and humans respectively, epidermal turnover, collagen and elastin structure, vascular distribution, orientation, and size, not having panniculus carnosus, developed rete-ridges, as well as abundant subdermal adipose tissue [21]. The subepidermal plexus and elastic content in pigs are less than in humans but still more similar compared to other species. Schmook et al. demonstrated that pig skin is a more effective model for testing the in-vitro skin penetration of candidate drugs compared to reconstructed human skin or epidermis [22]. Unlike rats, which are resistant to atherosclerosis induction [23], pigs with natural mutations in for apolipoproteins B (Lpb5) and U (Lpu1) can develop atherosclerosis and hypercholesterolemia in the iliac, femoral, and coronary arteries, which are major factors in the progression of PAD ulcers in humans [24]. Unlike rats’ injury-sensitive skin, the prolonged application of pressure does not damage the hind limbs of pigs enough to induce pressure ulcers, a condition more akin to humans [25]. The previous comparative analysis demonstrated that the concordance between the results of wound healing studies in humans, large animal models like pigs, small animal models, and in vitro models are 78 %, 53 %, and 57 %, respectively [21].
Despite the similarities in cutaneous wound healing between humans and pigs, the pig models come with multiple drawbacks compared to other animals. Pigs are notably more expensive to purchase and maintain, and their considerable size makes handling challenging and demands specialized housing facilities. Anesthetizing pigs is also more complicated, requiring appropriate assessment for the correct depth [26]. There is comparatively limited access to certain reagents in pigs, such as antibodies and PCR arrays, in comparison to other animal species [27].
3. Acute wound models
In scientific literature, the demarcation between acute and chronic wound models is frequently blurred, and some researchers do not make this distinction when presenting their studies [28]. A substantial portion of earlier literature is focused on acute wound healing in healthy animals. Only in recent decades has there been an increasing interest in preclinical models for addressing the healing of chronic wounds with comorbidities [29].
An acute wound is primarily characterized by its origin from a short-term external trauma, and its healing process is not hindered by accompanying diseases that could delay or halt the recovery, turning the wound into a chronic condition [30]. The healing process of acute wounds consists of four overlapping phases: hemostasis, inflammation, proliferation, and remodeling, generally lasting between 4 and 6 weeks [31,32]. The absence of comorbidities affecting wound healing is a hallmark of acute wound models. Typically, these experiments utilize young, healthy animals in clean and consistent environments [28], which may limit their clinical relevance.
Various acute wound models have been developed in rats, such as incision [33], partial-thickness [34], and full-thickness wounds [35]. Typically, an incision wound is made on the rat’s back using a scalpel blade, reaching the subcutaneous tissue. Evaluating such wounds is valuable for assessing the presence of surgical site infections, examining the effectiveness of surgical suturing tools, and investigating the mechanical properties of healed skin tissue. Partial-thickness excisional wounds involve the removal of the top skin layers, specifically the stratum corneum and stratum granulosum. This exposes the keratinocyte-basal cell layer, often achieved through tape-stripping techniques. Researchers use this model to study epidermis repair and regeneration, replicate inflammatory skin conditions like atopic dermatitis, and model superficial skin infections [20]. The full-thickness wound model is the most commonly used when studying acute wound healing in rats. It entails the removal of multiple layers: the epidermis, dermis, subcutaneous fat, and the panniculus carnosus, typically using a biopsy punch on the rat’s back [36].
A significant limitation of the full thickness wound models in rats is the healing through the contraction [20]. Wound contraction by inward dermal migration is the primary healing mechanism in rats, as opposed to re-epithelialization in humans [37,38]. To mitigate this limitation, some researchers suggest using square wounds to eliminate the contraction factor [16]. In square wounds, myofibroblasts disappear at the corners, causing fibrosis and earlier scarring, thereby leading to asymmetrical contraction, as opposed to symmetrical contraction in round wounds. However, Mawaki et al. showed that three days after wounding, the sides of square wounds stretch, and the corners become round. By day seven, the wound area had contracted by approximately 50 %, indicating that using a square wound shape does not significantly mitigate contraction effects [39].
Wound splinting in rat models has demonstrated effective results in slowing down contraction and in promoting wound healing primarily through granulation and re-epithelialization. This technique is applicable to wounds of any shape, whether they are square or circular [38,39]. Park et al. evaluated the effects of different adhesives (tissue adhesive vs. Krazy glue) for fixing splints on mouse skin and found that using Krazy glue significantly increased collagen deposition and re-epithelialization due to greater splint success [40]. Therefore, it is strongly recommended that the severity of elongation and fixation of wound corners be consistent in all groups to obtain homogenous and valid data. Splints are available in different materials, such as silicones (polysiloxane or polydimethylsiloxane) and metals. While silicone is affordable and can be easily tailored into desired sizes and shapes, it is susceptible to damage from self-grooming or collisions with cage walls during cutaneous implantation. Consequently, silicone splints are often implanted subcutaneously, a procedure that demands advanced surgical skills [38]. In contrast, metal splints are more challenging to cut and reshape but are resistant to physical damage [38,41,42].
4. Chronic wounds and comorbidities
A wound is categorized as chronic if it stalls at one of the intermediate healing stages, usually inflammation [43]. The healing progression of chronic wounds diverges from that of acute wounds in terms of duration and sequence of skin restoration. Fig. 1 illustrates a comparative overview of the wound healing phases for both acute and chronic wounds. Chronic wounds do not follow the typical timeline of cellular and molecular processes observed in the healing of a normal acute wound [44], as shown in Table 1. The failure to reduce inflammation in chronic wounds is often attributed to reduced blood flow caused by localized tissue ischemia [5,45]. In humans, the average healing duration for chronic wounds ranges from 12 to 13 months, and in some instances, it may span decades. Furthermore, the recurrence rate for chronic wounds is strikingly high, affecting approximately 60–70 % of patients [2].
Fig. 1.
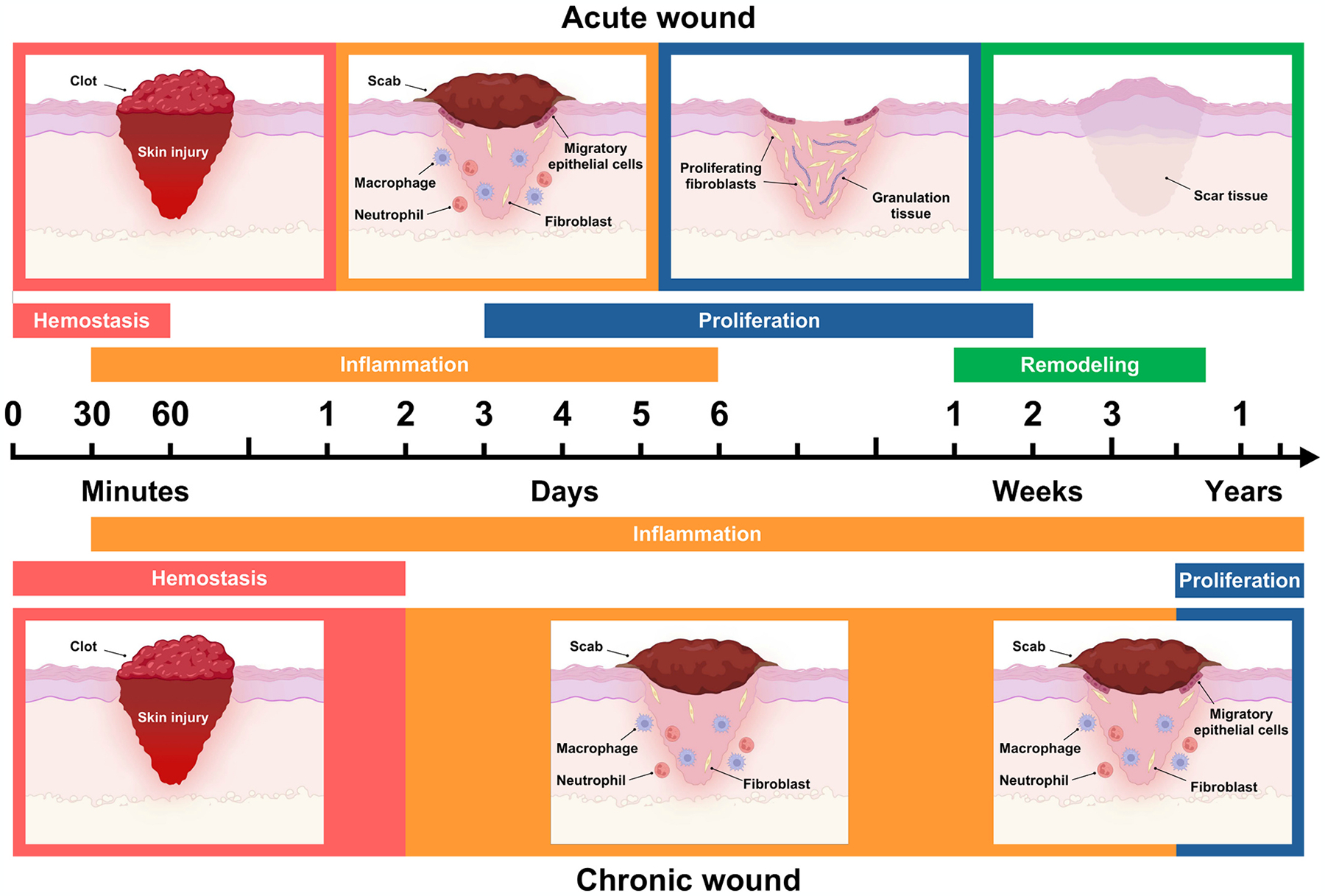
Phases of acute and chronic wound healing. Both types of wound healing encompass overlapping stages; however, the duration of each stage varies significantly due to distinct pathological characteristics. Additionally, the remodeling phase in chronic wounds may be prolonged for years or indefinitely stalled, reflecting the inherent resistance of chronic wounds to heal.
Table 1.
Molecular and cellular differences between acute and chronic wounds.
| Acute wounds | Chronic wounds | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Phases | Phases | References | |||||||
| Hemostasis | Inflammation | Proliferation | Remodeling | Hemostasis | Inflammation | Proliferation | Remodeling | ||
| Approximate phase time | A few minutes – 1 h | A few minutes – 3 weeks | 3 – 12 days | 3 days - 6 months | A few days | A few minutes - several years | A few months -several years | Several years | [49–53] |
| Dominant involved cells | Platelets | Mast Cells, dendritic cells, neutrophils, macrophages, lymphocytes, and keratinocytes | Fibroblasts, keratinocytes, M2 anti-inflammatory macrophages, Mast Cells, endothelial cells, Tregs pericytes | Fibroblasts, myofibroblasts, keratinocytes | Platelets | Increase in the ratio of neutrophils to lymphocytes (in DFUs), T helper 1, 17 and 22, M1 macrophages, mast cells, monocytes | Fibroblasts, myofibroblasts, keratinocytes | Deactivated fibroblasts (in DFUs) | [49–52,54] |
| Dominant involved molecules | PDGF, TGFβ, VEGF, cytokines, prostaglandins, leukotrienes, thromboxane | MCP, TGFβ, TNF-α, histamine, IL-6. IL-8, VEGF, EGF, leukotrienes, tryptase, cathepsin-G | MMP1, IL8, CXCR2, IL1, IL6, IL8, IL10, IL18, or IL20, MCP1, RANTES, IL8, IP10, MIG, VEGF, FGF, ANG 1, TSP | FGF-2, TGFβ, PDGF | PDGF, TGFβ, VEGF, cytokines, prostaglandins, leukotrienes, thromboxane | Impaired regulation of the TNF-α, IL-1β, IRAK1, and TRAF6 pathways. High expression of IL-18 and elevated levels of proteases. Decreased synthesis of healing-promoting agents, such as IL-10 and TGF-β. High expression of CXCR3 in T helper 1 cells. Upregulation of the NF-κB pathway. Overexpression of MMP-9 and overproduction of ROS. | Inhibition of FOXM1 and STAT3. Decrease of PDGF, FGF and EGF levels. | FGF-2, TGFβ, PDGF | [49,51,52,54] |
| Healing events | Release of Von Willebrand Factor from injured endothelial cells, causing platelet activation and coagulation. Vasoconstriction due to increased level of calcium ions at the SMCs. This ischemia causes an increase of vasoactive metabolites and vasodilation. | Release of histamine or serotonin via mast cells activation causing vasodilation, diapedesis of neutrophil, granulocytes and monocytes. Phagocytosis of pathogens and damaged cells. | Formation of granulation tissue. Keratinocyte migration, re-epithelialization, and vascularization. | Transformation of collagen type III to collagen type I to increase skin’s tensile strength. Wound contraction. Creation of skin structures such as sweat glands, sebaceous glands, and hair follicles. Increase of growth factor levels. | Hemostasis phase in chronic wounds is similar to that in acute wounds | Reduced migration of regulatory T cells and enhanced infiltration of T helper 17 cells. Excessive neutrophil infiltration with a distinct phenotype. In DFUs, monocytes predominantly polarize toward the M1 type. Fibroblasts exhibit signs of senescence, characterized by a reduced ability to migrate and a lack of responsiveness to growth factors. Unrestrained protease activity contributes to a proinflammatory cycle. Differences in MMP secretion prolong the inflammatory phase in chronic wounds compared to acute wounds. | Reduced angiogenesis and epithelialization. Excessive presence of ROS. Decrease of FOXM1 and STAT3 factors (in DFUs). | Decreased collagen deposition. Reduction of the activity of growth factor levels such as TGFβ pathway. | [44,49,51,54,55] |
Platelet-Derived Growth Factor (PDGF); Transforming growth factor-beta (TGFβ); Vascular Endothelial Growth Factor (VEGF); Smooth muscle cells (SMCs); Monocyte chemoattractant protein (MCP); Tumor necrosis factor-α;); Interleukin (IL); Epidermal Growth Factor (EGF); T regulatory cells (Tregs); Matrix metalloproteinases (MMPs); CXC chemokine receptors (CXCR); Regulated upon activation, normal T cell expressed and secreted (RANTES); Interferon-gamma-inducible protein (IP-10); Monokine-induced Gamma Interferon (MIG); Fibroblast growth factor (FGF); Angiopoietin (ANG) 1; Thrombospondin (TSP); Diabetic foot ulcers (DFUs); Interleukin-1 receptor activated kinases (IRAKs); Tumor necrosis factor receptor (TNFR)-associated factor 6 (TRAF6); Nuclear factor-κB (NF-κB); Reactive oxygen species (ROS); Forkhead box protein M1 (FOXM1); Signal transducer and activator of transcription 3 (STAT3).
Chronic wounds are manifestations of human-specific systemic disorders, and their development is influenced by the severity of the underlying comorbidities and by wound factors such as age, sex, and medicine used. Generally, wound comorbidities refer to underlying conditions that either directly lead to chronic wounds or transform acute wounds into chronic ones [46]. Wound factors, on the other hand, are secondary conditions that, while not causing chronic wounds directly, can influence their healing process [47].
Etiologically, chronic wounds have been classified into four main types by the underlying comorbidity: arterial, diabetic, venous, and pressure ulcers [43]. All these types of wounds can share common features such as susceptibility to infection, microcirculation disorders, inability to respond to reparative stimuli, and high levels of proinflammatory cytokines [43,48]. Additionally, wound infections play an ambiguous role, as they can lead to the chronic progression of an acute wound, thereby acting as comorbidities, or they can occur alongside primary comorbidities as an additional wound factor that exacerbates the severity of chronic wounds.
To date, rat models that precisely replicate the gradual development of chronic wounds caused by comorbidities remain yet to be developed. This challenge arises from the intricacies of wound formation processes and the physiological distinctions between rats and humans. Currently, inducing an underlying disease along with a full-thickness excisional wound is a prevalent approach to creating chronic wound models, distinguished from acute models due to the specific relevance to human chronic wound conditions like tissue ischemia. There have been models established for the primary types of comorbidities, which include models directly related to chronic wounds or those primarily focused on simulating the diseases themselves. In the next section, we will examine rat models for chronic wounds of various etiologies and assess models of corresponding comorbidities that could be effective in simulating these specific wound types. Table 2 provides concise summaries of these models, each associated with different comorbidities.
Table 2.
