ABSTRACT
Objective
Magnetic resonance‐guided laser interstitial thermal therapy (MRgLITT) is a novel tool and a minimally invasive treatment to drug‐resistant epilepsy (DRE). The focus of this research was to evaluate the effectiveness and safety of the newly developed dual‐wavelength dual‐output MRgLITT system LaserRO within two probe trajectories in treating DRE patients.
Methods
This is a retrospective analysis conducted at a single center, examining patients with DRE who received treatment with the LaserRO MRgLITT system. The system utilizes a sophisticated laser technology that can be configured as conventional single output for single wavelength or innovative dual outputs for dual wavelengths. The study involved a comprehensive review of patient information, encompassing demographics, seizure history, details related to the surgical parameters, and the subsequent clinical results. Primary outcome was post‐operation seizure outcome defined as Engel Scale Class at the end of follow‐up time.
Results
This study included a total of eight DRE patients received MRgLITT surgery between August 2022 and October 2023. Out of these, there were four mesial temporal lobe epilepsy (MTLE), three focal cortical dysplasia (FCD), and one cavernous malformation (CM) patients. Within the two probe trajectories, seven patients had single wavelength (980 or 1064 nm) laser treatment and one patient had dual‐wavelength (980 and 1064 nm) laser treatment. The median age of the patients was 27 (22–31) years, with a median follow‐up period of 9.7 (8.4–12.1) months. The mean BMI was recorded at 20.24 ± 2.95 kg/m2, and epilepsy history was 13 ± 6 years. The median intraoperative blood loss was 5 (5–9) mL, operation time was 231 (169–254) minutes, and length of stay (LOS) was 3 (3–5) days. The mean ablation volume ratio was 96.52% ± 3.67%. In terms of outcomes, over a median follow‐up time of 9.7 (range 8.4–12.1) months, there were two patients got Engel I, five patients got seizure‐free, and one patient decreased 75% seizure. Importantly, no serious complications following the procedures occurred.
Conclusions
The preliminary results indicate that the MRgLITT procedure, which operates dual‐output laser with single or dual wavelengths (980/1064 nm) within the two trajectories, is both effective and safe as a minimally invasive approach for different types of DRE patients.
Keywords: Epilepsy, intraoperative MRI, laser ablation, laser interstitial thermal therapy, seizure
The newly developed dual‐wavelength dual‐output MRgLITT system LaserRO within two probe trajectories in treating drug‐resistant epilepsy patients.
1. Introduction
Epilepsy is a chronic brain disorder caused by a variety of factors, characterized by excessive firing of brain neurons, resulting in recurrent, paroxysmal, and transitory central nervous system dysfunction. Epilepsy affects people of all ages, races, and geographies, while its prevalence is higher in children and teenagers [1]. Over 65 million individuals worldwide suffer from epilepsy [2] and the estimated number of individuals with epilepsy is 10 million in China [3]. This chronic neurological disorder not only has a negative long‐term impact on people's quality of life, but it also places a significant load on healthcare systems and families.
At present, the common therapeutic approaches can be divided into: (1) drug treatment; (2) epilepsy surgery (including neuromodulation therapy); (3) ketogenic diet. Among them, surgical resection of epileptic lesions had been recommended for drug resistance epilepsy, which affects around one‐third of patients with epilepsy [4]. While classical craniotomy for epileptogenic lesion resection carries substantial risks and numerous complications. Magnetic resonance‐guided laser interstitial thermal therapy (MRgLITT) has gained popularity as a minimally invasive alternative to surgical resections since FDA approval in 2007 [5]. Compared with the open surgery, MRgLITT is link to reduced index hospitalization expenses, a shorter length of stay (LOS), and a higher likelihood of discharge home [6]. Over the past 10 years, there has been an exponential increase in the application of MRgLITT for DRE in numerous epilepsy center [6, 7, 8, 9, 10].
However, there have been few reports on the use of a dual‐wavelength dual‐output within two probe trajectories to treat DRE patients. This retrospective study aimed to evaluate the effectiveness and safety of the newly designed dual‐wavelength dual‐output MRgLITT system LaserRO in treating DRE at our epilepsy center.
