Abstract
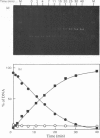
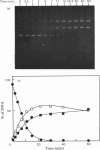
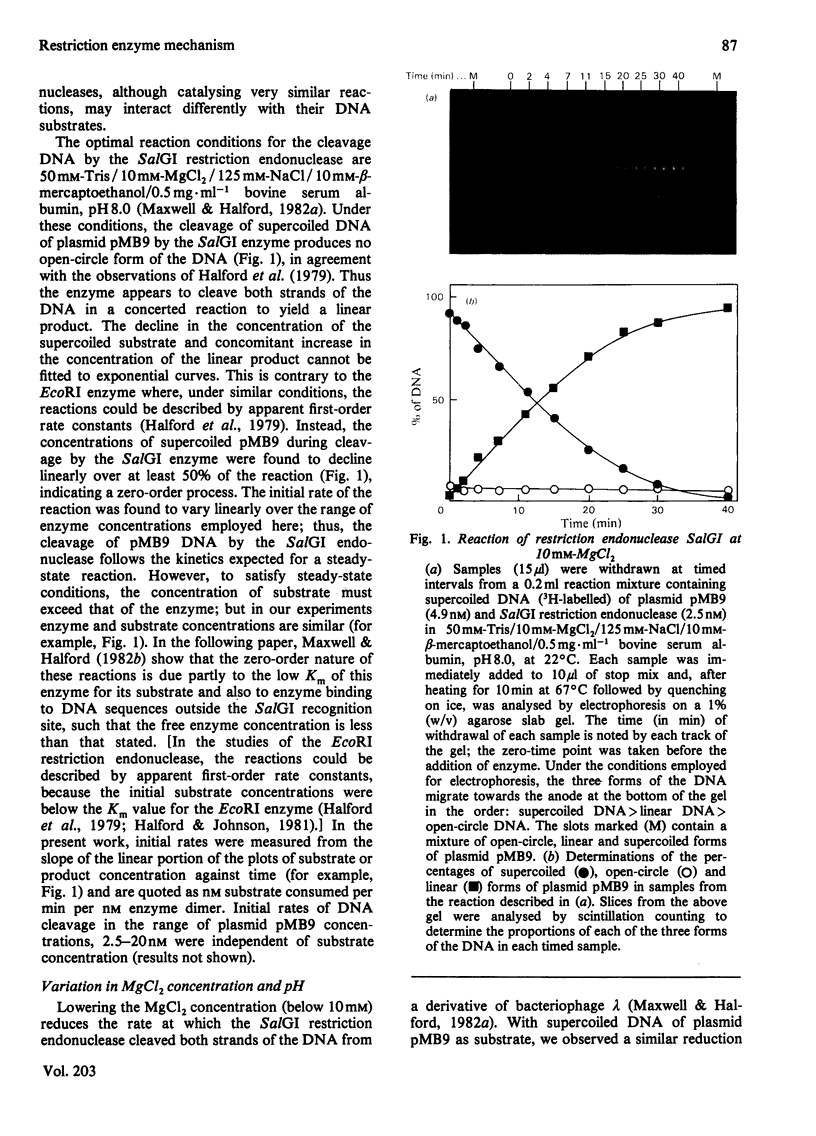
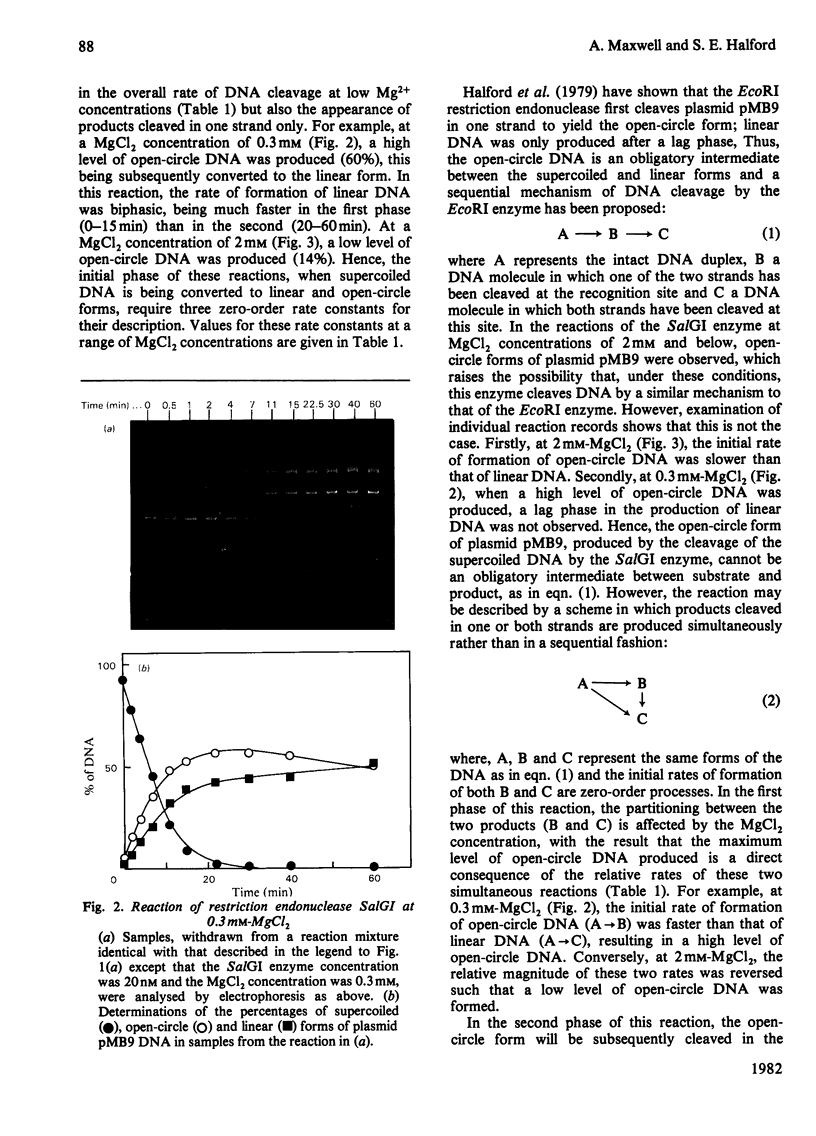
The cleavage of supercoiled DNA of plasmid pMB9 by restriction endonuclease SalGI has been studied. Under the optimal conditions for this reaction, the only product is the linear form of the DNA, in which both strands of the duplex have been cleaved at the SalGI recognition site. DNA molecules cleaved in one strand at this site were found to be poor substrates for the SalGI enzyme. Thus, both strands of the DNA appear to be cleaved in a concerted reaction. However, under other conditions, the enzyme cleaves either one or both strands of the DNA; the supercoiled substrate is then converted to either open-circle or linear forms, the two being produced simultaneously rather than consecutively. We propose a mechanism for the SalGI restriction endonuclease which accounts for the reactions of this enzyme under both optimal and other conditions. These reactions were unaffected by the tertiary structure of the DNA.
