Abstract
Introduction:
The levator veli palatini (LVP) muscle has two segments with distinct roles in velopharyngeal (VP) function. Previous research suggests longer extravelar segments with shorter intravelar segments may lead to a more advantageous mechanism for VP closure. The purpose of this study was to examine whether the distribution of the LVP intravelar and extravelar segments differs between children with cleft palate with and without VPI and controls.
Methods:
The study included 97 children: 37 with cleft palate +/− lip with VPI, 37 controls, and 19 with cleft palate with normal resonance. Measures included mean LVP length, mean extravelar LVP length, and intravelar LVP length.
Results:
Overall mean LVP length was similar (p=0.267) between controls and children with cleft palate (with and without VPI). However, there was a significant difference (p < 0.001) between group for both intravelar and extravelar LVP lengths: the intravelar segment was significantly longer in those with VPI compared to controls and children with cleft palate and normal resonance; and the extravelar segment was significantly shorter in those with VPI compared to controls and children with cleft palate and normal resonance.
Conclusions:
Results from this study demonstrate a significant difference between the distribution of the functional segments of the LVP among children with VPI, with a more disadvantageous distribution of the muscle segments among those with VPI.
INTRODUCTION
The levator veli palatini (LVP) muscle is the primary muscle responsible for elevation and retraction of the velum during velopharyngeal closure. The LVP is a paired muscle with portions of the muscular sling that are external and internal to the body of the velum.1–4 Using definitions described by Huang et al.,5 Perry et al.4 described the intravelar portion as the region of the muscle that is entirely contained within the velum and the extravelar portion as the region that extends from the origin of the muscle at the cranial base to the insertion along the lateral margins of the velum. The intravelar and extravelar segments of the LVP have been described to have variable contributions to velopharyngeal function due to the differences in the muscle fiber directions and position relative to the body of the velum.4–6
Anderson et al.6 used computational modeling to examine the contributions of the separate segments of the LVP. The authors found that activation of the extravelar LVP segments in isolation created near-closure, achieving two-thirds of velopharyngeal closure along the midsagittal plane. However, activation of the extravelar segment alone was unable to achieve full velopharyngeal closure. When activated in isolation, the intravelar segment generated more than 90% of the total closure force between the palate and the pharyngeal wall. Full velopharyngeal closure was only achieved with activation of both the intravelar and extravelar muscle segments. These findings support earlier claims4,5 that the extravelar segment functions primarily to elevate and retract the velum whereas, the intravelar segment is necessary to create a prominent closure force along the velar bulge or eminence. Independent of each other, intravelar and extravelar segments cannot achieve full velopharyngeal closure demonstrating their vital and distinct roles to velopharyngeal function.6
Inouye and colleagues7 combined MRI and computational modeling techniques to examine the impact of changes in the lengths of the LVP segments on velopharyngeal closure force. The authors observed that the length of the intravelar segment (labeled as the VP distance variable) was the most significant contributing factor to velopharyngeal function. More specifically, when the intravelar segment was lengthened in the computational model, the velopharyngeal mechanism was at a mechanical disadvantage and the authors proposed that an imbalance in the LVP segments among patients may be a significant factor contributing to velopharyngeal insufficiency (VPI). The authors also showed that a shorter extravelar LVP produced a more disadvantaged velopharyngeal system for speech. In contrast, a shorter intravelar LVP and longer extravelar LVP created the most advantageous velopharyngeal model.
Based on these studies, it is hypothesized that a shorter intravelar and longer extravelar LVP is associated with normal resonance and velopharyngeal function whereas a longer intravelar and shorter extravelar LVP is associated with VPI. Preliminary evidence among six adults with repaired cleft palate and normal speech supports this hypothesis8 with all individuals displaying LVP segments that were aligned with the advantaged LVP model proposed by Inouye and colleagues.7 However, variations in the LVP segments have not been examined among children with cleft palate to determine if the previously described7 disadvantaged LVP model is associated with VPI.
