Abstract
Background: The interplay between breast cancer treatment and osteoporosis has important consequences for anticancer therapy and patient bone health. Many breast cancer therapies involve hormonal treatments that lower estrogen levels, which can lead to an increased risk of osteoporosis due to reduced bone mineral density. Aromatase inhibitors, chemotherapy, and surgeries such as oophorectomy can further aggravate bone loss, highlighting the necessity of prioritizing bone health during cancer treatment.
Objective: This review is aimed at investigating the complex relationship between breast cancer therapies and bone health by examining the molecular and cellular mechanisms through which anticancer treatments lead to bone loss. It also seeks to assess the effects of various treatment options, such as selective estrogen receptor modulators (SERMs) and bisphosphonates, on reducing bone loss and maintaining bone health during cancer therapy.
Method: The review explores the mechanisms underlying bone loss in breast cancer patients undergoing treatment, focusing on factors such as estrogen depletion, inflammatory cytokines, and changes in bone remodelling processes. Additionally, it evaluates the efficacy of different therapeutic interventions, including pharmacological treatments like bisphosphonates and third-generation SERMs, in mitigating bone-related side effects.
Results: The findings indicate a critical need to balance the effectiveness of breast cancer treatments with the preservation of bone health. Pharmacological treatments like bisphosphonates and denosumab have been identified as essential for managing bone health in breast cancer patients. Furthermore, third-generation SERMs show potential in reducing bone loss associated with cancer therapy.
Keywords: body mineral density (BMD), breast cancer, correlation, medication, osteoporosis
1. Introduction
Breast cancer (BC) is the most persistent malignancy and owns the second-largest death rate in women universally [1, 2]. Approximately 4.1 million women with a history of BC were calculated in 2022 with 339,250 active cases with 43,250 death cases [3]. BC begins in the breast tissue and, if untreated, has the potential to spread to other parts of the body [4]. It affects about one in eight women throughout their lifetime. According to the studies, BC patient goes through various psychological stages, transitions, transformations, and exploration in surviving and diagnosing the disease [5]. One of the major transformations is situated with the bone microarchitecture which is characterized by severe pain, reduction in bone mineral density (BMD), impaired mobility, bone breakability, and a proclivity to develop pathologic fractures, spinal cord compression, bone marrow aplasia, and hypercalcemia [6–9]. These conditions are the chief cause of morbidity which may result in osteopenia/osteoporosis (OST), bone malignancies (osteolytic and osteoblastic), and multiple myeloma (inside bone marrow) [10]. As per the cohort study, BC treatments can increase the risk of osteoporosis and fractures in patients, with postmenopausal women (generally aged 50 and above) being especially susceptible due to age-related decreases in bone density [11]. Additionally, younger patients who undergo premature menopause as a result of cancer treatments may also face a higher risk of fractures, though this occurs through different mechanisms [11]. An estimated 200 million people are affected by osteoporosis worldwide, and amongst them, 68% of people have a prevalence of BC-induced osteoporosis, and over 8.9 million osteoporosis-related fragility fractures of the hip, spine, and wrist are reported annually [12, 13]. Although BC and OST initially appear unconnected, there is evidence to support the possibility of a link between the two. During menopause due to decreased circulating hormones (estrogen), bone loss is distressing in women with a 3%–5% rise in 5 years, which is one of the few characteristics that demonstrate an association between BC and OST in postmenopausal women. Certain important therapies, such as chemotherapy (CT) and hormone therapy, cause a decline in bone density and a reduction in estrogen levels in BCs that have estrogen receptors (ERs) [14–16]. Additional reasons include vitamin D insufficiency and a significant lifestyle including smoking, drinking excessively, and being inactive, all of which raise the risk of BC-associated osteoporosis [17, 18]. Some genetic abnormalities also enhance the risk; for instance, mutations in the COL1A1 and COL1A2 genes are linked to an increased risk of osteoporosis, whereas mutations in the BRCA1 and BRCA2 genes are linked to an increased risk of BC [19, 20]. According to epidemiologic research, approximately 70% of whole patients dying of BC have evidence of metastatic bone disease [21].
The present state of knowledge on the morphological and molecular subtypes of BC is covered in this review. A deeper comprehension of the biological processes of BC underlying osteoporosis development, which is influenced by BC metastasis, could lead to more targeted therapeutic strategies that address both cancer progression and bone health simultaneously. Here, we analyze BC treatments, which are the vital cause of osteoporosis. The key to finding innovative anticancer therapy targets for heterogeneous BC that can avoid serious conditions like osteoporosis can be observed in this article.
2. Distribution of BC—Histological and Molecular Classification
BC is a diverse illness with a spectrum of different subtypes, each with distinct biological traits that influence how patients adapt to different therapeutic interventions and their clinical prognoses [22, 23]. Using a hierarchical clustering method, groundbreaking research examining the gene expression profiles of breast tumours has classified them into “intrinsic subtypes” or “molecular subtypes,” depending on how closely related their gene expression patterns are [24]. By identifying four molecular subtypes of BC—Luminal A, Luminal B, Human Epidermal Growth Factor Receptor 2 (HER2-enriched or c-ERBB2), and basal-like [25–27] gene expression profiling has fundamentally altered our understanding of the disease. These intrinsic subtypes differ significantly in incidence, response to therapy, disease progression, survival, and imaging characteristics. But based on “histological” and “molecular” characteristics, there is a diminutive alteration in cataloguing the subtypes. Breast tumours that express the ER and/or progesterone receptor (PR) are quoted to be “hormone receptor-positive” BCs, whereas those that do not express the ER, PR, or HER2 are referred to as “triple-negative breast cancers” (TNBC) which is well-thought-out to be the most antagonistic [1, 28–30]. In 2000, Perou et al. were the first to find four molecular subtypes, including luminal, HER2-enriched, basal-like, and normal breast-like, in their study of changes in the gene expression patterns of 8102 human genes from 40 distinct malignant human breast tissue samples [31]. Later, Prat and Perou, in their studies, subdivided luminal type into Luminal A and Luminal B [32]. Coherence analysis reinforced the identification of Luminal A, Luminal B, HER2-enriched, and basal-like as the four primary intrinsic subtypes of BC [33]. In addition, the fifth intrinsic subtype of BC, claudin-low BC, was identified in a combined investigation of human and rodent mammary cancers in 2007 [34, 35].
Wellings and Jensen [36] disproved the long-held belief that different histological types of BC would develop from different microanatomical structures of the normal breast by demonstrating that the vast majority of invasive BCs and their in situ precursors originate from the terminal duct-lobular unit regardless of the histological type. Microarray-based investigation of 11 unique varieties of BC has shown that each special type is made up of tumours that are more homogeneous than IDC-NSTs (invasive ductal carcinoma no special types) and invasive lobular carcinomas (ILCs) at the transcriptome level [29]. Except for apocrine carcinomas, hierarchical cluster analysis showed that each histological special type under study related to just one molecular subtype [37]. According to earlier research, adenoid cystic, medullary, and metaplastic carcinomas consistently presented a basal-like phenotype, while tubular, mucinous, and neuroendocrine carcinomas consistently demonstrated a luminal phenotype [1, 28, 38]. None of the tumours, however, belonged to the subtype that resembled a normal breast, and 6% of the rarer forms of BC had a molecular apocrine character (Table 1). According to the WHO (2012), breast malignancies fall into two major categories: carcinomas or sarcomas, depending on which cell origin is involved [37]. Breast tumours known as carcinomas develop from the cells that line the lobules and terminal ducts that produce milk in the breast, or the epithelial component of the breast. Breast stromal cells, such as myofibroblasts and blood vessel cells, are the source of sarcomas, a far more uncommon type of BC (1% of initial BC). The majority of breast malignancies are carcinomas [41]. Based on their degree of invasiveness about the primary tumour sites, the several forms of BC within the broad category of carcinomas are distinguished. “Noninvasive (or in situ),” “invasive,” and “metastatic breast” cancers are the three main categories of frequent BCs [1, 42, 43].
Table 1.
The spread of breast cancer metastasis to different organs.
| Metastasis to organs | Spreadability % | Types of intrinsic factors involved in metastasis | References |
|---|---|---|---|
| Bone | 67% | Luminal A—79% | [1, 26, 39] |
| Luminal B—75% | |||
| HER2+—60% | |||
| Basal-like—40% | |||
|
| |||
| Liver | 40.8% | Basal-like (TNBC) and HER2+ are more frequent than others | [1, 25, 39] |
|
| |||
| Lung | 36.9% | Luminal A—25% | [32, 34, 39] |
| Luminal B—30% | |||
| HER2+—45% | |||
| Basal-like—35% | |||
|
| |||
| Brain | 12.6% | Luminal A—< 10% | [25, 26, 40] |
| Luminal B—10% | |||
| HER2+—30% | |||
| Basal-like—25% | |||
|
| |||
| Auxillary lymph nodes | 30%–50% | Not specified | [39, 40] |
| Mamary internal chain lymph nodes | 10%–40% | ||
| Supraclavicular lymph nodes | 1%–4% | ||
| Contralateral breast | 6% | ||
3. Noninvasive (or In Situ) BC
The term “intraductal carcinoma” also refers to ductal carcinoma in situ (DCIS). DCIS is a noninvasive or preinvasive BC that spreads through ducts and distorts ductal architecture [43]. Even though DCIS is not an invasive cancer, in situ carcinomas have a high potential to progress to an invasive cancer (unilateral); hence, it is crucial to treat the patient effectively and promptly if they want to avoid acquiring invasive cancer [44, 45].
4. Invasive or Infiltrating BC
Invasive breast tumours penetrate the stromal tissue surrounding the lobules and ducts with cancer cells that also spread outside of those structures. Two-thirds of women, or those over 55, are affected by this aggressive variant. Two subgroups of invasive BCs are recognised: invasive ductal carcinoma (IDC): about 80% of all BCs are IDCs, and the most prevalent kind of BC. It develops from DCIS with a fibrous response to produce a mass and metastasizes through lymphatics and blood. Several subtypes in the IDC classification include cribriform carcinoma of the breast, papillary carcinoma of the breast, mucinous carcinoma (MC) of the breast, and tubular carcinoma of the breast [44, 45]. ILC: ILC is the second most frequent type that ranges between 10% and 15% of all carcinomas. It develops from isolated tumour cells or in CDH 1 mutation with minimal fibrous response and metastasizes through viscera. ILC can distress women of any age but is more widespread in older women [46, 47]. Invasive subtypes collectively account for 90%–95% of all BC cases. Invasive ductal and lobular carcinomas with different pathologic characteristics as well as distinctive molecular and genetic abnormalities develop as single cells or in sheets. It is crucial to distinguish between lobular and ductal carcinomas since they may have various prognoses and available treatments that differ from one another [48].
5. Metastatic BC
Late-stage BC that has spread to other body organs are metastatic or stage IV malignancies. Metastatic BC can spread to distant areas such as the lung, liver, bone, and brain as well as to lymph nodes under the armpit [44, 46–48]. Micrometastases may still be present in the body even after the original tumour has been removed, allowing cancer to spread and come back. Clinically, individuals may be diagnosed with metastatic illness (or de novo metastatic BCs) at the time of their initial therapy, or they may experience metastases months or years later. Unfortunately, about 90%–95% of instances of BC are detected during their early stages, but 30% of these women develop a metastatic disease that is still incurable today [49].
6. Inflammatory Breast Cancer (IBC)
IBC, which contributes 1%–6% of all BCs, is a rare and aggressive kind [50]. Breast swelling resembling inflammation, skin that is purple or red, and pitting or thickening of the breast skin are major symptoms of IBC [43, 45, 46]. These symptoms are likely brought on by cancer cells obstructing lymphatic veins in the skin. Mammograms usually fail to detect IBC, which frequently does not present as a breast lump. IBCs are performing more proactively, expanding rapidly, and enlarging [51]. IBC is initially identified when the BC cells have already penetrated the skin locally [50, 51].
7. Paget's Disease of Breast
Paget's disease is a noninvasive epidermal BC, which affects the nipple-areolar complex and is frequently misdiagnosed as benign breast ailments [52]. Around 1%–3% of all BC cases are caused by this rare type of BC, which starts in the breast ducts and travels to the skin around the nipple before reaching the area around the areola [53]. The majority of the cells are positive for the HER2 protein, and about half of the cells exhibit ER+ and PR+ positivity. Usually, a tissue sample is required to detect cancer; occasionally, the diagnosis is corroborated using a mammogram, ultrasound, or MRI [52–54].
