Abstract
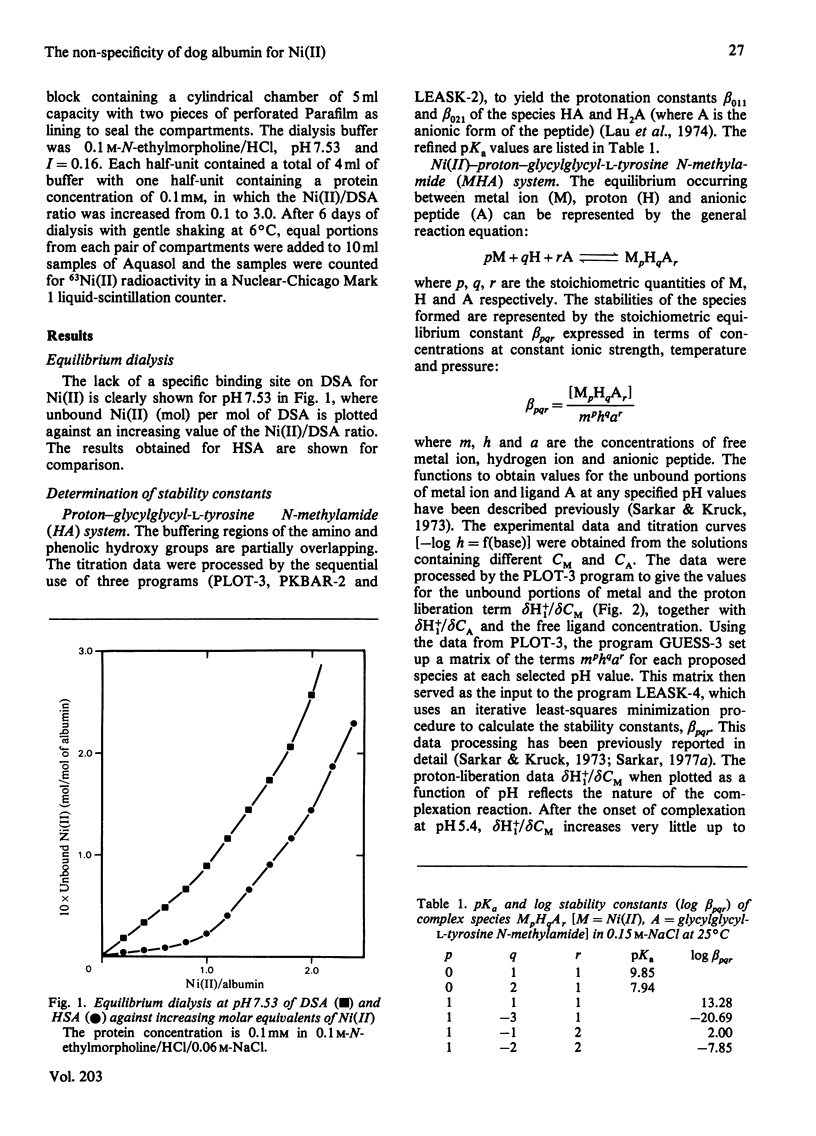
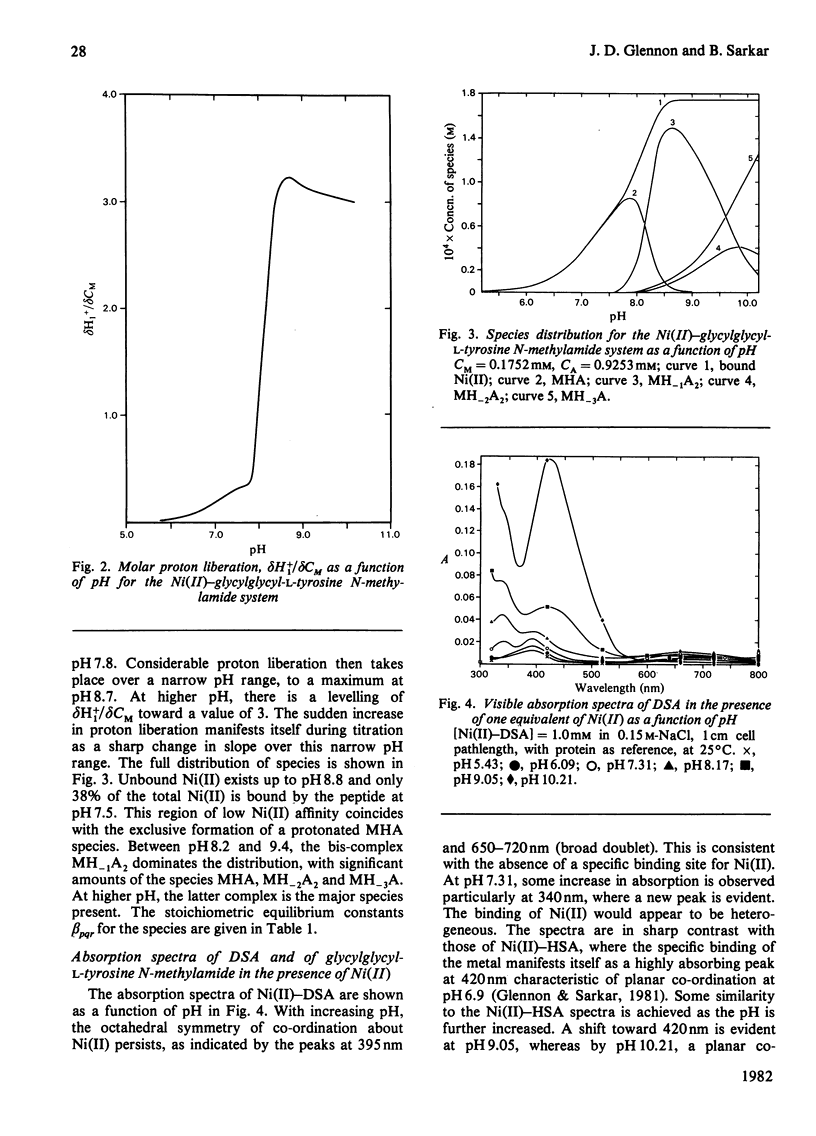
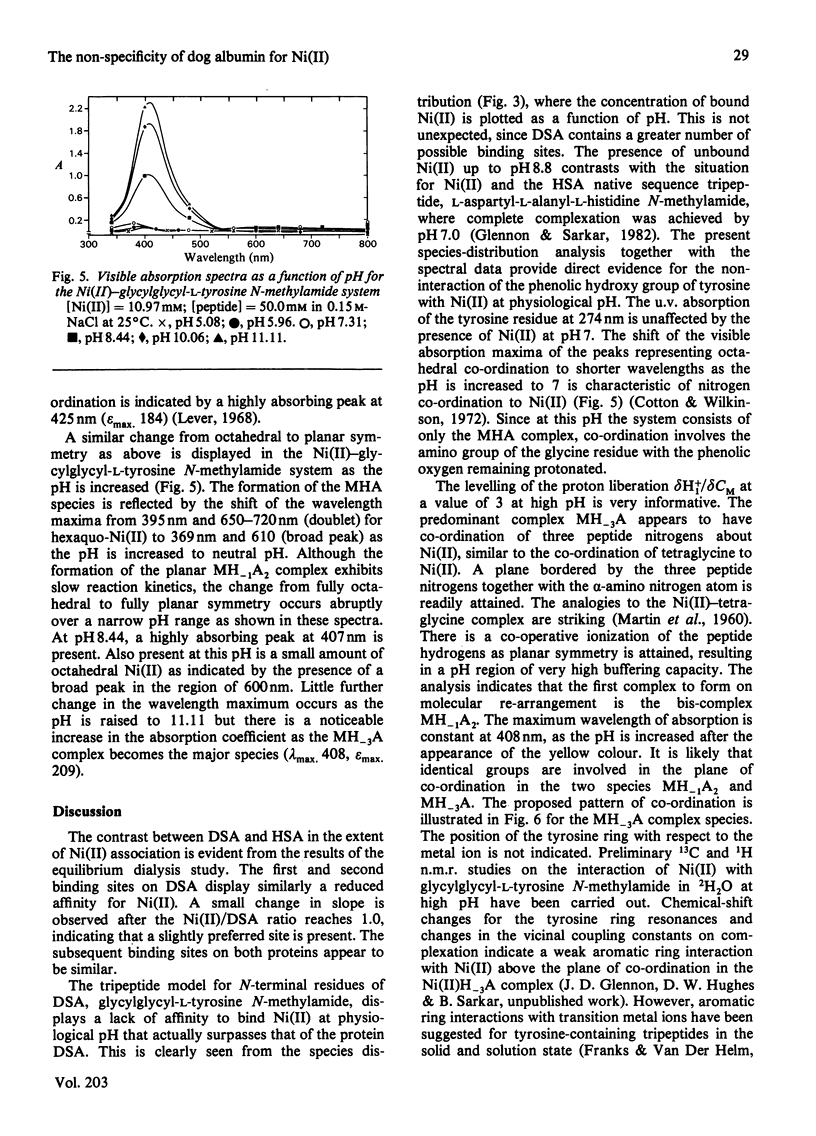
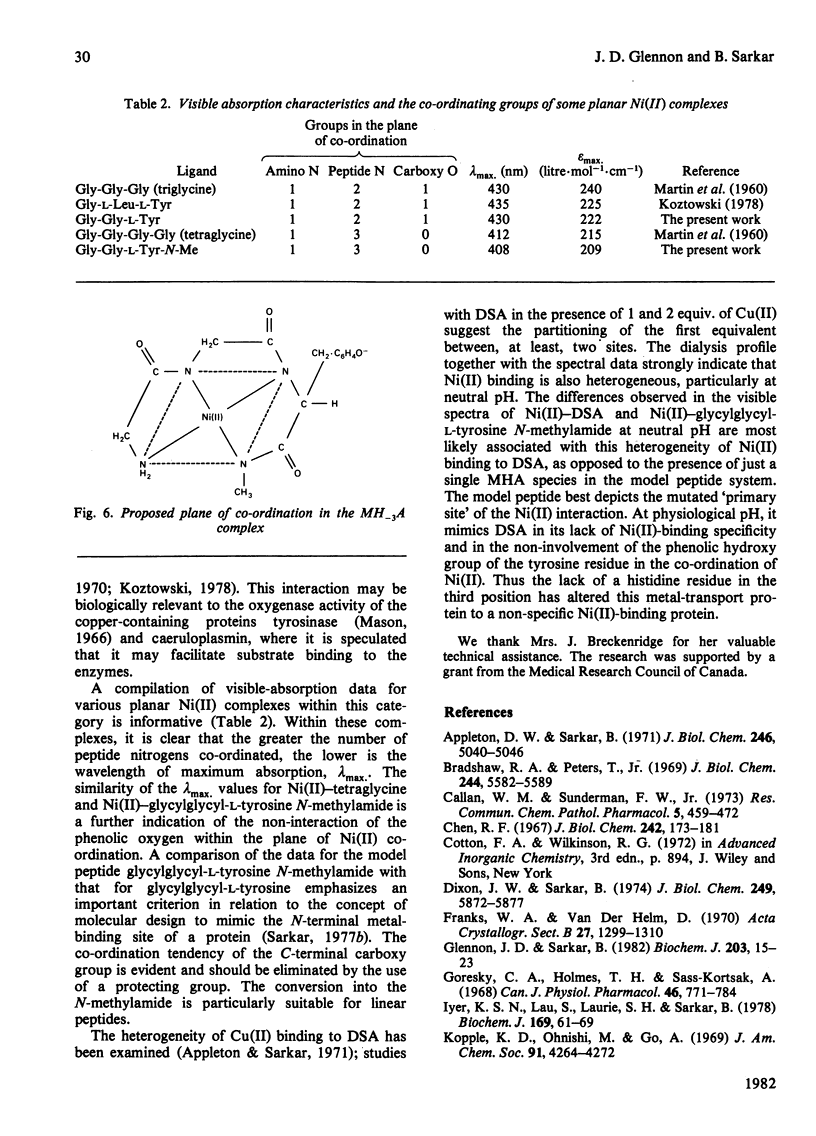
Equilibrium dialysis of dog serum albumin (DSA) against Ni(II) in 0.1 M-N-ethylmorpholine/HCl, pH 7.53, demonstrates the absence of a specific Ni(II)-binding site in DSA. To evaluate at the molecular level the influence of the genetic substitution of L-tyrosine for L-histidine at the N-terminal of DSA, a simple model tripeptide of the N-terminal residues, glycylglycyl-L-tyrosine N-methylamide, was synthesized and its Ni(II)-binding properties studied. A comparison of the visible absorption characteristics of Ni(II)-DSA with those of Ni(II)-glycylglycyl-L-tyrosine N-methylamide reveals a similar change from octahedral to planar co-ordination as the pH is increased. Both systems exhibit a low Ni(II)-binding affinity at physiological pH, with DSA binding a greater percentage of Ni(II) owing to the availability of at least two binding sites of similar affinities. The complex equilibria between