Abstract
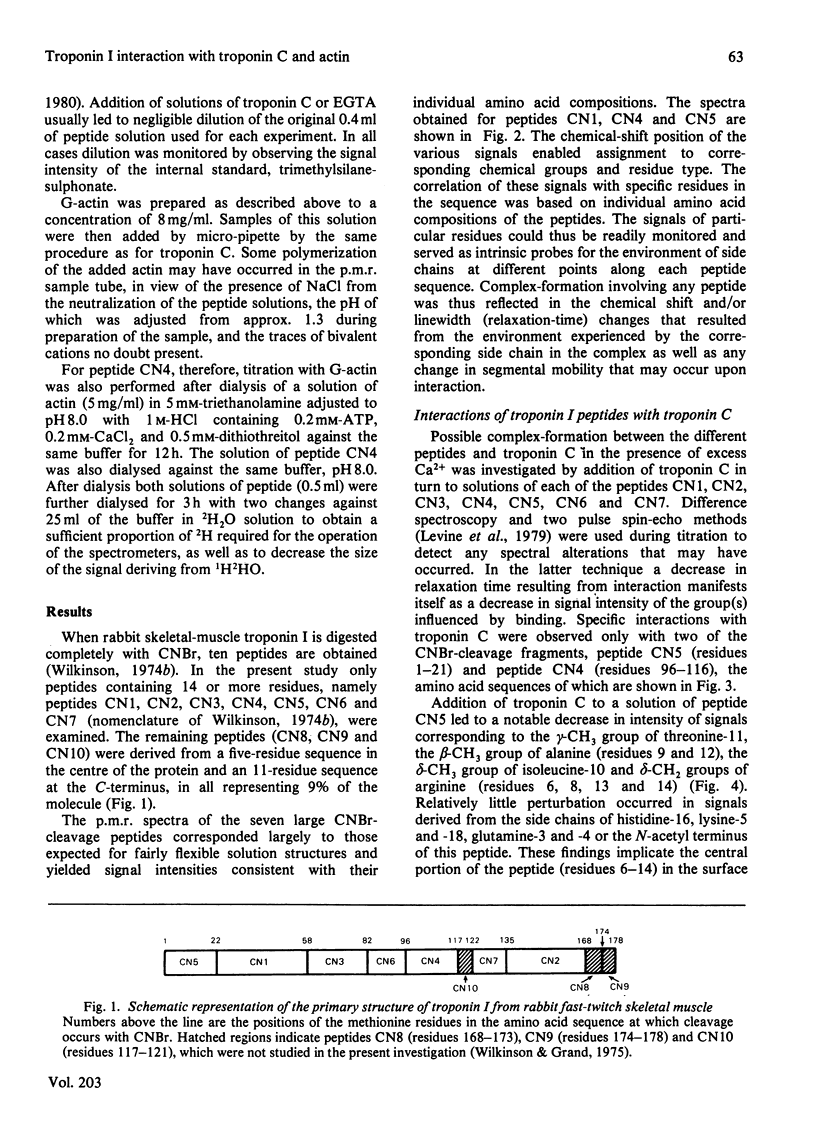
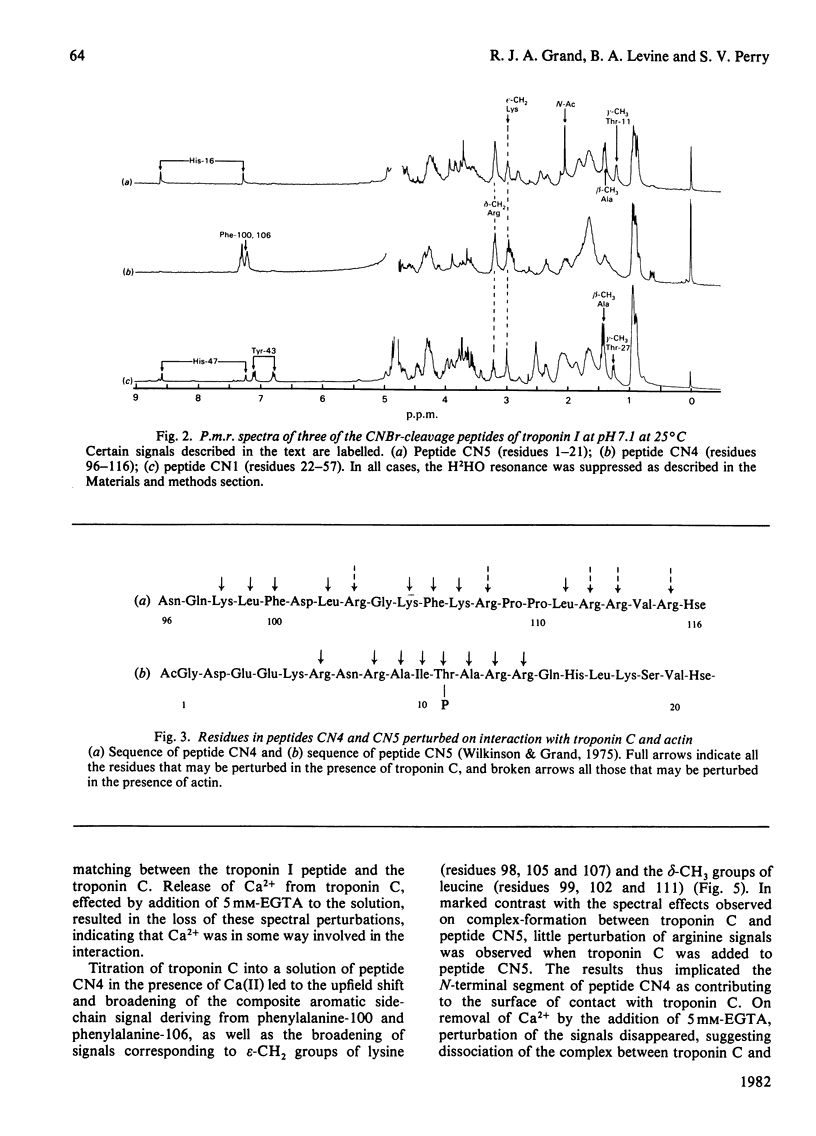
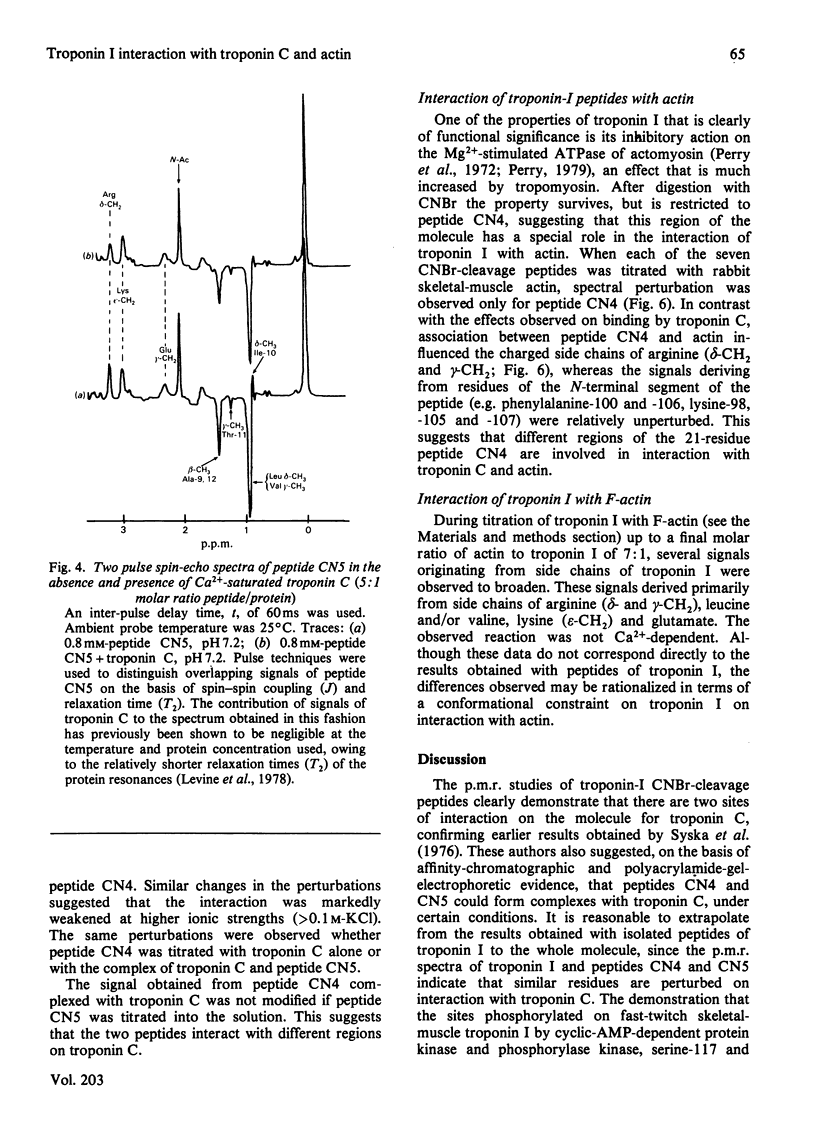
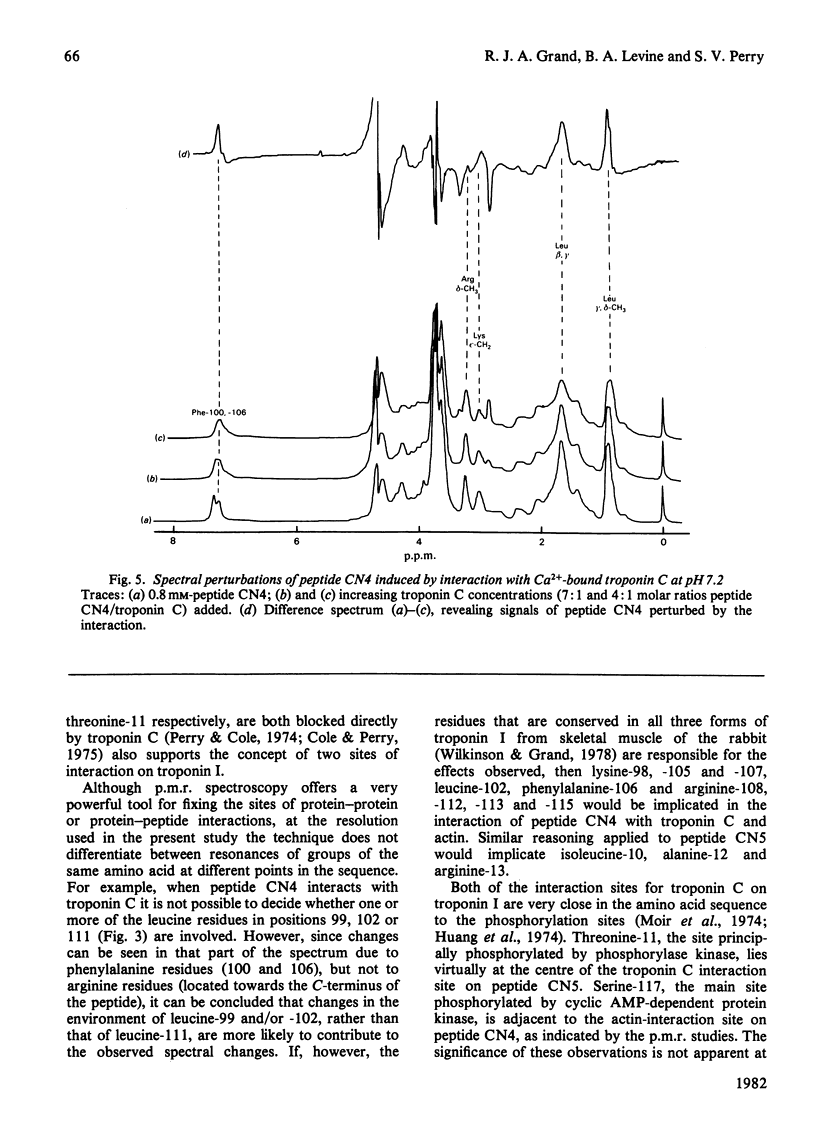
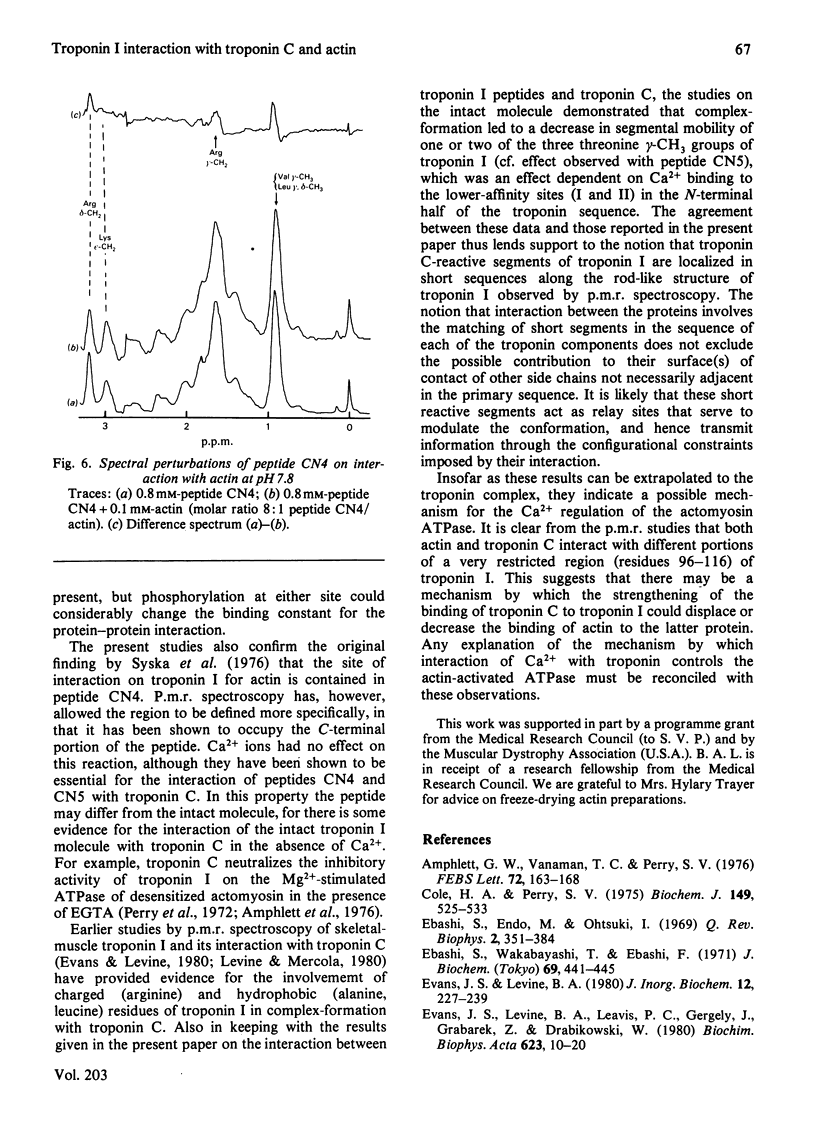
1. The p.m.r. spectra of the larger CNBr-cleavage peptides of troponin I from rabbit fast-twitch skeletal muscle corresponded largely to those of fairly flexible solution structures. 2. On addition of troponin C to each of the CNBr-cleavage peptides in turn, perturbations of side chains were noted only for peptides CN5 (residues 1-21) and CN4 (residues 96-116). 3. In the presence of Ca2+, troponin C induced perturbations of the side chains of threonine-11, alanine, isoleucine and arginine residues of peptide CN5. 4. In the presence of Ca2+, troponin C induced perturbations of the side chains of phenylalanine, lysine and leucine residues of peptide CN4. 5. Irrespective of the presence or absence of Ca2+, specific interaction with actin was observed only with peptide CN4. In this case the side chains of arginine residues were perturbed. 6. It is concluded that actin interacts with the C-terminal region of peptide CN4, whereas troponin C interacts with the N-terminal region of peptide CN4 and with peptide CN5.