8. Papillary Carcinoma
Papillary carcinoma is yet another extremely unusual kind of BC, contributing to almost 0.5%–1% of cases overall [55, 56]. With or without invasion, papillary carcinoma cells are commonly arranged in cellular proliferations (finger-like projections) that surround fibrovascular centres [57]. The majority of papillary carcinomas are invasive and are managed similarly to IDCs. The prognosis of invasive papillary carcinoma is typically better than other types of invasive BC [58].
9. Phyllodes Tumours (PTs)/Carcinoma
PTs, a different uncommon BC, are fibroepithelial neoplasms (2.5%), which often form in the breast stromal cells and have a unique morphology resembling leaves [59, 60]. 0.3%–1% of all BCs are caused by these tumours, of which 25% are aggressive and 60% are benign. Li–Fraumeni syndrome sufferers and women under the age of 40 had a higher risk of developing PT [61].
10. Angiosarcoma of the Breast
Angiosarcomas, a rare and aggressive kind of tumour with epithelial cells that cover blood or lymphatic veins, make up less than 0.05% of malignant tumours. This lesion's invasive tendencies, poor prognosis, and lack of typical radiologic signals are all features of the condition [62, 63].
11. Medullary Breast Carcinoma (MBC)
Medullary carcinoma, a rare, peculiar, and exceptional form of invasive BC, has certain histopathological characteristics [64]. Approximately 3%–5% of all breast carcinomas are MCBs, which have lymphoplasmacytic infiltration, syncytial growth (less than 75%), Grade 2/3 nuclei, a low likelihood of metastasis, and a good prognosis [65]. Higher BRCA1/2 mutation rates and a larger absence of the ER, PR, and HER2 receptors are found in MCBs, which are more curable than high-grade infiltrative carcinomas [66].
12. MC
MC, a rare and unusual histological disease, is MC, also referred to as colloid carcinoma. Most mucus-producing cancer cells that cause MC, which accounts for around 4% of all invasive BC, leak mucus. MC has a better prognosis than a variety of ductal and lobular carcinoma types. Women who are pre- and postmenopausal are more likely to have MC [67, 68].
13. Tubular Carcinoma
Tubular carcinoma is a distinct, well-differentiated tumour that is a low-proliferative Grade 1 neoplasm. It has a favourable prognosis and a low rate of local recurrence. TC, a member of the Luminal A type with high levels of ERs, accounts for between 1% and 4% of all incidences of BC [69–71].
14. Molecular or Intrinsic BC
Gene expression studies have identified a variety of distinct BC subtypes, and these subtypes differ significantly in terms of prognosis and the therapeutic targets that are present in the cancer cells. As previously mentioned, Prat and Perou, Naderi et al., and Sørlie concluded that BC subtypes can be divided into four categories based on the immunohistochemistry expression of hormone receptors [32–34]. This was supported by the cohort analysis. Several gene clusters associated with the expression of the ER (the luminal cluster), the expression of the HER2, proliferation, and a distinct gene cluster known as the basal cluster are now included in the list of intrinsic genes that distinguish these subtypes.
15. Luminal A
Between 50% and 60% of BCs are of the Luminal A subtype. Typically, these tumours have good prognoses and distinct histological types such as tubular, invasive cribriform, mucinous, and lobular. Additionally, they frequently have low nuclear pleomorphism, poor mitotic activity, and low histological grade. Luminal A has higher ER and lower levels of proliferation-related genes [23, 72].
16. Luminal B
The Luminal A subtype makes up 50%–60% of all BCs. These tumours often have good outcomes and distinctive histological types like tubular, invasive cribriform, mucinous, and lobular. They frequently also exhibit low nuclear pleomorphism, low histological grade, low mitotic activity, and low nuclear activity. Luminal A has increased ER and decreased levels of proliferation-related genes [23, 72].
17. HER2-Positive
HER2 is a member of this family and is one of four membrane tyrosine kinases. The HER2 receptor is produced by the HER2 gene, a protooncogene located on chromosome 17q21. As a result of ligand attachment to their extracellular domains, HER proteins undergo dimerization and extracellular domain transphosphorylation. The family or homodimerization itself is activated when produced at very high levels.
18. TNBC
TNBCs, having BRCA1/2 germline and PALB 2 mutations, express aberrant levels of PR, ER, and HER2 and are the most aggressive types [73]. This IDC made for 15%–20% of all incident BC, with a maximal 5-year survival rate and the worst prognosis [74]. Based on the results of gene expression profiling carried out on tumour samples from 587 TNBC patients, Lehmann et al. classified TNBC into six subgroups in 2011: basal-like 1 (BL1), basal-like 2 (BL2), mesenchymal (M), mesenchymal stem–like (MSL), immunomodulatory (IM), and luminal androgen receptor (LAR) [75, 76]. Additionally, they performed gene profiling on TNBC cell lines, analysed them, and split them into six subgroups, producing a solid cell model for clinical TNBC treatment [77, 78]. Figure 1 depicts different histological and molecular types of BC.
Figure 1.
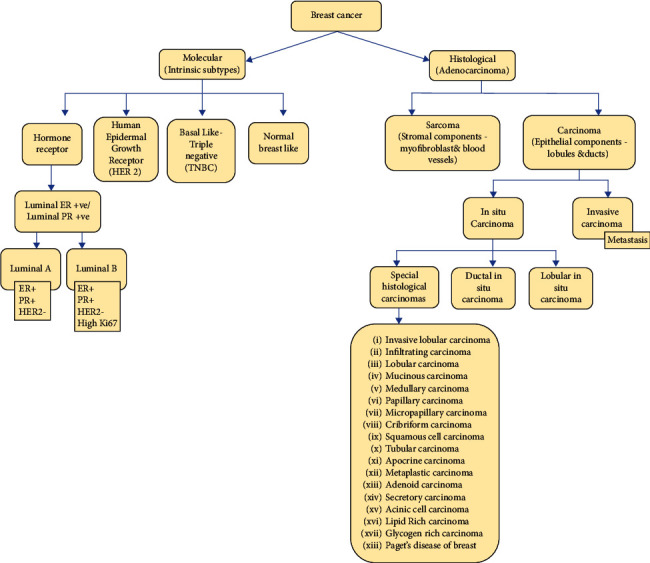
Image depicts different histological and molecular types of breast cancer.
19. Metastasis of Different Types of BC to Bones
In its early stages, BC can harm the liver, lungs, brain, peritoneum, auxiliary lymph nodes, bones, and peritoneum. The most typical site of metastases in BC patients is the bone [79, 80]. Even after it has gone to the bones, cancer can typically still be treated to stop it from growing [81]. However, bone deterioration brought on by BC considerably worsens skeletal problems and lowers the quality of life. Up to 53.71% of BC patients with Stages I–III would develop bone disorders at 15 years of follow-up [82, 83]. BC metastasis to the bones has a high recurrence rate, with approximately 67% of BC tumours metastasizing to the bones. Breast tumours with Luminal B (79%) and Luminal A (70%), as opposed to HER2+ or TNBC (basal-like), are more likely to develop bone disease. Advanced BC will impact the liver and lungs in around 37% of cases, and the auxiliary lymph nodes in between 30% and 50% of cases [84]. It is uncertain how many instances had bone metastases [85]. The relative incidence of bone metastasis in individuals with advanced metastatic sickness varies by cancer type and is 65%–75% in BC, 65%–70% in the prostate, 60% in the thyroid, 30%–40% in the lung, 40% in the bladder, 20%–25% in renal cell carcinoma, and 14%–45% in melanoma. In different types of carcinomas, the median time to death after the diagnosis of bone metastasis is 6 months in the case of melanomas, 6–7 months for lung cancer, 6–9 months for bladder cancer, 12 months for renal cell carcinoma, 12–53 months for prostate cancer, 19–25 months for BC, and 48 months for thyroid cancer [84, 86–88]. Excruciating pain, limited mobility, pathologic fractures, spinal cord compression, and bone metastases are all symptoms of bone metastases, which are the primary cause of morbidity [85, 89].
20. Bone Remodelling
The lifelong process of bone remodelling maintains a balance between bone formation (osteogenesis) by osteoblasts (OBs) and resorption by osteoclasts (OCs), resulting in a mature, dynamic bone structure to safeguard internal organs [90, 91]. Remodelling also happens when a fractured bone is reshaped or when a small crack is repaired [92]. Progenitor stem cells which are housed by the periosteum (bone surface layer) are responsible for the production of “OBs” and “chondroblasts” [93].
20.1. OBs or Bone Formation
In nontumour-containing bone, remodelling begins when OBs sense microcracks or when bones are bearing much weight. At the site of resorption lacunae, a team of OBs produces an extracellular matrix containing Type 1 collagen and various noncollagenous proteins, such as osteopontin, osteocalcin, and osteonectin [93]. Vitamin D, calcium, and alkaline phosphatase help this matrix mineralize. The OB activates M-CSF (macrophage colony-stimulating factor), which helps to stimulate myeloid cells like monocytes [95, 96]. OB also produces a substance called RANKL (receptor activator of nuclear factor κB ligand) which binds to RANK receptors on the surface of nearby monocytes. RANKL induces those monocytes to fuse to form a multinucleated OC cell [97, 98]. Furthermore, to keep bone resorption under control, the OBs also secrete “osteoprotegerin (OPG),” which binds to RANKL, prevents RANK receptors, and ultimately slows down the activation of OCs [99]. Osteoprogenitor, preosteoblast, and OB are the three separate steps of increased differentiation in OBs toward the skeletal lineage. With the assistance of osteoprogenitor cells, the transcription factor SOX-9 is first expressed and regulates the differentiation of chondrocytes based on their cell fate [100]. The sole cell type present in healthy cartilage is the chondrocyte, which creates a cartilaginous matrix made of collagen and proteoglycans. Additionally, the osteoprogenitor cell's preosteoblast has subsequently expressed the Runt-Related Transcription Factor 2 (RUNX2). On the other hand, preosteoblasts are impacted by WNT—catenin signalling throughout the development stage to encourage OBs [101, 102].
20.2. OCs or Bone-Resorbing Cells
OC origin depends upon a series of factors: (1) haemopoietic stem cell of bone marrow, (2) M-CSF which activates monocytes (a myeloid cell), (3) RANKL (a member of the tumour necrosis factor [TNF]) which helps multinucleated OCs (by fusion of monocytes) to mature, and (4) OPG to form a balance between bone formation and resorption [100, 103]. The OC starts secreting proteolytic enzymes mostly cathepsin K (collagen) and MMP9, which digest the collagen protein in the organic matrix [95, 104]. This drill pits on the bone surface known as “Howship's lacunae.” OCs within the sealing zone on the bone matrix start producing hydrochloric acid, which dissolves hydroxyapatite into soluble calcium (Ca2+) and phosphate ions (PO43−), and these ions get released into the bloodstream [100, 104]. Following bone resorption, the OC starts secreting an “osteoid” seam (a substance mainly made up of collagen) to fill in the lacunae, created by OCs. Calcium and phosphate begin to deposit on the seam, forming hydroxyapatite. Also, as OBs keep producing new bony material, many get trapped within tiny lacunae within the bony matrix and turn into osteocytes [90, 105]. Bone remodelling is affected by various hormones, that is, the parathyroid glands. The parathyroid hormone travels to the bones and stimulates the OB to release RANKL which triggers bone resorption [106, 107]. This allows calcium ions (Ca2+) to be released into the bloodstream, and that corrects the deficiency. Now, when the blood calcium level is higher than normal, the parathyroid gland releases less parathyroid hormone to have less bone resorption [108]. In addition, “parafollicular cells” in the thyroid gland produce a hormone called “calcitonin.” High calcitonin levels inhibit bone resorption which results in lower blood calcium levels [109, 110]. Another factor in bone remodelling is mechanical stress. That is why bones that bear a lot of weight remodel at such a high rate—a phenomenon called “Wolff's law” [111]. Vitamin D also stimulates intestinal absorption of calcium, which then causes calcium levels to increase and inhibits bone resorption [112, 113]. General factors that increase the risk of osteoporosis and fractures are given in Table 2.