2. Methods
2.1. Study design
Each admitted patient comprehended and agreed with the informed consent. Retrospective evaluation was conducted on DRE patients treated with MRgLITT utilizing a surgical laser ablation system between August 2022 and October 2023. Every procedure was carried out at our hospital. The research ethics review committee at our hospital approved this study. Prior to the procedure, informed consent forms were signed by each patient or their legal guardian. Data on outcomes, intraoperative procedures, and demographics were gathered and analyzed.
2.2. Patient selection
Preoperative evaluations were comprehensive and included 3.0‐T magnetic resonance imaging (MRI) epilepsy imaging protocol and scalp video electrography (VEEG) monitoring. As supplemental testing, positron emission tomography‐computed tomography (PET‐CT) were also employed. In order to assist in locating the epileptic foci, some patients also had electrode installation for invasive EEG monitoring and stereotactic electroencephalography (SEEG). After all data collecting was finished, neurologists and functional neurosurgeons at epilepsy center in our hospital reviewed all of the results after this evaluation to decide on the best way to treat seizures. We selected the DRE patients who received MRgLITT using two trajectories laser for treatment to further analysis in our hospital.
The MRgLITT inclusion criteria were as follows:
From the age of 2 to 70 years
Drug‐resistant epilepsy diagnosis
Focal seizure is the form of epilepsy
The epileptogenic focus is confined and appropriate for surgical intervention
Informed permission papers were completed and signed by the patients and their families.
The MRgLITT exclusion criteria were as follows:
MRI and craniotomy contraindications
Epilepsy medication has changed within a month
Progressive neurological diseases
Sever coagulation disorders
Acute infection or chronic infection;
pregnant or nursing women
2.3. Surgical process
Prior to the procedure, the target position, size, and optic fiber trajectory were planned using contrast‐enhanced MRI in order to produce temporary target coordinates and avoid significant vessels.
All the surgical process were performed after general anesthesia. Either the Leksell stereotactic frame or the Remebot neurosurgical robot (NOVOLIFE, Shandong, China) were used for stereotaxy. The target position, size, and optic fiber trajectory were preoperatively planned using Stereotactic Surgery Planning Software (V1.0, Genlight, Hangzhou, Zhejiang, China) to producing temporary target coordinates. The skin was cut and the skull was drilled by electric drill. Then the dura was burned by electrocoagulation. Fix the skull bone anchor, calculate the depth of electrode insertion, and after the hard cleaning rod breaks into the brain to build a channel, insert and fix two laser optical fiber in sequence. The patient then underwent an initial intraoperative MRI (Discovery MR750w 3.0T, GE Medical System, US) scan to check the accurate positioning of optical fiber (Figure 1).
FIGURE 1.
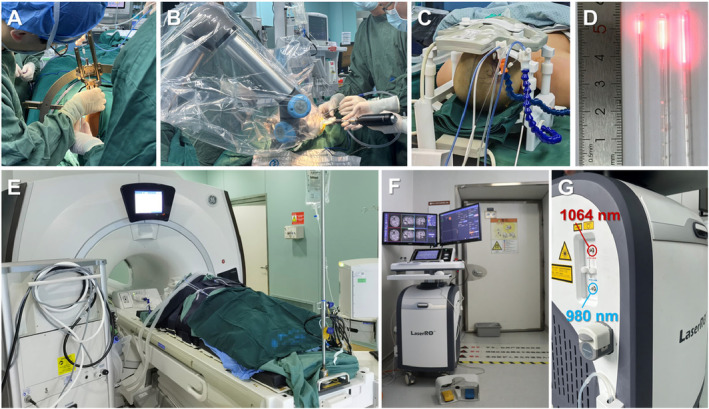
LITT procedure was performed in the intraoperative MRI room. (A) Optical fiber implantation under Leksell frame; (B) neurosurgical robot assisted optical fiber insertion; (C) intraoperative MR coil fixation after optical fiber insertion; (D) Fiber‐optic catheter outer (length 4, 10, 15 mm); (E) patients in the intraoperative MRI room; (F, G) the LaserRO system equipped with a dual‐wavelength laser, operating at 980 and 1064 nm, for MRgLITT.
