Abstract
Inflammasomes are multiprotein complexes that regulate the bioactive production of IL-1β and IL-18, being implicated in the inflammatory response of different diseases. The inflammasome formed by the cytosolic sensor NLRP3 is highly promiscuous, as it could be activated by different pathogen- and sterile-signals. However, few models have studied the implication of NLRP3 in tissue damage-induced inflammation, particularly the implication of NLRP3 in tendinopathies. Here, we aimed to investigate the implication of NLRP3 in a mouse model of tendinopathy by collagenase degradation of the extracellular matrix in the Achilles’ mice tendon. We found that NLRP3 was involved in the production of IL-1β, but another ASC-dependent inflammasome was required to produce IL-18 during sterile tissue damage. Our study suggests that in the immune response to extracellular matrix degradation different inflammasomes, probably expressed in different cell compartments, were able to differentially control IL-1β and IL-18 production in vivo. These results suggest the potential use of therapies targeting ASC as beneficial in the treatment of tendinopathies.
Keywords: ASC, Collagenase, inflammasome, NLRP3, sterile tissue damage, tendon
Introduction
The inflammasome is a multiprotein complex that facilitates the activation of caspase-1 and the subsequent production of mature pro-inflammatory cytokines interleukin (IL)-1β and IL-18 after proteolytically cleavage [1]. Different inflammasome sensor proteins are triggered in different cells in response to different activators, most of them belong to the nucleotide-binding oligomerization domain and leucine-rich repeat receptor (NLR)-family of proteins. Upon activation, most of the NLRs induce the oligomerization of the inflammasome adaptor protein ASC (Apoptosis-associated speck-like protein containing a caspase activation domain) to recruit and activate caspase-1 [2]. Meanwhile, all inflammasomes could be triggered in response to different pathogens, NLRP1 and NLRP3 inflammasomes could be also activated upon recognition of endogenous host-related danger signals [2]. While human NLRP1 expression is restricted to epithelial cells, NLRP3 is mainly expressed in myeloid cells. NLRP3 forms the most promiscuous inflammasome since it could be triggered by a wide range of stimuli, such as particulate matter, crystals, or extracellular ATP, as well as extracellular matrix degradation [2–4]. Almost all of the different NLRP3 activators result in a decrease of intracellular K+ that changes NLRP3 conformation towards an active oligomeric complex [5,6]. The NLRP3 inflammasome has been implicated in the pathophysiological process of several chronic inflammatory, metabolic, and degenerative diseases [7], including several models of sterile inflammation induced by uric acid crystals, silica particles, or Alum [5,8,9]. Injuries of tendons in the food and ankle by overuse are the most common type of tendinopathies, constituting in some cases a chronic injury [10]. The pathogenesis of tendinopathies includes degradation of extracellular matrix and inflammation [11,12], however, the role of the NLRP3 inflammasome has not being studied in the context of tendinopathies. In this study, we investigated the implication of NLRP3 in an in vivo model of tendinopathy, by inducing a sterile tendon damage injecting collagenase in the Achilles’ mice tendon and found that while NLRP3 controls IL-1β production, other ASC-dependent inflammasome was required for IL-18 production. Our study potentially proposes a beneficial use of ASC blockers to treat tendinopathies-associated inflammation.
Methods
Animals and procedures
Mice procedures were approved by the University of Murcia ethical committee and the Animal Health Service of the General Directorate of Fishing and Farming of the Council of Murcia (#A13160702). C57/BL6 mice were obtained from Jackson Laboratories, and Nlrp3−/− and Pycard−/− in C57/BL6 background were already described [13]. Mice were bred in specific pathogen-free conditions at the animal house of the Hospital Virgen Arrixaca with a 12:12 h light–dark cycle and used between 8 and 10 weeks of age. Sterile tissue damage was performed at the animal house of the University of Murcia with an injection of 20 µl of collagenase A (10 µg/µl, Sigma–Aldrich) on one paw of isoflurane-anesthetized mouse. The other paw was either non-injected or injected with 20 µl of saline solution and was used as a control. Different days after injection (from 1 to 21) the animals were sacrificed with CO2 inhalation and the calcaneal tendon was dissected for RT-qPCR studies, or the zone between gastrocnemius and calcaneus, including the tendon, adipose tissue, tibia and peroneus, was dissected for ELISA and histology.
