Abstract
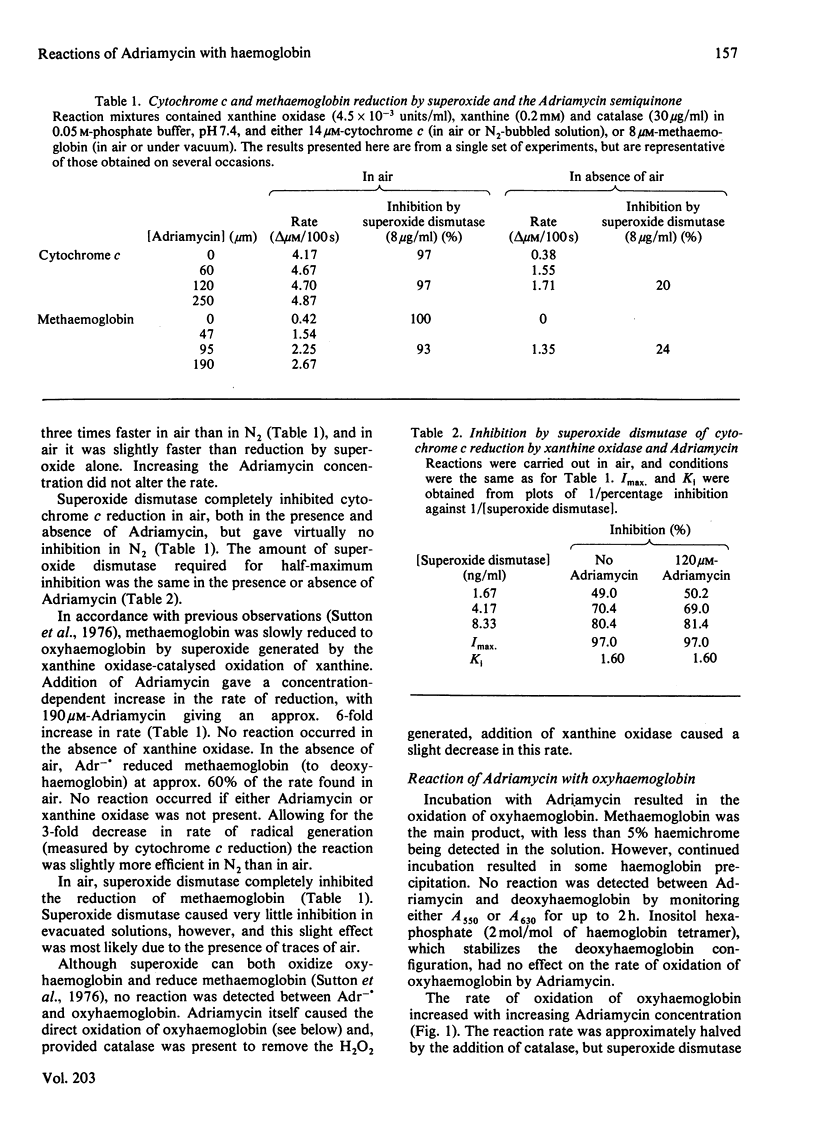
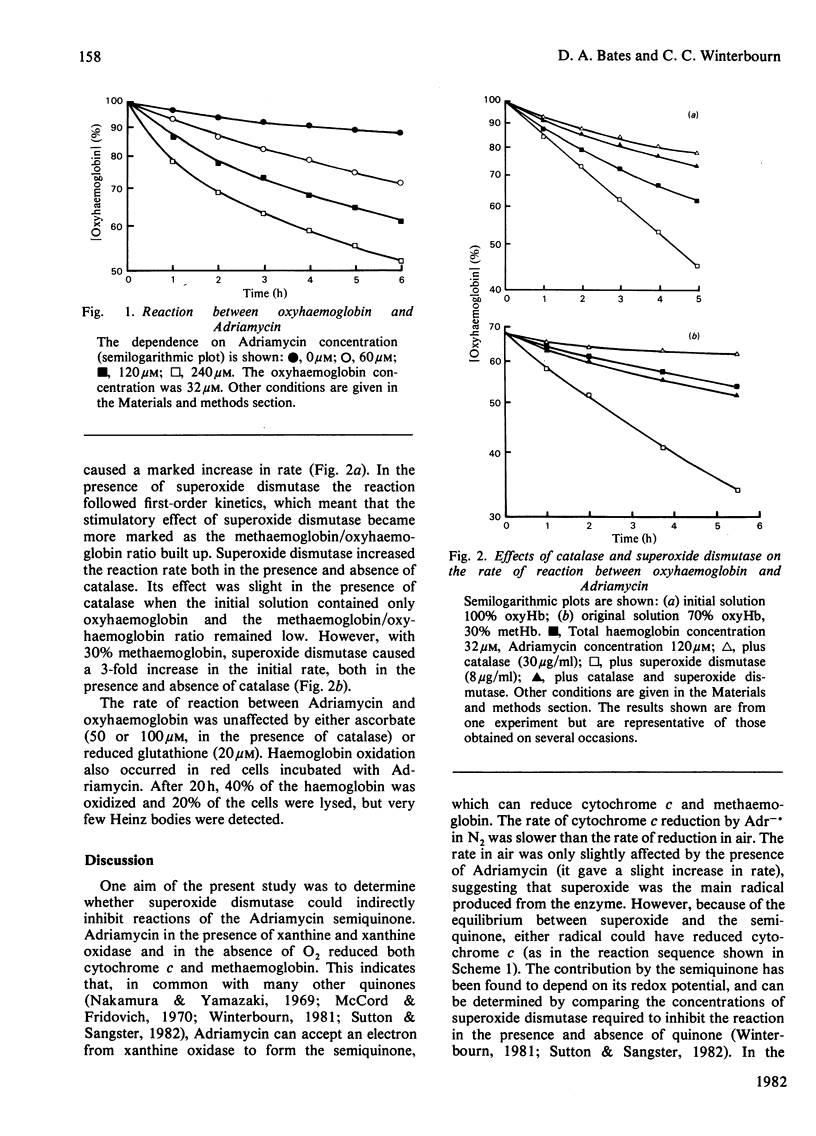
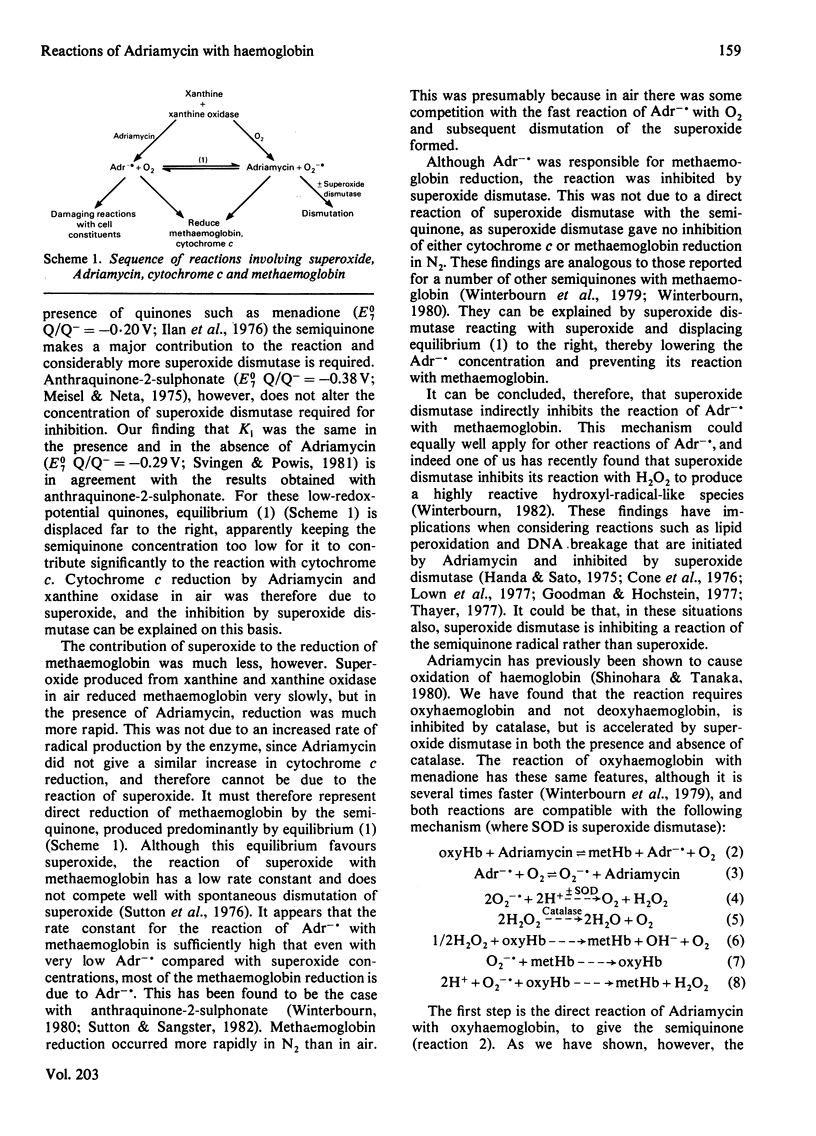
The Adriamycin semiquinone produced by the reaction of xanthine oxidase and xanthine with Adriamycin has been shown to reduce both methaemoglobin and cytochrome c. In air, but not N2, both reactions were inhibited by superoxide dismutase. With cytochrome c, superoxide formed by the rapid reaction of the semiquinone with O2, was responsible for the reduction. However, even in air, methaemoglobin was reduced directly by the Adriamycin semiquinone. Superoxide dismutase inhibited this reaction by removing superoxide and hence the semiquinone by displacing the equilibrium: Semiquinone + O2 in equilibrium or formed from quinone + O2-. to the right. This ability to inhibit indirectly reactions of the semiquinone could have wider implications for the protection given by superoxide dismutase against the cytotoxicity of Adriamycin. Oxidation of haemoglobin by Adriamycin has been shown to be initiated by a reversible reaction between the drug and oxyhaemoglobin, producing methaemoglobin and the Adriamycin semiquinone. Reaction of the semiquinone with O2 gives superoxide and H2O2, which can also react with haemoglobin. Catalase, by preventing this reaction of H2O2, inhibits oxidation of oxyhaemoglobin. Superoxide dismutase, however, accelerates oxidation, by inhibiting the