Chronic wound models associated with different comorbidities.
| Model | Methodology | Utility | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| 1. Peripheral artery disease (ischemic) ulcers | |||||
| 1a. Ischemic skin flap and graft models | |||||
| McFarlane flap | A 10 cm × 4 cm skin flap is formed on the rat’s dorsum. The flap is detached from the surrounding tissues but remains attached at its rear end. | The model is useful for investigating angiogenesis and ischemia-reperfusion. The entire flap can be used as a tissue sample, or it can be sectioned to study the effects of different oxygen levels and biochemical factors. |
|
|
[124] |
| Flap chamber | Improves the McFarlane flap by introducing a flap chamber that obstructs circulation from basal and lateral sides of the flap tissue. | The model has been employed for investigating angiogenesis and ischemia-reperfusion. The chamber isolates the dorsal skin flap from surrounding skin for more consistent and replicable results. |
|
|
[125] |
| H-shaped double flap | An 8-cm long H-shaped double flap is created on the back of each animal by making perpendicular cuts through the skin and panniculus carnosus. After elevating the flaps and cutting the central vein’s perforating branches, the flaps are sutured back into position. | The model is effective for examining tissue ischemia with progressively recovering blood flow. Initially, blood perfusion drops to 7 % of its normal rate on the first postoperative day, and then gradually returns to normal by day 16. |
|
|
[126] |
| Bipedicle flap | A long rectangular flap of skin is created by making incisions along the long sides, while the short sides were left intact, forming a bipedicle flap. The long sides of the raised flap are secured with surgical staples. The full-thickness excisional wounds are made using the biopsy punch close to the flap center. | The model is useful for studying delayed healing of full-thickness wounds, with wounds remaining open for 12 or more days after injury. |
|
|
[127,128] |
| Bipedicle flap with silicone sheeting | This bipedicle flap model incorporates the use of a silicone sheet placed beneath the flap to prevent reattachment and revascularization from the underlying tissue. Full-thickness wounds are induced at the center of the flap. | The model has been employed in investigating ischemic wound healing. The bipedicle flap remained viable throughout the entire ischemic wound healing period of about 28 days, in contrast to non-ischemic wounds which healed within approximately 10 days |
|
|
[129] |
| Skin graft | A square of skin, along with the panniculus carnosus, is excised from the back’s midline. The graft is then sutured back onto the underlying muscle fascia using nonabsorbable stitches. | Pharmacological studies, stem cells therapy, angiogenesis research |
|
|
[130] |
| 1b. Arterial insufficiency PAD models | |||||
| Sodium laurate-induced limb necrosis | A pharmacological model for PAD. Sodium laurate solution is injected into the right femoral arteries of rats to trigger ischemia. | This model is used to assess to evaluate the effects of long-term drug administration on hindlimb muscle circulation. |
|
|
[131] |
| Iliac and femoral artery ligation | A midline laparotomy is performed, ligating all branches from the left side of the aorta beyond the renal arteries and all branches originating from the left iliac artery using resorbable sutures. In a subsequent operation via the left inguinal incision, the femoral artery near the origin of the superficial epigastric artery is ligated. | This model is effective for studying limb ischemia over a prolonged duration. Reperfusion decreased from day 1 to week 4 compared to a control group. |
|
|
[69] |
| Ipsilateral femoral/iliac artery ligation | First, a right iliac artery ligation approximately 5 mm below its bifurcation from the aorta is performed using a 3-0 silk suture. After 3 weeks, the right femoral artery below the branching of the arteria profunda femoris is also ligated. | The model is designed to mimic the impairment of nutritive perfusion observed in patients with PAD. |
|
|
[65] |
| Ameroid constrictor | Utilizes a 0.75-mm internal diameter ameroid constrictor around the left common iliac artery and left femoral artery. | The model is aimed at simulating ischemic conditions in PAD by gradually narrowing vessel diameter. |
|
|
[70] |
| Apolipoprotein deficiency/ligations/ameroid constrictor | A modified, three-stage method for inducing chronic hindlimb ischemia and peripheral artery disease (PAD) in mice. Stage 1: Use of apolipoprotein E-deficient (apoE−/−) animals. Stage 2: Ameroid constrictor applied to the femoral artery. Stage 3: 14 days later, ligation of the femoral artery and its side branches near the inguinal ligament and popliteal bifurcation. |
The model is designed for studying severe, prolonged ischemia in older mice (20 months), providing insights into long-term ischemic effects without natural recovery. |
|
|
[71] |
| Lower limb artery removal | Multiple ligations are performed on the following arteries: common femoral artery, common external iliac, proximal common femoral, external iliac and saphenous arteries at knee level, common iliac and proximal common femoral arteries, common iliac and common femoral artery at knee level. Subsequent arterial resection involves complete excision from the proximal common femoral to the saphenous artery, external iliac to the saphenous artery, and common iliac to the saphenous artery. Bilaterally full thickness wounds (1.2 × 0.8 cm) were created on the dorsal of the feet of the rats. | The model is useful to assess the impact of ischemia on open wound healing. The foot dorsum was chosen for wounding due to its distal location relative to the arterial lesion, its fixed and flat surface, and its similarity to human condition. | In the ischemic limb, blood flow dramatically drops to 9.3 % of the control limb’s level immediately post-insult and stabilizes to about 85 % after 34 days.
|
|
[72] |
| 2. Diabetic wounds | |||||
| McFarlane flap model | STZ is injected intraperitoneally into rats. After several days, animals exhibiting elevated blood glucose levels are identified as diabetic. Subsequently, a McFarlane flap is surgically created on the backs of these diabetic rats. | This model is employed to assess the effectiveness of drugs in mitigating distal necrosis of flaps in diabetic rats. |
|
|
[87] |
| Skin graft | Diabetes is induced through an injection of STZ. A square skin section, including the panniculus carnosus, is surgically removed from the dorsal midline. The panniculus carnosus is then carefully excised to create a full-thickness skin graft, which is subsequently reattached to the underlying muscle fascia. | The model is designed for evaluating graft survival and preventing necrosis. |
|
|
[132] |
| Ischemic Foot Model | Hyperglycemia is induced in rats using STZ injection, followed by the resection of the external iliac, femoral, and saphenous arteries. Through a shaved longitudinal incision in the inguinal region, the external iliac and femoral arteries are dissected from the common iliac to the saphenous arteries. | The model is effective for researching critical limb ischemia. |
|
|
[61] |
| Excisional diabetic wound model | Diabetes was induced by intraperitoneal STZ injection. A full-thickness excision wound, spanning an area of 200 mm2 and 0.2 cm in depth, was created on the dorsum using a surgical blade and pointed scissors. | The model is used for assessing the efficacy of a topical therapy on diabetic wounds. |
|
|
[133] |
| 3. Venous stasis ulcers | |||||
| Vein obstruction | The model includes two phases:
|
The model is effective to study an intercellular adhesion molecule in venous disease. |
|
|
[90] |
| Arteriovenous fistula | An incision is made on the femoral artery (0.5 mm) to induce an arteriovenous fistula, followed by the creation of a femoral vein ligation above the fistula. | The model is useful to examine the inflammatory process in valve remodeling associated with acute and chronic venous hypertension. |
|
|
[134] |
| Vertical position/gravity model | Rats are placed in head-up and head-down positions using tube-like plastic cages that support the rat’s body in a tilted position. | The model aims to investigate physiological responses to gravitational loading or unloading of extremity veins. |
|
|
[93,135] |
| Occlusion and gravity | This model combines gravity and the occlusion of the saphenous vein. A plastic clip was applied to partially occlude the saphenous vein just below its confluence with the deep femoral vein. For the final four weeks prior to sacrifice, rats were housed in tube-like cages inclined at a 45-degree angle in a head-up position. | The model aims to investigate physiological responses to gravitational loading or unloading of extremity veins. |
|
|
[94] |
| Skin flap and venous congestion model | An abdominal skin flap measuring 3 × 6 cm, supplied by the epigastric artery and vein, is elevated and subjected to a 2-h occlusion of both the epigastric artery and vein. Following a period of 24 h, a secondary, 5-h occlusion is specifically applied to the vein. During the secondary ischemia phase, the flaps were resutured. After the 5-h period of secondary ischemia, the venous clamp was removed. | The model aims to investigate techniques for relieving venous congestion through targeted fluid transport interventions. |
|
|
[95] |
| 4. Pressure ulcers (PU) | |||||
| Weight-induced pressure | A steel plate is inserted into the rat’s peritoneal cavity, and constant pressure is applied to the abdominal wall using metal ingots for a duration of 2–4 h before being removed. | This model is employed to investigate deep tissue injury markers by analyzing exudate. |
|
|
[101,136] |
| Piston-induced external pressure | A flat plastic piston with an elastic spring is used to deliver constant, quasi-static pressure to the gracilis muscle in proximal limbs. Two different experimental approaches are used. In long-term experiments (lasting 120, 240, or 360 min), pressure is exerted externally on the skin to reduce the risk of cell death from infection. For shorter durations (up to 80 min), the pressure is directly applied to the gracilis muscle following skin retraction. | Study of muscle tissue damage in rats subjected to different pressure magnitudes and durations. |
|
|
[25] |
| Magnet-induced pressure | The model involves implanting a neodymium magnet in the peritoneal cavity after a transverse lower abdominal incision. A stronger neodymium magnet is then placed on the skin. The pressure was applied for 2–4 h and then removed, followed by monitoring the rats for up to one week. | This model is designed to study the pathogenesis of pressure ulcers. |
|
|
[101] |
| Cyclic magnet compression | A sterile steel disk (15 mm diameter, 0.3 mm thickness) is placed under the muscle through a 2 cm incision, which was then sutured. On post-operative day 3, a magnetic disk (15 mm diameter, 1500 G) is placed externally on the skin. Two hours after the initial pressurization, the magnets were removed for 30 min to allow for ischemia reperfusion. This pressurization cycle was repeated 5 times a day. | The model is utilized to simulate the development of stage II pressure ulcers. |
|
|
[103] |
| Prolonged magnet compression | This model is an adaptation of the previous one, extending the duration to up to 6 days. A steel plate is inserted into the rat’s back muscle. A magnet was positioned externally on the skin for 2 h, repeated 5 times daily over 6 days, to simulate a deep tissue injury. | The model is designed to investigate the impact of secondary factors (in particular, smoking) on the wound healing process of stage 4 pressure ulcers. |
|
|
[108] |
| Deep tissue injury at bony prominences | A hemispherical implant is placed on and beneath the lateral surface of the tibia, affecting the tibialis anterior muscle. Four weeks post-implantation, pressure is applied to the tissue above the implant for 24 h. | These models simulate the development of pressure ulcers when constant pressure applied on bony prominences. |
|
|
[105] |
| Spinal cord injury | In the SCI model, a complete transverse spinal cord cut at T10, along with a laminectomy at T9, is performed to induce SCI. Six weeks post SCI induction, pressure is applied to the skin surface over the midpoint of the tibialis anterior muscle belly using a custom-made device for 24 h. | These models simulate the development of pressure ulcers under chronic SCI condition. |
|
|
[105] |
| 5. In vivo biofilm models | |||||
| Infected full thickness wound model | A 6 mm diameter full-thickness wound is created on rats using a biopsy punch and inoculated with Pseudomonas aeruginosa strain PAO1. Wounds are harvested at 8 h, and 1-, 3-, and 7-days post-wounding. | The model tests the contribution of biofilm in delaying wound healing. |
|
|
[121] |
| Infected full thickness wound model | Round 2-cm diameter full-thickness wounds are created on rat flanks with sterile scissors. Two bacterial concentrations are used for colonization and infection groups, respectively. Wounds are harvested on day 6 post-wounding. |
|
|
|
[123] |
| Modified Walker-Mason model for full-thickness scald burns | A full-thickness burn is inflicted on male rats using an insulated mold with an opening, which exposes approximately 10 % of the total body surface area, to 99 °C water for 6 s.” Wounds are inoculated with P. aeruginosa, applying two distinct concentrations for different animal groups. Tissues are harvested on days 1, 3, 7, and 11 post-wounding. | The model is designed to assess the healing process of contaminated full-thickness scald burns. |
|
|
[137] |
| Modified Walker-Mason model for partial-thickness scald burns | Partial-thickness burn covering 10 % of total body surface area is induced by immersing in 99 °C water for 3 and 6 s, respectively. Three bacterial inoculum sizes are applied for different animal groups. | The model is designed to assess the healing process of contaminated partial-thickness scald burns. |
|
|
[138,139] |
| Infected pressure wound | To induce pressure ulcers in rats, incisions is made on the lateroabdominal region, and a metal plate is inserted subperitoneally. Ischemic wounds are produced by applying an indenter for 3 h. P. aeruginosa strains PAO-1, PAO-MW1, and PAO1- MP3 are used for inoculation. Skin samples were harvested on day 3. | The model is developed to study the infected pressure ulcers. |
|
|
[140] |
| Infected diabetic wound | Diabetes was induced by intraperitoneal STZ injection. A punch biopsy instrument is used to create a full-thickness, round wound through the panniculus carnosus in the interscapular region on the upper back of each rat. The skin flap is then excised using iris scissors. An oval-shaped silicone splint is affixed to the skin using immediate-bonding cyanoacrylate and reinforced with interrupted nylon sutures to secure its position. These wounds are subsequently inoculated with bacterial suspensions of either Staphylococcus aureus or Pseudomonas aeruginosa.The animals are sacrificed on day 11. | The model is used for evaluation of topical antimicrobial therapy in diabetic wound infections. |
|
|
[141] |
4.1. Peripheral arterial disease (ischemic) wounds
Peripheral arterial disease (PAD) is characterized by poor arterial perfusion in the lower extremities, typically due to atherosclerotic plaques narrowing the arteries’ lumen. Major risk factors, such as diabetes, smoking, high cholesterol, obesity, and a family history of PAD, can escalate the prevalence of this disorder. PAD ranks as the third leading cause of death among cardiovascular patients, following stroke and coronary heart disease [56]. Over 200 million adults globally suffer from PAD, with a prevalence of 20 % among individuals over 70 years of age [57]. Patients with mild PAD usually have adequate resting blood flow, but symptoms worsen during activities such as walking. In severe cases, inadequate blood flow can result in non-healing ischemic ulcers or gangrene, leading to amputation [57].
When researching chronic wounds of any origin, small animal models like rats provide significant benefits, including easy handling, quick reproduction cycles, and cost-effective care and maintenance. However, wild-type rats cannot successfully model PAD pharmacologically due to their natural resistance to atherogenesis and atherosclerosis [23,24]. High-fat diets do not induce atherosclerosis or hypercholesterolemia, and even a choline-deficient diet only provokes early-stage atherosclerosis formation, which is ineffective for inducing ischemic arterial perfusion. Transgenic rats may develop obesity, hyperlipidemia, hyperinsulinemia, and cardiac dysfunction, but myocardial dysfunction is associated more with microvascular disorders than atherosclerosis [23]. Researchers, due to the lack of suitable genetic and pharmacological rat models for PAD, rely heavily on surgical methods to induce limb ischemia [58].
4.1.1. Skin flap ischemic wound models
The ischemia and reperfusion of the skin have been mostly evaluated by the skin flap model [59]. Flaps are regions of the skin that partially detach from their origin and form a partial hypoxic zone due to declined blood flow. A full-thickness wound is usually induced on the flapped skin to emulate an ischemic wound. The healing timeframe of this type of wound is about 14–21 days vs. 10–12 in control groups [30]. To prevent reperfusion of this ischemic site, revascularization can be inhibited by putting a barrier like a silicone sheet between the flapped skin and the body. Since their invention in the 1960s, various modifications of skin flaps have been introduced.
While ischemic wound models using skin flaps and grafts mimic the limited blood supply to the wounded skin area, they fail to correctly depict the blockage in arteries leading to insufficient blood circulation in PAD. Additionally, small animals like rats display variances in granular tissue formation from head to tail, complicating the comparison of the results. Another downside of skin flap and graft models is the short ischemic duration in flap tissue, with blood flow normalizing within 14–16 days after inducing the ischemia. The use of silicone sheeting in these models poses issues of immune reactions, infection, and variability in outcomes, making the flap skin model hard to standardize due to reproducibility challenges. Finally, the typical positioning of the flaps on large regions of the animal’s body, like the head, back, and abdomen, does not correspond with the ulcer localization caused by limb ischemia in PAD. Thus, models simulating limb ischemia due to arterial insufficiency may be more representative of actual PAD conditions in humans.
4.1.2. Arterial insufficiency models
The iliac, femoral, and popliteal arteries are the most affected blood vessels by PAD in humans [57]. Typically, models of arterial insufficiency in rats involve inducing ischemia through incisions and/or ligations in the hind leg arteries, such as the common iliac, femoral, popliteal, and saphenous arteries (Fig. 2) [60–62]. Different ligation or incision locations on the arteries can yield varied outcomes [58,63]. Full restoration of blood perfusion only occurs seven days post-single coagulation in either the iliac or femoral artery, while double coagulation in both arteries delays recovery significantly, with just 54 % perfusion achieved after 28 days [64]. Simultaneous ligation of the femoral (from proximal to distal), popliteal, and a third of the saphenous arteries (proximal side) using titanium “S” shape clips in male Wistar rats showed persistently low tissue perfusion from day 0 (ischemic induction day) until day 30 [62]. Arterial vasodilator capacity and functions related to shear stress in the microcirculatory endothelium are impaired in double ligations of femoral and iliac arteries compared to a single ligation (iliac artery) [65].
Fig. 2.
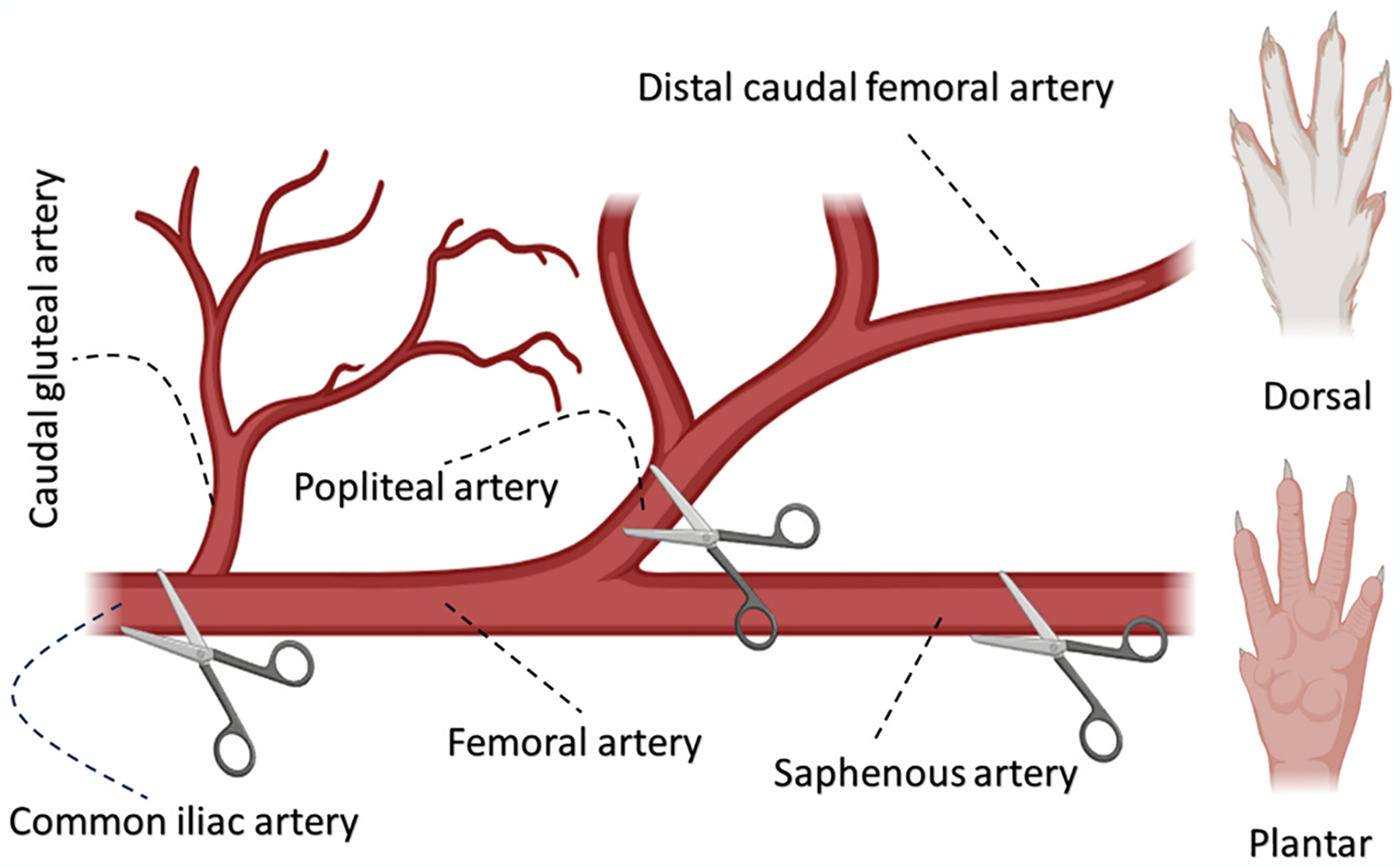
Schematic representation of an arterial incision on a rat’s hind leg as a model for limb ischemia. The common method for reducing blood perfusion and creating ischemia involves incisions or ligations in different arteries and their branches near the iliac artery, in the proximal part of the femoral artery, extending to the saphenous and/or popliteal arteries of the lower extremities.
