ABSTRACT
Background
Respiratory syncytial virus (RSV) and influenza virus are major viral etiologies of pediatric lower respiratory tract infection, but comparative data on inpatient burden are lacking.
Methods
Using a large‐scale health claims database in Japan, we identified patients under 5 years of age with a confirmed RSV or influenza diagnosis as an outpatient or inpatient between 2011 and 2022. Hospitalization rate, inpatient characteristics, various in‐hospital outcomes/complications, and healthcare resource utilization were described.
Results
A total of 176,911 RSV‐confirmed outpatients, 153,383 influenza‐confirmed outpatients, 90,413 RSV‐confirmed hospitalizations, and 11,186 influenza‐confirmed hospitalizations were identified. Among outpatients, 24.7% of RSV infection and 2.8% of influenza cases required hospitalization within 1 week. There was no co‐morbidities/prematurity for 95.0% of RSV hospitalizations and 96.5% of influenza hospitalizations. Proportions of in‐hospital outcomes/complications were (RSV infection vs. influenza): oxygen use 47.6% vs. 14.8%, mechanical ventilation 2.1% vs. 0.7%, pneumonia 33.6% vs. 12.8%, otitis media 7.7% vs. 2.3%, febrile seizure 1.5% vs. 34.4%, encephalitis/encephalopathy 0.1% vs. 0.5%, myocarditis < 0.1% vs. 0.6%, antibiotics prescription 48.0% vs. 24.4%. The mean inpatient stay was 6.1 vs. 4.3 days at direct medical costs of 435,744 vs. 315,809 JPY/patient. These trends held true in age‐stratified data. In‐hospital death occurred in 31 RSV infection and 6 influenza cases.
Conclusions
Although both infections resulted in substantial burden, RSV infection led to more frequent hospitalizations, worse in‐hospital outcomes, longer inpatient stays, higher medical costs, and more frequent antibiotics prescription compared to influenza. Most RSV hospitalizations occurred among healthy term children, emphasizing the need for prevention measures in all children.
Keywords: hospitalization, influenza, Japan, pediatrics, respiratory syncytial virus infections
1. Introduction
Lower respiratory tract infections (LRTIs) are major public health threats among young children. Among pediatric LRTIs, respiratory syncytial virus (RSV) and influenza virus are major viral etiologies with high burden globally [1, 2]. In Japan, the National Epidemiological Surveillance of Infectious Diseases (NESID) is conducted by the National Institute of Infectious Diseases (NIID) and Ministry of Health, Labour and Welfare (MHLW) [3]. For RSV infection and influenza, under the NESID, sentinel surveillance provides information on trends and levels of epidemics to inform policy, risk communication, and (for RSV infection) timing to initiate prophylaxis to individuals with underlying health conditions or prematurity [4, 5]. However, the NESID data only provide age‐stratified aggregate data regardless of clinical severity. Therefore, detailed and comprehensive burden data on severe cases require special studies, and such data are scarce in Japan. Most previous reports on severe cases of LRTI are based on small studies that describe single‐center/single‐prefecture experience or studies with only a brief description of in‐hospital outcomes [6, 7, 8]. Furthermore, although RSV and influenza share several clinical features in young children, comparative data on inpatient characteristics, inpatient outcomes, complications, and healthcare resource utilization (HCRU) are lacking globally. Finally, epidemiology of RSV infection and influenza has been disrupted globally due to the coronavirus disease (COVID‐19) pandemic and further data especially around epidemic shift in age group are warranted [9, 10]. Therefore, we used one of the largest health claims databases in Japan to comprehensively describe RSV infection and influenza in children with the aim to elucidate hospitalization rate among infected individuals, inpatient characteristics, inpatient outcomes, and HCRU.
2. Methods
2.1. Database and Study Population
This retrospective cohort study was conducted using data from the Medical Data Vision (MDV) database, one of the largest electronic health records databases in Japan covering approximately 450 hospitals (25% of acute care hospitals in Japan). The present study population was composed of patients under 5 years of age with a confirmed index diagnosis of RSV or influenza either outpatient or inpatient based on the International Classification of Diseases 10th Revision (ICD‐10) diagnostic codes (Table S1) within an identification period of April 1, 2011, to July 31, 2022. Diagnoses that occurred within 6 months of the index diagnosis of the same infection (wash‐out period) were excluded to avoid duplicating the same episode. Patients with a record of another infection with different pathogen within 30 days after the index date (e.g., suspected co‐infection) were also excluded.
2.2. Baseline Characteristics
Data on age at diagnosis, sex, diagnosis date, and hospital size were collected. A 12‐month look‐back period from the index date was used to define the baseline period during which presence of co‐morbidities/prematurity and palivizumab use were ascertained. Co‐morbidities included in this study were those eligible to receive palivizumab in Japan (ICD‐10 codes in Table S1) [11, 12, 13, 14].
2.3. Definitions of Hospitalized Patients, Clinical Outcomes, Healthcare Resource Utilization, and Hospitalization Rates
To describe inpatient characteristics including baseline characteristics, inpatient outcomes, and HCRU, we included all patients who had a confirmed diagnosis in the inpatient setting regardless of where or whether an outpatient RSV diagnosis was made (to ensure inclusion of patients who were diagnosed with RSV infection at different healthcare facilities such as clinics and patients who were never diagnosed before being hospitalized and diagnosed after hospitalization). To be identified as a relevant hospitalization, the infection had to have been coded as any of the following: (1) the disease that required the most medical resources, (2) primary disease for admission, or (3) disease that triggered the hospitalization. Patients who were admitted to the hospital were followed to examine in‐hospital clinical outcomes and HCRU beginning from the index date and ending at the time of in‐hospital death, discharge, the last record available for the patient, or the end of the study period (August 31, 2022), whichever occurred first. Variables for examination included intravenous (IV) fluid use, oxygen use, high flow‐nasal cannula oxygen therapy use, use of mechanical ventilation, intensive care unit (ICU) admissions, in‐hospital death, antibiotics use, in‐hospital complications (pneumonia, otitis media, febrile seizure, encephalitis or encephalopathy, and myocarditis). Finally, length of inpatient stay and direct medical costs during hospitalization for each patient were calculated by including all procedures, clinical exams, imaging, and medications (in Japanese yen).