Table 2.
| Primary factors of osteoporosis | |
| Nonmodifiable risk factors | |
| Factors | Possible cause/risk |
| Age | Personal history of the previous fracture |
| Gender | Genetic (family history) |
| Ethnicity (Asian and Caucasian) | Caucasian and Asian women are at highest risk |
| Modifiable risk factors | |
| Factors | Possible cause/risk |
| Low levels of physical activity (prolonged immobilization and/or sedentary lifestyle) | Estrogen deficiency (early menopause, prolonged amenorrhea periods) |
| Smoking | Low calcium intake or malnutrition |
| Alcohol consumption (≥ 3 units/day) | Osteoporosis secondary to chronic or consumptive diseases |
| Low weight (< 58 kg or 127 lb) | Chronic glucocorticoid use |
| Drugs used in oncology | |
| Aromatase inhibitors (BC) | Chemotherapy |
| Steroidal (exemestane) | Alkylating agents |
| Nonsteroidal (anastrozole and letrozole) | Anthracyclines and docetaxel |
| GnRH agonists (BC: goserelin and triptorelin) | Doxorubicin and excessive alcohol consumption |
| Selective ER modulators (BC) | 5-Fluorouracil |
| LHRH analogues (goserelin, buserelin, leuprorelin, and triptorelin) | Antidepressants and serotonin reuptake inhibitors |
| LHRH antagonists (goserelin) | Oral antidiabetics (thiazolidinediones) |
| Antiandrogens (enzalutamide, bicalutamide, flutamide, and nilutamide) | |
| Other osteopenizing drugs | |
| Methotrexate | NSAID category drugs |
| Megestrol acetate | Estramustine |
| Platinum compounds | Ifosfamide |
| Cyclophosphamide | Radiotherapy and hypogonadism |
| Interferon alfa | Combination of chemotherapy regimens |
| Cyclosporine | Valproic acid |
|
| |
| Secondary factors of osteoporosis | |
| Amenorrhoea | Malabsorption |
| Long-term or high-dose oral corticosteroid use | Smoking |
| Hyperthyroidism | Female hypogonadism |
| Anorexia nervosa | Male hypogonadism |
| Malignant disease | Hyperprolactinaemia |
| Multiple sclerosis/chronic inflammatory arthritis | Cushing's syndrome |
| Immobilization | Thyroxine treatment |
| Rheumatoid arthritis | Chronic inflammatory bowel disease |
| Myeloma | Excessive alcohol intake |
| Treatments for endometriosis | Chronic liver disease |
Abbreviations: BC, breast cancer; ER, estrogen receptor; GnRH, gonadotropin-releasing hormone; kg, kilograms; lb pounds; LHRH, luteinizing hormone-releasing hormone; NSAIDs, nonsteroidal anti-inflammatory drugs; PC, prostate cancer.
21. BC and Osteoporosis
The primary causes of osteoporosis in BC involve estrogen deprivation brought on by CT and hormone treatment (HT), and more particularly, the use of nonsteroidal aromatase inhibitors (AIs) [116]. By attaching to ERs alpha and beta (ER and ER, respectively), which are expressed in both OBs and OCs, estrogens play a crucial role in maintaining the health of bones by reducing bone resorption and bone loss [117, 118]. By increasing the synthesis of OPG and decreasing the synthesis of OC differentiation and proliferation factor RANKL, estrogens increase the proliferation and activity of OBs, decrease the apoptosis of osteocytes (involved in bone formation), and reduce the differentiation and maturation of osteoclastic precursors [119–121]. Rapid bone loss, especially in trabecular bone, can result from CT (especially CT involving alkylating agents and/or 5-fluorouracil) or following HT (especially HT using nonsteroidal AIs). There is a great cellular link between osteoporosis caused by BC treatment, especially CTs. BC CTCs enter the bone via the blood arteries that nourish the bone marrow [24, 122–124]. CTCs undergo a mesenchymal-to-epithelial transition (MET) and begin producing parathyroid hormone-related peptide (PTHrP), at which point they bind to specialized stromal cells lining the bone facing the marrow [125]. PTHrP stimulates the expression of receptor activators of NF-κB ligand (RANKL) and OPG in neighbouring OBs [126]. As a result, OC precursors mature into functional OCs that perform osteolysis, demineralizing the bone and exposing its extracellular matrix. During this action, transforming growth factor, calcium, bone morphogenetic proteins, fibroblast growth factors, and insulin-like growth factor-1 (IGF-1) are all produced, encouraging cancer cell proliferation and survival [127, 128]. The backbiting cycle of bone metastasis is caused by a self-sustaining positive feedback loop powered by TGF, which leads BC cells to produce more PTHrP as they multiply [129, 130]. Whereas hormone-induced ovarian failure, particularly in young women, can be restored months after withdrawal, CT-induced ovarian failure has more acute and difficult-to-reverse effects. Premenopausal women with CT-induced menopause treated with gonadotropin-releasing hormone (GnRH) agonists, women initially treated with tamoxifen (TAM) and then treated with AI, and ultimately women treated alone with AI, particularly those aged 70 years, had the highest risk of osteoporosis [131, 132]. Figure 2 is the pictorial representation of the generation of osteoporosis in patients with BC patients receiving different treatments.
Figure 2.
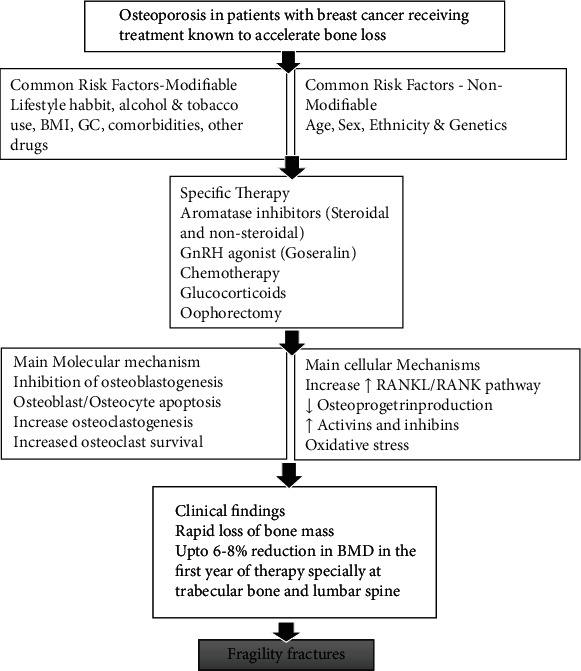
Pictorial representation of the generation of osteoporosis in patients with breast cancer receiving different treatments.
22. From Pathophysiology and Evidence
There is a strong link between osteoporosis and BC that can be attributed to the metabolism of estrogen [133, 134]. It was established in the 1980s that, in addition to a family predisposition, the traditional risk factors for BC (early menarche, late menopause, obesity, nulliparity, and advanced age at first childbirth) are associated with an increased endogenous or exogenous estrogen exposure duration. The risk variables linked to estrogen exposure should be protective against low bone density and the onset of osteoporosis, which, on the other hand, supports the development of BC [135]. Key TJ and group in 2011 proved that circulating higher concentrations of estrogens and androgens (sex hormones) after menopause may function as a mediator to explain how these factors affect BC risk. The potential of estrogen to sustain human bone cells when administered directly through an ER was well established in the 1990s [136]. OBs and OCs are direct receptor-transmitters for the effects of estrogens on bone metabolism. Leptin, neuropeptide Y, TNF, IGF-1, interleukin (IL)-1, and IL-6 are all cytokines and mediators that have a role in estrogen's indirect effects on bone metabolism [137–139]. Because they have a proliferative effect on breast tissue, these mediators and cytokines are regarded as risk factors for the development of BC and its therapy. On the other hand, bone density and bone quality are two key characteristics that are integrated to form bone strength. BMD T-scores, which represent a measured value 2.5 standard deviations below the average of a control group of young women and men, are also used to characterize osteoporosis [134]. BC survivors and their doctors are increasingly concerned about osteoporosis and osteopenia, which are frequently linked to therapy. Several BC treatments, including AIs, chemotherapy-induced ovarian failure (CIOF), and antagonists or agonists of the GnRH, cause bone loss in certain women that are significant enough to lead to osteoporosis and fractures [139]. The predicted 25 million cancer survivors in the United States by 2040, most of whom will be in their sixth, seventh, and eighth decades, are very relevant to osteoporosis [140]. The presence of cancer and several oncology treatments (glucocorticoids, hormone therapy, radiotherapy, and CT) all pose distinct risks for the onset of osteoporosis, bone loss, and fractures [140, 141].
23. Menopause or Postmenopausal Incidences
There is a thorough balance between osteoblastic bone synthesis and osteoclastic bone resorption in the healthy reproductive state. After menopause, there is a considerable change in this process. In addition to the classic symptoms of hot flashes and mucosal shrinkage brought on by physiologically reduced plasma levels of estriol, bone health suffers severely. Despite individual differences, healthy postmenopausal women lose 1% of their bone mass per year [85].
24. Treatments in Postmenopausal Women With BC
In women with BC, CT and endocrine treatments play a major role in intensifying osteoporosis, as they are well known to harm bone metabolism.
24.1. CT
In BC patients, bone density is negatively impacted by new chemotherapeutic drugs, and bone remodelling is negatively impacted by AIs, which can reduce plasma estradiol, estrone, and estrone sulphate concentrations by up to 98% [142–144]. Increased OC activation, increased OC survival and recruitment, increased osteoblastic apoptosis, and an increase in the depth and quantity of bone remodelling units are all consequences of estrogen deprivation [145, 146] (Table 3).
Table 3.
| Therapy | Mechanism | Stage of clinical development |
|---|---|---|
| Bisphosphonates | Block bone resorption; might block tumour-cell mitosis and stimulate tumour-cell apoptosis; alleviate bone pain | On the market |
| Denosumab | A human antibody that is effective in preventing bone loss and bone deterioration | On the market |
| Targeted therapy | ||
| (a) Monoclonal antibodies (palbocilib, ribociclib, and abemaciclib) | Trigger an immune system response that can destroy the outer wall of a cancer cell | On the market |
| (b) With HER2 negative breast cancer (olaparib and talazoparib) | Trap the PARP-1 protein at a single-stranded break and disrupt its catalytic cycle, leading to replication fork progression and double-strand breaks | Phase III |
| (c) With metastatic breast cancer (trastuzumab deruxtecan) | Inhibits HER2 homodimerization, thereby preventing HER2-mediated signalling | Phase III |
| Osteoprotegerin | Prevents RANKL from binding its receptor and stimulating osteoclasts | Phase II |
| RANK-Fc | Prevents RANKL from binding its receptor and stimulating osteoclasts | Phase I |
| PTHrP antibodies | Neutralize PTHrP | Phase III |
| Vitamin D analogues | Decrease PTHrP production | Phase III |
| Hormonal therapy (tamoxifen, anastrozole, exemestane, letrozole, gasorelin, fulvestrant, and elacestrant) | Restore the balance between bone resorption and formation, slowing bone loss and increasing bone mass | On the market (generic) |
Abbreviations: PTHrP, parathyroid-hormone-related peptide; RANK, receptor activator of nuclear factor-κB; RANKL, receptor activator of nuclear factor-κB ligand.
Chemotherapeutic drugs including methotrexate, doxorubicin, taxane, cyclophosphamide, 5-fluorouracil, and cisplatin have been shown to have an inhibitory effect on bone formation and promote bone resorption without increasing bone metastases both in vitro and in animal models [150–152]. Additionally, glucocorticoids such as prednisone and dexamethasone increase bone resorption, decrease osteoblastic activity, alter muscular strength, alter calcium absorption and excretion, and disrupt the pathways for growth hormone and growth factor production [153]. Regardless of age or menopausal state, long-term glucocorticoid (> 7.5–15 mg of prednisone per day for > 5 years) use may lead to a 30% increase in osteoporotic fracture [154]. In animal models, the trabecular bone structure was reduced by 60%. These side effects of CT are in addition to the elevated OC activity seen in BC patients' bones [155, 156]. In case-control research involving 352 postmenopausal women, it was found that women with primary BC had a 2.8-fold higher relative risk and a fivefold higher incidence of fractures annually. Even at the start of the research, women with recurrent BC had a sixfold higher fracture incidence, a 23-fold higher incidence yearly, and a 24.5% higher relative risk [157].