reaction of the semiquinone with methaemoglobin by the mechanism described above. Although superoxide dismutase has a detrimental effect on haemoglobin oxidation, it may protect the red cell against more damaging reactions of the Adriamycin semiquinone.
Full text
PDF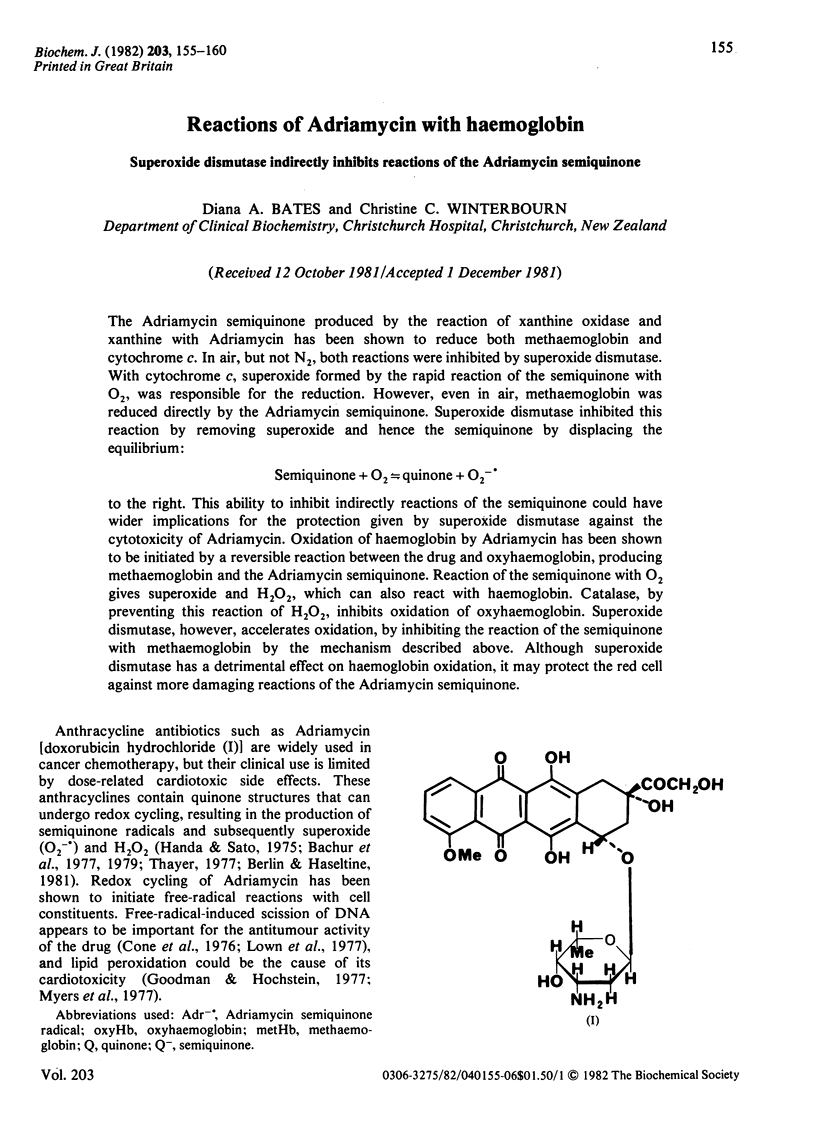


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachur N. R., Gordon S. L., Gee M. V. Anthracycline antibiotic augmentation of microsomal electron transport and free radical formation. Mol Pharmacol. 1977 Sep;13(5):901–910. [PubMed] [Google Scholar]
- Bachur N. R., Gordon S. L., Gee M. V., Kon H. NADPH cytochrome P-450 reductase activation of quinone anticancer agents to free radicals. Proc Natl Acad Sci U S A. 1979 Feb;76(2):954–957. doi: 10.1073/pnas.76.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benesch R. E., Benesch R., Yung S. Equations for the spectrophotometric analysis of hemoglobin mixtures. Anal Biochem. 1973 Sep;55(1):245–248. doi: 10.1016/0003-2697(73)90309-6. [DOI] [PubMed] [Google Scholar]
- Berlin V., Haseltine W. A. Reduction of adriamycin to a semiquinone-free radical by NADPH cytochrome P-450 reductase produces DNA cleavage in a reaction mediated by molecular oxygen. J Biol Chem. 1981 May 25;256(10):4747–4756. [PubMed] [Google Scholar]
- Cone R., Hasan S. K., Lown J. W., Morgan A. R. The mechanism of the degradation of DNA by streptonigrin. Can J Biochem. 1976 Mar;54(3):219–223. doi: 10.1139/o76-034. [DOI] [PubMed] [Google Scholar]