The purpose of this study was to examine whether the distribution of the LVP intravelar and extravelar segments differs between children with cleft palate and VPI and children without cleft palate presenting with normal resonance. A preliminary comparison was conducted by including a third group, children with cleft palate who have normal resonance and no history of VPI, to determine if any differences observed between groups was related to cleft palate regardless of VPI. Understanding differences in the distribution of the LVP segments among individuals with VPI compared to those with normal resonance may provide insights to better inform surgical approaches used for primary palatoplasty and/or palatal reconstruction surgeries to treat VPI.
METHODS
Institutional Review Board (IRB) approval was obtained prior to the start of the study. MRI studies for participants with cleft palate were collected as part of the standard of care at the clinical site and IRB approval allowed for retrospective review of clinical MRI data for the research study.
Participants
A total of 93 children were enrolled in the study, including: 37 children with repaired non-syndromic cleft palate (+/− cleft lip) and VPI (group 1), 37 children who were controls with normal velopharyngeal anatomy and resonance (group 2), and 19 children with repaired non-syndromic cleft palate (+/− cleft lip) with no history of VPI and normal resonance (group 3). The control group and cleft palate with VPI group were matched by age (+/− 6 months), sex, and race. The age ranged from 3–19 years of age with a mean age of 7.3 years. Each group included 20 boys and 17 girls, 36 White children, and one Asian child. Participants were matched by these key variables as literature has reported that velopharyngeal variables, including levator muscle length, are significantly impacted by age, race, and sex.9–11 Specifically, studies have demonstrated that the most significant differences in levator muscle length begins at around age seven. Furthermore, sex differences begin to be observed during childhood, with males having significantly greater levator muscle lengths when compared to their female counterparts. Sexual dimorphism effects continue across the lifespan and become most prevalent during pubertal ages.9,11 Literature also reports significant race effects occur across the lifespan for a variety of VP variables such as levator length, velar length, and velar thickness, with Black individuals often presenting with more advantageous velopharyngeal mechanisms.9,12 Given there were only 19 children in the cleft palate without VPI group, data from this group was used for preliminary comparison to determine if the any observed differences in the intravelar and extravelar segments were attributed to a history of cleft palate, VPI, or both. The mean age for this group was 5.2 years with children ranging from 3–8 years of age, including 8 males and 11 females. Ten were White, 1 was Black, 3 were Asian, and 5 were biracial.
Participants with non-syndromic cleft palate and VPI met the following criteria: (1) primary diagnosis of non-syndromic cleft palate; (2) presence of hypernasality and/or audible nasal emission on perceptual speech evaluation; (3) velopharyngeal MRI successfully completed as part of VPI evaluation; and (4) no prior VPI management. Those without VPI met the same criteria with the exception (2) and instead children presented with normal resonance as determined by a perceptual speech evaluation conducted by a speech-language pathologist with over 15 years of experience rating cleft speech.
Clinical Speech Evaluation.
Participants were seen by a cleft team speech-language pathologist (SLP) for a clinical visit during which they completed a perceptual speech evaluation. The evaluation included an audio-recorded speech sample with (1) 2–3 minutes of elicited conversation using prompts such as, “Tell me about your favorite movie or TV show” and “Tell me about your family;” (2) counting 1–10 or 1–20 depending on age; (3) a sentence or phrase repetition task using the American English Sentence Sample or American English Phrase Sample as a part of the Cleft Audit Protocol for Speech-Augmented-Americleft Modification (CAPS-A-AM).13,14 Hypernasality was rated on a 5-point ordinal scale using the CAPS-A-AM as follows: none, minimal, mild, moderate, severe. Participants were referred for MRI if there was diagnosed hypernasality in clinic and the participant and caregivers wanted to pursue imaging for surgical planning.
Magnetic Resonance Imaging.