25. Adjuvant CT
Even in the absence of clinically obvious disease, adjuvant systemic CT is frequently utilised in women with early BC after their original tumour has been treated with surgery and radiation. Unfortunately, premenopausal women who receive adjuvant CT run the danger of suffering significant bone loss and premature ovarian failure [158]. Because premenopausal women who get adjuvant CT but do not go through menopause do not experience a significant drop in BMD, it is possible that the estrogen shortage in these women is mostly to blame for bone loss [159, 160]. However, CT can directly damage bones. The fact that 75% of BC patients are postmenopausal at the time of diagnosis makes it crucial to determine if adjuvant CT poses a threat to bone health in addition to the one already posed by estrogen deprivation.
26. AIs
AIs reduce estrogen levels by preventing the aromatase enzyme, which is found in adipose tissue, from converting other hormones into estrogen [161]. These medications do not prevent estrogen production by the ovaries [162]. The cytochrome P450 enzyme aromatase (CYP19), which is found in a variety of tissues including the brain, fat, bone, healthy breast, and BC, transforms androgens from the adrenal gland into estrogens in postmenopausal women [163, 164]. When aromatase is inhibited, estrogen levels fall even further than the already abnormally low levels experienced by postmenopausal women [165]. The three selective AIs used to treat postmenopausal women with breast malignancies expressing the ER and/or PR are anastrozole, exemestane, and letrozole. Women receiving AIs experience a lumbar spine BMD loss of 2%–3% [147]. Randomized studies have shown that women with BC taking AIs have higher fracture rates than those taking TAM [150], and recent retrospective research has found that women taking AIs and bisphosphonates had decreased fracture risks [141]. The fracture risk remains high for the first 5 years of AI treatment and then declines at a rate comparable to that of women taking TAM from Years 5 through 10 [148].
27. Endocrine Treatment
It is generally established that TAM therapy increases BMD in postmenopausal women with BC. An increase in BMD was seen in women who took TAM compared to a yearly decline in the control group (0.6% vs. 1.0%, respectively), according to a randomized placebo-controlled double-blinded experiment. In the same study, women with BC who had TAM treatment had a significantly lower incidence of fractures [166, 167]. Along with the higher BMD, a notable improvement in bone structure was also seen. Regarding the mode of action, AIs work by preventing the activity of the aromatase enzyme, which lowers the level of estradiol in the blood. A decrease in BMD, an increased risk of fracture, and higher markers of bone resorption are all effects of AI-induced estrogen deprivation. Regardless of the kind of AI used, aromatase inhibitor-associated bone loss (AIBL) occurs at a rate that is at least twofold higher than the loss of BMD reported in healthy, age-matched postmenopausal women, leading to a significantly increased incidence of fractures [141, 149]. After 2 years of anastrozole treatment, bone structure as measured by the trabecular bone score (TBS) also considerably declined at the lumbar spine and the hip [168].
28. GnRH Antagonist, Oophorectomy, and CIOF
A GnRH antagonist is a compound that prevents the pituitary gland from producing the hormones, namely, luteinizing hormone (LH) and follicle-stimulating hormone (FSH). This stops the testicles in men from producing testosterone [169]. Treatment with a GnRH agonist (or antagonist) causes bone loss, “chemical menopause,” and is sometimes paired with TAM or AI [170]. Adjuvant CT causes primary ovarian failure, which accelerates bone loss that starts as soon as 6 months after treatment begins and continues to accelerate after 12 months [171]. In the lumbar spine, the extent of bone loss caused by GnRH agonists and CIOF is 6%–8% [172]. While degarelix primarily functions as an antagonist, leuprolide, goserelin, triptorelin, and histrelin are all regarded as GnRH agonists [173]. Because of the reduced ovarian reserve brought on by a decline in the quantity and quality of follicles, the risk of CIOF rises with age. The risk of CIOF is highest with alkylating drugs, such as cyclophosphamide, and lowest with platinum agents, anthracyclines, and taxanes. Increased risk of CIOF is associated with higher cyclophosphamide cumulative doses [174].
29. Selective Estrogen Receptor Modulators (SERMs)
One of three types of HT drugs called SERMs is used to treat ER-positive BC in both men and women, including those who are pre- and postmenopausal. SERMs prevent your body's natural estrogen from interacting with BC cells [175]. SERMs include TAM and raloxifene. Selective ER modulators bind to the ER, acting as an estrogen agonist or antagonist depending on the target tissue. TAM is an estrogen agonist in the bone in preclinical models [176]. TAM, however, prevents bone loss in postmenopausal women while it slightly increases bone loss in premenopausal women [14]. Based on the observation that women on TAM do not experience a reduction in the frequency of fragility fractures, the drug's bone-protective characteristics are rather ineffective [177]. Raloxifene, a medication for osteoporosis and BC prevention approved by the US FDA, raises BMD and lowers spine fractures but does not affect nonspinal fractures [178]. There are stronger and more efficient medications available to treat osteoporosis in women [179].
30. Other Factors for the Induction of Osteoporosis
30.1. Menopause and Aging
Beginning in middle age, bone loss is frequently caused solely by aging and occurs at a rate of 0.5%–1% per year in both men and women [180]. Women lose bone at a rate of 3%–5% per year for the first 5 years after menopause, which is caused mostly by a decrease in the amount of circulating estrogens. Osteoporosis affects one in every three postmenopausal women worldwide, according to estimates [181–183]. Geriatric illnesses such as a predisposition to falls, delirium, dementia, and incontinence can predispose both to osteoporotic fractures, in addition to increasing morbidity and mortality and delaying the time it takes for fractures to heal [184].
30.2. Vitamin D Deficiency
Other than menopause and age, secondary causes of bone loss include disease conditions that might induce osteoporosis [185]. These diseases, if present, have the potential to exacerbate the detrimental effects of BC treatment on bone health [14]. The most frequent secondary cause identified in both groups in this study was a vitamin D deficiency. These results are in line with those of other researchers who have discovered that vitamin D deficiency may be the primary cause of speeding up bone loss in people on an AI [186].
30.3. Idiopathic Hypercalciuria and Primary Hyperparathyroidism
The BC group exhibited a slightly greater prevalence of idiopathic hypercalciuria than the non-BC group. This disease is characterized by elevated urine calcium excretion in the absence of the use of diuretics or other disorders that cause hypercalcemia, such as high calcium or vitamin D consumption, primary hyperparathyroidism, sarcoidosis, or malignancies. Hypercalcemia affects up to 4% of the population and has been associated with cancer, primary hyperparathyroidism, eating too much calcium or vitamin D, ectopic production of 1,25-dihydroxy vitamin D (1,25(OH)2D), and inadequate 1,25(OH)2D degradation. Excessive vitamin D3 (or D2) consumption can result in hypercalcemia and hypercalciuria [187]. In healthy people, blood calcium, phosphorus, and 25 OHD levels are normally normal; however, as calcium consumption declines, serum calcium levels fall, which may increase PTH levels. Reduced PTH levels produce an increase in sclerostin, Cyp27b1, and S 1,25(OH)2D levels, which appears to promote ductal tubule calcium reabsorption and cause bone resorption or bone demineralization, resulting in osteopenia and osteoporosis [188]. Secondary causes of bone loss affect between 30% and 50% of women with osteoporosis. Vitamin D deficiency, idiopathic hypercalciuria, glucocorticoid excess, primary and secondary hyperparathyroidism, hyperthyroidism, hypogonadism, drugs, malabsorption, medication side effects, gastrointestinal disorders, hematologic disorders, Cushing's syndrome, solid organ (lung, liver, heart, and kidney) failure, and transplantation are some of the more prevalent causes of secondary osteoporosis [189, 190].
31. Management of Osteoporosis
Regarding osteoporosis, adequate calcium intake (1000 mg/day) and additional vitamin D (1000–2000 units/day) administration to postmenopausal women with osteopenia to prevent bone loss are well-proven [191]. Calcium intake over a lifetime has been shown to reduce the risk of osteoporosis by up to 20%. A review of 45 studies involving patients in nursing homes found that those who take calcium and vitamin D supplements have a lower likelihood of hip fractures [192]. A meta-analysis of postmenopausal-aged women and men (n = 45,509) revealed a lower fracture risk of 18%, which supported this finding [192]. Administration of calcium and vitamin D, however, is insufficient to stop AIBL. Apart from supplements (calcium and vitamin D) intake, certain lifestyle changes are mandatory for curing osteoporosis. Amongst all the management procedures for osteoporosis, bisphosphonates grab the first position as a first-line treatment for curing bone fragility issues. Alendronate, zoledronate, risedronate, and ibandronate are some of the bisphosphonates which are drugs used to treat fragile bones [193]. However, every treatment has its sort of side effects, and one of them is situated with bisphosphonates. When taking bisphosphonates orally with a glass full of water, a person should sit in an upright position, not lying on the bed, as it causes severe ulcers throughout the oesophagus and stomach [194, 195]. Similarly, with denosumab treatment, skin eczema, flatulence, cellulitis, and osteonecrosis of the jaw were seen [196]. TAM and raloxifene also have some severe adverse reactions. TAM depicts some severe pharmacodynamic effects, and a noncardioprotective drug causes thromboembolism and fatty liver. It also increases the oxygen species pathway within mitochondria, leading to cell apoptosis and induced aging [197]. Likewise, raloxifene leads to blood clots and increases the risk of death due to stroke [198]. Figure 3 demonstrates the complex interaction between estrogen signalling and BC development, focusing on how estradiol (E2), produced by adipose tissue, is a key driver of cancer cell proliferation. Estradiol binds to its receptors, initiating the activation of transcription factors such as ERK, which regulates cell cycle progression (G1, G2, and G3 phases) and contributes to tumour growth by enhancing processes like proliferation, angiogenesis, metastasis, and the reduction of apoptosis.
Figure 3.
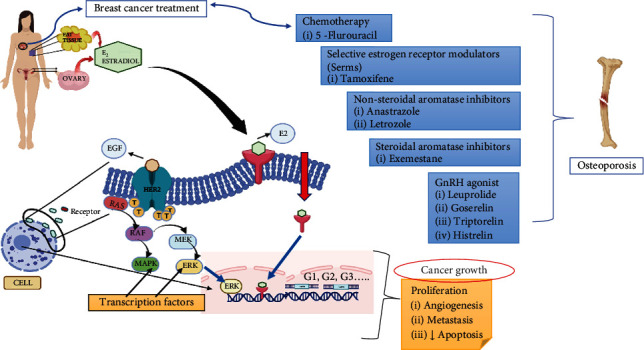
Image showing molecular and genetic level transitions in breast cancer patients that may cause osteoporosis.
To counteract this, BC therapies often target the estrogen pathway. These treatments include CT agents like 5-fluorouracil, SERMs such as TAM, and AIs (both nonsteroidal like anastrozole and letrozole and steroidal like exemestane). Additionally, GnRH agonists such as leuprolide and goserelin are employed to decrease estrogen production. However, one significant side effect of these estrogen-blocking treatments is an increased risk of osteoporosis, as estrogen is essential for bone health. Figure 3 effectively illustrates the connection between these therapies and their side effects, underscoring the challenge of balancing cancer treatment with the preservation of bone integrity.
32. Discussion
BC treatment can cause osteoporosis. At the same time, studies have reported that most of the anticancerous drugs used in the treatment of BC are well known to produce osteoporotic effects. In 2012, Hadji testified that several generations of SERMs have been developed for the prevention and treatment of postmenopausal osteoporosis. Third-generation SERMs (lasofoxifene, bazedoxifene, and raloxifene) exhibit ER agonist and antagonist activity depending on the target tissue and thereby improve bone health. According to the study, it is stated that a perfect SERM would shield bone without triggering breast or endometrial activity and only bazedoxifene reaches clinical use. Apart from that, first-generation SERMs (TAM), which is a first-line treatment for ER-positive BC, depict ER antagonistic activity in the breast and endometrial. Osteoporosis in cancer patients has now become a common phenomenon. This is due to anticancer drugs that, in some cases, when taken for a longer period, can cause bone-related diseases, and one of them is osteoporosis. All of these lead to therapy that involves the simultaneous administration of anticancer and antiosteoporotic drugs. Since osteoporosis is a progressive disease, cumulative targeted approaches in the early stage of disease development are necessary.