- French J. K., Winterbourn C. C., Carrell R. W. Mechanism of oxyhaemoglobin breakdown on reaction with acetylphenylhydrazine. Biochem J. 1978 Jul 1;173(1):19–26. doi: 10.1042/bj1730019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. J Biol Chem. 1970 Aug 25;245(16):4053–4057. [PubMed] [Google Scholar]
- Goodman J., Hochstein P. Generation of free radicals and lipid peroxidation by redox cycling of adriamycin and daunomycin. Biochem Biophys Res Commun. 1977 Jul 25;77(2):797–803. doi: 10.1016/s0006-291x(77)80048-x. [DOI] [PubMed] [Google Scholar]
- Handa K., Sato S. Generation of free radicals of quinone group-containing anti-cancer chemicals in NADPH-microsome system as evidenced by initiation of sulfite oxidation. Gan. 1975 Feb;66(1):43–47. [PubMed] [Google Scholar]
- Henderson C. A., Metz E. N., Balcerzak S. P., Sagone A. L., Jr Adriamycin and daunomycin generate reactive oxygen compounds in erythrocytes. Blood. 1978 Nov;52(5):878–885. [PubMed] [Google Scholar]
- Ilan Y. A., Czapski G., Meisel D. The one-electron transfer redox potentials of free radicals. I. The oxygen/superoxide system. Biochim Biophys Acta. 1976 May 14;430(2):209–224. doi: 10.1016/0005-2728(76)90080-3. [DOI] [PubMed] [Google Scholar]