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amphlett G. W., Vanaman T. C., Perry S. V. Effect of the troponin C-like protein from bovine brain (brain modulator protein) on the Mg2+-stimulated ATPase of skeletal muscle actinomyosin. FEBS Lett. 1976 Dec 15;72(1):163–168. doi: 10.1016/0014-5793(76)80836-8. [DOI] [PubMed] [Google Scholar]
- Cole H. A., Perry S. V. The phosphorylation of troponin I from cardiac muscle. Biochem J. 1975 Sep;149(3):525–533. doi: 10.1042/bj1490525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S., Endo M., Otsuki I. Control of muscle contraction. Q Rev Biophys. 1969 Nov;2(4):351–384. doi: 10.1017/s0033583500001190. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Wakabayashi T., Ebashi F. Troponin and its components. J Biochem. 1971 Feb;69(2):441–445. doi: 10.1093/oxfordjournals.jbchem.a129486. [DOI] [PubMed] [Google Scholar]
- Evans J. S., Levine B. A., Leavis P. C., Gergely J., Grabarek Z., Drabikowski W. Proton magnetic resonance studies on proteolytic fragments of troponin-C. Structural homology with the native molecule. Biochim Biophys Acta. 1980 May 29;623(1):10–20. doi: 10.1016/0005-2795(80)90003-3. [DOI] [PubMed] [Google Scholar]