Cytokine evaluation
Tendons were homogenized in homobuffer (70 mM saccharose, 220 mM mannitol, 2 mM Tris-HCl, 0.1 mM EDTA, 0.1% bovine serum albumin, and pH = 7.4, supplemented with protease inhibitors) and were used in ELISA for mouse IL-1β (high-sensitivity, Invitrogen), following the manufacturer’s instructions and read in a Synergy Mx plate reader (BioTek). Multiplexing for IL-6, IL-18, TNFα, and CXCL10 was performed using the Luminex colour-coded antibody-immobilized beads from Invitrogen, and the results were analyzed in a Luminex MAGPIX instrument (Luminex Corporation).
Quantitative reverse transcriptase-polymerase chain reaction (RT-qPCR) analysis
Tendons were homogenized using an Omni THQ homogenizer in Qiazol (Qiagen). Then left for 5 min at room temperature and centrifuged at 12000 × g for 15 min at 4°C. The upper phase was mixed with one volume of 70% ethanol and loaded in RNeasy Mini Kit columns (Qiagen). Total RNA was isolated after DNase I (Qiagen) treatment following the manufacturer’s instructions. Reverse transcription was performed using the iScript cDNA Synthesis kit (BioRad). The mix SYBR Green Premix ExTaq (Takara) was used for qPCR in an iCyclerMyiQ thermocycler (BioRad). Specific primers were from Sigma (KiCqStart SYBR Green Primers) and relative expression of Il6 and Il1b was normalized to the housekeeping gene Actb using the 2−ΔCt method.
Histopathology
Mice paws were fixed in 4% p-formaldehyde (Sigma–Aldrich) for 24 h, processed, paraffin-embedded, and sections stained with hematoxylin and eosin. Quantification of polymorphonuclear cells was performed by nuclear morphology in an AxioScope AX10 microscope (Carl Zeiss) and pictures were taken with an AxioCam506 Color (Carl Zeiss).
Statistics
Statistical analyses were performed using Prism (GraphPad). Data were transformed using Log2 for statistical analysis. Unpaired t-test was used for comparisons of two groups and two-way ANOVA for two variable comparisons between different groups. The test used in each panel is mentioned in the figure legend. Data are shown as mean values and error bars represent standard error from the number of independent assays indicated in the figure legend, which are also overlaid in the histograms as dots. Significant differences were annotated as ****P<0.0001, ***P<0.001, **P<0.01, *P<0.05, ns for P>0.05, not significant.
Results
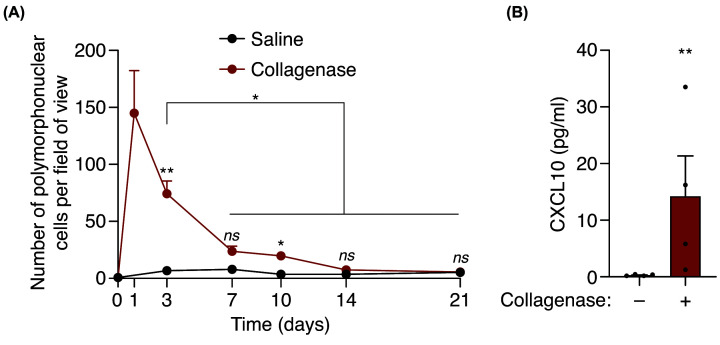
We initially examined the infiltration of polymorphonuclear (PMN) cells in the periphery of the tendon after extracellular matrix degradation by collagenase. PMN cells peaked at 1 day after local collagenase administration and decreased afterwards, reaching basal levels after 14 days (Figure 1A). This result shows that the inflammatory response was resolved within 1 month after local collagenase administration. After collagenase injection, we also found an increase in the chemokine CXCL10 (Figure 1B), suggesting a potential chemoattractant function of this chemokine towards PMN to injury.