For the purpose of calculating hospitalization rate among outpatients, we divided the number of patients with a confirmed index outpatient diagnosis by the number of patients who had a confirmed inpatient diagnosis in the same hospital. This was done to ensure that patients included in the numerator (hospitalized cases) were also in the denominator (outpatient cases). The basis of this algorithm was the assumption that almost all patients diagnosed in an outpatient clinic of a hospital will be admitted to the same hospital if the patient's condition deteriorates to the point of requiring inpatient care, as per clinical practice in Japan. The date of first admission was used to estimate the percentage of eligible patients hospitalized within 0–6 days, 0–13 days, and 0–29 days of the index date. Per cent hospitalizations within these time intervals were also calculated specifically for patients who required oxygen or intravenous (IV) fluids to restrict to those requiring medical attention.
2.4. Analyses
We took a descriptive approach to understand baseline characteristics, hospitalizations rates, in‐hospital clinical outcomes, and HCRU. We concluded that it would not be appropriate to estimate the relative measures of association (e.g., odds ratios) to compare such parameters between RSV infection and influenza, as various confounders (e.g., testing intensity) may exist. Instead, whenever possible, we presented data for the overall population, as well as data stratified by age since age is an important cofounder that influences various outcomes. Age categories used were less than 6 months, 6 months to 1 year, 1–2 years, and 2–4 years of age (summary data were also presented by month for less than 1 year). Continuous variables were summarized with means/standard deviations (SD) and median/interquartile range (IQR). Categorical variables were summarized using counts and percentages.
Epidemic curves for outpatients and inpatients for RSV infection and influenza were created to describe temporal trends. Finally, to understand the influence of the COVID‐19 pandemic on epidemiology, analyses were also performed and stratified by time period, including 2017–2019 (pre‐COVID‐19), 2020–2022 (during‐COVID‐19), and the 2021 season (as this season showed a unique RSV epidemic curve [3]). All analyses were performed using SAS software (version 9.4).
3. Results
During the study period between April 2011 and July 2022, 176,911 RSV‐confirmed cases and 153,383 influenza‐confirmed cases were identified in the outpatient setting (Figure 1; Tables S2–S3). Furthermore, 90,413 RSV‐confirmed hospitalizations and 11,186 influenza‐confirmed hospitalizations were identified (overall Table 1; healthy children Table S4). For both RSV infection and influenza, there were more males than females. Among hospitalized RSV cases, 39.0% were less than 6 months, 20.8% were 6 months to 1 year, 26.4% were 1–2 years, and 13.7% were 2–4 years. Among hospitalized influenza cases, 19.1% were less than 6 months, 10.6% were 6 months to 1 year, 25.4% were 1–2 years, and 44.8% were 2–4 years. Among inpatients, 85,896/90,413 (95.0%) of RSV cases and 10,789/11,186 (96.5%) of influenza cases occurred in patients with no record of relevant co‐morbidities or prematurity. Table S5 shows the data among infants by month of age.
FIGURE 1.
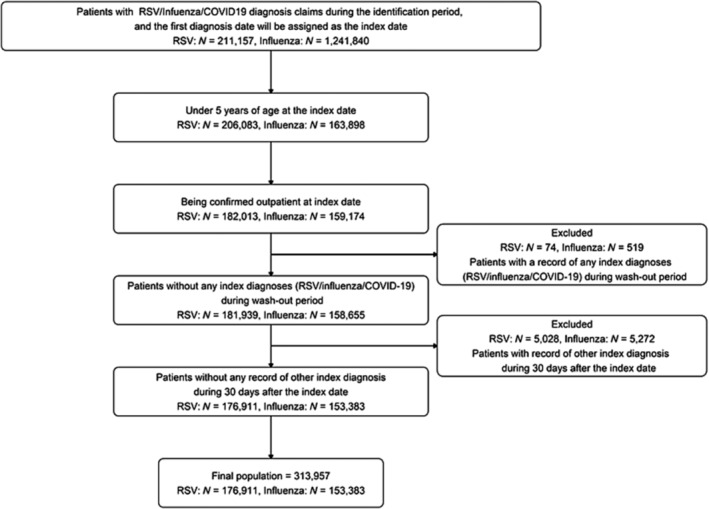
Description of the flow for assembling the outpatient population used to calculate hospital admission rates.
TABLE 1.
Baseline characteristics for all children hospitalized with RSV infection and influenza overall and by age categories.
| RSV | Influenza | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Age < 6 months | Age 6–11 months | Age 1 < 2 years | Age 2 < 5 years | Total | Age < 6 months | Age 6–11 months | Age 1 < 2 years | Age 2 < 5 years | |
| Total number of patients, n (relative % by age group) | 90,413 (100) | 35,273 (39.0) | 18,827 (20.8) | 23,898 (26.4) | 12,415 (13.7) | 11,186 (100) | 2141 (19.1) | 1188 (10.6) | 2845 (25.4) | 5012 (44.8) |
| Sex, n (%) | ||||||||||
| Female | 40,958 (45.3) | 15,564 (44.1) | 8030 (42.7) | 11,133 (46.6) | 6231 (50.2) | 4890 (43.7) | 986 (46.1) | 517 (43.5) | 1282 (45.1) | 2105 (42.0) |
| Male | 49,455 (54.7) | 19,709 (55.9) | 10,797 (57.4) | 12,765 (53.4) | 6184 (49.8) | 6296 (56.3) | 1155 (54.0) | 671 (56.5) | 1563 (54.9) | 2907 (58.0) |
| Age, months | ||||||||||
| Mean (SD) | (11.8, 11.1) | (2.5, 1.4) | (8.4, 1.7) | (16.7, 3.4) | (33.9, 8.7) | (24.0, 17.4) | (2.6, 1.3) | (8.6, 1.7) | (17.4, 3.3) | (40.7, 10.6) |