Participants completed a whole head MRI using a fully awake, non-sedated, non-contrast protocol. Participants from the control group were imaged at one of two research scanners using either a 1.5 Tesla Siemens MRI scanner or a 3.0 Tesla Siemens. Participants with cleft palate and VPI were all imaged using a 3 Telsa Phillips MRI scanner. Imaging protocols were consistent across sites using MR sequences that were closely aligned in spatial and temporal image resolution as well as overall duration of scan. At all sites, the imaging protocol included a high-resolution T2-weighted turbo-spin-echo 3D sequence obtained at rest following similar parameters as described by Perry et al (2022). Scan time varied by scanner from 3 and half minutes to 5 minutes in duration.
MRI Measurement.
Measurements of intravelar length and extravelar length were measured using Amira Visualization software. The measures were obtained from an oblique coronal slice through the long axis of the LVP muscle. The primary rater for the study had over 15 years’ experience in MRI evaluations of velopharyngeal anatomy. The other two raters received training from the primary rater and had at least two years’ experience with velopharyngeal MRI analysis. Definitions of overall LVP length at rest, mean extravelar length, and intravelar LVP length were consistent with previously published definitions and are provided in Figure 1.
Figure 1.
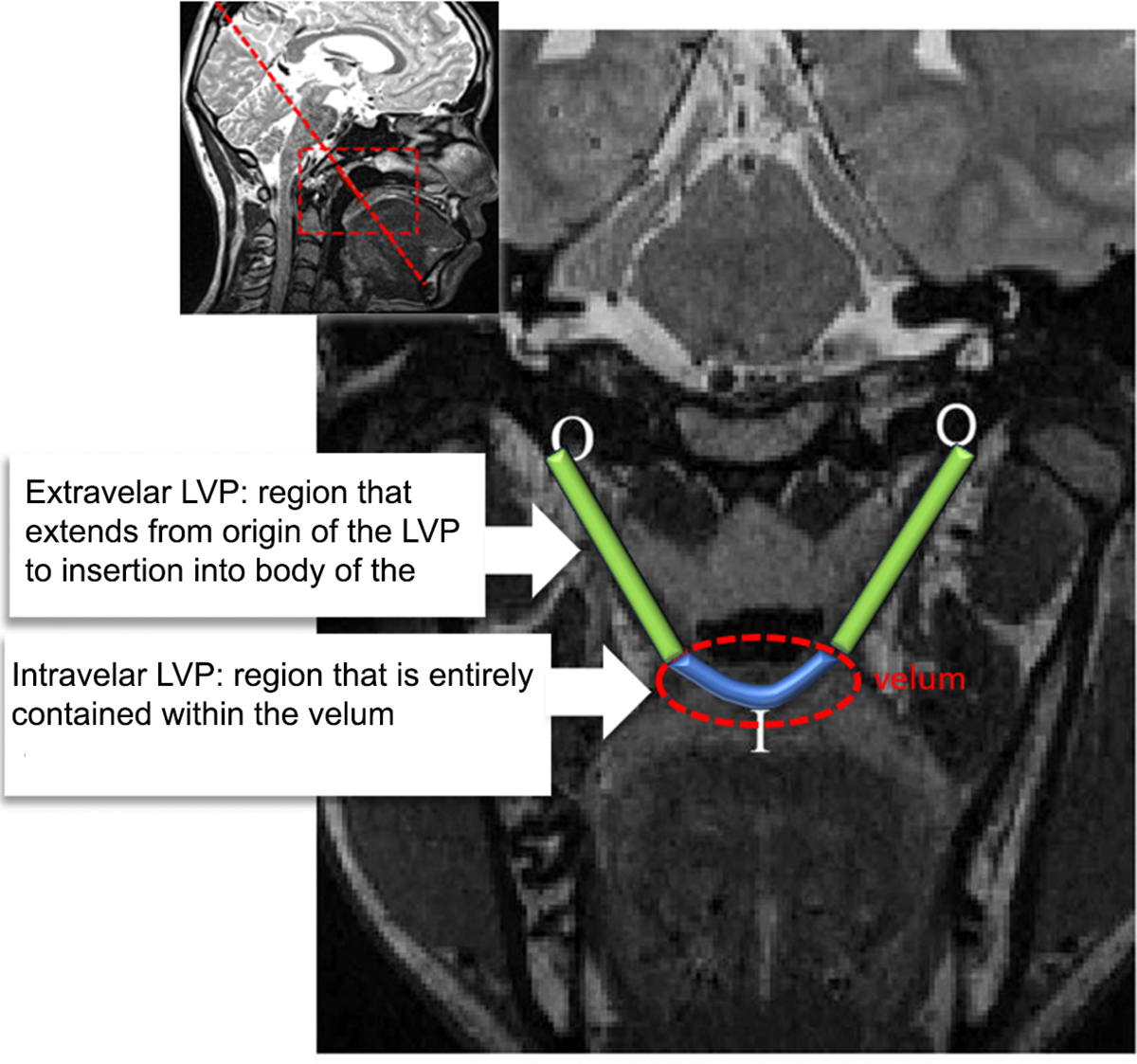
Demonstration of the LVP measures
Statistical Analysis
All data analyses were conducted using IBM SPSS Statistics (Version 28, Armonk, NY: IBM Corp). Independent samples t-tests were used to examine the differences between groups for the three LVP muscle measures: LVP length, intravelar LVP length, and mean extravelar LVP length. The normality assumption was met for all variables as assessed by Shapiro-Wilk’s test (p > 0.05).