Figure 1. Collagenase-induced tissue damage provokes a local inflammatory response.
(A) Quantification of polymorphonuclear cells per field of view of calcaneal tendon sections treated or not with collagenase for the indicated time. Centre values represent the mean (n = 3–15 independent animals per time point) and error bars represent s.e.m. Two-way ANOVA, *P<0.05, **P<0.005, ns P>0.05. (B) CXCL10 detection from calcaneal tendons of wild-type mice treated or not with collagenase after 3 days. Centre values represent the mean (n = 4 independent animals) and error bars represent s.e.m. Unpaired t-test, **P<0.005.
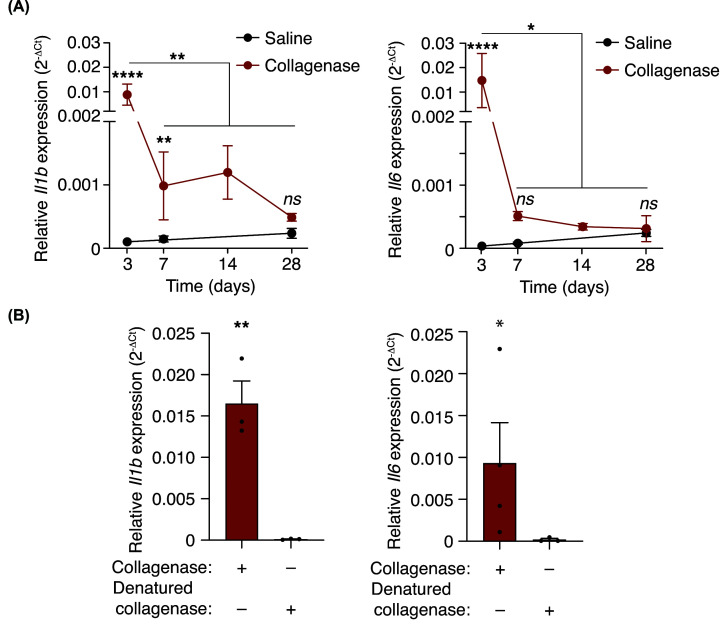
After tissue injury, we also found an increase in the expression of the pro-inflammatory cytokines Il1b and Il6 (Figure 2A). The induction of Il1b and Il6 decreased with the time after collagenase administration (Figure 2A) and was not observed when denatured collagenase was used (Figure 2B). Therefore, collagenase activity, probably by degradation of the extracellular matrix, was required to induce sterile tissue damage driven by an inflammatory infiltrate of PMN cells and an increase in pro-inflammatory cytokines.
Figure 2. Tissue damage provokes an elevation of IL-1β and IL-6.
(A,B) Quantitative PCR for Il1b and Il6 in the calcaneal tendons of wild-type mice after different times of collagenase injection as indicated (A) or after 3 days of denatured collagenase injection (B). Centre values represent the mean (n = 3–6 independent animals) and error bars represent s.e.m.; Two-way ANOVA (A) or unpaired t-test (B) ****P<0.0001, **P<0.01, *P<0.05, and ns P>0.05.