| Median (IQR) | 9.0 (3.0–17.0) | 2.0 (1.0–4.0) | 8.0 (7.0–10.0) | 16.0 (14.0–19.0) | 32.0 (27.0–40.0) | 21.0 (9.0–38.0) | 2.0 (2.0–3.0) | 9.0 (7.0–10.0) | 17.0 (14.0–20.0) | 40.0 (31.0–50.0) |
| Season by year, n (%) | ||||||||||
| 2011 (May 2011 to April 2012) | 2000 (2.2) | 724 (2.1) | 451 (2.4) | 547 (2.3) | 278 (2.2) | 360 (3.2) | 70 (3.3) | 38 (3.2) | 79 (2.8) | 173 (3.5) |
| 2012 (May 2012 to April 2013) | 3609 (4.0) | 1367 (3.9) | 845 (4.5) | 940 (3.9) | 457 (3.7) | 517 (4.6) | 99 (4.6) | 44 (3.7) | 129 (4.5) | 245 (4.9) |
| 2013 (May 2013 to April 2014) | 5259 (5.8) | 2252 (6.4) | 1191 (6.3) | 1227 (5.1) | 589 (4.8) | 933 (8.4) | 147 (6.9) | 99 (8.4) | 223 (7.9) | 464 (9.3) |
| 2014 (May 2014 to April 2015) | 8057 (8.9) | 3223 (9.1) | 1793 (9.5) | 2038 (8.5) | 1003 (8.1) | 1142 (10.2) | 262 (12.3) | 117 (9.9) | 275 (9.7) | 488 (9.8) |
| 2015 (May 2015 to April 2016) | 9532 (10.6) | 3834 (10.9) | 2057 (10.9) | 2464 (10.3) | 1177 (9.5) | 1575 (14.1) | 231 (10.8) | 202 (17.1) | 403 (14.2) | 739 (14.8) |
| 2016 (May 2016 to April 2017) | 8926 (9.9) | 3626 (10.3) | 2057 (10.9) | 2302 (9.6) | 941 (7.6) | 1378 (12.4) | 302 (14.1) | 128 (10.8) | 348 (12.3) | 600 (12.0) |
| 2017 (May 2017 to April 2018) | 13,434 (14.9) | 5148 (14.6) | 2987 (15.9) | 3588 (15.0) | 1711 (13.8) | 1677 (15.0) | 303 (14.2) | 174 (14.7) | 396 (13.9) | 804 (16.1) |
| 2018 (May 2018 to April 2019) | 12,356 (13.7) | 5105 (14.5) | 2754 (14.6) | 3148 (13.2) | 1349 (10.9) | 1895 (17.0) | 423 (19.8) | 186 (15.7) | 490 (17.3) | 796 (16.0) |
| 2019 (May 2019 to April 2020) | 11,576 (12.8) | 4380 (12.4) | 2382 (12.7) | 3313 (13.9) | 1501 (12.1) | 1660 (14.9) | 296 (13.9) | 194 (16.4) | 491 (17.3) | 679 (13.6) |
| 2020 (May to December) | 802 (0.9) | 304 (0.9) | 166 (0.9) | 238 (1.0) | 94 (0.8) | 3 (0.0) | 1 (0.1) | 2 (0.2) | 0 (0.0) | 0 (0.0) |
| 2021 (January to December) | 12,396 (13.7) | 4456 (12.6) | 1771 (9.4) | 3438 (14.4) | 2731 (22.0) | 6 (0.1) | 1 (0.1) | 1 (0.1) | 3 (0.1) | 1 (0.0) |
| 2022 (January to July) | 2410 (2.7) | 832 (2.4) | 362 (1.9) | 638 (2.7) | 578 (4.7) | 4 (0.0) | 1 (0.1) | 0 (0.0) | 3 (0.1) | 0 (0.0) |
| Co‐morbidity during baseline period, n (%) | ||||||||||
| Down syndrome | 387 (0.4) | 123 (0.4) | 91 (0.5) | 74 (0.3) | 99 (0.8) | 20 (0.2) | 3 (0.1) | 4 (0.3) | 2 (0.1) | 11 (0.2) |
| Congenital heart disease | 1817 (2.0) | 902 (2.6) | 501 (2.7) | 264 (1.1) | 150 (1.2) | 119 (1.1) | 36 (1.7) | 33 (2.8) | 29 (1.0) | 21 (0.4) |
| Bronchopulmonary dysplasia | 110 (0.1) | 40 (0.1) | 42 (0.2) | 18 (0.1) | 10 (0.1) | 5 (0.0) | 1 (0.1) | 2 (0.2) | 1 (0.0) | 1 (0.0) |
| Prematurity | 1979 (2.2) | 1288 (3.7) | 546 (2.9) | 122 (0.5) | 23 (0.2) | 79 (0.7) | 35 (1.6) | 36 (3.0) | 6 (0.2) | 2 (0.0) |
| Immunodeficiency | 169 (0.2) | 68 (0.2) | 45 (0.2) | 35 (0.2) | 21 (0.2) | 29 (0.3) | 4 (0.2) | 6 (0.5) | 5 (0.2) | 14 (0.3) |
| Pulmonary hypoplasia | 19 (0.0) | 10 (0.0) | 4 (0.0) | 2 (0.0) | 3 (0.0) | 2 (0.0) | 1 (0.1) | 1 (0.1) | 0 (0.0) | 0 (0.0) |
| Airway stenosis | 36 (0.0) | 9 (0.0) | 9 (0.1) | 13 (0.1) | 5 (0.0) | 2 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.1) | 0 (0.0) |
| Esophageal atresia | 3 (0.0) | 1 (0.0) | 0 (0.0) | 2 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Congenital metabolic disorder | 378 (0.4) | 82 (0.2) | 100 (0.5) | 115 (0.5) | 81 (0.7) | 93 (0.8) | 3 (0.1) | 9 (0.8) | 32 (1.1) | 49 (1.0) |
| Neuromuscular disorder | 358 (0.4) | 68 (0.2) | 77 (0.4) | 106 (0.4) | 107 (0.9) | 100 (0.9) | 1 (0.1) | 13 (1.1) | 30 (1.1) | 56 (1.1) |
| Cystic fibrosis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| No record of these co‐morbidities | 85,896 (95.0) | 32,977 (93.5) | 17,671 (93.9) | 23,251 (97.3) | 11,997 (96.6) | 10,789 (96.5) | 2064 (96.4) | 1100 (92.6) | 2750 (96.7) | 4875 (97.3) |
| Palivizumab use, n (%) | ||||||||||
| Yes | 1559 (1.7) | 706 (2.0) | 453 (2.4) | 354 (1.5) | 46 (0.4) | 82 (0.7) | 16 (0.8) | 28 (2.4) | 33 (1.2) | 5 (0.1) |
| No record of use | 88,854 (98.3) | 34,567 (98.0) | 18,374 (97.6) | 23,544 (98.5) | 12,369 (99.6) | 11,104 (99.3) | 2125 (99.3) | 1160 (97.6) | 2812 (98.8) | 5007 (99.9) |
| Size of healthcare facilities, n (%) | ||||||||||
| Less than 200 beds | 5252 (5.8) | 1781 (5.1) | 1209 (6.4) | 1454 (6.1) | 808 (6.5) | 551 (4.9) | 68 (3.2) | 55 (4.6) | 156 (5.5) | 272 (5.4) |
| 200 to 499 beds | 50,876 (56.3) | 19,056 (54.0) | 11,029 (58.6) | 13,859 (58.0) | 6932 (55.8) | 6090 (54.4) | 1138 (53.2) | 640 (53.9) | 1578 (55.5) | 2734 (54.6) |
| 500 or more beds | 34,285 (37.9) | 14,436 (40.9) | 6589 (35.0) | 8585 (35.9) | 4675 (37.7) | 4545 (40.6) | 935 (43.7) | 493 (41.5) | 1111 (39.1) | 2006 (40.0) |
Note: Rounding may create totals slightly over or below 100% in some cases.