33. Conclusion
The text highlights the current state of research on BC diagnosis and treatment, emphasizing the need for further research on early detection. Despite recent developments in techniques for detection and therapy guidance, these techniques have limitations that prevent their use as standalone tools. To plan effective treatment, it is important to understand the molecular heterogeneity of the tumour, and acute stratification is necessary to identify different groups and subgroups, each with its own unique prognosis and systemic therapy requirements. The tone is objective and professional, geared toward a scientific scholar audience. BC and osteoporosis are two significant health issues affecting a large number of people worldwide. Research has established that there is a link between these two conditions. Osteoporosis is more prevalent in females, and the decline in estrogen levels during menopause hastens the condition. Women who have had BC or are at high risk of developing it are often not advised to undergo hormone replacement therapy, which increases the risk of osteoporosis. BC treatments that reduce bone density also increase the risk of osteoporosis and fractures. Studies have suggested that CT-induced estrogen deprivation could be a possible cause of the link between BC and osteoporosis. Further research is needed to identify the underlying mechanisms and pathways of this connection.
Acknowledgments
The authors are thankful to the MVN University and Amity University for providing the necessary facilities to carry out the above-stated work.
Data Availability Statement
The data will be made available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
There is no funding associated with the work featured in this article.
References
- 1.Łukasiewicz S., Czeczelewski M., Forma A., Baj J., Sitarz R., Stanisławek A. Breast cancer—epidemiology, risk factors, classification, prognostic markers, and current treatment strategies—an updated review. Cancers . 2021;13(17):p. 4287. doi: 10.3390/cancers13174287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yedjou C. G., Sims J. N., Miele L., et al. Health and racial disparity in breast cancer. Advances in Experimental Medicine and Biology . 2019;1152:31–49. doi: 10.1007/978-3-030-20301-6_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giaquinto A. N., Sung H., Miller K. D., et al. Breast cancer statistics, 2022. CA: A Cancer Journal for Clinicians . 2022;72(6):524–541. doi: 10.3322/caac.21754. [DOI] [PubMed] [Google Scholar]
- 4.Mehrotra R., Yadav K. Breast cancer in India: present scenario and the challenges ahead. World Journal of Clinical Oncology . 2022;13(3):209–218. doi: 10.5306/wjco.v13.i3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ataollahi M., Sharifi J., Paknahad M., Paknahad A. Breast cancer and associated factors: a review. Journal of Medicine and Life . 2015;8(Special Issue 4):6–11. [PMC free article] [PubMed] [Google Scholar]
- 6.Ciria-Suarez L., Jiménez-Fonseca P., Palacín-Lois M., et al. Breast cancer patient experiences through a journey map: a qualitative study. PloS One . 2021;16(9, article e0257680) doi: 10.1371/journal.pone.0257680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zarrer J., Haider M.-T., Smit D. J., Taipaleenmäki H. Pathological crosstalk between metastatic breast cancer cells and the bone microenvironment. Biomolecules . 2020;10(2):p. 337. doi: 10.3390/biom10020337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rordorf T., Hassan A. A., Azim H., et al. Bone health in breast cancer patients: a comprehensive statement by CECOG/SAKK Intergroup. The Breast . 2014;23(5):511–525. doi: 10.1016/j.breast.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 9.Li B. T., Wong M. H., Pavlakis N. Treatment and prevention of bone metastases from breast cancer: a comprehensive review of evidence for clinical practice. Journal of Clinical Medicine . 2014;3(1):1–24. doi: 10.3390/jcm3010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drake M. T. Osteoporosis and cancer. Current Osteoporosis Reports . 2013;11(3):163–170. doi: 10.1007/s11914-013-0154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramin C., May B. J., Roden R. B. S., et al. Evaluation of osteopenia and osteoporosis in younger breast cancer survivors compared with cancer-free women: a prospective cohort study. Breast Cancer Research . 2018;20(1):p. 134. doi: 10.1186/s13058-018-1061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Villiers T. J., Goldstein S. R. Bone health 2022: an update. Climacteric . 2022;25(1):1–3. doi: 10.1080/13697137.2021.1965408. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro C. L. Osteoporosis: a long-term and late-effect of breast cancer treatments. Cancers . 2020;12(11):p. 3094. doi: 10.3390/cancers12113094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taxel P., Faircloth E., Idrees S., Van Poznak C. Cancer treatment–induced bone loss in women with breast cancer and men with prostate cancer. Journal of the Endocrine Society . 2018;2(7):574–588. doi: 10.1210/js.2018-00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer V., Haffner-Luntzer M. Interaction between bone and immune cells: implications for postmenopausal osteoporosis. Seminars in Cell & Developmental Biology . 2022;123:14–21. doi: 10.1016/j.semcdb.2021.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Cheng C.-H., Chen L.-R., Chen K.-H. Osteoporosis due to hormone imbalance: an overview of the effects of estrogen deficiency and glucocorticoid overuse on bone turnover. International Journal of Molecular Sciences . 2022;23(3):p. 1376. doi: 10.3390/ijms23031376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Martinis M., Allegra A., Sirufo M. M., et al. Vitamin D deficiency, osteoporosis and effect on autoimmune diseases and hematopoiesis: a review. International Journal of Molecular Sciences . 2021;22(16):p. 8855. doi: 10.3390/ijms22168855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LeBoff M. S., Greenspan S. L., Insogna K. L., et al. The clinician’s guide to prevention and treatment of osteoporosis. Osteoporosis International . 2022;33(10):2049–2102. doi: 10.1007/s00198-021-05900-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Augusciak-Duma A., Witecka J., Sieroń A. L., et al. Mutations in COL1A1 and COL1A2 genes associated with osteogenesis imperfecta (OI) types I or III. Acta Biochimica Polonica . 2018;65(1):79–86. doi: 10.18388/abp.2017_1612. [DOI] [PubMed] [Google Scholar]
- 20.Abu-Helalah M., Azab B., Mubaidin R., et al. BRCA1 and BRCA2 genes mutations among high risk breast cancer patients in Jordan. Scientific Reports . 2020;10(1):p. 17573. doi: 10.1038/s41598-020-74250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen N., Akter S., Akhter H., et al. TOR1B: a predictor of bone metastasis in breast cancer patients. Scientific Reports . 2023;13(1):p. 1495. doi: 10.1038/s41598-023-28140-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masood S. Breast cancer subtypes: morphologic and biologic characterization. Womens Health . 2016;12(1):103–119. doi: 10.2217/whe.15.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yersal O., Barutca S. Biological subtypes of breast cancer: prognostic and therapeutic implications. World Journal of Clinical Oncology . 2014;5(3):412–424. doi: 10.5306/wjco.v5.i3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grover M., Behl T., Virmani T., et al. Exploration of cytotoxic potential of longifolene/junipene isolated from chrysopogon zizanioides. Molecules . 2022;27(18):p. 5764. doi: 10.3390/molecules27185764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strehl J. D., Wachter D. L., Fasching P. A., Beckmann M. W., Hartmann A. Invasive breast cancer: recognition of molecular subtypes. Breast Care . 2011;6(4):258–264. doi: 10.1159/000331339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y., Li G., Bian W., et al. Value of genomics- and radiomics-based machine learning models in the identification of breast cancer molecular subtypes: a systematic review and meta-analysis. Annals of Translational Medicine . 2022;10(24):p. 1394. doi: 10.21037/atm-22-5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hon J. D. C., Singh B., Sahin A., et al. Breast cancer molecular subtypes: from TNBC to QNBC. American Journal of Cancer Research . 2016;6(9):1864–1872. [PMC free article] [PubMed] [Google Scholar]
- 28.Zubair M., Wang S., Ali N. Advanced approaches to breast cancer classification and diagnosis. Frontiers in Pharmacology . 2021;11, article 632079 doi: 10.3389/fphar.2020.632079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weigelt B., Geyer F. C., Reis-Filho J. S. Histological types of breast cancer: how special are they? Molecular Oncology . 2010;4(3):192–208. doi: 10.1016/j.molonc.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weigelt B., Reis-Filho J. S. Histological and molecular types of breast cancer: is there a unifying taxonomy? Nature Reviews Clinical Oncology . 2009;6(12):718–730. doi: 10.1038/nrclinonc.2009.166. [DOI] [PubMed] [Google Scholar]
- 31.Perou C. M., Sørlie T., Eisen M. B., et al. Molecular portraits of human breast tumours. Nature . 2000;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 32.Prat A., Perou C. M. Deconstructing the molecular portraits of breast cancer. Molecular Oncology . 2011;5(1):5–23. doi: 10.1016/j.molonc.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naderi S., Rezaei H. R., Taberlet P., et al. Large-scale mitochondrial DNA analysis of the domestic goat reveals six haplogroups with high diversity. PLoS ONE . 2007;2(10):p. e1012. doi: 10.1371/journal.pone.0001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sørlie T., Perou C. M., Tibshirani R., et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proceedings of the National Academy of Sciences of the United States of America . 2001;98(19):10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herschkowitz J. I., Simin K., Weigman V. J., et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biology . 2007;8(5):p. R76. doi: 10.1186/gb-2007-8-5-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wellings S. R., Jensen H. M. On the Origin and Progression of Ductal Carcinoma in the Human Breast. Journal of the National Cancer Institute . 1973;50(5):1111–1118. doi: 10.1093/jnci/50.5.1111. [DOI] [PubMed] [Google Scholar]
- 37.The Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature . 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyai K., Schwartz M. R., Divatia M. K., et al. Adenoid cystic carcinoma of breast: recent advances. World Journal of Clinical Cases . 2014;2(12):732–741. doi: 10.12998/wjcc.v2.i12.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dissanayake R., Towner R., Ahmed M. Metastatic breast cancer: review of emerging nanotherapeutics. Cancers . 2023;15(11):p. 2906. doi: 10.3390/cancers15112906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rostami R., Mittal S., Rostami P., Tavassoli F., Jabbari B. Brain metastasis in breast cancer: a comprehensive literature review. Journal of Neuro-Oncology . 2016;127(3):407–414. doi: 10.1007/s11060-016-2075-3. [DOI] [PubMed] [Google Scholar]
- 41.Grover M., Behl T., Sehgal A., et al. In vitro phytochemical screening, cytotoxicity studies of Curcuma longa extracts with isolation and characterisation of their isolated compounds. Molecules . 2021;26(24):p. 7509. doi: 10.3390/molecules26247509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makki J. Diversity of breast carcinoma: histological subtypes and clinical relevance. Clinical Medicine Insights: Pathology . 2015;8:23–31. doi: 10.4137/CPath.S31563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng Y., Spezia M., Huang S., et al. Breast cancer development and progression: risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes & Diseases . 2018;5(2):77–106. doi: 10.1016/j.gendis.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dieci M. V., Criscitiello C., Goubar A., et al. Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Annals of Oncology . 2014;25(3):611–618. doi: 10.1093/annonc/mdt556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Collaborative Group on Hormonal Factors in Breast Cancer. Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58 209 women with breast cancer and 101 986 women without the disease. The Lancet . 2001;358(9291):1389–1399. doi: 10.1016/S0140-6736(01)06524-2. [DOI] [PubMed] [Google Scholar]
- 46.Veronesi U., Boyle P., Goldhirsch A., Orecchia R., Viale G. Breast cancer. The Lancet . 2005;365(9472):1727–1741. doi: 10.1016/S0140-6736(05)66546-4. [DOI] [PubMed] [Google Scholar]