- Lown J. W., Sim S. K., Majumdar K. C., Chang R. Y. Strand scission of DNA by bound adriamycin and daunorubicin in the presence of reducing agents. Biochem Biophys Res Commun. 1977 Jun 6;76(3):705–710. doi: 10.1016/0006-291x(77)91557-1. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. The utility of superoxide dismutase in studying free radical reactions. II. The mechanism of the mediation of cytochrome c reduction by a variety of electron carriers. J Biol Chem. 1970 Mar 25;245(6):1374–1377. [PubMed] [Google Scholar]
- Myers C. E., McGuire W. P., Liss R. H., Ifrim I., Grotzinger K., Young R. C. Adriamycin: the role of lipid peroxidation in cardiac toxicity and tumor response. Science. 1977 Jul 8;197(4299):165–167. doi: 10.1126/science.877547. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Yamazaki I. One-electron transfer reactions in biochemical systems. IV. A mixed mechanism in the reaction of milk xanthine oxidase with electron acceptors. Biochim Biophys Acta. 1969 Sep 16;189(1):29–37. doi: 10.1016/0005-2728(69)90221-7. [DOI] [PubMed] [Google Scholar]
- Sagone A. L., Jr, Burton G. M. The effect of BCNU and adriamycin on normal and G6PD deficient erythrocytes. Am J Hematol. 1979;7(2):97–106. doi: 10.1002/ajh.2830070202. [DOI] [PubMed] [Google Scholar]
- Shinohara K., Tanaka K. R. The effects of adriamycin (doxorubicin HCl) on human red blood cells. Hemoglobin. 1980;4(5-6):735–745. doi: 10.3109/03630268008997741. [DOI] [PubMed] [Google Scholar]