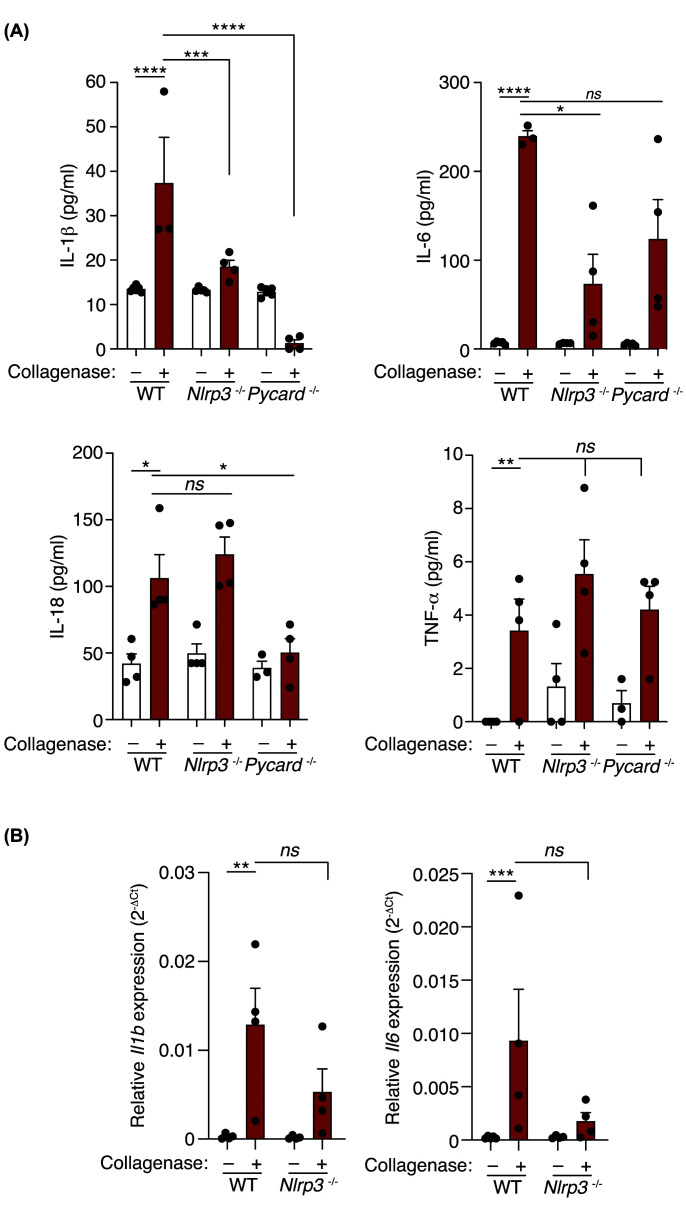
We next aimed to assess if the inflammatory response induced by collagenase was dependent on the NLRP3 inflammasome and found that both IL-1β and IL-6 were reduced in NLRP3-deficient mice (Figure 3A). However, IL-18 and TNFα were not affected in the Nlrp3−/− mice (Figure 3A), but Il1b and Il6 gene expression was reduced in tendons of the Nlrp3−/− mice (Figure 3B). To confirm a role for active inflammasomes and assess the NLRP3-independent production of IL-18, we used mice deficient in the common inflammasome adaptor protein ASC. Collagenase administration in Pycard−/− mice resulted in a reduction of IL-1β, IL-6, and IL-18, but not of TNFα (Figure 3A). This suggests that IL-18 production after tissue injury is dependent on the activation of an ASC-inflammasome.
Figure 3. Pro-inflammatory cytokine production in response to collagenase-induced tissue damage is controlled by different ASC-dependent inflammasomes.
(A) IL-1β, IL-6, IL-18, and TNF-α detection in calcaneal tendons of wild type, Nlrp3−/− and Pycard−/− mice treated or not with collagenase for 3 days. Centre values represent the mean (n = 3–6 independent animals) and error bars represent s.e.m.; Two-way ANOVA, ****P<0.0001, ***P<0.001, **P<0.01, *P<0.05, ns P>0.05. (B) Quantitative PCR for Il1b and Il6 in the calcaneal tendons of wild type and Nlrp3−/− mice treated as in (A). Centre values represent the mean (n = 4–5 independent animals) and error bars represent s.e.m.; Two-way ANOVA, ***P<0.001, **P<0.01, ns P>0.05.