All ablations were performed using the LaserRO MRI‐guided laser ablation system (Genlight Inc. Hangzhou, Zhejiang, China), comprises preoperative planning and intraoperative ablation. Preoperative planning involves inputting the ablation surgical plan (target and trajectory information, ablation parameter planning) into the surgical platform. Intraoperative procedure is based on the ablation platform, fiber kit and surgical tools, using real‐time images from the MR equipment to perform the treatment. The system outputs cooling water to the fiber probe through the cooling module. Throughout the treatment process, the ablation platform acquires real‐time images to detect the temperature of the target and its surrounding tissues to ensure safe and effective ablation (Figures 2, 3, 4).
FIGURE 2.
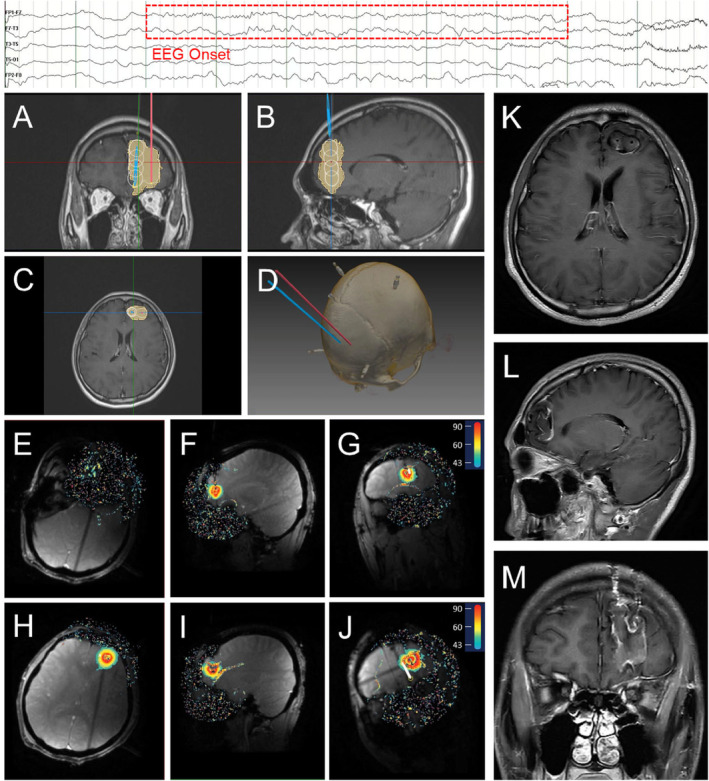
Representative FCD patient's preoperative ablation trajectory planning, intraoperative ablation real‐time thermal monitoring, and postoperative MRI. The top of the figure indicates EEG onset. (A–C) Ablation trajectory planning in coronal, sagittal, and axial plane. (White indicates the planned ablation and yellow indicates the actual ablation); (D) thin‐slice head CT with reconstruction; (E–G) ablation along the first trajectory in axial, sagittal, and coronal plane; (H–J) ablation along the second trajectory in axial, sagittal, and coronal plane; (K–M) postoperative 3‐month MRI in axial, sagittal, and coronal plane.
FIGURE 3.