- 47.Colditz G. A., Wolin K. Y., Gehlert S. Applying what we know to accelerate cancer prevention. Science Translational Medicine . 2012;4(127):p. 127rv4. doi: 10.1126/scitranslmed.3003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Polyak S. J., Morishima C., Shuhart M. C., Wang C. C., Liu Y., Lee D. Y.-W. Inhibition of T-cell inflammatory cytokines, hepatocyte NF-kappaB signaling, and HCV infection by standardized silymarin. Gastroenterology . 2007;132(5):1925–1936. doi: 10.1053/j.gastro.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 49.Allison K. H. Molecular pathology of breast Cancer. American Journal of Clinical Pathology . 2012;138(6):770–780. doi: 10.1309/AJCPIV9IQ1MRQMOO. [DOI] [PubMed] [Google Scholar]
- 50.Pulido A., Chen L., Kaczorowski T., et al. Functional materials discovery using energy-structure-function maps. Nature . 2017;543(7647):657–664. doi: 10.1038/nature21419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mamouch F., Berrada N., Aoullay Z., El Khanoussi B., Errihani H. Inflammatory breast cancer: a literature review. World Journal of Clinical Oncology . 2018;9(5–6):129–135. doi: 10.14740/wjon1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Markarian S., Holmes D. R. Mammary Paget’s disease: an update. Cancers . 2022;14(10):p. 2422. doi: 10.3390/cancers14102422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scardina L., di Leone A., Magno S., et al. Paget’s disease of the breast: our 20 years’ experience. Frontiers in Oncology . 2022;12, article 995442 doi: 10.3389/fonc.2022.995442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feng S., Fragiadaki M., Souilhol C., Ridger V., Evans P. C. Response by Feng et al. to letter regarding article, ‘mechanical activation of hypoxia-inducible factor 1α drives endothelial dysfunction at atheroprone sites. Arteriosclerosis, Thrombosis, and Vascular Biology . 2017;37(12):e199–e200. doi: 10.1161/ATVBAHA.117.310341. [DOI] [PubMed] [Google Scholar]
- 55.Pal S. K., Lau S. K., Kruper L., et al. Papillary carcinoma of the breast: an overview. Breast Cancer Research and Treatment . 2010;122(3):637–645. doi: 10.1007/s10549-010-0961-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ingle S. B., Murdeshwar H. G., Siddiqui S. Papillary carcinoma of breast: minireview. World Journal of Clinical Cases . 2016;4(1):20–24. doi: 10.12998/wjcc.v4.i1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kulka J., Madaras L., Floris G., Lax S. F. Papillary lesions of the breast. Virchows Archiv . 2022;480(1):65–84. doi: 10.1007/s00428-021-03182-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jadhav T., Prasad S. S., Guleria B., Tevatia M. S., Guleria P. Solid papillary carcinoma of the breast. Autopsy and Case Reports . 2022;12, article e2021352 doi: 10.4322/acr.2021.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lissidini G., Mulè A., Santoro A., et al. Malignant phyllodes tumor of the breast: a systematic review. Pathologica . 2022;114(2):111–120. doi: 10.32074/1591-951X-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Demian G. A., Fayaz S., el-Sayed Eissa H., et al. Phyllodes tumors of the breast: analysis of 35 cases from a single institution. Journal of the Egyptian National Cancer Institute . 2016;28(4):243–248. doi: 10.1016/j.jnci.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 61.Rayzah M. Phyllodes tumors of the breast: a literature review. Cureus . 2020;12(9, article e10288) doi: 10.7759/cureus.10288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bordoni D., Bolletta E., Falco G., et al. Primary angiosarcoma of the breast. International Journal of Surgery Case Reports . 2016;20(Supplement):12–15. doi: 10.1016/j.ijscr.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim Y.-J., Ryu J. M., Lee S. K., et al. Primary angiosarcoma of the breast: a single-center retrospective study in Korea. Current Oncology . 2022;29(5):3272–3281. doi: 10.3390/curroncol29050267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dai D., Shi R., Wang Z., et al. Competing risk analyses of medullary carcinoma of breast in comparison to infiltrating ductal carcinoma. Scientific Reports . 2020;10(1):p. 560. doi: 10.1038/s41598-019-57168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee M., Jara-Lazaro A. R., Cheok P. Y., Thike A. A. Medullary breast carcinoma: a pathogenic review and immunohistochemical study using tissue microarray. Singapore Medical Journal . 2022;63(7):394–401. doi: 10.11622/smedj.2021031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Akram M., Iqbal M., Daniyal M., Khan A. U. Awareness and current knowledge of breast cancer. Biological Research . 2017;50(1):p. 33. doi: 10.1186/s40659-017-0140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marrazzo E., Frusone F., Milana F., et al. Mucinous breast cancer: a narrative review of the literature and a retrospective tertiary single-centre analysis. The Breast . 2020;49:87–92. doi: 10.1016/j.breast.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Budzik M. P., Fudalej M. M., Badowska-Kozakiewicz A. M. Histopathological analysis of mucinous breast cancer subtypes and comparison with invasive carcinoma of no special type. Scientific Reports . 2021;11(1):p. 5770. doi: 10.1038/s41598-021-85309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sheppard D. G., Whitman G. J., Fornage B. D., Stelling C. B., Huynh P. T., Sahin A. A. Tubular carcinoma of the Breast. American Journal of Roentgenology . 2000;174(1):253–257. doi: 10.2214/ajr.174.1.1740253. [DOI] [PubMed] [Google Scholar]
- 70.Metovic J., Cascardi E., Uccella S., et al. Neuroendocrine neoplasms of the breast: diagnostic agreement and impact on outcome. Virchows Archiv . 2022;481(6):839–846. doi: 10.1007/s00428-022-03426-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wen C., Xu W., Qin G., et al. Pure and mixed tubular carcinoma of the breast: mammographic features, clinicopathological characteristics and prognostic analysis. Technology in Cancer Research & Treatment . 2021;20, article 153303382110451 doi: 10.1177/15330338211045198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smolarz B., Nowak A. Z., Romanowicz H. Breast cancer-epidemiology, classification, pathogenesis and treatment (review of literature) Cancers . 2022;14(10):p. 2569. doi: 10.3390/cancers14102569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Almansour N. M. Triple-negative breast cancer: a brief review about epidemiology, risk factors, signaling pathways, treatment and role of artificial intelligence. Frontiers in Molecular Biosciences . 2022;9, article 836417 doi: 10.3389/fmolb.2022.836417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zagami P., Carey L. A. Triple negative breast cancer: pitfalls and progress. NPJ Breast Cancer . 2022;8(1):p. 95. doi: 10.1038/s41523-022-00468-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sandhu G. S., Erqou S., Patterson H., Mathew A. Prevalence of triple-negative breast cancer in India: systematic review and meta-analysis. JCO Global Oncology . 2016;2(6):412–421. doi: 10.1200/JGO.2016.005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Howard F. M., Olopade O. I. Epidemiology of triple-negative breast Cancer. The Cancer Journal . 2021;27(1):8–16. doi: 10.1097/PPO.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 77.Yin L., Duan J.-J., Bian X.-W., Yu S. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Research . 2020;22(1):p. 61. doi: 10.1186/s13058-020-01296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lehmann B. D., Bauer J. A., Chen X., et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. Journal of Clinical Investigation . 2011;121(7):2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kennecke H., Yerushalmi R., Woods R., et al. Metastatic behavior of breast cancer subtypes. Journal of Clinical Oncology . 2010;28(20):3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 80.Wu Q., Li J., Zhu S., et al. Breast cancer subtypes predict the preferential site of distant metastases: a SEER based study. Oncotarget . 2017;8(17):27990–27996. doi: 10.18632/oncotarget.15856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Macedo F., Ladeira K., Pinho F., et al. Bone metastases: an overview. Oncology Reviews . 2017;11(1):p. 321. doi: 10.4081/oncol.2017.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pulido C., Vendrell I., Ferreira A. R., et al. Bone metastasis risk factors in breast cancer. ecancermedicalscience . 2017;11:p. 715. doi: 10.3332/ecancer.2017.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang J.-F., Zheng X. Q., Sun X. L., et al. Association between bone mineral density and severity of chronic kidney disease. International Journal of Endocrinology . 2020;2020:11. doi: 10.1155/2020/8852690.8852690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tufail M., Cui J., Wu C. Breast cancer: molecular mechanisms of underlying resistance and therapeutic approaches. American Journal of Cancer Research . 2022;12(7):2920–2949. [PMC free article] [PubMed] [Google Scholar]
- 85.Coleman R., Hadji P., Body J. J., et al. Bone health in cancer: ESMO Clinical Practice Guidelines. Annals of Oncology . 2020;31(12):1650–1663. doi: 10.1016/j.annonc.2020.07.019. [DOI] [PubMed] [Google Scholar]
- 86.Grover M., Behl T., Virmani T. Phytochemical screening, antioxidant assay and cytotoxic profile for different extracts of Chrysopogon zizanioides roots. Chemistry & Biodiversity . 2021;18(8, article e2100012) doi: 10.1002/cbdv.202100012. [DOI] [PubMed] [Google Scholar]
- 87.Chin C. J., Franklin J. H., Moussa M., Chin J. L. Metastasis from renal cell carcinoma to the thyroid 12 years after nephrectomy. CMAJ . 2011;183(12):1398–1399. doi: 10.1503/cmaj.092152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fomchenko E. I., Bayley J. C., Alvarez-Breckenridge C., Rhines L. D., Tatsui C. E. Spinal metastases and the evolving role of molecular targeted therapy, chemotherapy, and immunotherapy. Neurospine . 2022;19(4):978–993. doi: 10.14245/ns.2244290.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tsuzuki S., Park S. H., Eber M. R., Peters C. M., Shiozawa Y. Skeletal complications in cancer patients with bone metastases. International Journal of Urology . 2016;23(10):825–832. doi: 10.1111/iju.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bolamperti S., Villa I., Rubinacci A. Bone remodeling: an operational process ensuring survival and bone mechanical competence. Bone Research . 2022;10(1):p. 48. doi: 10.1038/s41413-022-00219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang L., You X., Zhang L., Zhang C., Zou W. Mechanical regulation of bone remodeling. Bone Research . 2022;10(1):p. 16. doi: 10.1038/s41413-022-00190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Loi F., Córdova L. A., Pajarinen J., Lin T., Yao Z., Goodman S. B. Inflammation, fracture and bone repair. Bone . 2016;86:119–130. doi: 10.1016/j.bone.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Solidum J. G. N., Jeong Y., Heralde F., Park D. Differential regulation of skeletal stem/progenitor cells in distinct skeletal compartments. Frontiers in Physiology . 2023;14, article 1137063 doi: 10.3389/fphys.2023.1137063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin X., Patil S., Gao Y.-G., Qian A. The bone extracellular matrix in bone formation and regeneration. Frontiers in Pharmacology . 2020;11:p. 757. doi: 10.3389/fphar.2020.00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Florencio-Silva R., Sasso G. R., Sasso-Cerri E., Simões M. J., Cerri P. S. Biology of bone tissue: structure, function, and factors that influence bone cells. BioMed Research International . 2015;2015(1) doi: 10.1155/2015/421746.421746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xu H., Wang W., Liu X., et al. Targeting strategies for bone diseases: signaling pathways and clinical studies. Signal Transduction and Targeted Therapy . 2023;8(1):p. 202. doi: 10.1038/s41392-023-01467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lampiasi N., Russo R., Kireev I., Strelkova O., Zhironkina O., Zito F. Osteoclasts differentiation from murine RAW 264.7 cells stimulated by RANKL: timing and behavior. Biology . 2021;10(2):p. 117. doi: 10.3390/biology10020117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fernandes M. H., Gomes P. D. S. Bone cells dynamics during peri-implantitis: a theoretical analysis. Journal of Oral & Maxillofacial Research . 2016;7(3):p. e6. doi: 10.5037/jomr.2016.7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McDonald M. M., Khoo W. H., Ng P. Y., et al. Osteoclasts recycle via osteomorphs during RANKL-stimulated bone resorption. Cell . 2021;184(5):1330–1347.e13. doi: 10.1016/j.cell.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Salhotra A., Shah H. N., Levi B., Longaker M. T. Mechanisms of bone development and repair. Nature Reviews Molecular Cell Biology . 2020;21(11):696–711. doi: 10.1038/s41580-020-00279-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vlashi R., Zhang X., Wu M., Chen G. Wnt signaling: essential roles in osteoblast differentiation, bone metabolism and therapeutic implications for bone and skeletal disorders. Genes & Diseases . 2023;10(4):1291–1317. doi: 10.1016/j.gendis.2022.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Karner C. M., Long F. Wnt signaling and cellular metabolism in osteoblasts. Cellular and Molecular Life Sciences . 2017;74(9):1649–1657. doi: 10.1007/s00018-016-2425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Russell P. K., Clarke M. V., Cheong K., et al. Androgen receptor action in osteoblasts in male mice is dependent on their stage of maturation. Journal of Bone and Mineral Research . 2015;30(5):809–823. doi: 10.1002/jbmr.2413. [DOI] [PubMed] [Google Scholar]