Regarding the epidemic curves for influenza, a clear seasonal pattern in wintertime was observed with no epidemic occurring in late 2020–2022 (Figure 2; Table S6). In contrast, RSV infection also had epidemic peaks, but with considerable year‐to‐year variability. Specifically, before and during 2016, the peaks were from fall to winter. In 2017–2019, peaks began and ended earlier spanning summer to fall. There was no epidemic in 2020, but the largest wave was observed in 2021 peaking in early summer. These findings were in line with the corresponding NESID data. Also, importantly, trends and levels of epidemics were similar between inpatients and outpatients for both RSV infection and influenza, respectively.
FIGURE 2.
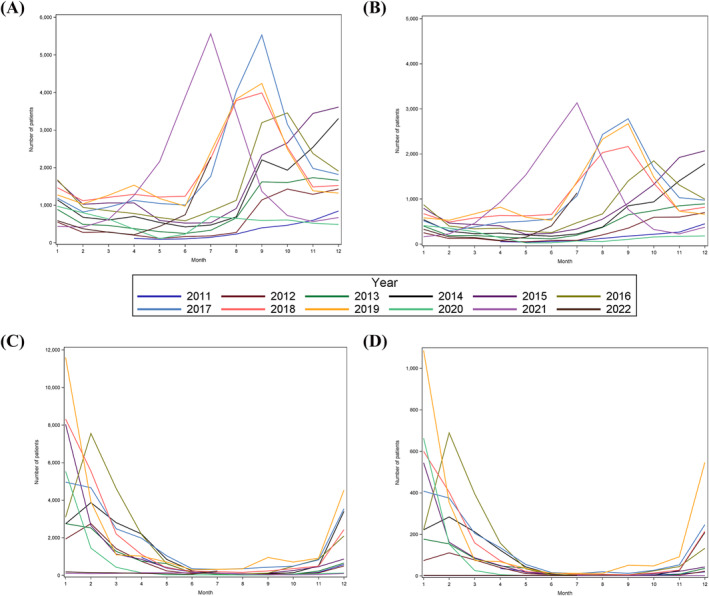
Epidemic curve for RSV and influenza identified in outpatient and inpatient settings. (A) Outpatient RSV infection, (B) inpatient RSV infection, (C) outpatient influenza, (D) inpatient influenza.
Hospitalization rate among confirmed outpatient RSV diagnosis was 43,637/176,911 (24.7%) within 6 days of diagnosis (Table 2). This rate was similar when restricting to hospitalization requiring oxygen/IV usage (42,277/176,911; 23.9%). When stratified by age group, the highest percentage of hospitalization was observed among the 1–2 years age category (11,995/42,485; 28.2%), although this could be due to less outpatient testing (Supplementary Methods). Also, hospitalization rate within 6 days of diagnosis among healthy children was higher (41,666/141,843; 29.4%) than the non‐restricted population and the highest percentage was observed for the less than 6 months age category within 29 days of diagnosis (15,717/45,318; 34.7%). In contrast, hospitalization rate among confirmed outpatient influenza diagnosis was much lower at 4247/153,383 (2.8%) within 6 days of diagnosis. When stratified by age group, the highest percentage of hospitalization was observed among those less than 6 months age (736/8741; 8.4%).
TABLE 2.
Percentage of first hospitalizations after confirmed diagnosis of RSV or influenza at the outpatient setting in Japan among the overall population and after restricting to healthy children.
| RSV | Influenza | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Age < 6 months | Age 6–11 months | Age 1 < 2 years | Age 2 < 5 years | Total | Age < 6 months | Age 6–11 months | Age 1 < 2 years | Age 2 < 5 years | |
| All children | ||||||||||
| Total confirmed diagnoses at outpatient setting, n (relative % by age group) | 176,911 (100) | 70,021 (39.6) | 39,311 (22.2) | 42,485 (24.0) | 25,094 (14.2) | 153,383 (100) | 8741 (5.7) | 16,295 (10.6) | 39,218 (25.6) | 89,129 (58.1) |
| Overall hospitalizations after diagnosis (n, % of outpatient diagnoses) | ||||||||||
| Admissions within 0–6 days | 43,637 (24.7) | 16,560 (23.7) | 8903 (22.7) | 11,995 (28.2) | 6179 (24.6) | 4247 (2.8) | 736 (8.4) | 441 (2.7) | 1126 (2.9) | 1944 (2.2) |
| Admissions within 0–13 days | 43,805 (24.8) | 16,645 (23.8) | 8949 (22.8) | 12,024 (28.3) | 6187 (24.7) | 4270 (2.8) | 739 (8.5) | 444 (2.7) | 1135 (2.9) | 1952 (2.2) |
| Admissions within 0–29 days | 44,281 (25.0) | 16,869 (24.1) | 9090 (23.1) | 12,116 (28.5) | 6206 (24.7) | 4327 (2.8) | 740 (8.5) | 448 (2.8) | 1156 (3.0) | 1983 (2.2) |
| Hospitalizations with oxygen/IV usage (n, % of outpatient diagnoses) | ||||||||||
| Admissions within 0–6 days | 42,277 (23.9) | 15,713 (22.4) | 8715 (22.2) | 11,841 (27.9) | 6008 (23.9) | 4089 (2.7) | 695 (8.0) | 426 (2.6) | 1092 (2.8) | 1876 (2.1) |
| Admissions within 0–13 days | 42,421 (24.0) | 15,787 (22.6) | 8755 (22.3) | 11,865 (27.9) | 6014 (24.0) | 4112 (2.7) | 698 (8.0) | 429 (2.6) | 1101 (2.8) | 1884 (2.1) |
| Admissions within 0–29 days | 42,881 (24.2) | 15,998 (22.9) | 8894 (22.6) | 11,956 (28.1) | 6033 (24.0) | 4167 (2.7) | 699 (8.0) | 433 (2.7) | 1122 (2.9) | 1913 (2.2) |
| Healthy children a | ||||||||||
| Overall hospitalizations after diagnosis, n (relative % by age group) | 141,843 (100) | 45,318 (31.9) | 33,225 (23.4) | 39,545 (27.9) | 23,755 (16.7) | 149,529 (100) | 8197 (5.5) | 15,277 (10.2) | 38,019 (25.4) | 88,036 (58.9) |
| Overall hospitalizations after diagnosis, n (% of outpatient diagnoses) | ||||||||||
| Admissions within 0–6 days | 41,666 (29.4) | 15,717 (34.7) | 8443 (25.4) | 11,573 (29.3) | 5933 (25.0) | 4020 (2.7) | 694 (8.5) | 386 (2.5) | 1066 (2.8) | 1874 (2.1) |
| Admissions within 0–13 days | 41,814 (29.5) | 15,794 (34.9) | 8479 (25.5) | 11,600 (29.3) | 5941 (25.0) | 4042 (2.7) | 697 (8.5) | 389 (2.5) | 1075 (2.8) | 1881 (2.1) |