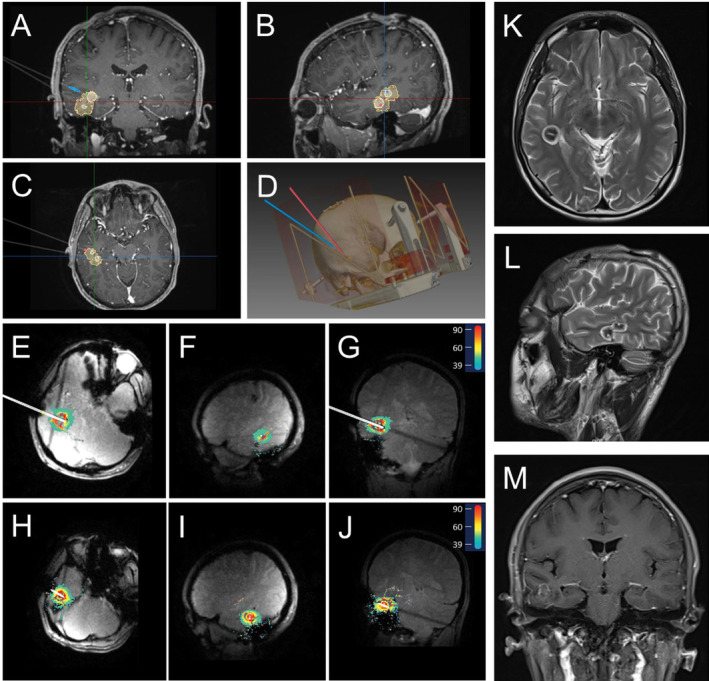
Representative CM patient's preoperative ablation trajectory planning, intraoperative ablation real‐time thermal monitoring, and postoperative MRI. This patient shows no typical EEG onset. (A–C) Ablation trajectory planning in coronal, sagittal, and axial plane. (White indicates the planned ablation and yellow indicates the actual ablation); (D) thin‐slice head CT with reconstruction; (E–G) ablation along the first trajectory in axial, sagittal, and coronal plane; (H–J) ablation along the second trajectory in axial, sagittal, and coronal plane; (K–M) postoperative 3‐month MRI in axial, sagittal, and coronal plane.
FIGURE 4.
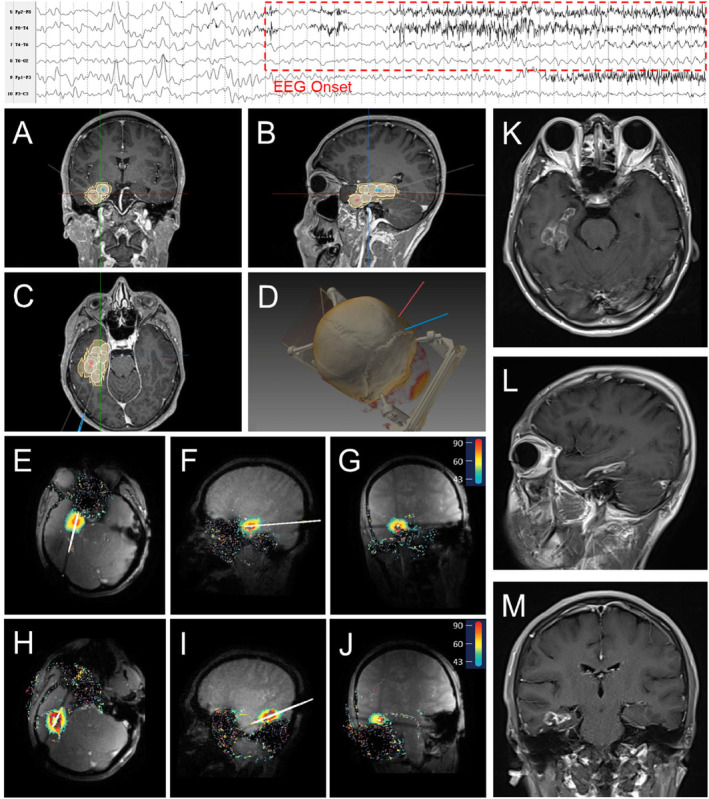
Representative MTLE patient's preoperative ablation trajectory planning, intraoperative ablation real‐time thermal monitoring, and postoperative MRI. The top of the figure indicates EEG onset. (A–C) Ablation trajectory planning in coronal, sagittal, and axial plane. (White indicates the planned ablation and yellow indicates the actual ablation); (D): thin‐slice head CT with reconstruction; (E–G) ablation along the first trajectory in axial, sagittal, and coronal plane; (H–J) ablation along the second trajectory in axial, sagittal, and coronal plane; (K–M) postoperative 3‐month MRI in axial, sagittal, and coronal plane.
The LaserRO system has unique technical specifications in its design. In terms of main performance, it employs dual‐wavelength light sources of 980 and 1064 nm, with a maximum laser power of up to 15 W. The probe comes in two outer diameters: 1.55 and 2.3 mm, with three different diffusing tip lengths: 4, 10, and 15 mm. The cooling system provides real‐time flow and residual monitoring. The inserted fiber probe is compatible with 1.5 and 3.0T MRI environments, with a 3.5‐second MRI temperature detection interval and a temperature measurement accuracy of ±2°C.