- Sutton H. C., Roberts P. B., Winterbourn C. C. The rate of reaction of superoxide radical ion with oxyhaemoglobin and methaemoglobin. Biochem J. 1976 Jun 1;155(3):503–510. doi: 10.1042/bj1550503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svingen B. A., Powis G. Pulse radiolysis studies of antitumor quinones: radical lifetimes, reactivity with oxygen, and one-electron reduction potentials. Arch Biochem Biophys. 1981 Jun;209(1):119–126. doi: 10.1016/0003-9861(81)90263-0. [DOI] [PubMed] [Google Scholar]
- Thayer W. S. Adriamycin stimulated superoxide formation in submitochondrial particles. Chem Biol Interact. 1977 Dec;19(3):265–278. doi: 10.1016/0009-2797(77)90050-3. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C. Cytochrome c reduction by semiquinone radicals can be indirectly inhibited by superoxide dismutase. Arch Biochem Biophys. 1981 Jun;209(1):159–167. doi: 10.1016/0003-9861(81)90268-x. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C., French J. K., Claridge R. F. Superoxide dismutase as an inhibitor of reactions of semiquinone radicals. FEBS Lett. 1978 Oct 15;94(2):269–272. doi: 10.1016/0014-5793(78)80953-3. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C., French J. K., Claridge R. F. The reaction of menadione with haemoglobin. Mechanism and effect of superoxide dismutase. Biochem J. 1979 Jun 1;179(3):665–673. doi: 10.1042/bj1790665. [DOI] [PMC free article] [PubMed] [Google Scholar]