| Admissions within 0–29 days | 42,228 (29.8) | 15,970 (35.2) | 8610 (25.9) | 11,688 (29.6) | 5960 (25.1) | 4095 (2.7) | 698 (8.5) | 392 (2.6) | 1094 (2.9) | 1911 (2.2) |
| Hospitalizations with oxygen/IV usage (% of outpatient diagnoses) | ||||||||||
| Admissions within 0–6 days | 40,384 (28.5) | 14,919 (32.9) | 8276 (24.9) | 11,425 (28.9) | 5764 (24.3) | 3874 (2.6) | 657 (8.0) | 375 (2.5) | 1033 (2.7) | 1809 (2.1) |
| Admissions within 0–13 days | 40,515 (28.6) | 14,988 (33.1) | 8308 (25.0) | 11,449 (29.0) | 5770 (24.3) | 3896 (2.6) | 660 (8.1) | 378 (2.5) | 1042 (2.7) | 1816 (2.1) |
| Admissions within 0–29 days | 40,916 (28.8) | 15,154 (33.4) | 8437 (25.4) | 11,536 (29.2) | 5789 (24.4) | 3947 (2.6) | 661 (8.1) | 381 (2.5) | 1061 (2.8) | 1844 (2.1) |
Healthy children were defined as those with children without a record of co‐morbidity of interest and no palivizumab use.
Various in‐hospital outcomes/HCRU‐related factors were described (Table 3). IV fluid was used in 86,258/90,413 (95.4%) of RSV cases and 10,622/11,186 (95.0%) of influenza cases. Oxygen was required in 43,056/90,413 (47.6%) of RSV cases and 1656/11,186 (14.8%) of influenza cases. Mechanical ventilation was required in 1928/90,413 (2.1%) of RSV cases and 74/11,186 (0.7%) of influenza cases. In‐hospital death occurred in 31/90,413 (< 0.1%) of RSV cases and 6/11,186 (0.1%) of influenza cases. Antibiotics were prescribed in 43,418/90,413 (48.0%) of RSV cases and 2728/11,186 (24.4%) of influenza cases. The mean inpatient stays were 6.1 days and 4.3 days with direct medical costs of 435,744JPY/patient and 315,809JPY/patient for RSV infection and influenza, respectively. Regarding in‐hospital complications, pneumonia occurred in 30,376/90,413 (33.6%) of RSV cases and 1428/11,186 (12.8%) of influenza cases. Otitis media occurred in 6915/90,413 (7.7%) of RSV cases and 255/11,186 (2.3%) of influenza cases. Febrile seizure occurred in 1366/90,413 (1.5%) of RSV cases and 3843/11,186 (34.4%) of influenza cases. Encephalitis or encephalopathy occurred in 49/90,413 (0.1%) of RSV cases and 54/11,186 (0.5%) of influenza cases. Myocarditis occurred in 4/90,413 (< 0.1%) of RSV cases and 68/11,186 (0.6%) of influenza cases.
TABLE 3.
Clinical outcomes and healthcare resource utilization and cost for children hospitalized with RSV infection and influenza.
| RSV | Influenza | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Age < 6 months | Age 6–11 months | Age 1 < 2 years | Age 2 < 5 years | Total | Age < 6 months | Age 6–11 months | Age 1 < 2 years | Age 2 < 5 years | |
| Total number of patients, n (relative % by age group) | 90,413 (100) | 35,273 (39.0) | 18,827 (20.8) | 23,898 (26.4) | 12,415 (13.7) | 11,186 (100) | 2141 (19.1) | 1188 (10.6) | 2845 (25.4) | 5012 (44.8) |
| Intravenous fluids use | ||||||||||
| No | 4155 (4.6) | 2432 (6.9) | 656 (3.5) | 548 (2.3) | 519 (4.2) | 564 (5.0) | 124 (5.8) | 58 (4.9) | 122 (4.3) | 260 (5.2) |
| Yes | 86,258 (95.4) | 32,841 (93.1) | 18,171 (96.5) | 23,350 (97.7) | 11,896 (95.8) | 10,622 (95.0) | 2017 (94.2) | 1130 (95.1) | 2723 (95.7) | 4752 (94.8) |
| Oxygen use | ||||||||||
| No | 47,357 (52.4) | 19,575 (55.5) | 9106 (48.4) | 12,105 (50.7) | 6571 (52.9) | 9530 (85.2) | 2014 (94.1) | 1016 (85.5) | 2292 (80.6) | 4208 (84.0) |
| Yes | 43,056 (47.6) | 15,698 (44.5) | 9721 (51.6) | 11,793 (49.4) | 5844 (47.1) | 1656 (14.8) | 127 (5.9) | 172 (14.5) | 553 (19.4) | 804 (16.0) |
| High flow nasal cannula oxygen therapy use | ||||||||||
| No | 88,117 (97.5) | 33,791 (95.8) | 18,426 (97.9) | 23,614 (98.8) | 12,286 (99.0) | 11,162 (99.8) | 2137 (99.8) | 1185 (99.8) | 2841 (99.9) | 4999 (99.7) |
| Yes | 2296 (2.5) | 1482 (4.2) | 401 (2.1) | 284 (1.2) | 129 (1.0) | 24 (0.2) | 4 (0.2) | 3 (0.3) | 4 (0.1) | 13 (0.3) |
| Use of mechanical ventilation | ||||||||||
| No | 88,485 (97.9) | 34,118 (96.7) | 18,510 (98.3) | 23,593 (98.7) | 12,264 (98.8) | 11,112 (99.3) | 2133 (99.6) | 1183 (99.6) | 2826 (99.3) | 4970 (99.2) |
| Yes | 1928 (2.1) | 1155 (3.3) | 317 (1.7) | 305 (1.3) | 151 (1.2) | 74 (0.7) | 8 (0.4) | 5 (0.4) | 19 (0.7) | 42 (0.8) |
| Intensive care unit (ICU) admissions | ||||||||||
| No | 89,813 (99.3) | 34,842 (98.8) | 18,743 (99.6) | 23,846 (99.8) | 12,382 (99.7) | 11,147 (99.7) | 2138 (99.9) | 1186 (99.8) | 2831 (99.5) | 4992 (99.6) |
| Yes | 600 (0.7) | 431 (1.2) | 84 (0.5) | 52 (0.2) | 33 (0.3) | 39 (0.4) | 3 (0.1) | 2 (0.2) | 14 (0.5) | 20 (0.4) |