After the ablation process, an MRI scan was used to confirm the ablation range. The probe and skull bone anchor were then withdrawn, and the incision was closed with sutures. The patient was transported to the general ward for overnight observation, during which we routinely did a postoperative CT scan to detect hemorrhage.
2.4. Statistical analysis
The Shapiro–Wilk method was used to assess data in this investigation for normality test. Continuous variables were defined as mean ± standard deviation (SD) or median (interquartile range). Categorical variables were expressed as numbers (n) and percentages (%). The analyses were performed using SPSS version 25.0 (IBM Co., Armonk, NY).
3. Results
3.1. Demographic Data
From August 2022 and October 2023, eight patients at our hospital had MRgLITT surgery using two trajectories optical laser. The median age of the operation age was 27 (22–31) years, with 6 male (75%) and 2 female (25%). The mean BMI was recorded at 20.24 ± 2.95 kg/m2, and epilepsy history was 13 ± 6 years. There are four patients with mesial temporal lobe epilepsy (MTLE), three with focal cortical dysplasia (FCD), and one with a cavernous malformation (CM). Within the two trajectories, seven patients received single wavelength (980 or 1064 nm) laser treatment, while one received dual‐wavelength (980 and 1064 nm) laser treatment (Tables 1 and 2).
TABLE 1.
Drug‐resistant epilepsy patient data.
| No. | Sex | Age (years) | BMI (kg/m2) | DRE | Seizure histories (years) | Laser wavelength (nm) | Blood loss (mL) | Operation time (min) | Hospital stays (days) | Ablation volume rate (%) | Follow‐up time (months) | Engel scale class | AEDs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 27 | 16.53 | FCD | 24 | 980 & 1064 | 5 | 400 | 2 | 90.33 | 22.6 | I | LEV, CBZ |
| 2 | F | 30 | 21.63 | MTLE | 14 | 1064 | 10 | 250 | 3 | 92.05 | 12.4 | I | OXC, LCM |
| 3 | F | 31 | 20.31 | MTLE | 8 | 1064 | 10 | 210 | 3 | 99.73 | 11.4 | Seizure free a | LCM |
| 4 | M | 27 | 16.90 | CM | 4 | 980 | 5 | 155 | 3 | 98.37 | 10.0 | Seizure free a | OXC, LCM |
| 5 | M | 32 | 25.20 | MTLE | 11 | 1064 | 5 | 155 | 3 | 94.82 | 9.3 | Decrease 75% a | OXC, VPA, PB |
| 6 | M | 15 | 21.33 | FCD | 10 | 1064 | 5 | 250 | 5 | 99.41 | 9.1 | Seizure free a | OXC, LEV, PER |
| 7 | M | 27 | 22.04 | FCD | 19 | 1064 | 5 | 212 | 5 | 99.59 | 8.2 | Seizure free a | CBZ, VPA |
| 8 | M | 30 | 17.96 | MTLE | 10 | 1064 | 5 | 255 | 6 | 97.84 | 8.2 | Seizure free a | OXC, LEV |
Abbreviations: AED, antiepileptic drugs; CBZ, carbamazepine; CM, cavernous malformation; DRE, drug‐resistant epilepsy; F, female; FCD, focal cortical dysplasia; LCM, lacosamide; LEV, levetiracetam; M, male; MTLE, mesial temporal epilepsy; OXC, oxcarbazepine; PB, phenobarbital; PER, perampanel; VPA, valproic acid.
Follow‐up time less than 12 months.
TABLE 2.
Summary of demographic and outcome data.
| Patient | Data |
|---|---|
| Number | MTLE 4; FCD3; CM 1 |
| Age (years) | 27 (22–31) |
| Sex | Female 2; Male 6 |
| BMI (kg/m2) | 20.24 + 2.95 |
| Seizure histories (yeas) | 13 ± 6 |
| Blood loss (mL) | 5 (5–9) |
| Operation time (min) | 231 (169–254) |
| Hospital stays (days) | 3 (3–5) |
| Ablation volume rate (%) | 96.52 ± 3.67 |
| Follow‐up time (months) | 9.7 (8.4–12.1) |
| Outcome | |
| Engel I(n) | 2 |
| Seizure free (n) | 5 |
| Decrease 75% (n) | 1 |
Abbreviations: CM, cavernous malformation; FCD, focal cortical dysplasia; MTLE, mesial temporal epilepsy.