A multivariate analysis of variance with an alpha = 0.05 was used to examine the effect of group across the three dependent variables related to the LVP muscle. Tukey-Kramer tests were used to compare all possible pairs of mean values. The Tukey-Kramer test (extension of Tukey honest significance test to handle unequal sample sizes) allows for multiple comparisons while preserving the family-wise type I error rate at a level of .05 and does not require equal sample sizes. The Levene’s test of equality revealed a p value greater than 0.5, therefore concluding requirements for test of equality were met.
Raters remeasured approximately 20% of randomly selected participants for comparisons of intra and inter-rater reliability. Inter and intra-rater reliability for the measures of intravelar LVP muscle length and mean extravelar LVP muscle length were obtained using the Pearson product-moment correlation.
RESULTS
Intra-rater reliability for subjects with cleft palate (with and without VPI) was 0.97 and inter-rater reliability was 0.97. Intra-rater reliability for control subjects was 0.94 and inter-rater reliability was 0.84.
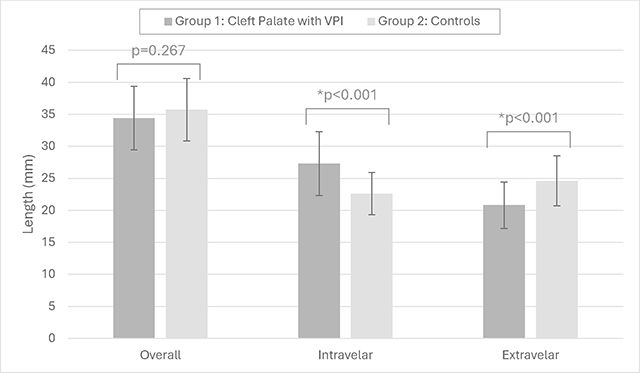
The primary comparison for this study was between children with cleft palate who presented with VPI and age, race, and sex matched controls. For this comparison, the overall mean LVP length was similar (p = 0.267) between children with cleft palate and VPI (mean = 34.4, SD = 4.96) and controls (mean = 35.7, SD = 4.87) (Table 1). However, there was a significant difference (p < .001) between groups for both intravelar and extravelar LVP lengths. The intravelar LVP segment was significantly longer in those with VPI (mean = 27.3 mm, SD = 5.0 mm) compared to controls (mean = 22.6 mm, SD = 3.3mm). The extravelar segment was significantly shorter in those with VPI (mean = 20.8 mm, SD = 3.63 mm) compared to controls (mean = 24.6 mm, SD = 3.9 mm). Five patients with VPI and matched controls are displayed in Figure 2 to demonstrate examples of the variations in the overall length of the intravelar and extravelar LVP segments.