| In‐hospital death | ||||||||||
| No | 90,382 (100) | 35,262 (100) | 18,818 (100) | 23,892 (100) | 12,410 (100) | 11,180 (100) | 2140 (100) | 1188 (100) | 2844 (100) | 5008 (99.9) |
| Yes | 31 (0.0) | 11 (0.0) | 9 (0.1) | 6 (0.0) | 5 (0.0) | 6 (0.1) | 1 (0.1) | 0 (0.0) | 1 (0.0) | 4 (0.1) |
| Antibiotics use | ||||||||||
| No | 46,995 (52.0) | 23,461 (66.5) | 8854 (47.0) | 9337 (39.1) | 5343 (43.0) | 8458 (75.6) | 1792 (83.7) | 866 (72.9) | 2067 (72.7) | 3733 (74.5) |
| Yes | 43,418 (48.0) | 11,812 (33.5) | 9973 (53.0) | 14,561 (60.9) | 7072 (57.0) | 2728 (24.4) | 349 (16.3) | 322 (27.1) | 778 (27.4) | 1279 (25.5) |
| In‐hospital complications | ||||||||||
| Pneumonia | 30,376 (33.6) | 6590 (18.7) | 6484 (34.4) | 11,353 (47.5) | 5949 (47.9) | 1428 (12.8) | 115 (5.4) | 158 (13.3) | 413 (14.5) | 742 (14.8) |
| Otitis media | 6915 (7.7) | 1047 (3.0) | 1876 (10.0) | 2911 (12.2) | 1081 (8.7) | 255 (2.3) | 17 (0.8) | 45 (3.8) | 102 (3.6) | 91 (1.8) |
| Febrile seizure | 1366 (1.5) | 67 (0.2) | 130 (0.7) | 761 (3.2) | 408 (3.3) | 3843 (34.4) | 44 (2.1) | 254 (21.4) | 1430 (50.3) | 2115 (42.2) |
| Encephalitis or encephalopathy | 49 (0.1) | 24 (0.1) | 2 (0.0) | 12 (0.1) | 11 (0.1) | 54 (0.5) | 4 (0.2) | 5 (0.4) | 24 (0.8) | 21 (0.4) |
| Myocarditis | 4 (0.0) | 1 (0.0) | 1 (0.0) | 1 (0.0) | 1 (0.0) | 68 (0.6) | 1 (0.1) | 5 (0.4) | 15 (0.5) | 47 (0.9) |
| Length of inpatient stay, days | ||||||||||
| Mean (SD) | 6.1 (3.3) | 6.3 (3.7) | 6.2 (2.9) | 5.9 (3.4) | 5.7 (2.5) | 4.3 (2.6) | 4.1 (1.8) | 4.5 (2.8) | 4.4 (2.5) | 4.3 (2.8) |
| Median (IQR) | 6.0 (4.0–7.0) | 6.0 (4.0–7.0) | 6.0 (4.0–7.0) | 5.0 (4.0–7.0) | 5.0 (4.0–7.0) | 4.0 (3.0–5.0) | 4.0 (3.0–4.0) | 4.0 (3.0–5.0) | 4.0 (3.0–5.0) | 4.0 (3.0–5.0) |
| Total costs during hospitalization (per patient) | ||||||||||
| Mean (SD) | 435,743.8 (271,703.1) | 453,731.5 (314,856.1) | 434,766.4 (248,195.8) | 420,035.9 (243,875.7) | 416,362.1 (215,192.6) | 315,809.4 (209,438.7) | 292,933.0 (151,420.1) | 327,031.6 (248,576.5) | 330,289.5 (234,427.2) | 314,702.2 (204,736.6) |
| Median (IQR) | 391,648.5 (286,581.3‐524,253.1) | 402,215.5 (291,487.7‐542,645.5) | 391,262.3 (286,157.9‐524,239.2) | 3847,67.1 (283,405.0‐506,331.2) | 380,722.7 (280,912.3–504,248.2) | 2821,85.4 (207,122.7‐371,700.4) | 272,527.0 (200,339.0‐346,756.2) | 287,527.1 (217,363.1‐377,209.5) | 295,797.2 (219,178.9‐391,632.7) | 279,503.3 (202,282.5‐372,237.4) |
Note: All costs are noted in Japanese Yen, Rounding may create totals slightly over or below 100% in some cases.
Abbreviations: IQR, interquartile range; SD, standard deviation.
Finally, we stratified the number of hospitalizations by COVID‐19 era (Tables S7–S8). There was a comparatively lower proportion of RSV hospitalizations in age < 6 months and age 6–11 months during COVID‐19 era (36.4%, 15.1%) and 2021 season (35.9%, 14.3%) compared with pre‐COVID‐19 era (39.3%, 21.9%). Conversely, there was a comparatively higher proportion of RSV hospitalizations in age 2 years to 4 years during COVID‐19 era (21.3%) and 2021 season (22.0%) compared with pre‐COVID‐19 era (12.0%). There were very few influenza hospitalizations during COVID‐19 era.
4. Discussion
We conducted a study using large electronic health records database covering approximately 25% of acute care hospitals in Japan to elucidate the comprehensive inpatient burden of RSV infection and influenza in young children. We found that a large number of RSV and influenza cases were diagnosed in both outpatient and inpatient settings with substantial in‐hospital outcomes/complications and HCRU during the 11‐year study period.
Similar to what has been observed in previous reports [1], over half of RSV hospitalizations occurred in infants less than 1 year of age. Approximately 95% of these RSV hospitalizations occurred in patients with no record of co‐morbidities or prematurity. We also observed hospitalization rates among children diagnosed with RSV infection to be 20%–30%, meaning that one in four medically attended RSV infections resulted in hospitalization. When restricting to patients without co‐morbidities/prematurity, the rates were even higher (up to 35%), which is likely to be due to unavailability of prevention measures in this population (unlike palivizumab for children with co‐morbidities/prematurity). These were in stark contrast to low hospitalization rates for influenza where only 2%–3% in children 6 months of age or older and 8%–9% among infants less 6 months of age.