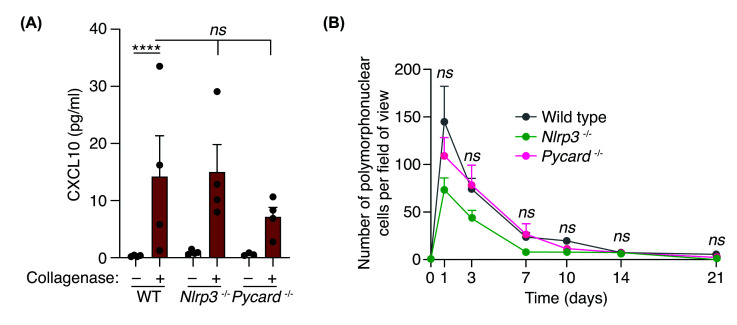
We finally examined if the inflammasome was affecting the production of the chemokine CXCL10 and the infiltration of PMN cells into the tissue, however, neither of these two parameters was significantly affected (Figure 4A,B) and the resolution of the inflammation, in terms of PMN cell infiltration, was neither significantly affected by the lack of NLRP3 or ASC (Figure 4B). Therefore, while inflammasomes control pro-inflammatory cytokine production during sterile tissue injury, it did not affect TNF-α, chemokine production or PMN infiltration.
Figure 4. Polymorphonuclear cell infiltration is independent on ASC-dependent inflammasomes.
(A) CXCL10 detection from calcaneal tendons of wild type, Nlrp3–/– and Pycard–/– mice treated or not with collagenase for 3 days. Centre values represent the mean (n = 3–4 independent animals) and error bars represent s.e.m.; Two-way ANOVA, ****P<0.0001, nsP>0.05. (B) Quantification of polymorphonuclear cells per field of view of calcaneal tendons sections of wild type, Nlrp3–/– and Pycard–/– mice treated as in (A) but for different times as indicated. Centre values represent the mean (n = 2–15 independent animals per time point) and error bars represent s.e.m.; Two-way ANOVA, nsP>0.05.
Discussion
In this study, we found that NLRP3 controls the production of IL-1β in a mouse model of tendinopathy by sterile extracellular matrix degradation, while an alternative ASC-dependent inflammasome controls IL-18 production. This result is surprising as IL-18 is a cytokine depending on caspase-1 activation for maturation and release [1], and although IL-6 is not an inflammasome-dependent cytokine, it is known that IL-1β blocking reduces the production of IL-6 in different pathologies [14]. Therefore, a reduction of IL-1β production in the Nlrp3−/− mice could be controlling IL-6 production in this in vivo model of sterile tissue injury. However, meanwhile ASC deficiency also reduce IL-1β production, IL-6 was not affected in the Pycard−/− mice. Therefore, that degradation of extracellular matrix could be inducing different ASC-dependent inflammasomes, that could be expressed in different cell types, being the production of IL-18 dependent from a non-NLRP3 but ASC-dependent inflammasome. While IL-1β is mainly produced by myeloid cells expressing NLRP3, IL-18 could be largely produced by epithelial cells that do not express NLRP3, but rather express NLRP1 and/or NLRC4 [2,15,16]. For example, autoinflammatory syndromes with gain-of-function mutations in NLRP3 are mainly driven by myeloid-production of IL-1β, while syndromes due to gain-of-function mutations in NLRC4 are associated with epithelial-production of IL-18 [17,18]. Also, IL-1β and IL-18 expression present different regulatory mechanisms [19], that could explain its differential production in different cells. However, differential production of IL-1β and IL-18 has not been always dissociated in in vivo models, and in allogenic transplantation induced a local production of both cytokines dependent on NLRP3 [20]. Therefore, our results confirm that in tendinopathies, differential IL-1β and IL-18 production could be controlled by different inflammasomes.