3.2. Perioperative and outcome data
The median intraoperative blood loss was 5 (5–9) mL, operation time was 231 (169–254) minutes, and length of stay was 3 (3–5) days. The mean ablation volume ratio was 96.52% ± 3.67%. In terms of outcomes, over a median follow‐up time of 9.7 (range 8.4–12.1) months, there are two patients got Engel I, five patients got seizure free, and one patient decreased 75% seizure. Importantly, there were no reports of serious complications such as prolonged edema, hemorrhage, and visual field loss following the procedures (Tables 1 and 2).
4. Discussion
With a relative decrease in the use of surgical resections and relative increases in usage of LITT for the treatment of DRE patients [11]. LITT is a both effective and safe approach to treat seizures without necessitating a craniotomy [12, 13, 14]. Our epilepsy center has conducted a preliminary exploration of LITT in the treatment of refractory epilepsy. As shown in Table 1, this study of eight patients with DRE who underwent MRgLITT treatment showed that all the patients had a high rate of postoperative epilepsy control. Seven patients did not experience any seizures and one MTLE patient experienced a considerable decrease in the rate of seizures during the median follow‐up period of 9.7 (8.4–12.1) months. Our preliminary early outcome demonstrated that the efficacy and safety of the newly developed dual‐wavelength dual‐output MRgLITT system LaserRO in treating DRE with no serious complications.
Among our eight DRE patients, there were four MTLE, three FCD, and one CM in our study. MTLE remains the most common type of DRE. The gold standard for MTLE is still anterior temporal lobectomy (ATL), which has been shown to be effective in two Class I trials and has rates of seizure freedom ranging from 60% to 80% after a 2‐year follow‐up [15]. While MRgLITT is becoming a minimally invasive alternative to open craniotomy for the remove of epileptic lesions. As for MTLE, a multicenter cohort study showed Engel I seizure freedom was achieved in 55.8% (149/267) at 1 year and 52.5% (126/240) at 2 years after MRgLITT treatment [7]. Other report has reported that the degree of cluster ablation in the amygdalohippocampal complex was substantially correlated with the seizure outcome following LITT in individuals with MTLE [16]. As for FCD and CM, 63.6% (7/11) children epilepsy patients with FCD underwent LITT achieved Engel Class I [17] and 83.3%(5/6) CM patients got Engel I seizure freedom [18]. In terms of outcomes at our epilepsy center, using dual‐wavelength dual‐output MRgLITT system, over a median follow‐up time of 9.7 (range 8.4–12.1) months, seven patients achieved seizure freedom during follow‐up after the procedure, and one MTLE patient got a considerable seizure decrease. Following the procedures, no serious complications occurred. The higher postoperative epilepsy control rate in our center is may related to the shorter follow‐up time. On the other hands, we applied the dual‐wavelength dual‐output MRgLITT system within the two trajectories may be more beneficial to the damage of lesions. Besides, safety is the main advantage of this minimally invasive surgery, which referring the 5 mL intraoperative blood loss and the 3 days length of stay approximately in our study. There is one reported that approximately 7.26 mL blood loss with average 4.95 days LOS [19] and another study showed the LOS was 1 night for 16 (89%) patients [20].
Our study also showed newly developed dual‐wavelength dual‐output MRgLITT system is feasible for the treatment of DRE without requiring a craniotomy. Different wavelengths laser differs in ablation range, heat transfer, and ablation duration. This MRgLITT system combines two laser wavelengths, 980 and 1064 nm, and can switch freely between the two wavelengths for ablation, making it better suited for lesions of different textures, shapes, and sizes. Besides, such as Figure 2 when the lesion area is large and a single optical fiber cannot meet the needs, dual or even multiple optical fibers need to be inserted. However, the ablation range of dual optical fibers is limited if only the 980 nm wavelength is used. Therefore, introducing 1064 nm wavelength for combined ablation can obtain a better ablation range. In summary, compared with other MRgLITT systems, (1) The dual‐output channel design allows wavelength switching during multi‐segment ablation to achieve better conformity; (2) The dual‐wavelength combination of 980 and 1064 nm can be used for larger lesions, reducing the number of optical fibers placed; (3) When the preoperative plan fails to correctly estimate the ablation range, switching wavelengths can avoid the risk of secondary optical fiber placement.