Ni(II) and glycylglycyl-L-tyrosine N-methylamide were studied by analytical potentiometry (0.15 M-NaCl, 25 degrees C). Four major complex species, MHA, MH-1A2, MH-2A2 and MH-3A [where M and A represent Ni(II) ion and anionic peptide respectively], were detected, MHA being the single species at physiological pH. There is no evidence for the involvement of the phenolic hydroxy group in the octahedral MHA complex, or within the plane of co-ordination in the high-pH species. The results provide direct evidence that the low Ni(II)-binding affinity of DSA is due to the genetic substitution of tyrosine for histidine at the N-terminal region of the protein.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleton D. W., Sarkar B. The absence of specific copper (II)-binding site in dog albumin. A comparative study of human and dog albumins. J Biol Chem. 1971 Aug 25;246(16):5040–5046. [PubMed] [Google Scholar]
- Bradshaw R. A., Peters T., Jr The amino acid sequence of peptide (1-24) of rat and human serum albumins. J Biol Chem. 1969 Oct 25;244(20):5582–5589. [PubMed] [Google Scholar]
- Callan W. M., Sunderman F. W., Jr Species variations in binding of 63 NI(II) by serum albumin. Res Commun Chem Pathol Pharmacol. 1973 Mar;5(2):459–472. [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Dixon J. W., Sarkar B. Isolation, amino acid sequence and copper(II)-binding properties of peptide (1-24) of dog serum albumin. J Biol Chem. 1974 Sep 25;249(18):5872–5877. [PubMed] [Google Scholar]
- Glennon J. D., Sarkar B. Nickel(II) transport in human blood serum. Studies of nickel(II) binding to human albumin and to native-sequence peptide, and ternary-complex formation with L-histidine. Biochem J. 1982 Apr 1;203(1):15–23. doi: 10.1042/bj2030015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goresky C. A., Holmes T. H., Sass-Kortsak A. The initial uptake of copper by the liver in the dog. Can J Physiol Pharmacol. 1968 Sep;46(5):771–784. doi: 10.1139/y68-120. [DOI] [PubMed] [Google Scholar]