It should be noted that the formation of PAD wounds is only one of the side effects of limb ischemia in PAD. Developing animal models of arterial insufficiency resulting in wound formation presents a significant challenge. Most arterial insufficiency models primarily target the investigation of tissue ischemia conditions rather than specifically studying and analyzing ischemic ulcers.
Although many current studies frequently utilize PAD surgical models [66], most of these models do not adequately replicate the chronic and gradual ischemia observed in humans, instead only emulating acute ischemia that recovers via compensatory arteriogenesis and angiogenesis phenomena [58]. Arteriogenesis is the transformation of existing arterio-arteriolar connections into fully developed and functional arteries through remodeling, while angiogenesis refers to the emergence of new capillaries from existing vessels, leading to the formation of novel capillary networks [67]. In PAD patients, the development of atherosclerotic vasoconstriction is a gradual process occurring over an extended period. In contrast, acute arterial occlusion by cutting or ligation in surgical models can increase blood flow and wall shear stress in collateral arteries, thereby accelerating arteriogenesis through cytokine stimulation [68].
Several techniques have been developed to refine surgical models of PAD by delaying and reducing arteriogenesis and angiogenesis, leading to progressive ischemia that mirrors the pathological conditions of PAD in humans. Lundberg et al. reported the emergence of collateral arteries after two weeks following double ligation (iliac and femoral arteries) [69]. Brown et al. performed an additional ligation of the ipsilateral femoral artery three weeks after a single iliac artery ligation [65]. This method resulted in a more sustained disruption of arterial perfusion than a single iliac artery ligation, which typically restores blood flow within 2–5 weeks.
In another approach, Tang et al. used an ameroid constrictor with an internal diameter of 0.75 mm to gradually narrow the diameter of the vessels, thereby creating chronic ischemia [70]. They observed a significant decrease in cutaneous blood flow in the group that applied the ameroid constrictor (chronic ischemia) compared to the group that underwent ligation in the iliac and left femoral arteries and all their branches (acute ischemia) 40 days post-operation. The angiogenesis score and vessel diameters were lower in rats with chronic ischemia compared to those with acute ischemia.
Krishna et al. modified this method for use in mice and included two preliminary stages [71]. Initially, the study employed apolipoprotein E-deficient (apoE−/−) mice with elevated plasma cholesterol, known to inhibit arteriogenesis more effectively than hyperinsulinemia or hyperglycemia [63]. This was followed by inducing ligations in the femoral artery and its side branches. Finally, 14 days after this second stage, an ameroid constrictor was applied to the femoral artery to gradually induce ischemia [71]. The severity and duration of hindlimb ischemia were higher in the group that followed all three stages (apolipoprotein E deficiency, femoral ligation, and gradual ischemia by ameroid constrictor) compared to the group that only completed two stages (Apolipoprotein E deficiency, femoral ligation). The expression of angiogenesis and shear stress markers also decreased in the three-stage group, indicating a reduction in arteriogenesis and angiogenesis.
Alizadeh et al. introduced a model involving multiple ligations and resection of peripheral arteries to induce limb ischemia, which included the induction of open wounds on the back of a rat’s paw [72]. Their research revealed that ischemia resulted in reduced wound contraction, a significant factor contributing to delayed ischemic wound healing.
4.2. Diabetic wounds
As of 2021, approximately 537 million adults, or 7 % of the global population, were living with diabetes, and projections suggest that this number will rise to 643 million by 2030 and 783 million by 2045 [73]. In the US, diabetic wounds account for the majority of non-traumatic lower extremity amputations, affecting about 25 % of patients with diabetes mellitus [74,75]. Key risk factors for developing diabetic foot ulcers include peripheral neuropathy and peripheral artery disease [76–78]. These factors can generally be reproduced in rats, potentially allowing for the creation of models for diabetic wounds [79,80]. Nevertheless, the development of an ideal animal model that accurately simulates diabetes and resulting diabetic wounds remains a challenge [81].
A recent study by Southam et al. in 2022 introduced a new model of type 2 diabetes in rats [79]. They combined a high-fat diet (HFD) with a streptozotocin (STZ) infusion administered over 14 days via a subcutaneously implanted osmotic mini-pump. Their findings suggested that this combination of a high-fat diet and a low streptozotocin dose induces an early-stage type 2 diabetes model, characterized by obesity, moderate hyperinsulinemia, and hyperglycemia with impaired glucose tolerance. On the other hand, using a high STZ dose led to late-stage type 2 diabetes, marked by pronounced hyperglycemia.
As a positive correlation exists between hyperglycemia intensity and diabetic wound severity, researchers need to carefully consider the STZ dose they employ and the resulting hyperglycemia severity [61]. Hyperglycemia has been noted to lead to sorbitol accumulation and protein glycation, which may trigger lower limb ulceration due to peripheral neuropathy [82]. In general, STZ doses ranging from 15 to 40 mg/kg have been employed alongside a high-fat diet containing 30–67 % of total kcal of fats to induce diabetes in rats [83]. One of the early adopters of the combined use of HFD and STZ was Reed et al., who demonstrated that administering 50 mg/kg streptozotocin intravenously alongside a 40 % high-fat diet could induce severe hyperglycemia in male Sprague-Dawley rats, with 41 % of the rats displaying serum glucose levels exceeding 450 mg/dL [84]. The diabetogenic impact of STZ varies with rat strains; Wistar-Kyoto rats show less sensitivity to the drug, while both Wistar and Sprague-Dawley rats display reliable sensitivity [83,85]. Also, intravenous streptozotocin administration results in more consistent hyperglycemia compared to intraperitoneal administration [83]. Thus, the severity of the induced diabetes can directly influence wound healing assessment results [86].
In addition to pharmacological models, some researchers also employ surgical methods to better simulate the pathological conditions of diabetic ulcers. For instance, skin flap models have been used to assess diabetic wounds with superficial ischemic arteries [87]. More precise and detailed models not only assist researchers in testing therapeutics but also enhance our understanding of chronic wound pathology. lévigne et al. highlighted the pivotal role of hyperglycemia in the development of diabetic wounds, rather than vascular ischemia, by inducing these parameters separately in a specific rat model. Their findings revealed that hyperglycemia increased susceptibility to necrosis, irrespective of ischemic lameness, compared to the group with a low glycemic index [61].
4.3. Venous stasis ulcers
Chronic Venous Insufficiency (CVI) often results from either valve insufficiency and reflux, or blockage of veins, leading to venous hypertension, edema, and skin ulcers in the lower extremities. The primary causes of this disorder include birth defects, biochemical changes in the venous wall, and inflammatory reactions due to venous damage [13,88]. Renowned risk factors contributing to CVI include old age, high body mass index, immobility, prolonged standing, loss of muscle mass, and ankle joint dysfunction [89].
In rat venous wound models, iliofemoral vein ligation and arteriovenous fistula (AVF) in the femoral vein are commonly employed to induce hypertension in the lower extremity veins, a characteristic clinical symptom of CVI. In a so-called vein obstructive model, the surgical procedure begins with a laparotomy, followed by ligation of the vena cava, then the common, external iliac, and femoral veins on both sides to eliminate collateral venous outflow (Fig. 3, right). Hahn et al. demonstrated that simple ligation with cotton threads can generate an acute hypertension model, whereas incorporating two-layer ligations with an absorbable suture can lead to a chronic CVI condition [90].
Fig. 3.
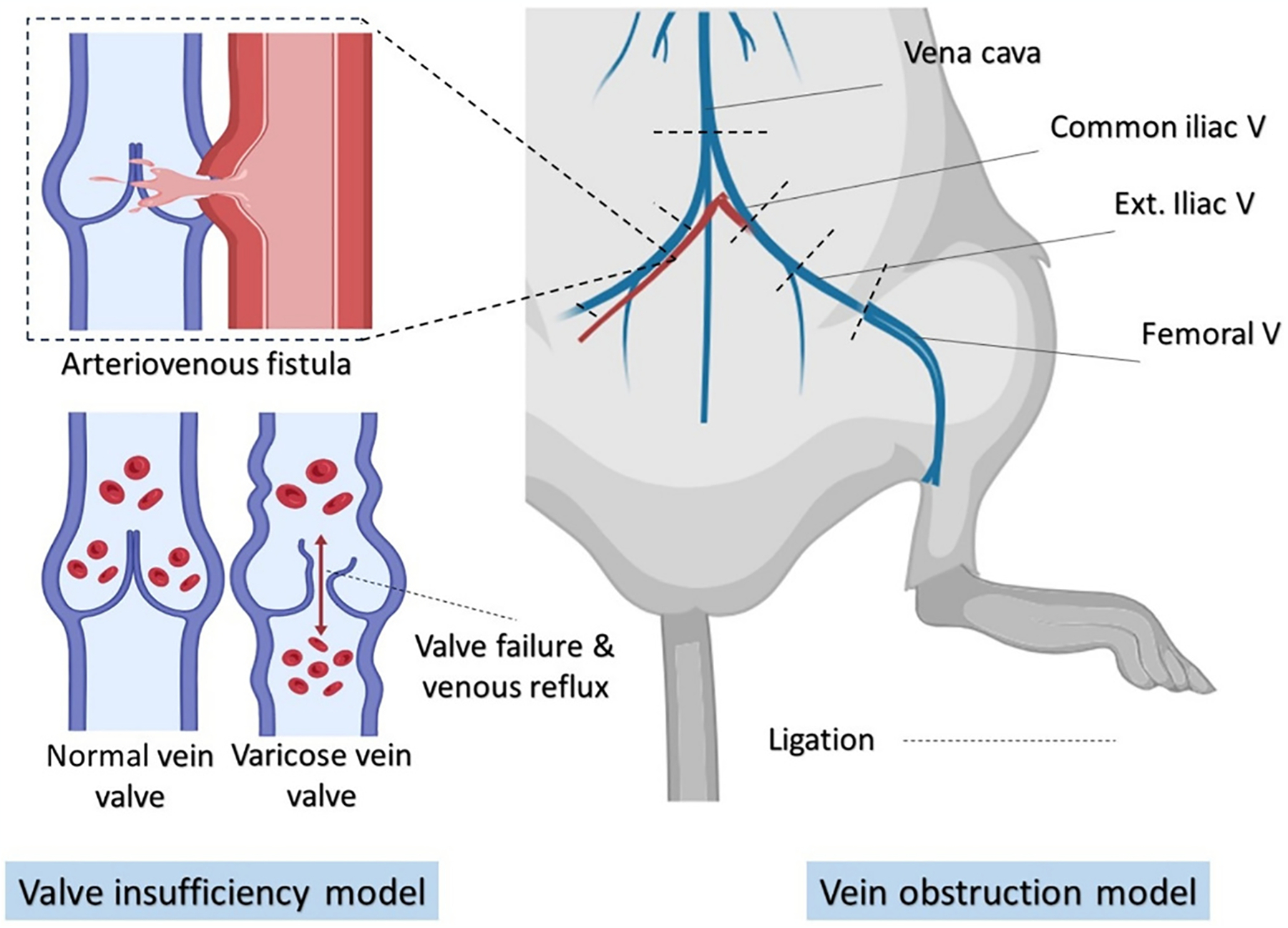
Models of valve insufficiency and vein obstruction for inducing chronic venous insufficiency in rats. In the obstructive model, ligation of the vena cava and bilateral ligation of the common, external iliac, and femoral veins are performed. In the valve insufficiency model, an arteriovenous anastomosis (fistula) is created between the femoral artery and vein to increase intravenous pressure, causing damage to the valves and the vein wall.
Another approach to inducing CVI involves creating a femoral arteriovenous fistula to elevate blood pressure and venous insufficiency (Fig. 3, left). In brief, rats are administered heparin (~1000 units/kg) to prevent coagulation. An arteriotomy of approximately 0.5 mm in length is performed, followed by the creation of an anastomosis between the vein and the artery using a 10-0 suture. A double ligation of the femoral vein proximal to the AVF and the superficial epigastric vein leads to hypertension in both the deep and superficial venous system [91].
Although venous pressure does rise after AVF creation, and initial venous valve damage is observed, the resultant blood pressure is excessively high and uncontrolled in intensity. Hence, this model does not adequately simulate the clinical condition of CVI in humans. In humans, the body’s vertical position leads to orthostatic complications, including large blood vessels, particularly in the veins of the lower extremities, as well as additional vascular wall stresses [92]. Positioning rats in this manner for four weeks disrupts blood flow and significantly increases leg venous system pressure, as well as pathologically alters the subcutaneous vein. Therefore, this rat model can reveal even the mechanisms of early and subsequent phases of CVI progression [93]. Dörnyei et al. merged the venous occlusion approach with the gravity model and discovered that this combination prompts adaptation in the saphenous vein network. They observed that the walls of veins undergoing flow-induced remodeling become fragile and more sensitive to gravitational stress. Furthermore, their findings suggest that exceeding inherent adaptation limits plays a critical role in the onset of lower extremity varicosity disease [94].
Similar to the PAD ulcer model, the animal model for assessing the wounds resulting from CVI has been mostly evaluated by the skin flap model [95]. Therefore, all the deficiencies can be generalized to the assessment of CVI ulcers as well.
4.4. Pressure ulcers
Pressure ulcers (PUs), also known as bedsores, are localized ischemic lesions of the skin and underlying tissues due to prolonged pressure on certain body areas resulting in blood flow obstruction. These ulcers mainly affect patients with impaired mobility and/or sensation who are bedridden or wheelchair-bound [96]. The incidence of pressure ulcers in emergency care is estimated at 10–18 % and in long-term treatment at 2.3-28 % according to existing medical literature [97]. Significant risk factors for pressure ulcers include impaired mobility, diabetes, malnutrition, emaciation, older age, a Braden score of less than 16, or a Waterlow score higher than 10. Pressure ulcers are typically classified into four stages [98]. The first stage involves skin erythema, which may be tender, soft, hard, warmer, or cooler. The second stage entails the loss of the entire epidermis and part of the superficial dermis. The third stage is a full-thickness lesion that may involve subcutaneous fat. The fourth stage involves muscle or bone along with a full-thickness ulcer. Depending on the anatomical location, the depth of pressure ulcers may vary. For instance, areas like the ear auricle, bridge of the nose, malleolus, and occiput, which lack subcutaneous adipose/fat tissue, usually have shallow ulcers and stage III pressure ulcers typically do not occur [99]. However, in areas with substantial fat content, pressure ulcers can be extremely deep at stage III [100].
Pressure ulcer research remains underdeveloped due to the disorder’s complex pathophysiology, which is associated with both intrinsic (e.g., fever, malnutrition, anemia, infection, hypoxemia, etc.) and extrinsic factors (e.g., duration of pressure, friction, immobility, etc.). However, animal models may offer a valuable tool for obtaining comparable and reliable data on etiological variables, histopathology, and the healing process in pressure ulcers [96].
Varying stages of Pressure Ulcers (PUs) in animal models can be induced by applying external pressure of different durations and magnitudes to the skin and muscles [25]. The capillary occlusion threshold in rats is estimated at 35 mmHg (4.6 kPa) when applied to an area. Cutaneous blood flow decreases to about 20 % of control at 50 mmHg (6.7 kPa), and prolonged, continuous application of this pressure induces skin necrosis [101]. Linder-Ganz et al. reported a sigmoid pressure versus time relationship for cell death in rats [25]. Specifically, the pressure magnitude is the dominant factor in tissue damage during a short initial period (less than 1 h). However, only partial cell death occurs even at high pressures (more than 32 kPa). With intermediate exposure time (1–2h), the level of cell damage depends significantly on time, and the required pressure decreases from 32 to 9 kPa. For periods exceeding 2 h, the pressure magnitude becomes the main factor in cell damage again. Blood reperfusion also contributes to cell damage in ischemic tissues with reduced metabolism, as it dramatically increases the level of free radicals, exceeding the normal free radical scavenging capacity. The cytotoxic activity of these free radicals leads to inflammation and impaired cell proliferation [96].
Hashimoto et al. conducted a comparative study between weight and magnet compression models in 8–9-week-old male Wistar rats to find a suitable model for PUs. They reported that tissue damage with weight compression was milder than with magnetic compression. Since the pressure magnitude and duration were the same in both methods, they hypothesized that sodium pentobarbital administered to rats in the weight model alleviated the compression damage compared to alert animals. Therefore, they suggested that the magnet model is a more reliable model for PUs than the weight model [101].
While ischemia has traditionally been considered a primary factor affecting PUs formation, recent studies have highlighted ischemia-reperfusion (I/R) as the foremost contributing factor. I/R triggers pathways such as leukocyte activation and oxidative stress, causing extensive cellular damage [102]. Qin et al. induced PUs by applying a magnet of 1500 gauss for 2-h intervals, followed by removing the magnet for 0.5 h to create reperfusion; this cycle was repeated five times per day, leading to stage II PUs after 2–3 days [103].
Deep tissue injury (DTI) is a type of pressure injury that occurs in the muscle adjacent to the bone and significantly impacts PU formation [104,105]. However, researchers have been unable to create a suitable experimental model of DTI, seemingly due to insufficient pressure exposure duration, which usually lasts from several hours to 4 days [106,107]. Song et al. increased the pressure application time (2 h each time, five times per day for six days) and successfully created stage III PUs in rats [108]. To develop a more clinically relevant model of PUs in rats, Lin et al. induced spinal cord injury (SCI) motivated by the 33 % prevalence of pressure ulcers in the SCI population and the differing responses to pressure injuries in SCI versus intact animals. They also introduced a model with an implant mimicking a bony protrusion, effectively inducing deep muscle tissue injuries, and offering insights into injury mechanisms in the SCI. [105].
4.5. Infected wounds
Infection is a major factor contributing to the chronic nature of wounds, impeding tissue repair, and altering inflammatory responses [109]. Several factors influence the risk of wound infection, including host characteristics such as age, immune status, malnutrition, and diabetes; the type, number, and synergistic interactions of microorganisms, including bacteria and fungi; elements of the wound environment like presence of necrotic tissue; and non-physical factors like practitioner skills [110].
Bacterial biofilms, composed of bacterial communities encased in self-produced exopolysaccharide matrices [111], shield the bacteria from physical and chemical treatments, as well as the host’s immune response, fostering antibiotic resistance [110]. This biofilm phenotype is distinct from planktonic bacteria, which are free-floating bacteria traditionally studied in microbiology. Historically, chronic wound infections were attributed to planktonic bacteria, but recent findings suggest that chronic wound infections are primarily associated with biofilms [112]. In acute and chronic wounds, the incidence of biofilm formation is approximately 10 % and 60 %, respectively [113].
Bacteria within biofilms employ multiple strategies to withstand the host immune system. They may adapt to low oxygen and nutrient conditions by slowing their metabolism, altering gene expression and protein synthesis, and reducing cell division [112]. Biofilm bacteria can also release toxins, causing tissue damage [114]. Biofilms compromise the effectiveness of immune cells, leading to delayed inflammatory responses, which in turn can inhibit tissue repair and remodeling. Furthermore, biofilms exhibit a remarkable resistance to antibiotics – up to 1000 times more than planktonic bacteria – enabling them to survive for extended periods and potentially initiate new infections [115]. Biofilms can also impede angiogenesis and collagen deposition, further delaying the wound healing process [116].