The NLRP3 inflammasome has been implicated in different diseases where the inflammatory response has an important role, being pharmacological targeting of NLRP3 an important area of development for the clinical treatment of different inflammatory conditions [7,21]. The results of our study propose that pharmacologic treatment of the inflammatory response associated to tendinopathy would be ideally treated with pan-inflammasome inhibitors [22] or therapies targeting ASC [23,24], rather than specific NLRP3 blockers or other inhibitors targeting single inflammasomes. This might be important for the clinical application of NLRP3 blockers in multi-factorial complex chronic diseases where the NLRP3 inflammasome is one of the contributing pathways [7,21].
Our study found that the inflammatory response driving the infiltration of PMN cells in our tendinopathy model after tendon injury was independent of ASC-dependent inflammasomes. This is in line with previous studies showing that in the absence of NLRP3, the application of galvanic currents in the mice tendon to activate NLRP3 did not affect the infiltration of PMN cells [13]. In contrast, another study shows that necrotic cells injected in the peritoneum of mice induces an NLRP3-dependent IL-1 production and control PMN cell infiltration into the peritoneum [25]. Also, peritoneal administration of uric acid crystals, Alum or silica particles, also induce an NLRP3-dependent PMN infiltration [5,8,9]. These differences could be due to the activation of different signalling pathways in the different models, the intraperitoneal or intratracheal administration of Alum or silica results in an inflammasome-dependent response mostly dependent on IL-1, since PMN infiltration depends on IL-1R signaling [8,9]. In our study there is a production of TNF-α and CXCL10 independently on the inflammasome, therefore CXCL10 could be recruiting PMN cells. Similarly, the effect of necrotic cells was dependent on ATP-derived from mitochondria of necrotic cells acting as a damage-associated signal that mainly activate the NLRP3 inflammasome [25]. In our tendinopathy model, we do not know the exact signal controlling NLRP3 and potentially other ASC-dependent inflammasomes. We could assume that in vivo, extracellular matrix degradation by collagenase could induce the presence of several damage-associated signals, as exposure of primed macrophages to collagenase-degraded collagen was unable to activate the inflammasome (not shown). ATP or other transient released damaged signals, as well as production of inflammatory mediators independent of the inflammasome (i.e. TNFα) might function as priming and activating signals for NLRP3 and other inflammasomes in vivo [26].
In conclusion, we found that the NLRP3/ASC-inflammasome controls the production of IL-1β, meanwhile, another ASC-dependent inflammasome is required to produce IL-18 in tendinopathy induced by sterile tissue degradation of the extracellular collagen matrix. However, the production of TNFα and CXCL10 chemokine and transient PMN cell infiltration in tendinopathies is independent of ASC inflammasomes. The understanding of the specific inflammatory microenvironment in tendinopathies will help to the development of novel pharmacological treatments.
Acknowledgements
We thank I. Couillin (INSERM, Orleans, France) for knock-out mice.
Abbreviations
- ASC
Apoptosis-associated speck-like protein containing a caspase activation domain
- IL
interleukin
- NLR
nucleotide-binding oligomerization domain and leucine-rich repeat receptor
- PMN
Polymorphonuclear cells
- TNFα
Tumor necrosis factor alpha
Data Availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Competing Interests
A.P-F. contract was supported by MVClinic Institute and Prim. P.P. declares that he is an inventor in a patent filed on March 2020 by the Fundación para la Formación e Investigación Sanitaria de la Región de Murcia (PCT/EP2020/056729) for a method to identify NLRP3-immunocompromised sepsis patients. P.P. and L.H-N. are co-founders of Viva in vitro Diagnostics SL. P.P. is a scientific consultant of Viva in vitro Diagnostics SL. The remaining authors declare no competing interests.
Funding
This work was supported by grants from FEDER/Ministerio de Ciencia, Innovación y Universidades—Agencia Estatal de Investigación [grant number PID2020-116709RB-I00 (to P.P.)]. A.P-F. was supported by MVClinic and Prim; L.H-N. was supported by the fellowship 21214/FPI/19 (Fundación Séneca, Región de Murcia, Spain).