- Iyer K. S., Lau S. J., Laurie S. H., Sarkar B. Synthesis of the native copper(II)-transport site of human serum albumin and its copper(II)-binding properties. Biochem J. 1978 Jan 1;169(1):61–69. doi: 10.1042/bj1690061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S. J., Kruck T. P., Sarkar B. A peptide molecule mimicking the copper(II) transport site of human serum albumin. A comparative study between the synthetic site and albumin. J Biol Chem. 1974 Sep 25;249(18):5878–5884. [PubMed] [Google Scholar]
- Lau S. J., Sarkar B. Ternary coordination complex between human serum albumin, copper (II), and L-histidine. J Biol Chem. 1971 Oct 10;246(19):5938–5943. [PubMed] [Google Scholar]
- Lucassen M., Sarkar B. Nickel(II)-binding constituents of human blood serum. J Toxicol Environ Health. 1979 Sep;5(5):897–905. doi: 10.1080/15287397909529799. [DOI] [PubMed] [Google Scholar]
- Sarkar B., Wigfield Y. Evidence for albumin--cu(II)--amino acid ternary complex. Can J Biochem. 1968 Jun;46(6):601–607. doi: 10.1139/o68-092. [DOI] [PubMed] [Google Scholar]
- Sunderman F. W., Jr A review of the metabolism and toxicology of nickel. Ann Clin Lab Sci. 1977 Sep-Oct;7(5):377–398. [PubMed] [Google Scholar]