Table 1.
Demonstration of the group mean measures between groups 1 and 2
|
Figure 2.
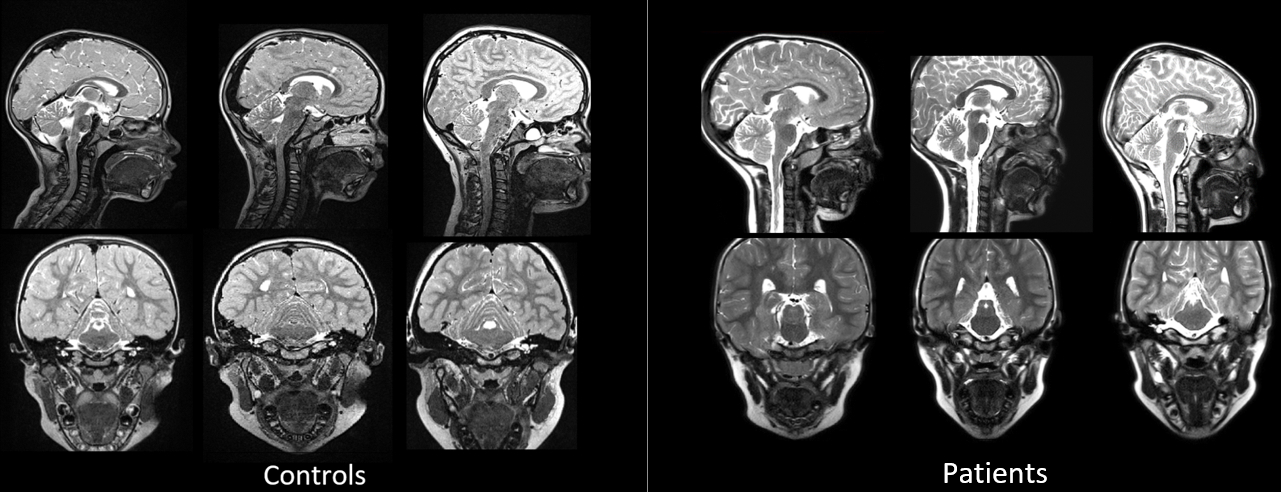
Examples of MR images obtained from controls compared to patients with cleft palate presenting with VPI
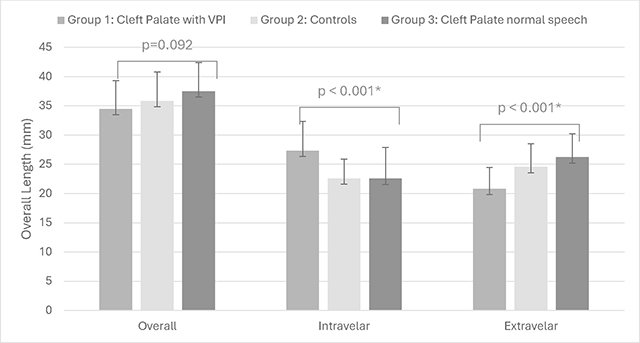
When examining all three groups, significant differences were observed for intravelar and extravelar LVP lengths with no significant difference in overall LVP muscle length. Participants in group 3 with cleft palate and normal resonance (with no history of VPI) displayed a mean LVP length of 37.5 mm (SD = 4.9 mm), intravelar length of 22.5 mm (SD = 5.3 mm), and mean extravelar length of 26.2 mm (SD = 4.0 mm) (Table 2).