The formation of biofilms is influenced by various factors, including the type of microorganisms present, the availability of nutrients, and physicochemical conditions. Ischemia, commonly associated with most chronic wounds, can impede efficient microbial elimination due to limited blood flow, thus prolonging inflammation and fostering biofilm development [117]. Although numerous studies have established a connection between biofilm formation, resistance to inflammatory processes, and delayed wound healing, the exact mechanisms remain poorly understood. A significant challenge in biofilm research is selecting an appropriate model that can accurately reflect biofilm infection dynamics, requiring careful consideration. Evaluating the interactions between biofilms and host cells within in vitro and in vivo models is critical and should be tailored to the specific research questions and objectives [109].
4.5.1. In vitro biofilm models
Numerous in vitro biofilm models have been developed to facilitate the study of bacteria within a controlled setting, mirroring the complexities of clinical scenarios [109]. Variations among these models include the types of surfaces or matrices they employ, the nutrient compositions in their mediums, and the decision to use either mono- or multispecies bacterial culture [111]. One example is the Lubbock chronic wound biofilm model, developed at the Medical Biofilm Research Institute in Lubbock, Texas, USA, focuses on three types of bacteria typically found in chronic wounds: S. aureus, P. aeruginosa, and Enterococcus faecalis. These bacteria are allowed to grow for 24 h in a medium comprising 50 % Bolton broth with heparinized bovine plasma and 5 % freeze-thaw laked horse red blood cells [118]. This model is instrumental in studying anaerobes, which are commonly present in chronic wounds. It allows for testing the efficacy of antimicrobial agents on wounds but has limitations in evaluating dynamic biofilm-host interactions due to the use of polypropylene pipette tips as a surface for biofilm formation. Werthén et al. developed a different model that enables biofilm development without a solid surface, using a medium that contains 50 % fetal calf serum and 0.1 % peptone [119]. Cell culture-based models utilize biotic surfaces, such as human epithelia, to examine the interactions between cells and biofilms.
Although in vitro models have increased our understanding of intercellular communication involving the quorum-sensing methods, processes of antimicrobial tolerance, the efficacy of different therapeutic agents, and biofilm formation, the interaction with the host immune system is not addressed by these models, hence, they fail to provide a significant clinically relevant information. Therefore, it is essential to validate the findings from even a well-established in vitro model using a suitable in vivo model.
4.5.2. In vivo biofilm models
Short-term in vitro infection models do not adequately replicate the prolonged host-biofilm interactions, which play a significant role in the development of chronic wounds [119,120]. Thus, recent focus has shifted toward in vivo models for chronic wound-biofilm studies.
A range of in vivo biofilm models related to chronic wounds have been studied using animal models, aiding in the understanding of the iterative processes involved in the development of chronicity. Typically, these protocols include creating a full-thickness wound and then incubating it with either mono- or multispecies bacterial strains [121]. It is important to note, though, that most natural biofilms differ from current in vivo models, as they are formed by multiple bacterial species interacting, competing, cooperating, and communicating within the biofilm. Therefore, understanding the mechanisms of multi-species biofilm formation is key to advancing strategies for managing bacterial biofilms in clinical settings [122].
Asada et al. found that removing factors like foreign bodies or diabetes mellitus and focusing on variables like bacterial size, rat age, and wound location are crucial for consistently producing visible wound infections [123]. They also noted that the thorough removal of the subcutaneous fat layer is key for achieving consistent infection results. Moreover, conducting the bacterial inoculation at least a day after the wounding process is essential to lower the risk of mortality due to bacteremia in the bleeding phase.
Most rat studies on biofilms use burn models, which are more common than other acute wound models. In the context of chronic wounds, the majority of published biofilm models explored are linked with diabetic wounds. Although rat biofilm models are less common compared to murine models, they provide valuable insights into chronic wound infections (see Table 2).
5. Wound factors
Although the studies mentioned above are valuable in identifying clinically relevant models of chronic ulcers, the data derived from rodent models of healing can be difficult to translate to the clinic, in part because rodent subjects typically used in these experiments are young/juvenile without comorbid disease. In addition, the literature lacks a comprehensive consensus on the materials and methods used to assess chronic wound healing, leading to inconsistent results and making it challenging to compare clinical outcomes across different studies. When analyzing chronic wound studies, considering concomitant wound factors like age, sex, anesthetics and analgesics used, and animal-specific wound healing mechanisms is among the most challenging tasks. In addition, the addition of comorbid conditions to otherwise healthy subjects in wound healing studies may increase the clinical relevance of the derived data. Understanding the contributions of these factors will assist researchers in choosing superior strategies to propose the optimal animal model for a specific chronic wound.
5.1. Age
Changes in the physiological state with age include impaired and delayed skin healing [142] due to a decrease in immune responses, antioxidant enzyme activity [143], cell renewal, elastin gene expression, and DNA repair [144–146]. The severity of ischemia-reperfusion injury in soft tissues increases with patient age [147]. Poor blood supply to the skin significantly affects skin function by reducing the number and proliferation of fibroblasts [143]. A direct relationship exists between the prevalence of chronic wounds and age: venous stasis, diabetes, and pressure ulcers occur more frequently in the 70s [148], 60s [149,150], and 60s [151] years of life, respectively. However, most experimental studies assessing chronic wounds use young adult rats [152,153], for example, Pascarella et al. used a 2-month-old rat to induce venous ulcer [134]. Therefore, ignoring the age factor and age-related changes in the experimental design can lead to inaccurate results since the wound condition in animal models does not accurately mimic the human situation [153,154]. Table 3 provides a qualitative comparison of some wound healing markers and parameters in young and old rats. Not only is old age a risk factor for major diseases, but it also slows down the wound-healing process [155]. In addition, age can significantly affect the protocol of animal model induction, for instance, the mass of pancreatic β-cells decreases with age in both humans and rats, necessitating a higher dose of STZ in young rats compared to older ones [83]. Hence, it is strongly recommended that the age of the rats in the model corresponds to the age at which a typical chronic wound is more common in humans.
Table 3.
Comparison of wound healing-related factors in young and old rats.
| Healing factors | Young rats | Old rats | References |
|---|---|---|---|
| Tumor necrosis factor (TNF-α) | + | +++ | [156] |
| Catalase | + | ++ | [143] |
| Vascular endothelial growth factor (VEGF) | ++ | + | [144,156] |
| Re-epithelialization | ++ | + | [142,157] |
| Vascularization | +++ | + | [144,158] |
The plus sign (+) represents the amount and intensity of the wound healing factors.
The exact relationship between human and rat ages remains a matter of debate. The anatomical and physiological growth of rats does not correlate linearly with that of a human, complicating direct age comparisons. Estimates have shown that during the feeding period, every 42.4 days in rat’s life are comparable to 1 human year, while in pre-pubertal and adult stages, this value is 4.3 days and 11.8 days, respectively. Fig. 4 illustrates an age scale for partially comparing rat ages with human ages in adulthood [11]. Typically, 3-month-old and 20-month-old rats are considered young adults and old rats, respectively [159,160].
Fig. 4.
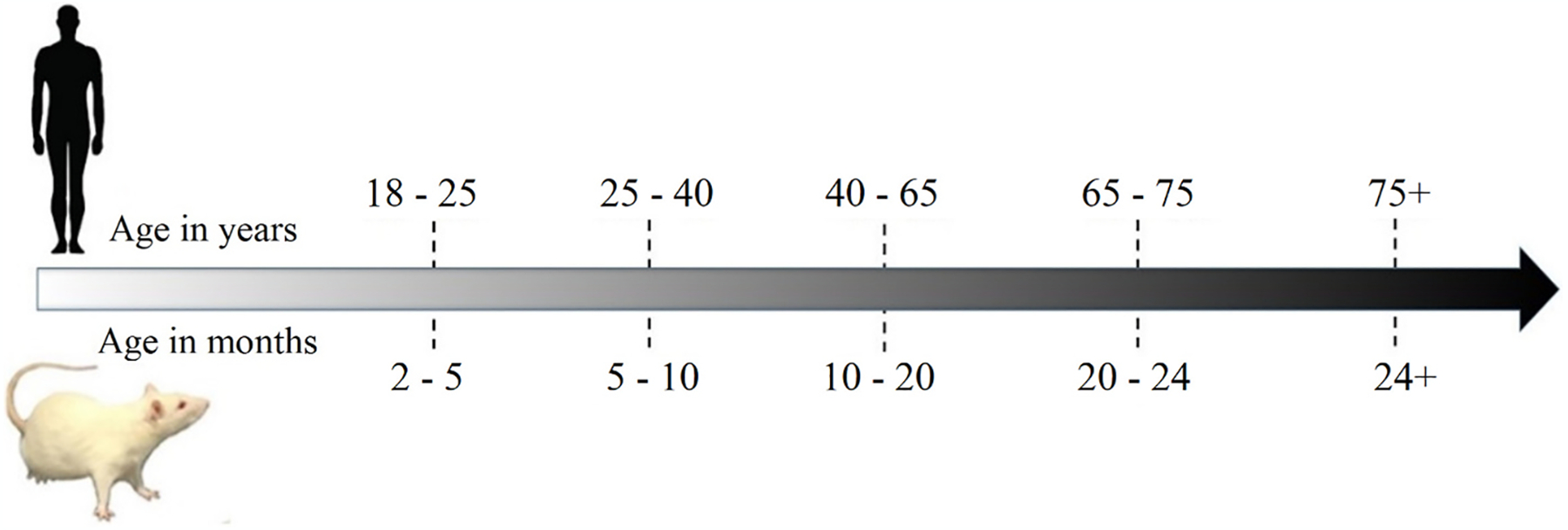
Timeline for comparing ages between humans and rats.
5.2. Sex
Given that the number of men and women in the human population is approximately equal, a better understanding of sex’s role in the pathology and treatment of chronic wounds is crucial. However, in animal models of chronic wounds, there is insufficient knowledge of sex differences between test subjects. The predominant use of male animals in preclinical studies results in a lack of data for females. This could potentially explain why women tend to be more vulnerable to drug side effects compared to men [161,162]. Furthermore, while women are epidemiologically more affected by ulcers (65–70 % of patients) [148,163] and other skin diseases than men, only a small percentage of experimental studies in this area focus on female animals. For example, in a cohort study that evaluated all original articles published in dermatological journals during 2012–2013, only 4.3 % of the studies were conducted on females compared to 32.1 % on males; in 60.4 % of the studies, the sex parameter was unstated, and 5.8 % of the experiments included both sexes [162]. Thus, an understanding of the biological basis of sex differences in the treatment of chronic wounds can be gained through experimental studies comparing both sexes simultaneously [162].
Wound healing exhibits sexual dimorphism associated with sex hormones; generally, while estrogens shorten skin wound healing time, androgens prolong this process [164]. Estrogens display anti-inflammatory activity and have positive effects on the proliferation of endothelial cells, migration, collagen production [165], and wound healing angiogenesis [166]. On the other hand, androgenic hormones promote inflammation and inhibit the healing process [167]. Therefore, it is strongly recommended to use both male and female rats in preclinical studies on wound healing. Many chronic wounds, like diabetic ulcers, predominantly affect the elderly [150], when the protective and restorative effect of female sex hormones is eliminated due to menopause, which occurs at an average age of 47 ± 4.2 years [168]. Post-menopausal women may experience reduced levels of hydroxyproline and superoxide dismutase, narrowing of papillary capillaries, degradation of skin vascularization, and skin thinning due to a decrease in estrogen levels [165]. Bilateral ovariectomy is a promising animal model for assessing chronic wound healing in the context of menopause [169,170]. Additionally, females have 6 % more β-cells compared to males; this results in the need for a higher dose of STZ in female rats than in males during diabetic model induction [171].
The variability of biological indicators in female rats compared to males is due to the different types of sex hormones and their level fluctuations throughout the estrous cycle [172,173]. The estrous cycle in rats, with a typical duration of four days, consists of a sequence of hormonal changes segmented into four stages: metestrus, diestrus, proestrus, and estrus [174], echoing the 28-day menstrual cycle in humans in its cyclical pattern. Some biological and histological skin parameters are significantly affected by the estrous cycle, such as epithelial thickness, sebaceous gland cells [80], and TNF-α [175], as shown in Fig. 5. Ideally, all female rats should be evaluated in the same phase of the estrous cycle to eliminate differences caused by the estrous cycle phases [176]. Estrous phase homogenization in female rats can be achieved using pharmacological methods, such as chorionic gonadotropin injection [177], or by selecting female rats in the same phase (typically the estrus phase). The latter method involves determining different phases of the estrous cycle in rats by staining vaginal cells in a vaginal smear [176,178].
Fig. 5.
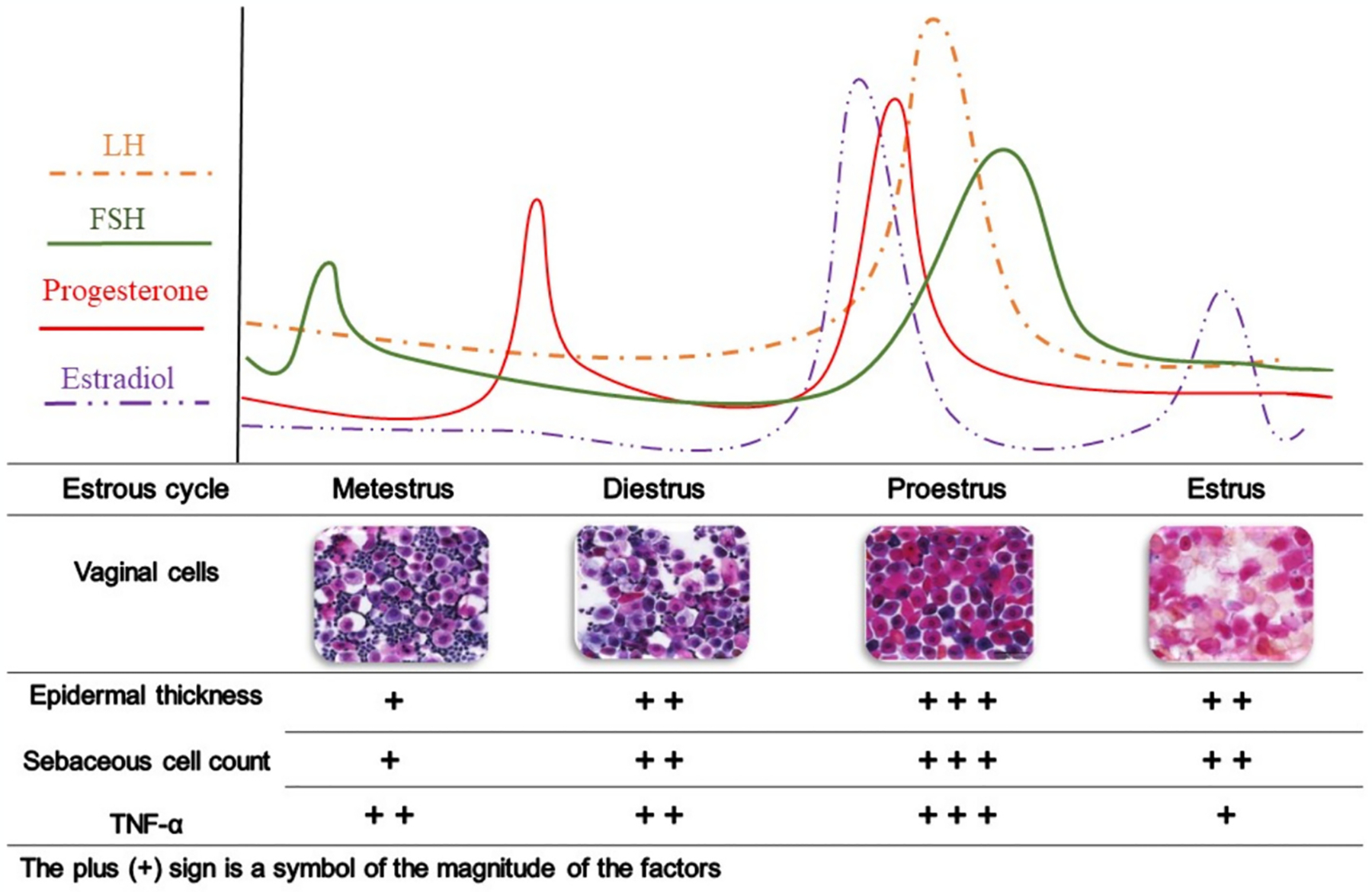
Changes in biological and histological characteristics of skin during the estrous cycle in rats. The images of the vaginal cells are reprinted from Hubscher et al. [180], with permission. Luteinizing hormone, LH; follicle-stimulating hormone, FSH; Tumor necrosis factor-alpha, TNF-α.
To address the longstanding oversight of female subjects in preclinical studies, the NIH mandates the consideration of sex as a biological variable in the design, analysis, and reporting phases of vertebrate animal and human studies it funds. For research proposals that focus exclusively on a single sex, the NIH demands comprehensive justification, which must be supported by scientific literature, preliminary data, or other relevant considerations [179].
5.3. Anesthetic and analgesic drugs
Before inflicting wounds on animals, anesthetic and analgesic drugs are administered to anesthetize and relieve pain. However, these drugs can have side effects that interfere with wound healing and affect the results. For instance, studies have demonstrated that the expression of many genes associated with wound healing is altered when rats are administered ketamine, xylazine, or thiopental, which are commonly used for anesthesia in skin wound examinations [181,182]. These affected genes include those linked to growth factors, extracellular matrix remodeling enzymes, inflammatory cytokines, and chemokines [183]. Huss et al. analyzed the literature to provide recommendations for using analgesic drugs with minimal side effects in rat wound healing models [184]. While opioid drugs such as morphine and fentanyl may affect wound healing, non-selective NSAIDs (e.g. diclofenac and flunixin meglumine), as well as topical drugs like lidocaine, did not appear to impact the healing process. Berg et al. evaluated the antibacterial effects of both EMLA and lidocaine and reported that EMLA has potent antibacterial properties that might lead to false negative results when evaluating bacterial cultures [185]. Additionally, the use of various anesthetics and analgesics, each with potential side effects on wound healing, introduces more variables, making it challenging to apply data from animal models to human clinical situations [16]. Therefore, researchers should choose the most appropriate drugs to minimize pain, unwanted effects, and variables in their wound healing models.