CRediT Author Contribution
Alejandro Peñín-Franch: Formal analysis, Investigation, Methodology, Writing—review & editing. Laura Hurtado-Navarro: Formal analysis, Investigation, Writing—review & editing. José Antonio García-Vidal: Investigation, Methodology, Writing—review & editing. Pilar Escolar-Reina: Methodology, Writing—review & editing. Francesc Medina-Mirapeix: Conceptualization, Resources, Supervision, Writing—review & editing. Pablo Pelegrín: Conceptualization, Resources, Supervision, Funding acquisition, Writing—original draft, Project administration, Writing—review & editing.
Ethics Approval
Mice procedures were approved by the University of Murcia ethical committee and the Animal Health Service of the General Directorate of Fishing and Farming of the Council of Murcia (#A13160702).
References
- 1.Chan A.H. and Schroder K. (2020) Inflammasome signaling and regulation of interleukin-1 family cytokines. J. Exp. Med. 217, e20190314 10.1084/jem.20190314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnett K.C., Li S., Liang K. and Ting J.P.Y. (2023) A 360° view of the inflammasome: Mechanisms of activation, cell death, and diseases. Cell 186, 2288–2312 10.1016/j.cell.2023.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamasaki K., Muto J., Taylor K.R., Cogen A.L., Audish D., Bertin J.et al. (2009) NLRP3/cryopyrin is necessary for interleukin-1 (IL-1) release in response to hyaluronan, an endogenous trigger of inflammation in response to injury. J. Biol. Chem. 284, 12762–12771 10.1074/jbc.M806084200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babelova A., Moreth K., Tsalastra-Greul W., Zeng-Brouwers J., Eickelberg O., Young M.F.et al. (2009) Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors. J. Biol. Chem. 284, 24035–24048 10.1074/jbc.M109.014266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tapia-Abellán A., Angosto-Bazarra D., Alarcón-Vila C., Baños M.C., Hafner-Bratkovič I., Oliva B.et al. (2021) Sensing low intracellular potassium by NLRP3 results in a stable open structure that promotes inflammasome activation. Sci Adv. 7, eabf4468 10.1126/sciadv.abf4468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hafner-Bratkovič I. and Pelegrin P. (2018) Ion homeostasis and ion channels in NLRP3 inflammasome activation and regulation. Curr. Opin. Immunol. 52, 8–17 10.1016/j.coi.2018.03.010 [DOI] [PubMed] [Google Scholar]
- 7.Coll R.C., Schroder K. and Pelegrín P. (2022) NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol. Sci. 43, 653–668 10.1016/j.tips.2022.04.003 [DOI] [PubMed] [Google Scholar]
- 8.Eisenbarth S.C., Colegio O.R., O'Connor W., Sutterwala F.S. and Flavell R.A. (2008) Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 453, 1122–1126 10.1038/nature06939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hornung V., Bauernfeind F., Halle A., Samstad E.O., Kono H., Rock K.L.et al. (2008) Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9, 847–856 10.1038/ni.1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sobhani S., Dekker R., Postema K. and Dijkstra P.U. (2013) Epidemiology of ankle and foot overuse injuries in sports: A systematic review. Scand. J. Med. Sci. Sports 23, 669–686 10.1111/j.1600-0838.2012.01509.x [DOI] [PubMed] [Google Scholar]
- 11.De Cesar Netto C., Godoy-Santos A.L., Augusto Pontin P., Natalino R.J.M., Pereira C.A.M., Lima F.D.D.O.et al. (2018) Novel animal model for Achilles tendinopathy: Controlled experimental study of serial injections of collagenase in rabbits. PloS ONE 13, e0192769 10.1371/journal.pone.0192769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sánchez-Sánchez J.L., Calderón-Díez L., Herrero-Turrión J., Méndez-Sánchez R., Arias-Buría J.L. and Fernández-de-las-Peñas C. (2020) Changes in gene expression associated with collagen regeneration and remodeling of extracellular matrix after percutaneous electrolysis on collagenase-induced