Table 2.
Demonstration of the group mean measures between groups 1, 2, and 3
|
Post-hoc analysis demonstrated that children in group 1, with cleft palate and VPI, had a significantly longer intravelar muscle length compared to those in group 2, the control group, (p < 0.001) and those in group 3, with cleft palate and normal resonance/velopharyngeal competence (p = 0.001). In addition, children with VPI (group 1) displayed a significantly shorter extravelar LVP muscle compared to controls in group 2 (p < 0.001) and children with cleft palate and normal resonance/velopharyngeal competence in group 3 (p < 0.001). There was no significant difference (p > 0.05) between group 3, children with cleft palate and normal resonance, and those in group 2, the control group, for any of the three LVP muscle measures. The intravelar and extravelar LVP length among children with cleft palate and normal resonance (group 3) was within 2 mm of the control group (group 2).
DISCUSSION
Results from this study demonstrate a significant difference between the distribution of the functional segments of the LVP among children with repaired cleft palate presenting with VPI (group 1), with a more disadvantageous distribution of the muscle segments when compared to controls (group 2). Specifically, children in group 1 (with repaired cleft palate and VPI) had significantly longer intravelar LVP segments and shorter extravelar LVP segments compared to children without cleft palate. A preliminary comparison among 19 children with cleft palate, no history of VPI, and normal resonance (group 3) was used to evaluate if differences observed among children with VPI was related to cleft palate anatomy, regardless of VPI. Results demonstrated that the observations of a longer intravelar LVP and shorter extravelar LVP are not observed in children with cleft palate with normal resonance and may be a unique feature related to and/or contributing to VPI. Of interest, the LVP intravelar and extravelar measures among children with cleft palate and normal resonance were similar to that of controls. These findings observed in clinical patients support the computational study assumptions made by Inouye and colleagues7 that a longer intravelar segment and shorter extravelar segment is associated with VPI.
The intravelar LVP segment within the velum is most responsible for creating a tight seal between the velum and the posterior pharyngeal wall, and the extravelar LVP primarily responsible for elevation and retraction of the velum. Data from the present study are reflective of static length differences and do not describe the functional contribution or involvement of these muscle segments during speech. However, combined with insights from prior investigations, these findings suggest a likely functional difference in the LVP segments when variations such as those in the present study are observed. Schleif and colleagues observed that the intravelar LVP, extravelar LVP, LVP origin to origin length, and velar thickness combined were significant predictors (p < .05) for LVP contraction velocity using dynamic MRI data during speech among twenty-two speakers. The authors report that these findings demonstrate that both the intravelar and extravelar lengths are critical to achieving normal velopharyngeal function and any variation to these distributions of LVP segments significantly alters the function of the velopharyngeal system. These findings are also supported by computational modeling studies which highlight the critical role of the different segments working in synergy for velopharyngeal function.6,7
The question of clinical significance based on these data is whether surgery can alter these anatomic differences and if so, which techniques may be more likely to create an advantageous system for normal velopharyngeal function. Inouye et al.7 showed that decreasing the length of the intravelar segment using a LVP overlapping intravelar veloplasty (IVV) technique by one standard deviation (3.57 mm) increases closure force and decreases minimal activation required more than any other velopharyngeal parameter. An overlap of intravelar LVP fibers by 10 mm resulted in maximum closure force achieved.15 The authors propose that techniques that shorten or overlap the LVP segments may be more favorable than an end-to-end approximation of the LVP muscle segments during primary palatoplasty. However, excessive overlap (defined as a 20 mm intravelar LVP overlap) was found to decrease closure force achieved, demonstrating an optimal length in the intravelar segment is ideal and excessive shortening may be unnecessary and even disadvantageous.15
Surgical procedures include a wide range of possible LVP overlap scenarios and some variations include a more radial dissection around the hamular process. It is likely that dissection and release of the cleft muscles from the hamular process would allow the intravelar segments to be overlapped to a greater extent without lateralized tethering the LVP intravelar segments near the hamular process. This may also serve to create a smaller intravelar segment and more advantageous mechanism for speech. Further research is needed to explore how varying degrees of muscle overlap and hamular dissection impact the LVP muscle segments and their contribution to overall velopharyngeal function. Lastly, surgical techniques that increase the velar eminence or velar thickness could serve as a substitute for functional deficit caused by an abnormal intravelar segment in patients with VPI. Future research should examine how surgical techniques during primary palatoplasty and during secondary surgery to treat VPI impact the LVP muscle segment distribution and how such alterations impact overall muscle function and speech outcomes.