Full text
PDF

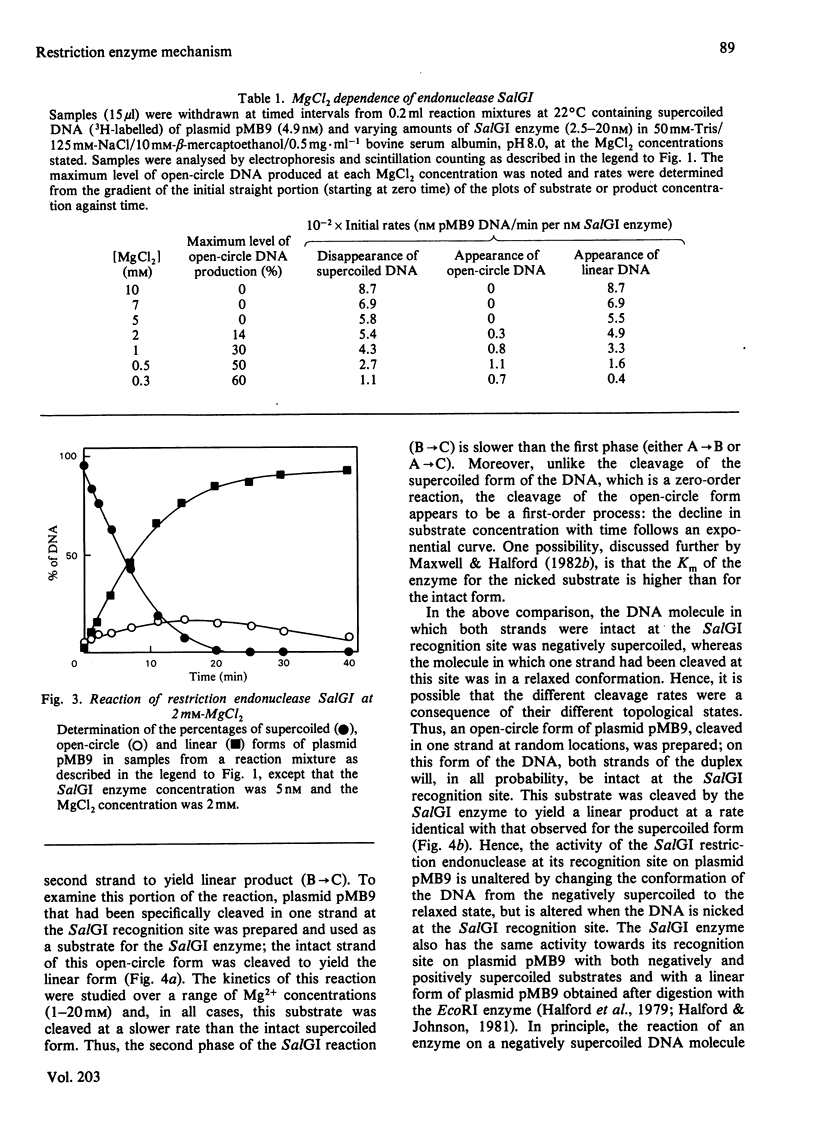
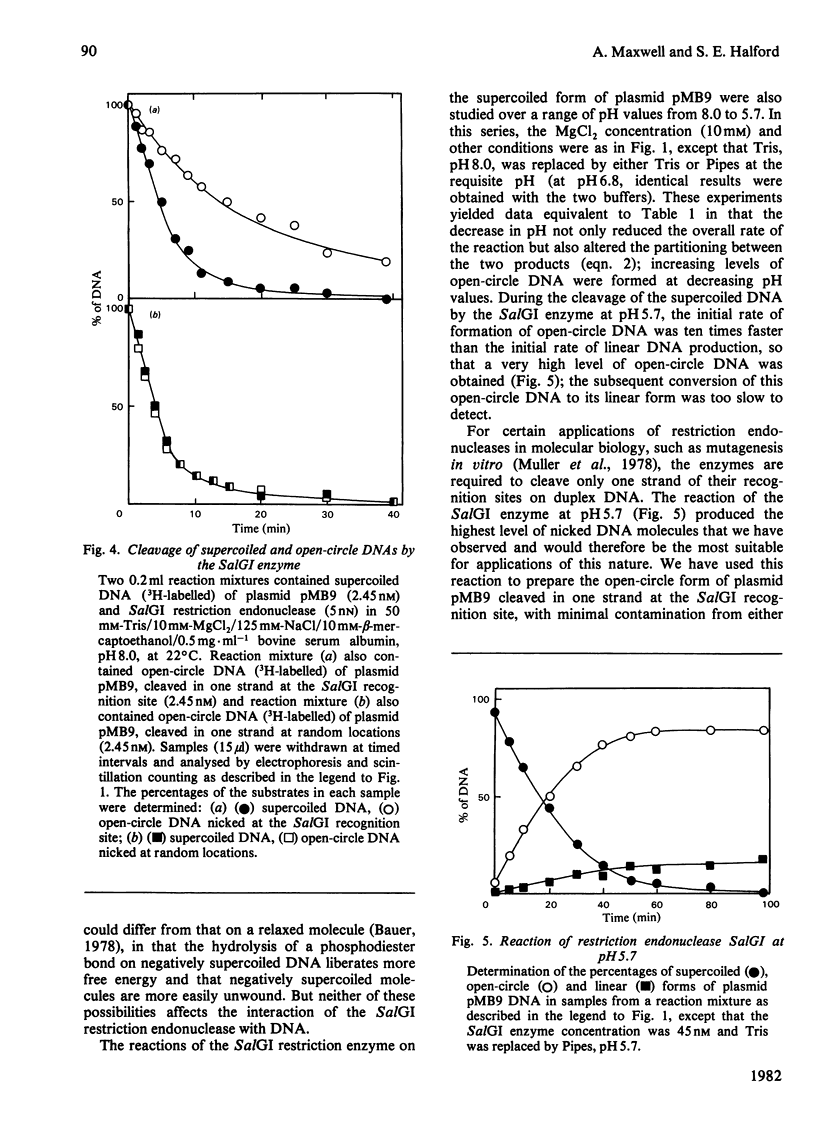


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer W. R. Structure and reactions of closed duplex DNA. Annu Rev Biophys Bioeng. 1978;7:287–313. doi: 10.1146/annurev.bb.07.060178.001443. [DOI] [PubMed] [Google Scholar]
- Halford S. E., Johnson N. P., Grinsted J. The EcoRI restriction endonuclease with bacteriophage lambda DNA. Kinetic studies. Biochem J. 1980 Nov 1;191(2):581–592. doi: 10.1042/bj1910581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford S. E., Johnson N. P., Grinsted J. The reactions of the EcoRi and other restriction endonucleases. Biochem J. 1979 May 1;179(2):353–365. doi: 10.1042/bj1790353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford S. E., Johnson N. P. The EcoRI restriction endonuclease, covalently closed DNA and ethidium bromide. Biochem J. 1981 Dec 1;199(3):767–777. doi: 10.1042/bj1990767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. H., Grossman L. I. Electrophoresis of DNA in agarose gels. Optimizing separations of conformational isomers of double- and single-stranded DNAs. Biochemistry. 1977 Sep 20;16(19):4217–4225. doi: 10.1021/bi00638a014. [DOI] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Smith H. O. A restriction enzyme from Hemophilus influenzae. II. J Mol Biol. 1970 Jul 28;51(2):393–409. doi: 10.1016/0022-2836(70)90150-6. [DOI] [PubMed] [Google Scholar]