achilles tendinopathy in an experimental animal model: A pilot study. J. Clin. Med. 9, 3316 10.3390/jcm9103316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peñin-Franch A., García-Vidal J.A., Martínez C.M., Escolar-Reina P., Martínez-Ojeda R.M., Gómez A.I.et al. (2022) Galvanic current activates the NLRP3 inflammasome to promote Type I collagen production in tendon. eLife 11, e73675 10.7554/eLife.73675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinarello C.A., Simon A. and van der Meer J.W.M. (2012) Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 11, 633–652 10.1038/nrd3800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drutman S.B., Haerynck F., Zhong F.L., Hum D., Hernandez N.J., Belkaya S.et al. (2019) Homozygous NLRP1 gain-of-function mutation in siblings with a syndromic form of recurrent respiratory papillomatosis. Proc. Natl. Acad. Sci. U. S. A. 116, 19055–19063 10.1073/pnas.1906184116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duncan J.A. and Canna S.W. (2018) The NLRC4 inflammasome. Immunol. Rev. 281, 115–123 10.1111/imr.12607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canna S.W., Girard C., Malle L., de Jesus A., Romberg N., Kelsen J.et al. (2017) Life-threatening NLRC4-associated hyperinflammation successfully treated with IL-18 inhibition. J. Allergy Clin. Immunol. 139, 1698–1701 10.1016/j.jaci.2016.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldbach-Mansky R., Dailey N.J., Canna S.W., Gelabert A., Jones J., Rubin B.I.et al. (2006) Neonatal-onset multisystem inflammatory disease responsive to interleukin-1β inhibition. N. Engl. J. Med. 355, 581–592 10.1056/NEJMoa055137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu Q. and Kanneganti T.D. (2017) Cutting edge: Distinct regulatory mechanisms control proinflammatory cytokines IL-18 and IL-1β. J. Immunol. 198, 4210–4215 10.4049/jimmunol.1700352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amores-Iniesta J., Barberà-Cremades M., Martínez C.M., Pons J.A., Revilla-Nuin B., Martínez-Alarcón L.et al. (2017) Extracellular ATP activates the NLRP3 inflammasome and is an early danger signal of skin allograft rejection. Cell Rep. 21, 3414–3426 10.1016/j.celrep.2017.11.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y., Ye X., Escames G., Lei W., Zhang X., Li M.et al. (2023) The NLRP3 inflammasome: Contributions to inflammation-related diseases. Cell. Mol. Biol. Lett. 28, 51 10.1186/s11658-023-00462-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Docherty C.A., Fernando A.J., Rosli S., Lam M., Dolle R.E., Navia M.A.et al. (2023) A novel dual NLRP1 and NLRP3 inflammasome inhibitor for the treatment of inflammatory diseases. Clin. Transl. Immunol. 12, e1455 10.1002/cti2.1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertheloot D., Wanderley C.W., Schneider A.H., Schiffelers L.D., Wuerth J.D., Tödtmann J.M.et al. (2022) Nanobodies dismantle post‐pyroptotic ASC specks and counteract inflammation in vivo. EMBO Mol. Med. 14, e15415 10.15252/emmm.202115415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soriano-Teruel P.M., García-Laínez G., Marco-Salvador M., Pardo J., Arias M., DeFord C.et al. (2021) Identification of an ASC oligomerization inhibitor for the treatment of inflammatory diseases. Cell Death Dis. 12, 1155 10.1038/s41419-021-04420-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iyer S.S., Pulskens W.P., Sadler J.J., Butter L.M., Teske G.J., Ulland T.K.et al. (2009) Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc. Natl. Acad. Sci. U. S. A. 106, 20388–20393 10.1073/pnas.0908698106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pelegrin P. (2011) Inflammasome activation by danger signals. In The Inflammasomes(Couillin I., Pétrilli V. and Martinon F., eds), pp. 101–121, Springer Basel, Basel: 10.1007/978-3-0348-0148-5_7 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.