5.4. Smoking
Smoking is an extremely common risk factor for poor wound healing in human patients. In 2020, the global prevalence of smoking among adult men and women was 32.6 % and 6.5 %, respectively [186]. Generally, smoke is categorized into two types: mainstream smoke, also known as “first-hand” smoke or active smoking, which is inhaled by the smoker, and side-stream smoke, or passive smoking, a significant part of environmental tobacco smoke, emitted from the burning tip of a cigarette between puffs. Both smoke types comprise a complex mixture of unstable acids, gases, and particulate matter [187]. Cigarette smoke contains over 4000 distinct toxins, including carbon monoxide and nicotine. Nicotine, the primary vasoactive component in cigarettes, induces vasoconstriction by activating the sympathetic nervous system, thereby reducing tissue perfusion [188]. The detrimental effects of smoking on tissue oxygen levels can manifest after consuming just one cigarette, regardless of an individual’s smoking history [189]. Fibroblasts, crucial in the repair process, are exposed to smoke components circulating in the bloodstream. Loss of skin elasticity is associated with abnormal fibroblast function due to smoke exposure [187]. Moreover, nicotine adversely affects wound re-epithelialization by inhibiting keratinocyte migration [190]. Smoking is linked to increased complications in wound healing, such as prolonged healing times, wound reopening, tissue flap necrosis, anastomotic leakage, weakened wound strength, and a higher risk of infections. These negative effects on inflammatory cells, such as neutrophils and macrophages, and the reduced oxygen delivery to tissues are mechanisms by which smoking exacerbates the pathobiology of chronic wounds [189].
There is no standardized and universally accepted model for cigarette smoke exposure [191]. An ideal animal model for studying smoking would require accurate assessment of risks associated with tobacco exposure, based on precise human measurements. However, many smokers are reluctant to disclose their smoking habits and tobacco exposure levels. Consequently, biomarkers have become the primary and objective method for determining nicotine exposure. Among these, cotinine is the preferred biomarker [192]. Discrepancies in models can arise from inaccurate methods of measuring cigarette smoke concentration, variations in inhalation chamber types, differing exposure durations, and other factors [191]. Common models include: (a) passive smoking using inhalation chambers of various shapes and sizes, along with cigarettes and an air [191,193]; (b) inducing nicotine dependence through intermittent subcutaneous nicotine injections [194,195]; and (c) in vitro models, where cultured cells are exposed to cigarette smoke. For example, Carnevali et al. cultured fibroblasts in a three-dimensional matrix of natural type I collagen fibers, exposing them to the smoke of two filterless cigarettes for 30 min. They observed that fibroblast contraction of collagen gels was related to a decrease in fibronectin synthesis by the fibroblasts [196].
5.5. Stress
Numerous studies have highlighted the significant impact of psychological stress on the wound healing process. Stressors activate the hypothalamic-pituitary-adrenal (HPA) and sympathetic-adrenal medullary (SAM) axes, leading to the release of hormones like cortisol, epinephrine, and norepinephrine from the pituitary and adrenal glands [197]. Furthermore, research has established a direct connection between the immune system and the sympathetic nervous system in lymphoid organs. Immune cells, including lymphocytes, granulocytes, and monocytes, possess receptors for neuroendocrine hormones, which can influence aspects such as cytokine expression, adhesion molecule presence, and the movement, proliferation, and differentiation of immune cells [198].
Limited research into the impact of anxiety and depression on the healing of venous ulcers and diabetic foot ulcers suggests that symptoms of these conditions negatively affect chronic wound healing, although the hormonal or immune mechanisms involved were not explored in detail [198]. Diabetic foot ulcers, affecting 25 % of diabetic patients at some point in their lives, provide a critical model for studying the influence of emotional distress on chronic wound healing [199]. Recent research indicates that neuropathy, a significant risk factor for foot ulcers, is associated with increased overall distress and specific emotional responses, such as fear of the consequences of ulcers and anger toward healthcare providers [198,200].
Experimental animal models have utilized two distinct stressors, social disruption (SDR) and physical restraint (RST), to trigger neuroendocrine reactions and investigate their effects on wound healing [201]. While psychological stress is known to hinder wound healing, the specific type of stressor and the organism’s response to it are crucial factors. For instance, Sheridan et al. found that the SDR stressor did not significantly reduce the healing process compared to RST [201].
The relationship between stress and wound healing has been more extensively studied in acute wounds than in chronic ones. However, chronic wounds respond differently to stress compared to acute wounds, and the molecular mechanisms influencing acute wound healing under stress are not directly applicable to chronic wounds. For instance, acute wounds show a decrease in matrix metalloproteinase-9 (MMP-9) activity under stress, which aids in their healing process. In contrast, chronic wounds, such as pressure sores and venous ulcers, experience an increase in MMP-9 activity, leading to a shift from tissue regeneration to extracellular matrix degradation and, consequently, impairing the healing process [198]. These differences underscore the need for targeted research and the development of sophisticated animal models to explore the impact of stress on the healing of chronic wounds, taking their unique biological and molecular characteristics into account.
5.6. Obesity
Clinically, overweight and obesity are defined by a Body mass index (BMI) of 25 kg/m2 and 30 kg/m2, respectively [202]. Since 1999, the prevalence of obesity among adults in the United States has steadily increased. By 2015–2016, the rates of obesity and overweight reached 38.0 % and 74.7 % in men, respectively, while in women, the rates stood at 41.5 % and 68.9 % [203]. It is estimated that by 2030, nearly 50 % of American adults will be classified as overweight or obese. Traditionally, white adipose tissue was primarily viewed as a storage site for energy. However, recent insights have revealed that fat cells are metabolically active, producing adipokines such as adiponectin and leptin, which have varied metabolic effects. These hormones target immune cells and inflammatory systems, and there is a positive correlation between the increase in white adipose tissue and pro-inflammatory agents like TNF-α and various interleukins, including IL-1b, IL-6, and IL-8 [204,205].
The changes in adipose tissue due to obesity highlight the potential for obesity to trigger and maintain a chronic low-level inflammatory response, positioning obesity as a wound factor. Obesity directly impacts comorbidities; for instance, the link between obesity and venous insufficiency is well-established [206]. Although the precise mechanisms behind wound disruptions are just beginning to be understood, increased intra-abdominal pressure may lead to heightened reflux, expanded vein diameter, increased venous pressure, and consequently, impaired venous function [207]. Regarding diabetes, excessive body fat can lead to type 2 diabetes [208].
Understanding the intrinsic changes that occur in the body due to an increase in adipose tissue mass, especially those affecting the wound healing process, is essential for developing animal models for chronic wounds further complicated by obesity. There are two primary categories of animal models used in obesity research: monogenic models, which involve mutations linked to a single gene, and polygenic models that are typically used to study diet-induced obesity. Diet-induced models have shown that a high-fat diet, consisting of 30 % fat for rats over 15 weeks, causes a delay in wound healing of about 14–21 days [205]. In contrast, mice on a reduced-calorie diet exhibited faster wound healing compared to both ovariectomized and intact mice on a high-fat diet [209]. The presence of estrogen partially mitigated the delay in wound healing compared to the absence of estrogen observed in menopause or in ovariectomized mice, which led to increased weight gain. Monogenic models mostly target the leptin gene, which plays a significant role in counteracting inflammation. Genetically obese mice naturally exhibit leptin resistance and a disturbed wound-healing process, but administering leptin to these animals accelerates wound healing by reducing polymorphonuclear leukocytes [210].
5.7. Infection as a wound factor
In the previous section, we discussed the rat models of the infection acting as a primary comorbidity that turns acute wounds into chronic ones. However, the considerable impact of infection as a complicating factor in the healing of chronic wounds arising from other comorbidities necessitates the development of specific animal models.
Infection is a leading factor contributing to the delayed healing process of chronic open wounds [211]. A meta-analysis revealed that infections and biofilm formations are common in 78.2 % of chronic wounds in humans [212], highlighting infection’s pivotal role in the pathophysiology of chronic wounds [213]. The progression of infection from contamination through localized infection to potentially life-threatening conditions such as systemic infection, sepsis, and multiple organ dysfunction syndrome underscores its grave risk to health [211].
The standard approach for studying the infection factor in chronic wound healing employs a two-step process, starting with the creation of a model based on a specific wound comorbidity, followed by inoculation of the wound with pathogens [140,141]. Significant differences were demonstrated between infected and non-infected chronic wounds regarding wound size, healing time, and severity of inflammation, among other factors [140]. Although the approach is straightforward, introducing an additional factor can lead to potential pitfalls that must be carefully evaluated. Bacteria can release virulent factors and toxins that disrupt host defenses, such as autophagy [214]. Furthermore, the polymicrobial nature of chronic wounds is common, indicating that the collaboration between aerobic and anaerobic organisms in causing chronic wound infections should be considered more clinically significant than infections caused by a single potential pathogen [215].
In contemporary medical practices, maintaining wound sterility and applying antiseptic measures are fundamental. Nonetheless, under certain conditions, an infection could potentially aid the healing process of wounds. Historically, before the advent of antibiotics, the presence of infection was often seen as beneficial in clinical medicine. The phenomenon of ‘laudable pus,’ a dense, white, odorless discharge indicative of a healing wound, was associated with infections by bacteria considered to be of comparatively low harm, such as Staphylococcus aureus [216]. Studies have demonstrated that infections by S. aureus can lead to the development of skin abscesses, marked by an influx of neutrophils. These immune cells are known for producing a strong superoxide reaction and for their role in carrying antimicrobial peptides, both of which are key in promoting the healing of wounds [217].
6. Effectiveness of rat models in translational research
Animal experimentation is widely recognized as a crucial component of biomedical progress in the development of new interventions [218]. Despite the ethical dilemmas it poses, the indispensable role of animal models in preclinical studies cannot be overstated. They are vital in uncovering the pathophysiology of various diseases and exploring new treatment options for both animal and human health issues [219]. Rats share many physiological and genetic characteristics with humans, making them a valuable model for studying human diseases, including the mechanisms of chronic wound healing progression and potential treatment strategies. Consequently, rat models have been effectively utilized in preclinical studies to assess the efficacy of various interventions in chronic wound healing.
For instance, an STZ-induced diabetic rat model with a full-thickness dorsal wound showed that a combination of decellularized porcine acellular dermal matrix (ADM) with autologous adipose-derived stem cells (ASCs) enhanced cell proliferation and regeneration, thus accelerating the wound healing process [220]. Additionally, the ADM reduced inflammation in diabetic wounds, promoting wound healing and tissue regeneration. The ADM used in this study is a commercial product currently undergoing clinical trials to assess safety and clinical performance in the treatment of corneal ulcers (ClinicalTrials.gov identifier: NCT04054817).
Another example involves the treatment of diabetic wounds with Rejuveinix (RJX), an intravenous pharmaceutical composition containing antioxidants and anti-inflammatory vitamins. Diabetic wound and burn rat models were established using a high-fat diet combined with a single injection of STZ, followed by either a full-thickness skin excision wound on the back or a burn injury via a heated brass probe. RJX was administered via intraperitoneal injections for three weeks. The results indicated that RJX, administered at a dosage more than tenfold lower than its clinical maximum tolerated dose (MTD), significantly accelerated the healing of both excision wounds and burn injuries in diabetic rats [221]. At the same time, the phase 1 clinical trials of RJX (ClinicalTrials.gov identifier: NCT03680105) evaluated its pharmacokinetics and pharmacodynamics, demonstrating a favorable safety profile and tolerability in healthy human subjects [222].
7. Limitations
The primary goal of animal models is to replicate the pathophysiological conditions of human chronic wounds and evaluate therapeutic outcomes. Creating rat models that accurately represent human chronic wounds is challenging due to the fundamental differences in physiology, anatomy, pathology, and healing mechanisms between rats and humans [21,223]. Differences include a dense hair layer, thin epidermis, dermis, and absence of apocrine glands in rats [224]. While chronic wounds are common among humans, they are relatively rare in animals, which adds further constraints to the use of animal models [225]. Despite the limitations in using rat models to induce chronic wounds, rats remain a vital resource in biomedical research. Their advantages include accessibility, a wealth of knowledge from previous studies, and low maintenance costs. While inherent differences prevent rat models from perfectly mimicking human pathophysiology, advancing these models can reduce redundant research, conflicting results, and unnecessary use of time and resources.
8. Conclusion
The morbidity and high costs associated with chronic wounds underscore the critical need for new treatment methods [226], and animal models continue to be powerful tools for testing new therapeutic agents. While current rat and other animal models for chronic wounds play a crucial role in biomedical research, there is a significant need for continued research and improvement in these models. Currently, the available models are not sufficiently comprehensive for all applications, highlighting the importance of this research area. Refining animal models is essential to advance our understanding of chronic wounds and develop better treatments. Enhanced models will more accurately reflect human conditions, improving the translation of research into clinical practice.
Acknowledgments
This work was supported in part by the NIH awards P20GM152301, P20GM109090, and HL125736. Figs. 1–4 were created using BioRender.com.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Mahboubeh Ghanbari: Data curation, Investigation, Visualization, Writing – original draft, Writing – review & editing. Yury Salkovskiy: Conceptualization, Funding acquisition, Investigation, Project administration, Supervision, Validation, Writing – review & editing. Mark A. Carlson: Conceptualization, Investigation, Methodology, Writing – review & editing.
References
- [1].Järbrink K, Ni G, Sönnergren H, Schmidtchen A, Pang C, Bajpai R, Car J, Prevalence and incidence of chronic wounds and related complications: a protocol for a systematic review, Syst. Rev 5 (2016), 10.1186/S13643-016-0329-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Frykberg RG, Banks J, Challenges in the treatment of chronic wounds, Adv. Wound Care (New Rochelle) 4 (2015) 560–582, 10.1089/wound.2015.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sen CK, Human wounds and its burden: an updated compendium of estimates, Adv. Wound Care (New Rochelle) 8 (2019) 39–48, 10.1089/WOUND.2019.0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Han G, Ceilley R, Chronic wound healing: a review of current management and treatments, Adv. Ther 34 (2017) 599–610, 10.1007/S12325-017-0478-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zindle JK, Wolinsky E, Bogie KM, A review of animal models from 2015 to 2020 for preclinical chronic wounds relevant to human health, J. Tissue Viability 30 (2021) 291–300, 10.1016/J.JTV.2021.05.006. [DOI] [PubMed] [Google Scholar]
- [6].Dorsett-Martin WA, Rat models of skin wound healing: a review, Wound Repair Regen 12 (2004) 591–599, 10.1111/J.1067-1927.2004.12601.X. [DOI] [PubMed] [Google Scholar]
- [7].Sun D, Gao W, Hu H, Zhou S, Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B 12 (2022) 3049, 10.1016/J.APSB.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dowden H, Munro J, Trends in clinical success rates and therapeutic focus, Nat. Rev. Drug Discov 18 (2019) 495–496, 10.1038/D41573-019-00074-Z. [DOI] [PubMed] [Google Scholar]
- [9].Takebe T, Imai R, Ono S, The current status of drug discovery and development as originated in United States academia: the influence of industrial and academic collaboration on drug discovery and development, Clin. Transl. Sci 11 (2018) 597, 10.1111/CTS.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Perrin S, Preclinical research: Make mouse studies work, Nature 507 (2014) 423–425, 10.1038/507423a, 2014 507:7493. [DOI] [PubMed] [Google Scholar]
- [11].Ghasemi A, Jeddi S, Kashfi K, The laboratory rat: age and body weight matter, EXCLI J 20 (2021) 1431–1445, 10.17179/EXCLI2021-4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ellenbroek B, Youn J, Rodent models in neuroscience research: is it a rat race? Dis. Model. Mech 9 (2016) 1079–1087, 10.1242/DMM.026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nunan R, Harding KG, Martin P, Clinical challenges of chronic wounds: searching for an optimal animal model to recapitulate their complexity, Dis. Model. Mech 7 (2014) 1205–1213, 10.1242/DMM.016782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Meijer MK, Sommer R, Spruijt BM, Van Zutphen LFM, Baumans V, Influence of environmental enrichment and handling on the acute stress response in individually housed mice, Lab. Anim 41 (2007) 161–173, 10.1258/002367707780378168. [DOI] [PubMed] [Google Scholar]
- [15].Iannaccone PM, Jacob HJ, Rats!, Dis. Model. Mech 2 (2009) 206–210, 10.1242/DMM.002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dorsett-Martin WA, Rat models of skin wound healing: a review, Wound Repair Regen 12 (2004) 591–599, 10.1111/J.1067-1927.2004.12601.X. [DOI] [PubMed] [Google Scholar]
- [17].Smith JR, Bolton ER, Dwinell MR, The rat: a model used in biomedical research, Methods Mol. Biol 2018 (2019) 1–41, 10.1007/978-1-4939-9581-3_1/COVER. [DOI] [PubMed] [Google Scholar]