Lastly, future research is needed to understand if the LVP differences found in this study among those with VPI are related to pre-existing conditions that are observed before primary cleft palate repair and whether surgery can be used to alter the anatomy to create a more advantageous system. If pre-existing conditions are persistent after surgery and appear to persist regardless of surgical procedure, muscle variations may be used to identify those who are at higher risk for VPI. These are questions that cannot be addressed in the present study but are important to understand the practical clinical implications of these findings.
Study Limitations
Observations from this study cannot confirm if this association between a longer intravelar LVP segment and VPI is the direct or independent cause of VPI among these children. There are multiple factors that impact velopharyngeal function for this patient group, including palate length, thickness, pharyngeal depth, and relative position and continuity of the LVP. Future research should consider all these potential influencing factors. Results from this study are specific to children with repaired cleft palate who are non-syndromic. Studies have demonstrated differences in the LVP among individuals with syndromes,16,17 including those with 22q11.2 deletion syndrome however, the relationship of these LVP differences to VPI among this population in addition to closure force and velopharyngeal competence is unclear. Future studies should include all cases of VPI to understand how findings generalize to the broader clinical population. Lastly, findings from this study are representative of a single racial ethnic group and future research should expand to include other racial-ethnic categories given the likely impact of race that has been reported on velopharyngeal variables.
CONCLUSION
Results from this study demonstrate a significant difference between the distribution of the functional segments of the LVP among children with VPI, with a more disadvantageous distribution of the muscle segments among those with VPI. While there are likely multiple factors that impact VP function for this patient group−such as palate length and pharyngeal depth−this study highlights that children with history of cleft palate repair and VPI have a significant anatomic difference in the key muscle responsible for velopharyngeal closure. The impact of surgical technique used during palatoplasty on intravelar and extravelar LVP length should be further examined.
Funding Sources:
We have no known conflicts of interest to disclose. We have strictly adhered to ethical guidelines and applicable regulations throughout the research process. Research reported in this publication was supported by the National Institute of Dental & Craniofacial Research of the National Institutes of Health under Award Numbers K23DE025023, U01DE029750, F31DE033236. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Cho JH, Kim JK, Lee H, Yoon J. Surgical anatomy of human soft palate. Laryngoscope. 2013;123(11):2900–2904. doi: 10.1002/lary.24067. [DOI] [PubMed] [Google Scholar]
- 2.Perry JL, Sutton BP, Kuehn DP, Gamage JK. Using MRI for assessing velopharyngeal structures and function. Cleft Palate Craniofac J. 2014;51(4):476–485. doi: 10.1597/12-083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuehn DP, Azzam NA. Anatomical characteristics of palatoglossus and the anterior faucial pillar. Cleft Palate J. 1978;15(4):349–359. [PubMed] [Google Scholar]