5. Conclusion
MRgLITT is a minimally invasive approach for ablation of DRE lesions with great safety, low complication rate, and satisfactory surgical outcomes, especially using dual‐output laser with single or dual wavelengths (980/1064 nm) within the two trajectories. It could potentially replace craniotomy for some DRE patients in the future. However, given the limitations of this investigation, preliminary small sample size results and short‐term follow‐up, a large sample prospective and multi‐center RCT of MRgLITT study are still needed to provide sufficient and necessary evidence.
Ethics Statement
Informed consent was obtained from the patient to publish his clinical imaging and data, and approval for this study was provided by the Institutional Review Board of the Second Affiliated Hospital of Zhejiang University School of Medicine (2022‐203).
Consent
All authors approved the final manuscript and the submission to this journal.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding: This study was supported by the “Pioneer” and “Leading Goose” R&D Program of Zhejiang (2024C03039, 2024C03093) and National Natural Science Foundation of China (82272112, 82271301).
Jingquan Lin, Wei Gao, and Yuqi Ying contributed equally to this work.
Contributor Information
Jianmin Zhang, Email: zjm135@zju.edu.cn.
Junming Zhu, Email: dr.zhujunming@zju.edu.cn.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Asadi‐Pooya A. A., Brigo F., Lattanzi S., and Blumcke I., “Adult Epilepsy,” Lancet 402, no. 10399 (2023): 412–424. [DOI] [PubMed] [Google Scholar]
- 2. Kanner A. M. and Bicchi M. M., “Antiseizure Medications for Adults With Epilepsy: A Review,” JAMA 327, no. 13 (2022): 1269–1281. [DOI] [PubMed] [Google Scholar]
- 3. Ding D., Zhou D., Sander J. W., Wang W., Li S., and Hong Z., “Epilepsy in China: Major Progress in the Past Two Decades,” Lancet Neurology 20, no. 4 (2021): 316–326. [DOI] [PubMed] [Google Scholar]
- 4. Perucca E., Perucca P., White H. S., and Wirrell E. C., “Drug Resistance in Epilepsy,” Lancet Neurology 22, no. 8 (2023): 723–734. [DOI] [PubMed] [Google Scholar]
- 5. Chen J. S., Lamoureux A. A., Shlobin N. A., et al., “Magnetic Resonance‐Guided Laser Interstitial Thermal Therapy for Drug‐Resistant Epilepsy: A Systematic Review and Individual Participant Data Meta‐Analysis,” Epilepsia 64, no. 8 (2023): 1957–1974. [DOI] [PubMed] [Google Scholar]
- 6. Sharma M., Ball T., Alhourani A., et al., “Inverse National Trends of Laser Interstitial Thermal Therapy and Open Surgical Procedures for Refractory Epilepsy: A Nationwide Inpatient Sample‐Based Propensity Score Matching Analysis,” Neurosurgical Focus 48, no. 4 (2020): E11. [DOI] [PubMed] [Google Scholar]
- 7. Youngerman B. E., Banu M. A., Khan F., et al., “Long‐Term Outcomes of Mesial Temporal Laser Interstitial Thermal Therapy for Drug‐Resistant Epilepsy and Subsequent Surgery for Seizure Recurrence: A Multi‐Centre Cohort Study,” Journal of Neurology, Neurosurgery, and Psychiatry 94, no. 11 (2023): 879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gupta K., Cabaniss B., Kheder A., et al., “Stereotactic MRI‐Guided Laser Interstitial Thermal Therapy for Extratemporal Lobe Epilepsy,” Epilepsia 61, no. 8 (2020): 1723–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aum D. J., Reynolds R. A., McEvoy S., et al., “Surgical Outcomes of Open and Laser Interstitial Thermal Therapy Approaches for Corpus Callosotomy In Pediatric Epilepsy,” Epilepsia 64, no. 9 (2023): 2274–2285. [DOI] [PubMed] [Google Scholar]