- [18].Masson-Meyers DS, Andrade TAM, Caetano GF, Guimaraes FR, Leite MN, Leite SN, Frade MAC, Experimental models and methods for cutaneous wound healing assessment, Int. J. Exp. Pathol 101 (2020) 21–37, 10.1111/IEP.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chien S, Ischemic rabbit ear model created by minimally invasive surgery, Wound Repair Regen 15 (2007) 928–935, 10.1111/J.1524-475X.2007.00285.X. [DOI] [PubMed] [Google Scholar]
- [20].Saeed S, Martins-Green M, Animal models for the study of acute cutaneous wound healing, Wound Repair Regen 31 (2023) 6–16, 10.1111/WRR.13051. [DOI] [PubMed] [Google Scholar]
- [21].Sullivan TP, Eaglstein WH, Davis SC, Mertz P, The pig as a model for human wound healing, Wound Repair Regen 9 (2001) 66–76, 10.1046/J.1524-475X.2001.00066.X. [DOI] [PubMed] [Google Scholar]
- [22].Schmook FP, Meingassner JG, Billich A, Comparison of human skin or epidermis models with human and animal skin in in-vitro percutaneous absorption, Int. J. Pharm 215 (2001) 51–56, 10.1016/S0378-5173(00)00665-7. [DOI] [PubMed] [Google Scholar]
- [23].Zhao Y, Qu H, Wang Y, Xiao W, Zhang Y, Shi D, Small rodent models of atherosclerosis, Biomed. Pharmacother 129 (2020), 10.1016/J.BIOPHA.2020.110426. [DOI] [PubMed] [Google Scholar]
- [24].Li X, Liu Y, Zhang H, Ren L, Li Q, Li N, Animal models for the atherosclerosis research: a review, Protein Cell 2 (2011) 189, 10.1007/S13238-011-1016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Linder-Ganz E, Engelberg S, Scheinowitz M, Gefen A, Pressure-time cell death threshold for albino rat skeletal muscles as related to pressure sore biomechanics, J. Biomech 39 (2006) 2725–2732, 10.1016/J.JBIOMECH.2005.08.010. [DOI] [PubMed] [Google Scholar]
- [26].Mirra A, Maidanskaia EG, Carmo LP, Levionnois O, Spadavecchia C, How is depth of anaesthesia assessed in experimental pigs? A scoping review, PLoS One 18 (2023), 10.1371/JOURNAL.PONE.0283511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Seaton M, Hocking A, Gibran NS, Porcine models of cutaneous wound healing, ILAR J 56 (2015) 127–138, 10.1093/ILAR/ILV016. [DOI] [PubMed] [Google Scholar]
- [28].Parnell LKS, Volk SW, The evolution of animal models in wound healing research: 1993–2017, Adv. Wound Care (New Rochelle) 8 (2019) 692–702, 10.1089/WOUND.2019.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sami DG, Heiba HH, Abdellatif A, Wound healing models: a systematic review of animal and non-animal models, Wound Medicine 24 (2019) 8–17, 10.1016/J.WNDM.2018.12.001. [DOI] [Google Scholar]
- [30].Tan MLL, Chin JS, Madden L, Becker DL, Challenges faced in developing an ideal chronic wound model, Expert Opin. Drug Discovery 18 (2023) 99–114, 10.1080/17460441.2023.2158809. [DOI] [PubMed] [Google Scholar]
- [31].Almadani YH, Vorstenbosch J, Davison PG, Murphy AM, Wound healing: a comprehensive review, Semin. Plast. Surg 35 (2021) 141–144, 10.1055/S-0041-1731791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wallace HA, Basehore BM, Zito PM, Wound Healing Phases, StatPearls, 2023. [PubMed] [Google Scholar]
- [33].Petersen H, Tavakoli F, Kruber S, Münscher A, Gliese A, Hansen NO, Uschold S, Eggert D, Robertson WD, Gosau T, Sehner S, Kwiatkowski M, Schlüter H, Schumacher U, Knecht R, Miller RJD, Comparative study of wound healing in rat skin following incision with a novel picosecond infrared laser (PIRL) and different surgical modalities, Lasers Surg. Med 48 (2016) 385–391, 10.1002/LSM.22498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Guo H-F, Ali RM, Hamid RA, Zaini AA, Khaza’ai H, A new model for studying deep partial-thickness burns in rats, Int. J. Burns Trauma 7 (2017) 107. [PMC free article] [PubMed] [Google Scholar]
- [35].Carlson MA, Longaker MT, Thompson JS, Granulation tissue regression induced by musculocutaneous advancement flap coverage, Surgery 131 (2002) 332–337, 10.1067/msy.2002.120673. [DOI] [PubMed] [Google Scholar]
- [36].Naldaiz-Gastesi N, Goicoechea M, Alonso-Martín S, Aiastui A, López-Mayorga M, García-Belda P, Lacalle J, San José C, Araúzo-Bravo MJ, Trouilh L, Anton-Leberre V, Herrero D, Matheu A, Bernad A, García-Verdugo JM, Carvajal JJ, Relaix F, Lopez de Munain A, García-Parra P, Izeta A, Identification and characterization of the dermal panniculus carnosus muscle stem cells, Stem Cell Rep 7 (2016) 411–424, 10.1016/J.STEMCR.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ren L, Zhou B, Chen L, Silicone ring implantation in an excisional murine wound model, Wounds 24 (2012) 36–42. [PubMed] [Google Scholar]
- [38].Davidson JM, Yu F, Opalenik SR, Splinting strategies to overcome confounding wound contraction in experimental animal models, Adv. Wound Care (New Rochelle) 2 (2013) 142–148, 10.1089/WOUND.2012.0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mawaki A, Nakatani T, Sugama J, Konya C, Relationship between the distribution of myofibroblasts, and stellar and circular scar formation due to the contraction of square and circular wound healing, Anat. Sci. Int 82 (2007) 147–155, 10.1111/J.1447-073X.2007.00179.X. [DOI] [PubMed] [Google Scholar]
- [40].Park SA, Covert J, Teixeira L, Motta MJ, Deremer SL, Abbott NL, Dubielzig R, Schurr M, Isseroff RR, McAnulty JF, Murphy CJ, Importance of defining experimental conditions in a mouse excisional wound model, Wound Repair Regen 23 (2015) 251–261, 10.1111/WRR.12272. [DOI] [PubMed] [Google Scholar]
- [41].Carlson MA, Longaker MT, Thompson JS, Wound splinting regulates granulation tissue survival, J. Surg. Res 110 (2003) 304–309, 10.1016/S0022-4804(02)00098-7. [DOI] [PubMed] [Google Scholar]
- [42].Carlson MA, Thompson JS, Wound splinting modulates granulation tissue proliferation, Matrix Biol 23 (2004) 243–250, 10.1016/j.matbio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- [43].Bowers S, Franco E, Chronic wounds: evaluation and management, Am. Fam. Physician 101 (2020) 159–166. [PubMed] [Google Scholar]
- [44].Martin P, Nunan R, Cellular and molecular mechanisms of repair in acute and chronic wound healing, Br. J. Dermatol 173 (2015) 370–378, 10.1111/BJD.13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Trujillo AN, Kesl SL, Sherwood J, Wu M, Gould LJ, Demonstration of the rat ischemic skin wound model, J. Vis. Exp 2015 (2015), 10.3791/52637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Valderas JM, Starfield B, Sibbald B, Salisbury C, Roland M, Defining comorbidity: implications for understanding health and health services, Ann. Fam. Med 7 (2009) 357–363, 10.1370/AFM.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Guo S, DiPietro LA, Factors affecting wound healing, J. Dent. Res 89 (2010) 219–229, 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Maksymova OS, German SM, Moskalenko PO, Yasenok VO, Gortynska OM, Hortynskyi KM, Tkach GF, Features of skin wounds healing under chronic hyperglycemia and improvement of their treatment methods, Wiad. Lek 74 (2021) 1174–1179, 10.36740/wlek202105124. [DOI] [PubMed] [Google Scholar]
- [49].Fernández-Guarino M, Hernández-Bule ML, Bacci S, Cellular and molecular processes in wound healing, Biomedicines 11 (2023), 10.3390/BIOMEDICINES11092526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Li Q, Wang D, Jiang Z, Li R, Xue T, Lin C, Deng Y, Jin Y, Sun B, Advances of hydrogel combined with stem cells in promoting chronic wound healing, Front. Chem 10 (2022), 10.3389/FCHEM.2022.1038839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Rabbani M, Rahman E, Powner MB, Triantis IF, Making sense of electrical stimulation: a meta-analysis for wound healing, Ann. Biomed. Eng 52 (2024) 153–177, 10.1007/S10439-023-03371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Raziyeva K, Kim Y, Zharkinbekov Z, Kassymbek K, Jimi S, Saparov A, Immunology of acute and chronic wound healing, Biomolecules 11 (2021), 10.3390/BIOM11050700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Landén NX, Li D, Ståhle M, Transition from inflammation to proliferation: a critical step during wound healing, Cell. Mol. Life Sci 73 (2016) 3861–3885, 10.1007/S00018-016-2268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M, Growth factors and cytokines in wound healing, Wound Repair Regen 16 (2008) 585–601, 10.1111/J.1524-475X.2008.00410.X. [DOI] [PubMed] [Google Scholar]
- [55].Eming SA, Krieg T, Davidson JM, Inflammation in wound repair: molecular and cellular mechanisms, J. Invest. Dermatol 127 (2007) 514–525, 10.1038/SJ.JID.5700701. [DOI] [PubMed] [Google Scholar]
- [56].Al-Zoubi NA, Shatnawi NJ, Gender variation in symptomatic peripheral arterial occlusive disease among type-2 diabetic patients, SAGE Open Med 7 (2019), 10.1177/2050312119840198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zemaitis MR, Boll JM, Dreyer MA, Peripheral arterial disease, in: StatPearls [Internet], StatPearls Publishing, Treasure Island (FL), 2023. [PubMed] [Google Scholar]
- [58].Lin JB, Phillips EH, Riggins TE, Sangha GS, Chakraborty S, Lee JY, Lycke RJ, Hernandez CL, Soepriatna AH, Thorne BRH, Yrineo AA, Goergen CJ, Imaging of small animal peripheral artery disease models: recent advancements and translational potential, Int. J. Mol. Sci 16 (2015) 11131–11177, 10.3390/IJMS160511131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ballestín A, Casado JG, Abellán E, Vela FJ, Alvarez V, Usón A, Lopez E, Marinaro F, Blazquez R, Sanchez-Margallo FM, Ischemia-reperfusion injury in a rat microvascular skin free flap model: a histological, genetic, and blood flow study, PloS One 13 (2018), 10.1371/JOURNAL.PONE.0209624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Beltran-Camacho L, Jimenez-Palomares M, Rojas-Torres M, Sanchez-Gomar I, Rosal-Vela A, Eslava-Alcon S, Perez-Segura MC, Serrano A, Antequera-González B, Alonso-Piñero JA, González-Rovira A, Extremera-García MJ, Rodriguez-Piñero M, Moreno-Luna R, Larsen MR, Durán-Ruiz MC, Identification of the initial molecular changes in response to circulating angiogenic cells-mediated therapy in critical limb ischemia, Stem Cell Res. Ther 11 (2020) 1–20, 10.1186/S13287-020-01591-0/FIGURES/6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lévigne D, Tobalem M, Modarressi A, Pittet-Cuénod B, Hyperglycemia increases susceptibility to ischemic necrosis, Biomed. Res. Int 2013 (2013), 10.1155/2013/490964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hoinoiu B, Jiga LP, Nistor A, Dornean V, Barac S, Miclaus G, Ionac M, Hoinoiu T, Chronic Hindlimb ischemia assessment; quantitative evaluation using laser Doppler in a rodent model of surgically induced peripheral arterial occlusion, Diagnostics (Basel) 9 (2019), 10.3390/DIAGNOSTICS9040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Aref Z, de Vries MR, Quax PHA, Variations in surgical procedures for inducing hind limb ischemia in mice and the impact of these variations on neovascularization assessment, Int. J. Mol. Sci 20 (2019), 10.3390/IJMS20153704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hellingman AA, Bastiaansen AJNM, De Vries MR, Seghers L, Lijkwan MA, Löwik CW, Hamming JF, Quax PHA, Variations in surgical procedures for hind limb Ischaemia mouse models result in differences in collateral formation, Eur. J. Vasc. Endovasc. Surg 40 (2010) 796–803, 10.1016/J.EJVS.2010.07.009. [DOI] [PubMed] [Google Scholar]
- [65].Brown MD, Kelsall CJ, Milkiewicz M, Anderson S, Hudlicka O, A new model of peripheral arterial disease: sustained impairment of nutritive microcirculation and its recovery by chronic electrical stimulation, Microcirculation 12 (2005) 373–381, 10.1080/10739680590934817. [DOI] [PubMed] [Google Scholar]
- [66].Li C, Nie F, Liu X, Chen M, Chi D, Li S, Pipinos II, Li X, Antioxidative and Angiogenic hyaluronic acid-based hydrogel for the treatment of peripheral artery disease, ACS Appl. Mater. Interfaces 13 (2021) 45224–45235, 10.1021/ACSAMI.1C11349. [DOI] [PubMed] [Google Scholar]
- [67].Heil M, Eitenmüller I, Schmitz-Rixen T, Schaper W, Arteriogenesis versus angiogenesis: similarities and differences, J. Cell. Mol. Med 10 (2006) 45–55, 10.1111/J.1582-4934.2006.TB00290.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Yang Y, Tang G, Yan J, Park B, Hoffman A, Tie G, Wang R, Messina LM, Cellular and molecular mechanism regulating blood flow recovery in acute versus gradual femoral artery occlusion are distinct in the mouse, Journal of vascular surgery: official publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery, North American Chapter 48 (2008) 1546, 10.1016/J.JVS.2008.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Lundberg G, Luo F, Blegen H, Kalin B, Wahlberg E, A rat model for severe limb ischemia at rest, Eur. Surg. Res 35 (2003) 430–438, 10.1159/000072228. [DOI] [PubMed] [Google Scholar]
- [70].Tang GL, Chang DS, Sarkar R, Wang R, Messina LM, The effect of gradual or acute arterial occlusion on skeletal muscle blood flow, arteriogenesis, and inflammation in rat hindlimb ischemia, J. Vasc. Surg 41 (2005) 312–320, 10.1016/j.jvs.2004.11.012. [DOI] [PubMed] [Google Scholar]
- [71].Krishna SM, Omer SM, Li J, Morton SK, Jose RJ, Golledge J, Development of a two-stage limb ischemia model to better simulate human peripheral artery disease, Scientific Reports 10 (2020) 1–16, 10.1038/s41598-020-60352-4, 2020 10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Alizadeh N, Pepper M, Alfo K, Montandon D, Gabbiani G, Bochaton-Piallat M, Pittet B, Persistent ischemia impairs myofibroblast development in wound granulation tissue a new model of delayed wound healing, Wound Repair Regen 13 (2005) A22, 10.1111/J.1067-1927.2005.130117V.X. [DOI] [PubMed] [Google Scholar]
- [73].IDF Diabetes Atlas | Tenth Edition. https://diabetesatlas.org/. [Google Scholar]
- [74].Hou X, Guo P, Cai F, Lin Y, Zhang J, Angiosome-guided endovascular revascularization for treatment of diabetic foot ulcers with peripheral artery disease, Ann. Vasc. Surg 86 (2022) 242–250, 10.1016/J.AVSG.2022.02.012. [DOI] [PubMed] [Google Scholar]
- [75].Okur ME, Bülbül EÖ, Mutlu G, Eleftherıadou K, Karantas ID, Okur NÜ, Siafaka PI, An updated review for the diabetic wound healing systems, Curr. Drug Targets 23 (2022) 393–419, 10.2174/1389450122666210914104428. [DOI] [PubMed] [Google Scholar]
- [76].Ogbera OA, Osa E, Edo A, Chukwum E, Common clinical features of diabetic foot ulcers: perspectives from a developing nation, Int. J. Low. Extrem. Wounds 7 (2008) 93–98, 10.1177/1534734608318236. [DOI] [PubMed] [Google Scholar]
- [77].Hokkam EN, Assessment of risk factors in diabetic foot ulceration and their impact on the outcome of the disease, Prim. Care Diabetes 3 (2009) 219–224, 10.1016/J.PCD.2009.08.009. [DOI] [PubMed] [Google Scholar]
- [78].Khalifa WA, Risk factors for diabetic foot ulcer recurrence: a prospective 2-year follow-up study in Egypt, Foot (Edinb.) 35 (2018) 11–15, 10.1016/J.FOOT.2017.12.004. [DOI] [PubMed] [Google Scholar]
- [79].Southam K, de Sousa C, Daniel A, Taylor BV, Foa L, Premilovac D, Development and characterisation of a rat model that exhibits both metabolic dysfunction and neurodegeneration seen in type 2 diabetes, J. Physiol 600 (2022) 1611–1630, 10.1113/JP282454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Vykoukal D, Davies MG, Vascular biology of metabolic syndrome, Journal of vascular surgery: official publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery, North American Chapter. 54 (2011) 819, 10.1016/J.JVS.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Goyal SN, Reddy NM, Patil KR, Nakhate KT, Ojha S, Patil CR, Agrawal YO, Challenges and issues with streptozotocin-induced diabetes - a clinically relevant animal model to understand the diabetes pathogenesis and evaluate therapeutics, Chem. Biol. Interact 244 (2016) 49–63, 10.1016/J.CBI.2015.11.032. [DOI] [PubMed] [Google Scholar]
- [82].Reiber GE, Lipsky BA, Gibbons GW, The burden of diabetic foot ulcers, Am. J. Surg 176 (1998) 5S–10S, 10.1016/S0002-9610(98)00181-0. [DOI] [PubMed] [Google Scholar]
- [83].Ghasemi A, Jeddi S, Streptozotocin as a tool for induction of rat models of diabetes: a practical guide, EXCLI J 22 (2023) 274–294, 10.17179/EXCLI2022-5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Reed MJ, Meszaros K, Entes LJ, Claypool MD, Pinkett JG, Gadbois TM, Reaven GM, A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat, Metabolism 49 (2000) 1390–1394, 10.1053/META.2000.17721. [DOI] [PubMed] [Google Scholar]
- [85].Furman BL, Streptozotocin-induced diabetic models in mice and rats, Curr. Protoc. Pharmacol 70 (2015), 10.1002/0471141755.PH0547S70, 5.47.1–5.47.20. [DOI] [PubMed] [Google Scholar]
- [86].Risk factors associated with healing chronic diabetic foot ulcers: the importance of hyperglycemia - PubMed, https://pubmed.ncbi.nlm.nih.gov/16567857/. [PubMed] [Google Scholar]
- [87].Kara M, Baykan H, Karabulut D, Investigation of the effect of sildenafil on flap survival in a diabetic rat model, Ann. Chir. Plast. Esthet 67 (2022) 232–238, 10.1016/J.ANPLAS.2022.02.004. [DOI] [PubMed] [Google Scholar]
- [88].Patterson D, Belch JJF, Venous insufficiency, Vascular Medicine: A Companion to Braunwald’s Heart Disease 785–793 (2022), 10.1016/B978-0-7216-0284-4.50062-2. [DOI] [Google Scholar]
- [89].Suehiro K, Morikage N, Yamashita O, Harada T, Samura M, Takeuchi Y, Mizoguchi T, Hamano K, Risk factors in patients with venous stasis-related skin lesions without major abnormalities on duplex ultrasonography, Ann. Vasc. Dis 9 (2016) 201, 10.3400/AVD.OA.16-00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Hahn TL, Whitfield R, Salter J, Granger DN, Unthank JL, Lalka SG, Evaluation of the role of intercellular adhesion molecule 1 in a rodent model of chronic venous hypertension, J. Surg. Res 88 (2000) 150–154, 10.1006/JSRE.1999.5766. [DOI] [PubMed] [Google Scholar]
- [91].Pascarella L, Schmid-Schönbein GW, Bergan J, An animal model of venous hypertension: the role of inflammation in venous valve failure, J. Vasc. Surg 41 (2005) 303–311, 10.1016/j.jvs.2004.10.038. [DOI] [PubMed] [Google Scholar]
- [92].Monos E, Raffai G, Dörnyei G, Nádasy GL, Fehér E, Structural and functional responses of extremity veins to long-term gravitational loading or unloading—lessons from animal systems, Acta Astronaut 60 (2007) 406–414, 10.1016/J.ACTAASTRO.2006.09.027. [DOI] [Google Scholar]
- [93].Monos E, Lóránt M, Dörnyei G, Bérczi V, Nádasy G, Long-term adaptation mechanisms in extremity veins supporting orthostatic tolerance, News Physiol. Sci 18 (2003) 210–214, 10.1152/NIPS.01447.2003. [DOI] [PubMed] [Google Scholar]
- [94].Dörnyei G, Hetthéssy J, Patai B, Balogh F, Németi Á, Jäckel M, Tőkes A, Fees A, Varady Z, Monos E, Nádasy GL, Combined effect of chronic partial occlusion and orthostatic load on the saphenous vein network: a varicosity model in the rat, Phlebology 35 (2020) 92–101, 10.1177/0268355519852557. [DOI] [PubMed] [Google Scholar]
- [95].Cottler P, Gampper TJ, Rodeheaver GT, Skalak TC, Evaluation of clinically applicable exsanguination treatments to alleviate venous congestion in an animal skin flap model, Wound Repair Regen 7 (1999) 187–195, 10.1046/J.1524-475X.1999.00187.X. [DOI] [PubMed] [Google Scholar]
- [96].Salcido R, Popescu A, Ahn C, Animal models in pressure ulcer research, J. Spinal Cord Med 30 (2007) 107–116, 10.1080/10790268.2007.11753921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Olivo S, Canova C, Peghetti A, Rossi M, Zanotti R, Prevalence of pressure ulcers in hospitalised patients: a cross-sectional study, J. Wound Care 29 (2020) S20–S28, 10.12968/JOWC.2020.29.SUP3.S20. [DOI] [PubMed] [Google Scholar]
- [98].Al Aboud AM, Manna B, Wound Pressure Injury Management, StatPearls, 2022. [PubMed] [Google Scholar]
- [99].Edsberg LE, Black JM, Goldberg M, McNichol L, Moore L, Sieggreen M, Revised national pressure ulcer advisory panel pressure injury staging system: revised pressure injury staging system, Journal of Wound, Ostomy, and Continence Nursing 43 (2016) 585, 10.1097/WON.0000000000000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Kottner J, Cuddigan J, Carville K, Balzer K, Berlowitz D, Law S, Litchford M, Mitchell P, Moore Z, Pittman J, Sigaudo-Roussel D, Yee CY, Haesler E, Pressure ulcer/injury classification today: an international perspective, J. Tissue Viability 29 (2020) 197–203, 10.1016/J.JTV.2020.04.003. [DOI] [PubMed] [Google Scholar]