We further examined in‐hospital outcomes and HCRU related to each infection. Important in‐hospital outcomes including oxygen use, ICU admission, and mechanical ventilation were more prevalent with RSV infections compared to influenza across all age groups. Still, ICU admission and mechanical ventilation among the RSV inpatient population were much lower compared to the data from the U.S., which could be due to a lower threshold of hospitalization in Japan and scarcity of pediatric ICUs in Japan (i.e., some specialized care including mechanical ventilation is routinely done in general wards) [15]. Although the numbers and proportions were small, a few in‐hospital deaths occurred for both infections. Antibiotic use was more prevalent for RSV infection than for influenza. This could be due to more severe in‐hospital outcomes for RSV infection and improved antimicrobial stewardship is warranted. Regarding in‐hospital complications, pneumonia and otitis media were more common in RSV infection than in influenza. In contrast, febrile seizures, encephalitis/encephalopathy, and myocarditis were more common in influenza than in RSV infection. Notably, approximately one‐third of influenza hospitalization were associated with febrile seizure. Longer inpatient stays (6.1 vs. 4.3 days) with higher direct medical costs (435,744 vs. 315,809 JPY/patient) were observed with RSV infection compared with influenza. Finally, analyses by COVID‐19 era revealed a slight shift in age of RSV hospitalizations to older age groups in 2021–2022, which is in line with lack of epidemic in 2020. This shift may recover to pre‐COVID‐19 trends after a few years of epidemics together with disrupted seasonal pattern as observed in the United States [10]
Given the substantial burden of RSV infection with considerable in‐hospital outcomes/complications and HCRU, the available RSV prophylaxis, palivizumab, which only covers children with specific co‐morbidities or prematurity, was not enough to protect the majority of children from hospitalization when infected. In contrast, for influenza, routine vaccination and antivirals are available and widely used, with vaccine uptake among children estimated to be approximately 50% [16, 17]. This may be playing a role in reducing influenza disease burden. Nevertheless, the availability of prevention/treatment for influenza and the unavailability of prevention/treatment for RSV infection are reflected in the real‐world disease burden from RSV disease. To mitigate this RSV burden in the pediatric population, novel RSV prevention measures to protect all children are needed. Recently, another monoclonal antibody with extended half‐life, nirsevimab, was approved for use as a preventative treatment for all infants entering their first RSV season in the United States and many other countries [18]. Maternal immunization is also approved for use, although only available in a seasonal manner with a narrow window period for immunization (pregnant individuals at 32–36 weeks gestational age) in the United States [19]. Nirsevimab was also approved in Japan in March 2024, but reimbursement under the national health insurance is only limited to a high‐risk population similar to palivizumab. Therefore, widespread use in all infants via, for example, inclusion in the National Immunization Program is needed to reduce the burden of RSV infection in all infants. There are other potential preventive measures for toddlers under development [20]. The current study highlights that the burden of severe RSV infection in children in Japan and other countries are similar. Therefore, improved awareness of disease burden as well as wide use of these preventive measures are highly anticipated.
4.1. Limitations
There are several limitations to this study. First, the current database is based on participating hospitals only, and there is no linkage of data across hospitals. Second, although both RSV and influenza testing is covered by NHI (Supplementary Methods), careful interpretation is necessary as testing intensity may differ between RSV infection and influenza as well as by age group (especially infants vs. young children > 1 years). Third, the database did not have information on influenza vaccination/anti‐viral use, which may have influenced study outcomes. Fourth, the database used did not include geographic information, and thus, regional variation in seasonality was not able to be assessed. Finally, similar to other database studies, medical coding may not always reflect true diagnosis or procedures.
5. Conclusions
A large number of RSV and influenza cases were diagnosed in both Japanese outpatient and inpatient settings during the study period. There were also substantial in‐hospital outcomes/complications and HCRU, many of which were more frequently observed with RSV infection compared to influenza. Furthermore, longer inpatient stays with more expensive direct medical costs were seen with RSV infection compared to influenza. Approximately one in four RSV infection resulted in hospitalization with most hospitalization occurring among healthy term children, emphasizing the need for RSV prevention measures in all children.
Author Contributions
Takeshi Arashiro: conceptualization, formal analysis, investigation, methodology, writing – original draft. Rolf Kramer: conceptualization, formal analysis, funding acquisition, methodology. Jing Jin: formal analysis, methodology. Munehide Kano: conceptualization, formal analysis, methodology. Fangyuan Wang: formal analysis, methodology. Isao Miyairi: conceptualization, formal analysis, investigation, methodology.
Ethics Statement
The study was conducted under the ethical principles consistent with the Japanese Ethical Guidelines for Medical and Biological Research Involving Human Subjects and the Declaration of Helsinki. The requirement for informed consent was waived as all personal and identifying data were anonymized in the administrative health claims dataset.
Conflicts of Interest
Takeshi Arashiro is a Sanofi employee and a cooperative researcher at the National Institute of Infectious Diseases, Japan. Rolf Kramer, Jing Jin, and Munehide Kano are Sanofi employees. Fangyuan Wang is a Syneos Health employee. Isao Miyairi received honoraria from Sanofi for lectures and advisory committees.
Supporting information
Table S1. Relevant ICD‐10 codes for conditions of interest.
Table S2. Baseline characteristics of all children < 5 years of age who were diagnosed in the outpatient setting with RSV infection and influenza.
Table S3. Baseline characteristics of healthy children < 5 years of age who were diagnosed in the outpatient setting with RSV infection and influenza.
Table S4. Baseline characteristics for healthy children hospitalized with RSV infection and influenza overall and by age categories.
Table S5. Distribution of infants < 1 year of age who are diagnosed in the outpatient setting, who are hospitalized, who required oxygen use, or who required mechanical ventilation with RSV infection or influenza in Japan by month of age.
Table S6. Annual number of cases by month.
Table S7. Baseline characteristics of children < 5 years of age who are hospitalized with RSV infection by age group and COVID‐19 era.
Table S8. Baseline characteristics of children < 5 years of age who are hospitalized with influenza in Japan by age group and COVID‐19 era.
Acknowledgements
We thank employees of Syneos Health for technical assistance in study design and data analysis. We also thank Ko Nakajo for technical assistance in study conception and design.