- 10. Mo J., Guo Z., Wang X., et al., “Magnetic Resonance‐Guided Laser Interstitial Thermal Therapy vs. Open Surgery for Drug‐Resistant Mesial Temporal Lobe Epilepsy: A Propensity Score Matched Retrospective Cohort Study,” International Journal of Surgery 110, no. 1 (2024): 306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ghaith A. K., El‐Hajj V. G., Sanchez‐Garavito J. E., et al. 2024. “Trends in the Utilization of Surgical Modalities for the Treatment of Drug‐Resistant Epilepsy: A Comprehensive 10‐Year Analysis Using the National Inpatient Sample.” Neurosurgery 94, no. 6: 1191–1200. [DOI] [PubMed] [Google Scholar]
- 12. Barot N., Batra K., Zhang J., et al., “Surgical Outcomes Between Temporal, Extratemporal Epilepsies and Hypothalamic Hamartoma: Systematic Review and Meta‐Analysis of MRI‐Guided Laser Interstitial Thermal Therapy for Drug‐Resistant Epilepsy,” Journal of Neurology, Neurosurgery, and Psychiatry 93, no. 2 (2022): 133–143. [DOI] [PubMed] [Google Scholar]
- 13. Ordaz J. D., Vishnubhotla R., Alfonso A., et al., “Single‐Institution Comparative Study of Magnetic Resonance‐Guided Laser Interstitial Thermal Therapy and Open Corpus Callosotomy,” World Neurosurgery 175 (2023): e326–e335. [DOI] [PubMed] [Google Scholar]
- 14. Candela‐Cantó S., Muchart J., Ramírez‐Camacho A., et al., “Robot‐Assisted, Real‐Time, Mri‐Guided Laser Interstitial Thermal Therapy for Pediatric Patients With Hypothalamic Hamartoma: Surgical Technique, Pitfalls, and Initial Results,” Journal of Neurosurgery: Pediatrics 29, no. 6 (2022): 681–692. [DOI] [PubMed] [Google Scholar]
- 15. Wu C., Schwalb J. M., Rosenow J. M., McKhann G. M., Neimat J. S., and American Society for Stereotactic and Functional Neurosurgeons , “The American Society for Stereotactic and Functional Neurosurgery Position Statement on Laser Interstitial Thermal Therapy for the Treatment of Drug‐Resistant Epilepsy,” Neurosurgery 90, no. 2 (2022): 155–160. [DOI] [PubMed] [Google Scholar]
- 16. Kim M. J., Hwang B., Mampre D., et al., “Ablation of Apparent Diffusion Coefficient Hyperintensity Clusters in Mesial Temporal Lobe Epilepsy Improves Seizure Outcomes After Laser Interstitial Thermal Therapy,” Epilepsia 64, no. 3 (2023): 654–666. [DOI] [PubMed] [Google Scholar]
- 17. Lewis E. C., Weil A. G., Duchowny M., Bhatia S., Ragheb J., and Miller I., “MR‐Guided Laser Interstitial Thermal Therapy for Pediatric Drug‐Resistant Lesional Epilepsy,” Epilepsia 56, no. 10 (2015): 1590–1598. [DOI] [PubMed] [Google Scholar]
- 18. Satzer D., Tao J. X., Issa N. P., et al., “Stereotactic Laser Interstitial Thermal Therapy for Epilepsy Associated With Solitary and Multiple Cerebral Cavernous Malformations,” Neurosurgical Focus 48, no. 4 (2020): E12. [DOI] [PubMed] [Google Scholar]
- 19. Niu H., Li K., Liang X., et al., “MR‐Guided Laser Interstitial Thermal Therapy for Drug‐Resistant Lesional Epilepsy: A Single‐Center Experience,” Chinese Neurosurgical Journal 9, no. 1 (2023): 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xu D. S., Chen T., Hlubek R. J., et al., “Magnetic Resonance Imaging‐Guided Laser Interstitial Thermal Therapy for the Treatment of Hypothalamic Hamartomas: A Retrospective Review,” Neurosurgery 83, no. 6 (2018): 1183–1192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