- [101].Hashimoto M, Kurose T, Kawamata S, Comparison between a weight compression and a magnet compression for experimental pressure ulcers in the rat. Histological studies and effects of anesthesia, Arch. Histol. Cytol 71 (2008) 303–316, 10.1679/AOHC.71.303. [DOI] [PubMed] [Google Scholar]
- [102].Wang Y, Pu L, Li Z, Hu X, Jiang L, Hypoxia-inducible factor-1α gene expression and apoptosis in ischemia-reperfusion injury: a rat model of early-stage pressure ulcer, Nurs. Res 65 (2016) 35–46, 10.1097/NNR.0000000000000132. [DOI] [PubMed] [Google Scholar]
- [103].Qin Z, Wang Y, Zhao W, Zhang Y, Tian Y, Sun S, Li X, Pressure ulcer healing promoted by adequate protein intake in rats, Exp. Ther. Med 15 (2018) 4173–4178, 10.3892/ETM.2018.5934/HTML. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Preston A, Rao A, Strauss R, Stamm R, Zalman D, Deep tissue pressure injury: a clinical review, Am. J. Nurs 117 (2017) 50–57, 10.1097/01.NAJ.0000516273.66604.C7. [DOI] [PubMed] [Google Scholar]
- [105].Lin F, Pandya A, Cichowski A, Modi M, Reprogle B, Lee D, Kadono N, Makhsous M, Deep tissue injury rat model for pressure ulcer research on spinal cord injury, J. Tissue Viability 19 (2010) 67–76, 10.1016/J.JTV.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Ahmed AK, Goodwin CR, Sarabia-Estrada R, Lay F, Ansari AM, Steenbergen C, Pang C, Cohen R, Born LJ, Matsangos AE, Ng C, Marti GP, Abu-Bonsrah N, Phillips NA, Suk I, Sciubba DM, Harmon JW, A non-invasive method to produce pressure ulcers of varying severity in a spinal cord-injured rat model, Spinal Cord 54 (2016) 1096–1104, 10.1038/sc.2016.61, 2016 54:12. [DOI] [PubMed] [Google Scholar]
- [107].Kwan MPC, Tam EWC, Lo SCL, Leung MCP, Lau RYC, The time effect of pressure on tissue viability: investigation using an experimental rat model, Exp. Biol. Med. (Maywood) 232 (2007) 481–487. [PubMed] [Google Scholar]
- [108].Song M, Li X, Liu S, Wang Y, Zhao Z, Wang Y, Chen Z, Effects of smoking on the wound healing of stage 4 pressure ulcers in rats, Chinese Journal of Burns 36 (2020) 953–958, 10.3760/CMA.J.CN501120-20190827-00361. [DOI] [PubMed] [Google Scholar]
- [109].Ganesh K, Sinha M, Mathew-Steiner SS, Das A, Roy S, Sen CK, Chronic wound biofilm model, Adv Wound Care (New Rochelle) 4 (2015) 382–388, 10.1089/WOUND.2014.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Hurlow J, Bowler PG, Acute and chronic wound infections: microbiological, immunological, clinical and therapeutic distinctions, J. Wound Care 31 (2022) 436–445, 10.12968/JOWC.2022.31.5.436. [DOI] [PubMed] [Google Scholar]
- [111].Reddersen K, Tittelbach J, Wiegand C, 3D biofilm models containing multiple species for antimicrobial testing of wound dressings, Microorganisms 10 (2022), 10.3390/MICROORGANISMS10102027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Vestby LK, Grønseth T, Simm R, Nesse LL, Bacterial biofilm and its role in the pathogenesis of disease, Antibiotics (Basel) 9 (2020), 10.3390/ANTIBIOTICS9020059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Clinton A, Carter T, Chronic wound biofilms: pathogenesis and potential therapies, Lab. Med 46 (2015) 277–284, 10.1309/LMBNSWKUI4JPN7SO. [DOI] [PubMed] [Google Scholar]
- [114].Cont A, Rossy T, Al-Mayyah Z, Persat A, Biofilms deform soft surfaces and disrupt epithelia, Elife 9 (2020) 1–22, 10.7554/ELIFE.56533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Versey Z, da Cruz Nizer WS, Russell E, Zigic S, DeZeeuw KG, Marek JE, Overhage J, Cassol E, Biofilm-innate immune Interface: contribution to chronic wound formation, Front. Immunol 12 (2021), 10.3389/FIMMU.2021.648554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Roy S, Santra S, Das A, Dixith S, Sinha M, Ghatak S, Ghosh N, Banerjee P, Khanna S, Mathew-Steiner S, Das Ghatak P, Blackstone BN, Powell HM, Bergdall VK, Wozniak DJ, Sen CK, Staphylococcus aureus Biofilm Infection Compromises Wound Healing by Causing Deficiencies in Granulation Tissue Collagen, Ann Surg 271 (2020) 1174–1185, 10.1097/SLA.0000000000003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Malone-Povolny MJ, Maloney SE, Schoenfisch MH, Nitric oxide therapy for diabetic wound healing, Adv. Healthc. Mater 8 (2019), 10.1002/ADHM.201801210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Sun Y, Dowd SE, Smith E, Rhoads DD, Wolcott RD, In vitro multispecies Lubbock chronic wound biofilm model, Wound Repair Regen 16 (2008) 805–813, 10.1111/J.1524-475X.2008.00434.X. [DOI] [PubMed] [Google Scholar]
- [119].Werthén M, Henriksson L, Jensen PØ, Sternberg C, Givskov M, Bjarnsholt T, An in vitro model of bacterial infections in wounds and other soft tissues, APMIS 118 (2010) 156–164, 10.1111/J.1600-0463.2009.02580.X. [DOI] [PubMed] [Google Scholar]
- [120].Zhao G, Usui ML, Underwood RA, Singh PK, James GA, Stewart PS, Fleckman P, Olerud JE, Time course study of delayed wound healing in a biofilm-challenged diabetic mouse model, Wound Repair Regen 20 (2012) 342–352, 10.1111/J.1524-475X.2012.00793.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Kanno E, Toriyabe S, Zhang L, Imai Y, Tachi M, Biofilm formation on rat skin wounds by Pseudomonas aeruginosa carrying the green fluorescent protein gene, Exp. Dermatol 19 (2010) 154–156, 10.1111/J.1600-0625.2009.00931.X. [DOI] [PubMed] [Google Scholar]
- [122].Yang L, Liu Y, Wu H, Høiby N, Molin S, Song ZJ, Current understanding of multi-species biofilms, Int. J. Oral Sci 3 (2011) 74–81, 10.4248/IJOS11027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Asada M, Nakagami G, Minematsu T, Nagase T, Akase T, Huang L, Yoshimura K, Sanada H, Novel models for bacterial colonization and infection of full-thickness wounds in rats, Wound Repair Regen 20 (2012) 601–610, 10.1111/J.1524-475X.2012.00800.X. [DOI] [PubMed] [Google Scholar]
- [124].McFarlane RM, DeYoung G, Henry RA, The design of a pedicle flap in the rat to study necrosis and its prevention, Plast. Reconstr. Surg 35 (1965) 177–182, 10.1097/00006534-196502000-00007. [DOI] [PubMed] [Google Scholar]
- [125].Hsueh YY, Wang DH, Huang TC, Chang YJ, Shao WC, Tuan TL, Hughes MW, Wu CC, Novel skin chamber for rat ischemic flap studies in regenerative wound repair, Stem Cell Res Ther 7 (2016), 10.1186/S13287-016-0333-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Quirinia A, Jensen FT, Viidik A, Ischemia in wound healing. I: Design of a flap model–changes in blood flow, Scand. J. Plast. Reconstr. Surg. Hand Surg 26 (1992) 21–28, 10.3109/02844319209035178. [DOI] [PubMed] [Google Scholar]
- [127].Schwarz DA, Lindblad WJ, Rees RS, Altered collagen metabolism and delayed healing in a novel model of ischemic wounds, Wound Repair Regen 3 (1995) 204–212, 10.1046/J.1524-475X.1995.30212.X. [DOI] [PubMed] [Google Scholar]
- [128].Chen C, Schultz GS, Bloch M, Edwards PD, Tebes S, Mast BA, Molecular and mechanistic validation of delayed healing rat wounds as a model for human chronic wounds, Wound Repair Regen 7 (1999) 486–494, 10.1046/J.1524-475X.1999.00486.X. [DOI] [PubMed] [Google Scholar]
- [129].Trujillo AN, Kesl SL, Sherwood J, Wu M, Gould LJ, Demonstration of the rat ischemic skin wound model, J. Vis. Exp 2015 (2015) 52637, 10.3791/52637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Wang J, Hao H, Huang H, Chen D, Han Y, Han W, The effect of adipose-derived stem cells on full-thickness skin grafts, Biomed. Res. Int 2016 (2016), 10.1155/2016/1464725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Yoshida H, Itoh S, Hara T, Sasaki Y, Kondo S, Nakagawa T, Asanuma A, Tanabe S, A phosphodiesterase 3 inhibitor, K-134, improves hindlimb skeletal muscle circulation in rat models of peripheral arterial disease, Atherosclerosis 221 (2012) 84–90, 10.1016/J.ATHEROSCLEROSIS.2011.12.044. [DOI] [PubMed] [Google Scholar]
- [132].Zografou A, Papadopoulos O, Tsigris C, Kavantzas N, Michalopoulos E, Chatzistamatiou T, Papassavas A, Stavropoulou-Gioka C, Dontas I, Perrea D, Autologous transplantation of adipose-derived stem cells enhances skin graft survival and wound healing in diabetic rats, Ann. Plast. Surg 71 (2013) 225–232, 10.1097/SAP.0B013E31826AF01A. [DOI] [PubMed] [Google Scholar]
- [133].Prasad YS, Miryala S, Lalitha K, Saritha B, Maheswari CU, Sridharan V, Srinandan CS, Nagarajan S, An injectable self-healing anesthetic glycolipid-based oleogel with antibiofilm and diabetic wound skin repair properties, Sci. Rep 10 (2020), 10.1038/S41598-020-73708-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Pascarella L, Schmid-Schönbein GW, Bergan J, An animal model of venous hypertension: the role of inflammation in venous valve failure, J. Vasc. Surg 41 (2005) 303–311, 10.1016/j.jvs.2004.10.038. [DOI] [PubMed] [Google Scholar]
- [135].Monos E, Lóránt M, Fehér E, Influence of long-term experimental orthostatic body position on innervation density in extremity vessels, Am. J. Physiol. Heart Circ. Physiol 281 (2001), 10.1152/AJPHEART.2001.281.4.H1606. [DOI] [PubMed] [Google Scholar]
- [136].Sari Y, Nakagami G, Kinoshita A, Huang L, Ueda K, Iizaka S, Sanada H, Sugama J, Changes in serum and exudate creatine phosphokinase concentrations as an indicator of deep tissue injury: a pilot study, Int. Wound J 5 (2008) 674–680, 10.1111/J.1742-481X.2008.00543.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Brandenburg KS, Weaver AJ, Karna SLR, You T, Chen P, Van Stryk S, Qian L, Pineda U, Abercrombie JJ, Leung KP, Formation of Pseudomonas aeruginosa biofilms in full-thickness scald burn wounds in rats, Sci. Rep 9 (2019), 10.1038/S41598-019-50003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Brandenburg KS, Weaver AJ, Qian L, You T, Chen P, Karna SLR, Fourcaudot AB, Sebastian EA, Abercrombie JJ, Pineda U, Hong J, Wienandt NA, Leung KP, Development of pseudomonas aeruginosa biofilms in partial-thickness burn wounds using a Sprague-Dawley rat model, J. Burn Care Res 40 (2019) 44–57, 10.1093/JBCR/IRY043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Weaver AJ, Brandenburg KS, Smith BW, Leung KP, Comparative analysis of the host response in a rat model of deep-partial and full-thickness burn wounds with Pseudomonas aeruginosa infection, Front. Cell. Infect. Microbiol 9 (2020), 10.3389/FCIMB.2019.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Nakagami G, Morohoshi T, Ikeda T, Ohta Y, Sagara H, Huang L, Nagase T, Sugama J, Sanada H, Contribution of quorum sensing to the virulence of Pseudomonas aeruginosa in pressure ulcer infection in rats, Wound Repair Regen 19 (2011) 214–222, 10.1111/J.1524-475X.2010.00653.X. [DOI] [PubMed] [Google Scholar]
- [141].Mendes JJ, Leandro CI, Bonaparte DP, Pinto AL, A rat model of diabetic wound infection for the evaluation of topical antimicrobial therapies, Comp. Med 62 (2012) 37. [PMC free article] [PubMed] [Google Scholar]
- [142].Kim DJ, Mustoe T, Clark RA, Cutaneous wound healing in aging small mammals: a systematic review, Wound Repair Regen 23 (2015) 318–339, 10.1111/WRR.12290. [DOI] [PubMed] [Google Scholar]
- [143].Jankovic A, Saso L, Korac A, Korac B, Relation of redox and structural alterations of rat skin in the function of chronological aging, Oxid. Med. Cell. Longev 2019 (2019), 10.1155/2019/2471312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Soybir OC, Gürdal SÖ, Oran EŞ, Tülübaş F, Yüksel M, Akyildiz AI, Bilir A, Soybir GR, Delayed cutaneous wound healing in aged rats compared to younger ones, Int. Wound J 9 (2012) 478, 10.1111/J.1742-481X.2011.00897.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Sgonc R, Gruber J, Age-related aspects of cutaneous wound healing: a mini-review, Gerontology 59 (2013) 159–164, 10.1159/000342344. [DOI] [PubMed] [Google Scholar]
- [146].Kazanci A, Kurus M, Atasever A, Analyses of changes on skin by aging, Skin Res. Technol 23 (2017) 48–60, 10.1111/SRT.12300. [DOI] [PubMed] [Google Scholar]
- [147].Liu M, Zhang P, Chen M, Zhang W, Yu L, Yang XC, Fan Q, Aging might increase myocardial ischemia / reperfusion-induced apoptosis in humans and rats, Age (Omaha) 34 (2012) 621, 10.1007/S11357-011-9259-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Berenguer Pérez M, López-Casanova P, Sarabia Lavín R, González de la Torre H, Verdú-Soriano J, Epidemiology of venous leg ulcers in primary health care: incidence and prevalence in a health centre-a time series study (2010–2014), Int. Wound J 16 (2019) 256–265, 10.1111/IWJ.13026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Cheng YJ, Imperatore G, Geiss LS, Wang J, Saydah SH, Cowie CC, Gregg EW, Secular Changes in the Age-Specific Prevalence of Diabetes Among U.S. Adults: 1988–2010, Diabetes Care 36 (2013) 2690, 10.2337/DC12-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Jacobs E, Rathmann W, Tönnies T, Arendt D, Marchowez M, Veith L, Kuss O, Brinks R, Hoyer A, Age at diagnosis of type 2 diabetes in Germany: a nationwide analysis based on claims data from 69 million people, Diabet. Med 37 (2020) 1723–1727, 10.1111/DME.14100. [DOI] [PubMed] [Google Scholar]
- [151].Margolis DJ, Bilker W, Knauss J, Baumgarten M, Strom BL, The incidence and prevalence of pressure ulcers among elderly patients in general medical practice, Ann. Epidemiol 12 (2002) 321–325, 10.1016/S1047-2797(01)00255-1. [DOI] [PubMed] [Google Scholar]
- [152].Hashimoto M, Kurose T, Kawamata S, Comparison between a weight compression and a magnet compression for experimental pressure ulcers in the rat. Histological studies and effects of anesthesia, Arch. Histol. Cytol 71 (2008) 303–316, 10.1679/AOHC.71.303. [DOI] [PubMed] [Google Scholar]
- [153].Tobalem M, Lévigne D, Modarressi A, Atashi F, Villard F, Hinz B, Pittet-Cuénod B, Hyperglycemia interacts with ischemia in a synergistic way on wound repair and myofibroblast differentiation, Plast. Reconstr. Surg. Glob. Open 3 (2015), 10.1097/GOX.0000000000000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Ghanbari M, Ghasemi A, Maternal hypothyroidism: an overview of current experimental models, Life Sci 187 (2017) 1–8, 10.1016/J.LFS.2017.08.012. [DOI] [PubMed] [Google Scholar]
- [155].Moor AN, Tummel E, Prather JL, Jung M, Lopez JJ, Connors S, Gould LJ, Consequences of age on ischemic wound healing in rats: altered antioxidant activity and delayed wound closure, Age (Dordr.) 36 (2014) 733–748, 10.1007/S11357-014-9617-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Saldías MP, Fernández C, Morgan A, Díaz C, Morales D, Jaña F, Gómez A, Silva A, Briceño F, Oyarzún A, Maldonado F, Cerda O, Smith PC, Cáceres M, Aged blood factors decrease cellular responses associated with delayed gingival wound repair, PloS One 12 (2017), 10.1371/JOURNAL.PONE.0184189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Kopcewicz M, Walendzik K, Bukowska J, Kur-Piotrowska A, Machcinska S, Gimble JM, Gawronska-Kozak B, Cutaneous wound healing in aged, high fat diet-induced obese female or male C57BL/6 mice, Aging 12 (2020) 7066–7111, 10.18632/AGING.103064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Gunin AG, Petrov VV, Vasil’eva OV, Golubtsova NN, Blood vessels in human dermis during aging, Adv. Gerontol 27 (2014) 54–61. [PubMed] [Google Scholar]
- [159].Sengupta P, The laboratory rat: relating its age with human’s, Int. J. Prev. Med 4 (2013) 624. [PMC free article] [PubMed] [Google Scholar]
- [160].Gholami H, Jeddi S, Zadeh-Vakili A, Farrokhfall K, Rouhollah F, Zarkesh M, Ghanbari M, Ghasemi A, Transient congenital hypothyroidism alters gene expression of glucose transporters and impairs glucose sensing apparatus in young and aged offspring rats, Cell. Physiol. Biochem 43 (2017) 2338–2352, 10.1159/000484386. [DOI] [PubMed] [Google Scholar]
- [161].Clayton JA, Collins FS, Policy: NIH to balance sex in cell and animal studies, Nature 509 (2014) 282–283, 10.1038/509282a, 2014 509:7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Kong BY, Haugh IM, Schlosser BJ, Getsios S, Paller AS, Mind the gap: sex bias in basic skin research, J. Invest. Dermatol 136 (2016) 12–14, 10.1038/JID.2015.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Díaz-Herrera MÁ, Martínez-Riera JR, Verdú-Soriano J, Capillas-Pérez RM, Pont-García C, Tenllado-Pérez S, Cunillera-Púertolas O, Berenguer-Pérez M, Gea-Caballero V, Multicentre study of chronic wounds point prevalence in primary health care in the southern metropolitan area of Barcelona, J. Clin. Med 10 (2021) 1–11, 10.3390/JCM10040797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Engeland CG, Sabzehei B, Marucha PT, Sex hormones and mucosal wound healing, Brain Behav. Immun 23 (2009) 629–635, 10.1016/J.BBI.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Farage MA, Neill S, MacLean AB, Physiological changes associated with the menstrual cycle: a review, Obstet. Gynecol. Surv 64 (2009) 58–72, 10.1097/OGX.0B013E3181932A37. [DOI] [PubMed] [Google Scholar]
- [166].El Mohtadi M, Whitehead K, Dempsey-Hibbert N, Belboul A, Ashworth J, Estrogen deficiency – a central paradigm in age-related impaired healing? EXCLI J 20 (2021) 99, 10.17179/EXCLI2020-3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Gushiken LFS, Beserra FP, Bastos JK, Jackson CJ, Pellizzon CH, Cutaneous wound healing: an update from physiopathology to current therapies, Life 11 (2021), 10.3390/LIFE11070665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Özdemir O, Çöl M, The age at menopause and associated factors at the health center area in Ankara, Turkey, Maturitas 49 (2004) 211–219, 10.1016/j.maturitas.2004.01.013. [DOI] [PubMed] [Google Scholar]
- [169].Medina-Contreras J, Villalobos-Molina R, Zarain-Herzberg A, Balderas-Villalobos J, Ovariectomized rodents as a menopausal metabolic syndrome model. A minireview, Mol. Cell Biochem 475 (2020) 261–276, 10.1007/S11010-020-03879-4. [DOI] [PubMed] [Google Scholar]
- [170].Ji MS, Yang XY, Hao Y, Shi J, Histomorphological and biochemical analysis of rat model of menopausal skin aging, Bull. Exp. Biol. Med 172 (2022) 377–380, 10.1007/S10517-022-05396-4. [DOI] [PubMed] [Google Scholar]
- [171].Marchese E, Rodeghier C, Monson RS, Mccracken B, Shi T, Schrock W, Martellotto J, Oberholzer J, Danielson KK, Enumerating β-cells in whole human islets: sex differences and associations with clinical outcomes after islet transplantation, Diabetes Care 38 (2015) e176, 10.2337/DC15-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Ajayi AF, Akhigbe RE, Staging of the estrous cycle and induction of estrus in experimental rodents: an update, Fertility Research and Practice 6 (2020) 1–15, 10.1186/S40738-020-00074-3, 2020 6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC, Estrous cycle influences the response of female rats in the elevated plus-maze test, Physiol. Behav 74 (2001) 435–440, 10.1016/S0031-9384(01)00593-5. [DOI] [PubMed] [Google Scholar]
- [174].Emanuele MA, Wezeman F, Emanuele NV, Alcohol’s effects on female reproductive function, Alcohol Res. Health 26 (2002) 274. [PMC free article] [PubMed] [Google Scholar]
- [175].Stapleton PA, McBride CR, Yi J, Abukabda AB, Nurkiewicz TR, Estrous cycle-dependent modulation of in vivo microvascular dysfunction after nanomaterial inhalation, Reprod. Toxicol 78 (2018) 20–28, 10.1016/J.REPROTOX.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Dundar SO, Ozcura F, Cetin ED, Beder N, Dundar M, Effects of estrogen replacement therapy on vascular endothelial growth factor expression in choroidal and retinal vasculature, Bratisl. Lek. Listy 111 (2010) 473–476. [PubMed] [Google Scholar]
- [177].Merkwitz C, Blaschuk O, Eplinius F, Winkler J, Prömel S, Schulz A, Ricken A, A simple method for inducing estrous cycle stage-specific morphological changes in the vaginal epithelium of immature female mice, Lab. Anim 50 (2016) 344–353, 10.1177/0023677215617387. [DOI] [PubMed] [Google Scholar]
- [178].Marcondes FK, Bianchi FJ, Tanno AP, Determination of the estrous cycle phases of rats: some helpful considerations, Braz. J. Biol 62 (2002) 609–614, 10.1590/S1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- [179].National Institutes of Health: NOT-OD-15-102: Consideration of Sex as a Biological Variable in NIH-funded Research, https://grants.nih.gov/grants/guide/notice-files/not-od-15-102.html.