Funding: This work was supported by Sanofi and AstraZeneca.
Data Availability Statement
Individual‐level data of patients included in this manuscript are considered sensitive and will not be shared. The study methods and statistical analyses are described in detail in Section 2 and throughout the manuscript.
References
- 1. Li Y., Wang X., Blau D. M., et al., “Global, Regional, and National Disease Burden Estimates of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Children Younger Than 5 Years in 2019: A Systematic Analysis,” Lancet 399, no. 10340 (2022): 2047–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang X., Li Y., O'Brien K. L., et al., “Global Burden of Respiratory Infections Associated With Seasonal Influenza in Children Under 5 Years in 2018: A Systematic Review and Modelling Study,” The Lancet Global Health 8, no. 4 (2020): e497–e510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. National Institute of Infectious Diseases . Weekly Infectious Disease Report. https://www.niid.go.jp/niid/ja/idwr.html. Accessed December 23, 2023. (In Japanese)
- 4. National Institute of Infectious Diseases . Influenza Epidemiological Information. https://www.niid.go.jp/niid/ja/diseases/a/flu.html. Accessed December 23, 2023. (In Japanese)
- 5. National Institute of Infectious Diseases , “RS Virus Infections 2018‐2021,” Infectious Agents Surveillance Report. 43, no. 4 (2022): 79–81 (In Japanese). [Google Scholar]
- 6. Nagasawa K. and Ishiwada N., “Disease Burden of Respiratory Syncytial Virus Infection in the Pediatric Population in Japan,” Journal of Infection and Chemotherapy 28, no. 2 (2022): 146–157. [DOI] [PubMed] [Google Scholar]
- 7. Kobayashi Y., Togo K., Agosti Y., and McLaughlin J. M., “Epidemiology of Respiratory Syncytial Virus in Japan: A Nationwide Claims Database Analysis,” Pediatrics International 64, no. 1 (2022): e14957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mashiba S., Takahashi T., Hayashi N., et al., “Features of Patients With Influenza Virus Infection Examined in the Emergency Department of a University Hospital in North‐Western Japan,” The Journal of International Medical Research 32, no. 3 (2004): 331–336. [DOI] [PubMed] [Google Scholar]
- 9. Bardsley M., Morbey R. A., Hughes H. E., et al., “Epidemiology of Respiratory Syncytial Virus in Children Younger Than 5 Years in England During the COVID‐19 Pandemic, Measured by Laboratory, Clinical, and Syndromic Surveillance: A Retrospective Observational Study,” The Lancet Infectious Diseases 23, no. 1 (2023): 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hamid S. W., Parikh R., Jones J., et al., “Seasonality of Respiratory Syncytial Virus — United States, 2017–2023,” Morbidity and Mortality Weekly Report (MMWR). 72, no. 14 (2023): 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gantenberg J. R., van Aalst R., Zimmerman N., et al., “Medically Attended Illness Due to Respiratory Syncytial Virus Infection Among Infants Born in the United States Between 2016 and 2020,” The Journal of Infectious Diseases 226, no. Suppl 2 (2022): S164–S174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bennett M. V., McLaurin K., Ambrose C., and Lee H. C., “Population‐Based Trends and Underlying Risk Factors for Infant Respiratory Syncytial Virus and Bronchiolitis Hospitalizations,” PLoS ONE 13, no. 10 (2018): e0205399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mori M., Yoshizaki K., Watabe S., et al., “Safety, Efficacy and Pharmacokinetics of Palivizumab in Off‐Label Neonates, Infants, and Young Children at Risk for Serious Respiratory Syncytial Virus Infection: A Multicenter Phase II Clinical Trial,” The Lancet Regional Health – Western Pacific 39 (2023): 39100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. AstraZeneca . Notification of Partial Change Approval Application for the Humanized Monoclonal Antibody Against RS Virus, “Synagis”. https://www.astrazeneca.co.jp/media/press‐releases1/2023/2023070301.html. Accessed December 23, 2023. (In Japanese)
- 15. Arriola C. S., Kim L., Langley G., et al., “Estimated Burden of Community‐Onset Respiratory Syncytial Virus‐Associated Hospitalizations Among Children Aged <2 Years in the United States, 2014‐15,” Journal of the Pediatric Infectious Diseases Society 9, no. 5 (2020): 587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nobuhara H., Watanabe Y., and Miura Y., “Estimated Influenza Vaccination Rates in Japan,” Japanese Journal of Public Health 61 (2014): 354–359 (In Japanese). [PubMed] [Google Scholar]
- 17. Okubo Y., Uda K., and Miyairi I., “Trends in Influenza and Related Health Resource Use During 2005‐2021 Among Children in Japan,” The Pediatric Infectious Disease Journal 42, no. 8 (2023): 648–653. [DOI] [PubMed] [Google Scholar]
- 18. Sanofi . Press Release: FDA Approves Beyfortus™ (Nirsevimab‐Alip) to Protect Infants Against RSV Disease. https://www.sanofi.com/en/media‐room/press‐releases/2023/2023‐07‐17‐17‐00‐00‐2705911. Accessed December 23, 2023.
- 19. U.S. Food & Drug Administration . ABRYSVO. https://www.fda.gov/vaccines‐blood‐biologics/abrysvo. Accessed December 23, 2023.
- 20. PATH . RSV Vaccine and mAb Snapshot. https://www.path.org/resources/rsv‐vaccine‐and‐mab‐snapshot/. Accessed December 23, 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Relevant ICD‐10 codes for conditions of interest.
Table S2. Baseline characteristics of all children < 5 years of age who were diagnosed in the outpatient setting with RSV infection and influenza.
Table S3. Baseline characteristics of healthy children < 5 years of age who were diagnosed in the outpatient setting with RSV infection and influenza.
Table S4. Baseline characteristics for healthy children hospitalized with RSV infection and influenza overall and by age categories.
Table S5. Distribution of infants < 1 year of age who are diagnosed in the outpatient setting, who are hospitalized, who required oxygen use, or who required mechanical ventilation with RSV infection or influenza in Japan by month of age.
Table S6. Annual number of cases by month.
Table S7. Baseline characteristics of children < 5 years of age who are hospitalized with RSV infection by age group and COVID‐19 era.
Table S8. Baseline characteristics of children < 5 years of age who are hospitalized with influenza in Japan by age group and COVID‐19 era.
Data Availability Statement
Individual‐level data of patients included in this manuscript are considered sensitive and will not be shared. The study methods and statistical analyses are described in detail in Section 2 and throughout the manuscript.