- 4.Kotlarek KJ, Perry JL, Fang X. Morphology of the levator veli palatini muscle in adults with repaired cleft palate. J Craniofac Surg. 2017;28(3):833–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang MHS, Lee ST, Rajendran NK. A fresh cadaveric study of the paratubal muscles: Implications for eustachian tube function in cleft palate. Plast Reconstr Surg (1963). 1997;100(4):833–842. doi: 10.1097/00006534-199709001-00003. [DOI] [PubMed] [Google Scholar]
- 6.Anderson P, Fels S, Stavness I, Pearson WGJ, Gick B. Intravelar and extravelar portions of soft palate muscles in velic constrictions: A three-dimensional modeling study. J Speech Lang Hear Res. 2019;62(4):802–814. doi: 10.1044/2018_JSLHR-S-17-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inouye JM, Pelland CM, Lin KY, Borowitz KC, Blemker SS. A computational model of velopharyngeal closure for simulating cleft palate repair. J Craniofac Surg. 2015;26(3):658–662. https://www.ncbi.nlm.nih.gov/pubmed/25974769 doi: 10.1097/SCS.0000000000001441. [DOI] [PubMed] [Google Scholar]
- 8.Perry JL, Kotlarek KJ, Sutton BP, et al. Variations in velopharyngeal structure in adults with repaired cleft palate. Cleft Palate Craniofac J. 2018;55(10):1409–1418. doi: 10.1177/1055665617752803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perry JL, Kollara L, Kuehn DP, Sutton BP, Fang X. Examining age, sex, and race characteristics of velopharyngeal structures in 4- to 9-year-old children using magnetic resonance imaging. Cleft PalatecCraniofac J. 2018;55(1):21–34. doi: 10.1177/1055665617718549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perry JL, Kollara L, Sutton BP, Kuehn DP, Fang X. Growth effects on velopharyngeal anatomy from childhood to adulthood. J Speech Lang Hear Res. 2019;62(3):682–692. doi: 10.1044/2018_JSLHR-S-18-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perry JL, Lee MK, Tahmasebifard N, Gilbert IR, Snodgrass TD, Shaffer JR, Schleif EP, Weinberg SM (2023). Sex differences in velopharyngeal anatomy of 9–10-year-old children. Journal of Speech, Language, and hearing Research, 66(12):4828–4837. doi: 10.1044/2023_JSLHR-23-00279. Epub 2023 Oct 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perry JL, Kuehn DP, Sutton BP, Gamage JK, Fang X. Anthropometric analysis of the velopharynx and related craniometric dimensions in three adult populations using MRI. The Cleft PalatecCraniofac J. 2016;53(1):1–13. doi: 10.1597/14-015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapman KL, Baylis A, Trost-Cardamone J, et al. The americleft speech project: A training and reliability study. Cleft PalatecCraniofac J. 2016;53(1):93–108. doi: 10.1597/14-027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams JL CK, Sitzman TJ. Assessing the agreement of hypernasality and audible nasal emission ratings between audio-recordings and a clinic setting. Cleft Palate Craniofac J. 2023. doi: 10.1177/10556656231185494. [DOI] [PubMed] [Google Scholar]
- 15.Inouye JPJ, Perry JP, Nyswonger J, Pelland C, Lin K, Borowitz K, Blemker. A mathematical model predicts that anatomical variability influences the efficacy of palate repair procedures. Proceedings of the 71st Annual Meeting and Symposia of the American Cleft Palate-Craniofacial Association. 2014:Abstract nr 132. [Google Scholar]
- 16.Perry JL, Williams JL, Snodgrass TD, Sitzman TJ. VPI management in SATB2 syndrome: Use of MRI to evaluate anatomy and physiology in non-cleft VPI. Cleft Palate Craniofac J. 2023;60(11):1499–1504. doi: 10.1177/10556656221106888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kollara L, Baylis AL, Kirschner RE, et al. Interaction of the craniofacial complex and velopharyngeal musculature on speech resonance in children with 22q11.2 deletion syndrome: An MRI analysis. J Plast Reconstr Aesthet Surg. 2021;74(1):174–182. doi: 10.1016/j.bjps.2020.08.005. [DOI] [PubMed] [Google Scholar]