- 104.Everts V., Jansen I. D. C., De Vries T. J. Mechanisms of bone resorption. Bone . 2022;163, article 116499 doi: 10.1016/j.bone.2022.116499. [DOI] [PubMed] [Google Scholar]
- 105.Kim J.-M., Lin C., Stavre Z., Greenblatt M. B., Shim J.-H. Osteoblast-osteoclast communication and bone homeostasis. Cells . 2020;9(9):p. 2073. doi: 10.3390/cells9092073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Siddiqui J. A., Partridge N. C. Physiological bone remodeling: systemic regulation and growth factor involvement. Physiology . 2016;31(3):233–245. doi: 10.1152/physiol.00061.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cannarella R., Barbagallo F., Condorelli R. A., Aversa A., La Vignera S., Calogero A. E. Osteoporosis from an endocrine perspective: the role of hormonal changes in the elderly. Journal of Clinical Medicine . 2019;8(10):p. 1564. doi: 10.3390/jcm8101564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Suva L. J., Friedman P. A. Chapter one-structural pharmacology of PTH and PTHrP. In: Litwack G., editor. Vitamins and Hormones . Vol. 120. Academic Press; 2022. (Parathyroid Hormone). [DOI] [PubMed] [Google Scholar]
- 109.Rolighed L., Rejnmark L., Sikjaer T., et al. Vitamin D treatment in primary hyperparathyroidism: a randomized placebo controlled trial. The Journal of Clinical Endocrinology and Metabolism . 2014;99(3):1072–1080. doi: 10.1210/jc.2013-3978. [DOI] [PubMed] [Google Scholar]
- 110.Hsiao C.-Y., Chen T.-H., Chu T.-H., Ting Y.-N., Tsai P.-J., Shyu J.-F. Calcitonin induces bone formation by increasing expression of Wnt10b in osteoclasts in ovariectomy-induced osteoporotic rats. Frontiers in Endocrinology . 2020;11:p. 613. doi: 10.3389/fendo.2020.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Frost H. M. Bone’s mechanostat: a 2003 update. The Anatomical Record . 2003;275A(2):1081–1101. doi: 10.1002/ar.a.10119. [DOI] [PubMed] [Google Scholar]
- 112.Holick M. F. Vitamin D deficiency. The New England Journal of Medicine . 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 113.Christakos S., Dhawan P., Porta A., Mady L. J., Seth T. Vitamin D and intestinal calcium absorption. Molecular and Cellular Endocrinology . 2011;347(1–2):25–29. doi: 10.1016/j.mce.2011.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Castañeda S., Casas A., González-del-Alba A., et al. Bone loss induced by cancer treatments in breast and prostate cancer patients. Clinical and Translational Oncology . 2022;24(11):2090–2106. doi: 10.1007/s12094-022-02872-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shapiro C. L. Cancer Survivorship. The New England Journal of Medicine . 2019;380(14):1380–1382. doi: 10.1056/NEJMc1900709. [DOI] [PubMed] [Google Scholar]
- 116.Goss P. E., Hadji P., Subar M., Abreu P., Thomsen T., Banke-Bochita J. Effects of steroidal and nonsteroidal aromatase inhibitors on markers of bone turnover in healthy postmenopausal women. Breast Cancer Research . 2007;9(4):p. R52. doi: 10.1186/bcr1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cheung A. M., Heisey R., Srighanthan J. Breast cancer and osteoporosis. Current Opinion in Endocrinology, Diabetes and Obesity . 2013;20(6):532–538. doi: 10.1097/01.med.0000436195.10599.dd. [DOI] [PubMed] [Google Scholar]
- 118.Roman-Blas J. A., Castañeda S., Largo R., Herrero-Beaumont G. Osteoarthritis associated with estrogen deficiency. Arthritis Research & Therapy . 2009;11(5):p. 241. doi: 10.1186/ar2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Handforth C., D’Oronzo S., Coleman R., Brown J. Cancer treatment and bone health. Calcified Tissue International . 2018;102(2):251–264. doi: 10.1007/s00223-017-0369-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rachner T. D., Coleman R., Hadji P., Hofbauer L. C. Bone health during endocrine therapy for cancer. The Lancet Diabetes & Endocrinology . 2018;6(11):901–910. doi: 10.1016/S2213-8587(18)30047-0. [DOI] [PubMed] [Google Scholar]
- 121.Maurizi A., Rucci N. The osteoclast in bone metastasis: player and target. Cancers . 2018;10(7):p. 218. doi: 10.3390/cancers10070218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Choksi P., Williams M., Clark P. M., Poznak C. V. Skeletal manifestations of treatment of breast cancer. Current Osteoporosis Reports . 2013;11(4):319–328. doi: 10.1007/s11914-013-0179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kumar S., Tiwari A., Tiwari V., et al. Synthesis, anticancer, and antimicrobial evaluation of integerrimide-A. BioMed Research International . 2023;2023(1) doi: 10.1155/2023/9289141.e9289141 [DOI] [Google Scholar]
- 124.Rastogi S., Nidhi S., Virmani T., Deshwal C., Gupta J. A review on naturally derived compounds for potential anticancer activity. Indian Drugs Journal . 2016;4:75–86. [Google Scholar]
- 125.Elango J., Rahman S. U., Henrotin Y., et al. Parathyroid hormone-related protein (PTHrP) accelerates soluble RANKL signals for downregulation of osteogenesis of bone mesenchymal stem cells. Journal of Clinical Medicine . 2019;8(6):p. 836. doi: 10.3390/jcm8060836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rashed F., Kamijyo S., Shimizu Y., et al. The effects of receptor activator of NF-κB ligand-binding peptides on bone resorption and bone formation. Frontiers in Cell and Developmental Biology . 2021;9, article 648084 doi: 10.3389/fcell.2021.648084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Landini L., Marini M., Souza Monteiro de Araujo D., et al. Schwann cell insulin-like growth factor receptor type-1 mediates metastatic bone cancer pain in mice. Brain, Behavior, and Immunity . 2023;110:348–364. doi: 10.1016/j.bbi.2023.03.013. [DOI] [PubMed] [Google Scholar]
- 128.Witsch E., Sela M., Yarden Y. Roles for growth factors in cancer progression. Physiology . 2010;25(2):85–101. doi: 10.1152/physiol.00045.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wong S. K., Mohamad N.-V., Giaze T. R., Chin K.-Y., Mohamed N., Ima-Nirwana S. Prostate cancer and bone metastases: the underlying mechanisms. International Journal of Molecular Sciences . 2019;20(10):p. 2587. doi: 10.3390/ijms20102587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Coughlin T. R., Romero-Moreno R., Mason D. E., et al. Bone: a fertile soil for cancer metastasis. Current Drug Targets . 2017;18(11):1281–1295. doi: 10.2174/1389450117666161226121650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hadji P., Gnant M., Body J. J., et al. Cancer treatment-induced bone loss in premenopausal women: a need for therapeutic intervention? Cancer Treatment Reviews . 2012;38(6):798–806. doi: 10.1016/j.ctrv.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 132.Zaman K., Thürlimann B., Huober J., et al. Bone mineral density in breast cancer patients treated with adjuvant letrozole, tamoxifen, or sequences of letrozole and tamoxifen in the BIG 1-98 study (SAKK 21/07) Annals of Oncology . 2012;23(6):1474–1481. doi: 10.1093/annonc/mdr448. [DOI] [PubMed] [Google Scholar]
- 133.Mills E. G., Yang L., Nielsen M. F., Kassem M., Dhillo W. S., Comninos A. N. The relationship between bone and reproductive hormones beyond estrogens and androgens. Endocrine Reviews . 2021;42(6):691–719. doi: 10.1210/endrev/bnab015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sözen T., Özışık L., Başaran N. Ç. An overview and management of osteoporosis. European Journal of Rheumatology . 2017;4(1):46–56. doi: 10.5152/eurjrheum.2016.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dall G. V., Britt K. L. Estrogen effects on the mammary gland in early and late life and breast cancer risk. Frontiers in Oncology . 2017;7:p. 110. doi: 10.3389/fonc.2017.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Samavat H., Kurzer M. S. Estrogen metabolism and breast cancer. Cancer Letters . 2015;356(2):231–243. doi: 10.1016/j.canlet.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kitaura H., Marahleh A., Ohori F., et al. Osteocyte-related cytokines regulate osteoclast formation and bone resorption. International Journal of Molecular Sciences . 2020;21(14):p. 5169. doi: 10.3390/ijms21145169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Harmer D., Falank C., Reagan M. R. Interleukin-6 interweaves the bone marrow microenvironment, bone loss, and multiple myeloma. Frontiers in Endocrinology . 2018;9:p. 788. doi: 10.3389/fendo.2018.00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hadji P., Gottschalk M., Ziller V., Kalder M., Jackisch C., Wagner U. Bone mass and the risk of breast cancer: the influence of cumulative exposure to oestrogen and reproductive correlates. Results of the Marburg breast cancer and osteoporosis trial (MABOT) Maturitas . 2007;56(3):312–321. doi: 10.1016/j.maturitas.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 140.Shapiro C. L. Management of osteoporosis in women with breast cancer. Breast Cancer Management . 2020;9(2) doi: 10.2217/bmt-2020-0012. [DOI] [Google Scholar]
- 141.Hadji P., Aapro M. S., Body J. J., et al. Management of aromatase inhibitor-associated bone loss (AIBL) in postmenopausal women with hormone sensitive breast cancer: joint position statement of the IOF, CABS, ECTS, IEG, ESCEO IMS, and SIOG. Journal of Bone Oncology . 2017;7:1–12. doi: 10.1016/j.jbo.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hyder T., Marino C. C., Ahmad S., Nasrazadani A., Brufsky A. M. Aromatase inhibitor-associated musculoskeletal syndrome: understanding mechanisms and management. Frontiers in Endocrinology . 2021;12, article 713700 doi: 10.3389/fendo.2021.713700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hadji P., Coleman R. E., Wilson C., et al. Adjuvant bisphosphonates in early breast cancer: consensus guidance for clinical practice from a European Panel. Annals of Oncology . 2016;27(3):379–390. doi: 10.1093/annonc/mdv617. [DOI] [PubMed] [Google Scholar]
- 144.Coleman R., Body J. J., Aapro M., Hadji P., Herrstedt J., ESMO Guidelines Working Group Bone health in cancer patients: ESMO Clinical Practice Guidelines. Annals of Oncology . 2014;25:iii124–iii137. doi: 10.1093/annonc/mdu103. [DOI] [PubMed] [Google Scholar]
- 145.Rachner T. D., Khosla S., Hofbauer L. C. Osteoporosis: now and the future. Lancet . 2011;377(9773):1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen X., Wang Z., Duan N., Zhu G., Schwarz E. M., Xie C. Osteoblast-osteoclast interactions. Connective Tissue Research . 2018;59(2):99–107. doi: 10.1080/03008207.2017.1290085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Eastell R., Adams J. E., Coleman R. E., et al. Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230. Journal of Clinical Oncology . 2008;26(7):1051–1057. doi: 10.1200/JCO.2007.11.0726. [DOI] [PubMed] [Google Scholar]
- 148.Coleman R. E., Banks L. M., Girgis S. I., et al. Skeletal effects of exemestane on bone-mineral density, bone biomarkers, and fracture incidence in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES): a randomised controlled study. The Lancet Oncology . 2007;8(2):119–127. doi: 10.1016/S1470-2045(07)70003-7. [DOI] [PubMed] [Google Scholar]
- 149.Hadji P., Body J. J., Aapro M. S., et al. Practical guidance for the management of aromatase inhibitor-associated bone loss. Annals of Oncology . 2008;19(8):1407–1416. doi: 10.1093/annonc/mdn164. [DOI] [PubMed] [Google Scholar]
- 150.Gnant M. F. X., Mlineritsch B., Luschin-Ebengreuth G., et al. Zoledronic acid prevents cancer treatment-induced bone loss in premenopausal women receiving adjuvant endocrine therapy for hormone-responsive breast cancer: a report from the Austrian Breast and Colorectal Cancer Study Group. Journal of Clinical Oncology . 2007;25(7):820–828. doi: 10.1200/JCO.2005.02.7102. [DOI] [PubMed] [Google Scholar]