- Evans J. S., Levine B. A. Protein-protein interaction sites in the calcium modulated skeletal muscle troponin complex. J Inorg Biochem. 1980 Jun;12(3):227–239. doi: 10.1016/s0162-0134(00)80204-4. [DOI] [PubMed] [Google Scholar]
- Hartley B. S. Strategy and tactics in protein chemistry. Biochem J. 1970 Oct;119(5):805–822. doi: 10.1042/bj1190805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T. S., Bylund D. B., Stull J. T., Krebs E. G. The amino acid sequences of the phosphorylated sites in troponin-I from rabbit skeletal muscle. FEBS Lett. 1974 Jun 15;42(3):249–252. doi: 10.1016/0014-5793(74)80738-6. [DOI] [PubMed] [Google Scholar]
- Leavis P. C., Rosenfeld S. S., Gergely J., Grabarek Z., Drabikowski W. Proteolytic fragments of troponin C. Localization of high and low affinity Ca2+ binding sites and interactions with troponin I and troponin T. J Biol Chem. 1978 Aug 10;253(15):5452–5459. [PubMed] [Google Scholar]
- Levine B. A., Thornton J. M., Fernandes R., Kelly C. M., Mercola D. Comparison of the calcium- and magnesium-induced structural changes of troponin--C. A proton magnetic resonance study. Biochim Biophys Acta. 1978 Jul 21;535(1):11–24. doi: 10.1016/0005-2795(78)90028-4. [DOI] [PubMed] [Google Scholar]
- Moir A. J., Wilkinson J. M., Perry S. V. The phosphorylation sites of troponin I from white skeletal muscle of the rabbit. FEBS Lett. 1974 Jun 15;42(3):253–256. doi: 10.1016/0014-5793(74)80739-8. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Perry S. V., Cole H. A. Phosphorylation of troponin and the effects of interactions between the components of the complex. Biochem J. 1974 Sep;141(3):733–743. doi: 10.1042/bj1410733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. V. The regulation of contractile activity in muscle. Biochem Soc Trans. 1979 Aug;7(4):593–617. doi: 10.1042/bst0070593. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Syska H., Wilkinson J. M., Grand R. J., Perry S. V. The relationship between biological activity and primary structure of troponin I from white skeletal muscle of the rabbit. Biochem J. 1976 Feb 1;153(2):375–387. doi: 10.1042/bj1530375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks R. A., Perry S. V. Characterization of a region of the primary sequence of troponin C involved in calcium ion-dependent interaction with troponin I. Biochem J. 1978 Aug 1;173(2):449–457. doi: 10.1042/bj1730449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson J. M., Grand R. J. Comparison of amino acid sequence of troponin I from different striated muscles. Nature. 1978 Jan 5;271(5640):31–35. doi: 10.1038/271031a0. [DOI] [PubMed] [Google Scholar]
- Wilkinson J. M., Grand R. J. The amino acid sequence of troponin I from rabbit skeletal muscle. Biochem J. 1975 Aug;149(2):493–496. [PMC free article] [PubMed] [Google Scholar]
- Wilkinson J. M., Perry S. V., Cole H. A., Trayer I. P. The regulatory proteins of the myofibril. Separation and biological activity of the components of inhibitory-factor preparations. Biochem J. 1972 Mar;127(1):215–228. doi: 10.1042/bj1270215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson J. M. The preparation and properties of the components of troponin B. Biochim Biophys Acta. 1974 Aug 8;359(2):379–388. doi: 10.1016/0005-2795(74)90238-4. [DOI] [PubMed] [Google Scholar]