- [180].Hubscher CH, Brooks DL, Johnson JR, A quantitative method for assessing stages of the rat estrous cycle, Biotech. Histochem 80 (2005) 79–87, 10.1080/10520290500138422. [DOI] [PubMed] [Google Scholar]
- [181].Mostafavinia A, Bidram M, Gomi Avili A, Mahmanzar M, Karimifard SA, Sajadi E, Amini A, Hadipour Jahromy M, Ghoreishi SK, Chien S, Bayat M, An improvement in acute wound healing in rats by the synergistic effect of photobiomodulation and arginine, Laboratory Animal Research 35 (2019) 1–11, 10.1186/S42826-019-0025-X, 2019 35:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Afzali H, Khaksari M, Norouzirad R, Jeddi S, Kashfi K, Ghasemi A, Acidified nitrite improves wound healing in type 2 diabetic rats: role of oxidative stress and inflammation, Nitric Oxide 103 (2020) 20–28, 10.1016/J.NIOX.2020.07.001. [DOI] [PubMed] [Google Scholar]
- [183].Altun MA, Ozaydin A, Arkan H, Demiryas S, Akbas F, Bahtiyar N, Onaran I, Anesthesia may alter mRNA expression of certain wound healing-associated genes in dermal wound environment of the rats, Mol. Biol. Rep 46 (2019) 2819–2827, 10.1007/S11033-019-04728-4. [DOI] [PubMed] [Google Scholar]
- [184].Huss MK, Felt SA, Pacharinsak C, Influence of pain and analgesia on orthopedic and wound-healing models in rats and mice, Comp. Med 69 (2019) 535–545, 10.30802/AALAS-CM-19-000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Berg JO, Mössner BK, Skov MN, Lauridsen J, Gottrup F, Kolmos HJ, Antibacterial properties of EMLA and lidocaine in wound tissue biopsies for culturing, Wound Repair Regen 14 (2006) 581–585, 10.1111/J.1743-6109.2006.00157.X. [DOI] [PubMed] [Google Scholar]
- [186].Dai X, Gakidou E, Lopez AD, Evolution of the global smoking epidemic over the past half century: strengthening the evidence base for policy action, Tob. Control 31 (2022) 129–137, 10.1136/TOBACCOCONTROL-2021-056535. [DOI] [PubMed] [Google Scholar]
- [187].Wong LS, Martins-Green M, Firsthand cigarette smoke alters fibroblast migration and survival: implications for impaired healing, Wound Repair Regen 12 (2004) 471–484, 10.1111/J.1067-1927.2004.12403.X. [DOI] [PubMed] [Google Scholar]
- [188].McRobert J, Smoking and its effects on the healing process of chronic wounds, Br. J. Community Nurs Suppl. (2013), 10.12968/BJCN.2013.18.SUP3.S18. [DOI] [PubMed] [Google Scholar]
- [189].McDaniel JC, Browning KK, Smoking, chronic wound healing, and implications for evidence-based practice, J. Wound Ostomy Continence Nurs 41 (2014) 415–423, 10.1097/WON.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Zia S, Ndoye A, Lee TX, Webber RJ, Grando SA, Receptor-mediated inhibition of keratinocyte migration by nicotine involves modulations of calcium influx and intracellular concentration, J. Pharmacol. Exp. Ther 293 (2000) 973. [PubMed] [Google Scholar]
- [191].Brito MVH, Yasojima EY, Silveira EL, Yamaki VN, Teixeira RKC, Feijó DH, Gonçalves TB, New experimental model of exposure to environmental tobacco smoke, Acta Cir. Bras 28 (2013) 815–819, 10.1590/S0102-86502013001200002. [DOI] [PubMed] [Google Scholar]
- [192].Florescu A, Ferrence R, Einarson T, Selby P, Soldin O, Koren G, Methods for quantification of exposure to cigarette smoking and environmental tobacco smoke: focus on developmental toxicology, Ther. Drug Monit 31 (2009) 14–30, 10.1097/FTD.0B013E3181957A3B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Trombitas V, Nagy A, Berce C, Tabaran F, Albu S, Effect of cigarette smoke on wound healing of the septal mucosa of the rat, Biomed. Res. Int 2016 (2016), 10.1155/2016/6958597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Liu Z, Liu X-W, Lu S-F, Yu A-L, Zhang Z-W, Effect of nicotine withdrawal on pain sensitivity in rats to mechanical stimulation and thermal stimulation, Eur. Rev. Med. Pharmacol. Sci 18 (2014) 2759–2765. [PubMed] [Google Scholar]
- [195].Caille S, Clemens K, Stinus L, Cador M, Modeling nicotine addiction in rats, Methods Mol. Biol 829 (2012) 243–256, 10.1007/978-1-61779-458-2_15. [DOI] [PubMed] [Google Scholar]
- [196].Carnevali S, Nakamura Y, Mio T, Liu X, Takigawa K, Romberger DJ, Spurzem JR, Rennard SI, Cigarette smoke extract inhibits fibroblast-mediated collagen gel contraction, Am. J. Physiol 274 (1998), 10.1152/AJPLUNG.1998.274.4.L591. [DOI] [PubMed] [Google Scholar]
- [197].Gouin JP, Kiecolt-Glaser JK, The impact of psychological stress on wound healing: methods and mechanisms, Immunol. Allergy Clin. North Am 31 (2011) 81–93, 10.1016/J.IAC.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Vileikyte L, Stress and wound healing, Clin. Dermatol 25 (2007) 49–55, 10.1016/J.CLINDERMATOL.2006.09.005. [DOI] [PubMed] [Google Scholar]
- [199].Burgess JL, Wyant WA, Abujamra BA, Kirsner RS, Jozic I, Diabetic wound-healing science, Medicina (Kaunas) 57 (2021), 10.3390/MEDICINA57101072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Lazzarini PA, Crews RT, van Netten JJ, Bus SA, Fernando ME, Chadwick PJ, Najafi B, Measuring plantar tissue stress in people with diabetic peripheral neuropathy: a critical concept in diabetic foot management, J. Diabetes Sci. Technol 13 (2019) 869–880, 10.1177/1932296819849092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Sheridan JF, Padgett DA, Avitsur R, Marucha PT, Experimental models of stress and wound healing, World J. Surg 28 (2004) 327–330, 10.1007/S00268-003-7404-Y. [DOI] [PubMed] [Google Scholar]
- [202].van Baak MA, Pramono A, Battista F, Beaulieu K, Blundell JE, Busetto L, Carraça EV, Dicker D, Encantado J, Ermolao A, Farpour-Lambert N, Woodward E, Bellicha A, Oppert JM, Effect of different types of regular exercise on physical fitness in adults with overweight or obesity: systematic review and meta-analyses, Obes. Rev 22 (2021), 10.1111/OBR.13239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Wang Y, Beydoun MA, Min J, Xue H, Kaminsky LA, Cheskin LJ, Has the prevalence of overweight, obesity and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic, Int. J. Epidemiol 49 (2020) 810–823, 10.1093/IJE/DYZ273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Pence BD, Woods JA, Exercise, obesity, and cutaneous wound healing: evidence from rodent and human studies, Adv. Wound Care (New Rochelle) 3 (2014) 71–79, 10.1089/WOUND.2012.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Nascimento AP, Costa AMA, Overweight induced by high-fat diet delays rat cutaneous wound healing, Br. J. Nutr 96 (2006) 1069–1077, 10.1017/BJN20061955. [DOI] [PubMed] [Google Scholar]
- [206].Deol ZK, Lakhanpal S, Franzon G, Pappas PJ, Effect of obesity on chronic venous insufficiency treatment outcomes, J. Vasc. Surg. Venous Lymphat. Disord 8 (2020) 617–628.e1, 10.1016/J.JVSV.2020.04.006. [DOI] [PubMed] [Google Scholar]
- [207].Pierpont YN, Dinh TP, Salas RE, Johnson EL, Wright TG, Robson MC, Payne WG, Obesity and surgical wound healing: a current review, ISRN Obes 2014 (2014) 1–13, 10.1155/2014/638936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Alawainati MA, Ayoob ZA, AlQari AA, Makhlooq F, Naser HS, Bukamal F, Prevalence and characteristics of obesity in patients with type-2 diabetes mellitus in primary care centers in Bahrain: a cross-sectional study, J. Family Community Med 30 (2023) 109–115, 10.4103/JFCM.JFCM_9_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Holcomb VB, Keck VA, Barrett JC, Hong J, Libutti SK, Núñez NP, Obesity impairs wound healing in ovariectomized female mice, In Vivo (Brooklyn) 23 (515) (2009). [PubMed] [Google Scholar]
- [210].Doulberis M, Papaefthymiou A, Polyzos SA, Katsinelos P, Grigoriadis N, Srivastava DS, Kountouras J, Rodent models of obesity, Minerva Endocrinol 45 (2020) 243–263, 10.23736/S0391-1977.19.03058-X. [DOI] [PubMed] [Google Scholar]
- [211].Leaper D, Assadian O, Edmiston CE, Approach to chronic wound infections, Br. J. Dermatol 173 (2015) 351–358, 10.1111/BJD.13677. [DOI] [PubMed] [Google Scholar]
- [212].Malone M, Bjarnsholt T, McBain AJ, James GA, Stoodley P, Leaper D, Tachi M, Schultz G, Swanson T, Wolcott RD, The prevalence of biofilms in chronic wounds: a systematic review and meta-analysis of published data, J. Wound Care 26 (2017) 20–25, 10.12968/JOWC.2017.26.1.20. [DOI] [PubMed] [Google Scholar]
- [213].Liu YF, Ni PW, Huang Y, Xie T, Therapeutic strategies for chronic wound infection, Chin. J. Traumatol 25 (2022) 11–16, 10.1016/J.CJTEE.2021.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Xu J, Ma Y, Zhu X, Zhang J, Cheng Z, Wu W, Wang P, Enhanced autophagy promotes the clearance of Pseudomonas aeruginosa in diabetic rats with wounds, Ann. Transl. Med 8 (2020) 1362, 10.21037/ATM-20-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Bowler PG, Davies BJ, The microbiology of infected and noninfected leg ulcers, Int. J. Dermatol 38 (1999) 573–578, 10.1046/J.1365-4362.1999.00738.X. [DOI] [PubMed] [Google Scholar]
- [216].Freiberg JA, The mythos of laudable pus along with an explanation for its origin, J. Community Hosp. Intern. Med. Perspect 7 (2017) 196–198, 10.1080/20009666.2017.1343077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Russell DG, Staphylococcus and the healing power of pus, Cell Host Microbe 3 (2008) 115–116, 10.1016/J.CHOM.2008.02.008. [DOI] [PubMed] [Google Scholar]
- [218].Kolimi P, Narala S, Nyavanandi D, Youssef AAA, Dudhipala N, Innovative treatment strategies to accelerate wound healing: trajectory and recent advancements, Cells 11 (2022), 10.3390/CELLS11152439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Domínguez-Oliva A, Hernández-Ávalos I, Martínez-Burnes J, Olmos-Hernández A, Verduzco-Mendoza A, Mota-Rojas D, The importance of animal models in biomedical research: current insights and applications, Animals (Basel) 13 (2023), 10.3390/ANI13071223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Chou PR, Lin YN, Wu SH, Lin SD, Srinivasan P, Hsieh DJ, Huang SH, Supercritical carbon dioxide-decellularized porcine acellular dermal matrix combined with autologous adipose-derived stem cells: its role in accelerated diabetic wound healing, Int. J. Med. Sci 17 (2020) 354–367, 10.7150/IJMS.41155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Uckun FM, Orhan C, Tuzcu M, Durmus AS, Ozercan IH, Volk M, Sahin K, RJX improves wound healing in diabetic rats, Front. Endocrinol. (Lausanne) 13 (2022), 10.3389/FENDO.2022.874291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Uckun FM, Carlson J, Orhan C, Powell J, Pizzimenti NM, van Wyk H, Ozercan IH, Volk M, Sahin K, Rejuveinix shows a favorable clinical safety profile in human subjects and exhibits potent preclinical protective activity in the lipopolysaccharide-galactosamine mouse model of acute respiratory distress syndrome and multi-organ failure, Front. Pharmacol 11 (2020), 10.3389/FPHAR.2020.594321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Linder-Ganz E, Engelberg S, Scheinowitz M, Gefen A, Pressure-time cell death threshold for albino rat skeletal muscles as related to pressure sore biomechanics, J. Biomech 39 (2006) 2725–2732, 10.1016/J.JBIOMECH.2005.08.010. [DOI] [PubMed] [Google Scholar]
- [224].Gurtner GC, Wong VW, Sorkin M, Glotzbach JP, Longaker MT, Surgical approaches to create murine models of human wound healing, J. Biomed. Biotechnol 2011 (2011), 10.1155/2011/969618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Tan MLL, Chin JS, Madden L, Becker DL, Challenges faced in developing an ideal chronic wound model, Expert Opin. Drug Discovery 18 (2023) 99–114, 10.1080/17460441.2023.2158809. [DOI] [PubMed] [Google Scholar]
- [226].Järbrink K, Ni G, Sönnergren H, Schmidtchen A, Pang C, Bajpai R, Car J, The humanistic and economic burden of chronic wounds: a protocol for a systematic review, Syst. Rev 6 (2017), 10.1186/S13643-016-0400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