- 151.Segovia-Mendoza M., García-Quiroz J., Díaz L., García-Becerra R. Combinations of calcitriol with anticancer treatments for breast cancer: an update. International Journal of Molecular Sciences . 2021;22(23):p. 12741. doi: 10.3390/ijms222312741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Park J. H., Im S. A., Byun J. M., et al. Cyclophosphamide, methotrexate, and 5-fluorouracil as palliative treatment for heavily pretreated patients with metastatic breast cancer: a multicenter retrospective analysis. Journal of Breast Cancer . 2017;20(4):347–355. doi: 10.4048/jbc.2017.20.4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rizzoli R., Biver E. Glucocorticoid-induced osteoporosis: who to treat with what agent? Nature Reviews Rheumatology . 2015;11(2):98–109. doi: 10.1038/nrrheum.2014.188. [DOI] [PubMed] [Google Scholar]
- 154.van Staa T. P., Leufkens H. G., Abenhaim L., Zhang B., Cooper C. Oral corticosteroids and fracture risk: relationship to daily and cumulative doses. Rheumatology . 2000;39(12):1383–1389. doi: 10.1093/rheumatology/39.12.1383. [DOI] [PubMed] [Google Scholar]
- 155.Kalder M., Hadji P. Breast cancer and osteoporosis–management of cancer treatment-induced bone loss in postmenopausal women with breast cancer. Breast Care . 2014;9(5):312–317. doi: 10.1159/000368843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Velentza L., Zaman F., Sävendahl L. Bone health in glucocorticoid-treated childhood acute lymphoblastic leukemia. Critical Reviews in Oncology/Hematology . 2021;168, article 103492 doi: 10.1016/j.critrevonc.2021.103492. [DOI] [PubMed] [Google Scholar]
- 157.Kanis J. A., Cooper C., Rizzoli R., Reginster J.-Y. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporosis International . 2019;30(1):3–44. doi: 10.1007/s00198-018-4704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shapiro S. L., Brown K. W., Thoresen C., Plante T. G. The moderation of mindfulness-based stress reduction effects by trait mindfulness: results from a randomized controlled trial. Journal of Clinical Psychology . 2011;67(3):267–277. doi: 10.1002/jclp.20761. [DOI] [PubMed] [Google Scholar]
- 159.Nisha Y., Dubashi B., Bobby Z., et al. Cytotoxic chemotherapy is associated with decreased bone mineral density in postmenopausal women with early and locally advanced breast cancer. Archives of Osteoporosis . 2023;18(1):p. 41. doi: 10.1007/s11657-023-01231-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Greep N. C., Giuliano A. E., Hansen N. M., Taketani T., Wang H.-J., Singer F. R. The effects of adjuvant chemotherapy on bone density in postmenopausal women with early breast cancer. The American Journal of Medicine . 2003;114(8):653–659. doi: 10.1016/s0002-9343(03)00127-x. [DOI] [PubMed] [Google Scholar]
- 161.Brodie A., Sabnis G., Jelovac D. Aromatase and breast cancer. The Journal of Steroid Biochemistry and Molecular Biology . 2006;102(1–5):97–102. doi: 10.1016/j.jsbmb.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cuzick J., Forbes J. F., Sestak I., et al. Long-term results of tamoxifen prophylaxis for breast cancer--96-month follow-up of the randomized IBIS-I trial. Journal of the National Cancer Institute . 2007;99(4):272–282. doi: 10.1093/jnci/djk049. [DOI] [PubMed] [Google Scholar]
- 163.Santen R. J., Simpson E. History of estrogen: its purification, structure, synthesis, biologic actions, and clinical implications. Endocrinology . 2019;160(3):605–625. doi: 10.1210/en.2018-00529. [DOI] [PubMed] [Google Scholar]
- 164.Qureshi R., Picon-Ruiz M., Aurrekoetxea-Rodriguez I., et al. The major pre- and postmenopausal estrogens play opposing roles in obesity-driven mammary inflammation and breast cancer development. Cell Metabolism . 2020;31(6):1154–1172.e9. doi: 10.1016/j.cmet.2020.05.008. [DOI] [PubMed] [Google Scholar]
- 165.Chumsri S., Howes T., Bao T., Sabnis G., Brodie A. Aromatase, aromatase inhibitors, and breast cancer. The Journal of Steroid Biochemistry and Molecular Biology . 2011;125(1–2):13–22. doi: 10.1016/j.jsbmb.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Waqas K., Lima Ferreira J., Tsourdi E., Body J.-J., Hadji P., Zillikens M. C. Updated guidance on the management of cancer treatment-induced bone loss (CTIBL) in pre- and postmenopausal women with early-stage breast cancer. Journal of Bone Oncology . 2021;28, article 100355 doi: 10.1016/j.jbo.2021.100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fisher B., et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. Journal of Clinical Oncology . 1998;16(8):2672–2685. doi: 10.1200/JCO.1998.16.8.2672. [DOI] [PubMed] [Google Scholar]
- 168.Malagrinò M., Zavatta G. Review of bone health in women with estrogen receptor-positive breast cancer receiving endocrine therapy. Womens Health . 2023;19, article 17455057221149493 doi: 10.1177/17455057221149493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Scott L. J. Enzalutamide: a review in castration-resistant prostate cancer. Drugs . 2018;78(18):1913–1924. doi: 10.1007/s40265-018-1029-9. [DOI] [PubMed] [Google Scholar]
- 170.Korde N., Roschewski M., Zingone A., et al. Treatment with carfilzomib-lenalidomide-dexamethasone with lenalidomide extension in patients with smoldering or newly diagnosed multiple myeloma. JAMA Oncology . 2015;1(6):746–754. doi: 10.1001/jamaoncol.2015.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lu S., Gong M., Zha Y., et al. Changes in bone mineral density after parathyroidectomy in patients with moderate to severe primary hyperparathyroidism. Journal of International Medical Research . 2020;48(10, article 0300060520964698) doi: 10.1177/0300060520964698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yin Q.-Q., Xu L.-H., Zhang M., Xu C. Muscarinic acetylcholine receptor M1 mediates prostate cancer cell migration and invasion through hedgehog signaling. Asian Journal of Andrology . 2018;20(6):608–614. doi: 10.4103/aja.aja_55_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhu J., Xing W., Li T., Lin H., Ou J. GnRH antagonist protocol versus GnRH agonist long protocol: a retrospective cohort study on clinical outcomes and maternal-neonatal safety. Frontiers in Endocrinology . 2022;13, article 875779 doi: 10.3389/fendo.2022.875779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Salama M., Woodruff T. K. Anticancer treatments and female fertility: clinical concerns and role of oncologists in oncofertility practice. Expert Review of Anticancer Therapy . 2017;17(8):687–692. doi: 10.1080/14737140.2017.1335199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Burstein H. J., Lacchetti C., Anderson H., et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO clinical practice guideline focused update. Journal of Clinical Oncology . 2019;37(5):423–438. doi: 10.1200/JCO.18.01160. [DOI] [PubMed] [Google Scholar]
- 176.Behjati S., Frank M. The effects of tamoxifen on immunity. Current Medicinal Chemistry . 2009;16(24):3076–3080. doi: 10.2174/092986709788803042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Coleman R. E., Collinson M., Gregory W., et al. Benefits and risks of adjuvant treatment with zoledronic acid in stage II/III breast cancer. 10 years follow-up of the AZURE randomized clinical trial (BIG 01/04) Journal of Bone Oncology . 2018;13:123–135. doi: 10.1016/j.jbo.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ensrud K. E. Central nervous system active medications and risk for fractures in older women. Archives of Internal Medicine . 2003;163(8):p. 949. doi: 10.1001/archinte.163.8.949. [DOI] [PubMed] [Google Scholar]
- 179.Cosman F., de Beur S. J., LeBoff M., et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporosis International . 2014;25(10):2359–2381. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Camacho P. M., Dayal A. S., Diaz J. L., et al. Prevalence of secondary causes of bone loss among breast cancer patients with osteopenia and osteoporosis. Journal of Clinical Oncology . 2008;26(33):5380–5385. doi: 10.1200/JCO.2008.17.7451. [DOI] [PubMed] [Google Scholar]
- 181.Nordin B. E., Need A. G., Steurer T., Morris H. A., Chatterton B. E., Horowitz M. Nutrition, osteoporosis, and aging. Annals of the New York Academy of Sciences . 1998;854(1):336–351. doi: 10.1111/j.1749-6632.1998.tb09914.x. [DOI] [PubMed] [Google Scholar]
- 182.VanderWalde A., Hurria A. Aging and osteoporosis in breast and prostate cancer. A Cancer Journal for Clinicians . 2011;61(3):139–156. doi: 10.3322/caac.20103. [DOI] [PubMed] [Google Scholar]
- 183.Cohen A. J., Roe F. J. Review of risk factors for osteoporosis with particular reference to a possible aetiological role of dietary salt. Food and Chemical Toxicology . 2000;38(2–3):237–253. doi: 10.1016/s0278-6915(99)00145-3. [DOI] [PubMed] [Google Scholar]
- 184.Dionyssiotis Y., Dontas I. A., Economopoulos D., Lyritis G. P. Rehabilitation after falls and fractures. Journal of Musculoskeletal and Neuronal Interactions . 2008;8(3):244–250. [PubMed] [Google Scholar]
- 185.Sawamoto K., Álvarez J. V., Herreño A. M., et al. Bone-specific drug delivery for osteoporosis and rare skeletal disorders. Current Osteoporosis Reports . 2020;18(5):515–525. doi: 10.1007/s11914-020-00620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Rizzoli R., Body J. J., Brandi M. L., et al. Cancer-associated bone disease. Osteoporosis International . 2013;24(12):2929–2953. doi: 10.1007/s00198-013-2530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tebben P. J., Singh R. J., Kumar R. Vitamin D-mediated hypercalcemia: mechanisms, diagnosis, and treatment. Endocrine Reviews . 2016;37(5):521–547. doi: 10.1210/er.2016-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Escribano J., Balaguer A., Figuls M. R., Feliu A., Ferre N. Dietary interventions for preventing complications in idiopathic hypercalciuria. Cochrane Database of Systematic Reviews . 2014;2 doi: 10.1002/14651858.CD006022.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Painter S. E., Kleerekoper M., Camacho P. M. Secondary osteoporosis: a review of the recent evidence. Endocrine Practice . 2006;12(4):436–445. doi: 10.4158/EP.12.4.436. [DOI] [PubMed] [Google Scholar]
- 190.Tannenbaum C., Clark J., Schwartzman K., et al. Yield of laboratory testing to identify secondary contributors to osteoporosis in otherwise healthy women. The Journal of Clinical Endocrinology and Metabolism . 2002;87(10):4431–4437. doi: 10.1210/jc.2002-020275. [DOI] [PubMed] [Google Scholar]
- 191.Dawson-Hughes B., Mithal A., Bonjour J. P., et al. IOF position statement: vitamin D recommendations for older adults. Osteoporosis International . 2010;21(7):1151–1154. doi: 10.1007/s00198-010-1285-3. [DOI] [PubMed] [Google Scholar]
- 192.Bischoff-Ferrari H. A., Dawson-Hughes B., Baron J. A., et al. Calcium intake and hip fracture risk in men and women: a meta-analysis of prospective cohort studies and randomized controlled trials. The American Journal of Clinical Nutrition . 2007;86(6):1780–1790. doi: 10.1093/ajcn/86.5.1780. [DOI] [PubMed] [Google Scholar]
- 193.Tu K. N., Lie J. D., Wan C. K. V., et al. Osteoporosis: a review of treatment options. Pharmacology & Therapeutics . 2018;43(2):92–104. [PMC free article] [PubMed] [Google Scholar]
- 194.Wiesner A., Szuta M., Galanty A., Paśko P. Optimal dosing regimen of osteoporosis drugs in relation to food intake as the key for the enhancement of the treatment effectiveness-a concise literature review. Foods . 2021;10(4):p. 720. doi: 10.3390/foods10040720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wright N. C., Foster P. J., Mudano A. S., et al. Assessing the feasibility of the effectiveness of discontinuing bisphosphonates trial: a pilot study. Osteoporosis International . 2017;28(8):2495–2503. doi: 10.1007/s00198-017-4073-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhang N., Zhang Z.-K., Yu Y., Zhuo Z., Zhang G., Zhang B.-T. Pros and cons of denosumab treatment for osteoporosis and implication for RANKL aptamer therapy. Frontiers in Cell and Developmental Biology . 2020;8:p. 325. doi: 10.3389/fcell.2020.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tang S., Mao X., Chen Y., Yan L., Ye L., Li S. Reactive oxygen species induce fatty liver and ischemia-reperfusion injury by promoting inflammation and cell death. Frontiers in Immunology . 2022;13, article 870239 doi: 10.3389/fimmu.2022.870239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Barrett-Connor E., Mosca L., Collins P., et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. The New England Journal of Medicine . 2006;355(2):125–137. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data will be made available on request.