- Maxwell A., Halford S. E. The SalGI restriction endonuclease. Enzyme specificity. Biochem J. 1982 Apr 1;203(1):93–98. doi: 10.1042/bj2030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell A., Halford S. E. The SalGI restriction endonuclease. Purification and properties. Biochem J. 1982 Apr 1;203(1):77–84. doi: 10.1042/bj2030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W., Weber H., Meyer F., Weissmann C. Site-directed mutagenesis in DNA: generation of point mutations in cloned beta globin complementary dna at the positions corresponding to amino acids 121 to 123. J Mol Biol. 1978 Sep 15;124(2):343–358. doi: 10.1016/0022-2836(78)90303-0. [DOI] [PubMed] [Google Scholar]
- Ruben G., Spielman P., Tu C. D., Jay E., Siegel B., Wu R. Relaxed circular SV40 DNA as cleavage intermediate of two restriction endonucleases. Nucleic Acids Res. 1977 Jun;4(6):1803–1813. doi: 10.1093/nar/4.6.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R. A., Modrich P. Substrate dependence of the mechanism of EcoRI endonuclease. Nucleic Acids Res. 1978 Aug;5(8):2991–2997. doi: 10.1093/nar/5.8.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Wilcox K. W. A restriction enzyme from Hemophilus influenzae. I. Purification and general properties. J Mol Biol. 1970 Jul 28;51(2):379–391. doi: 10.1016/0022-2836(70)90149-x. [DOI] [PubMed] [Google Scholar]