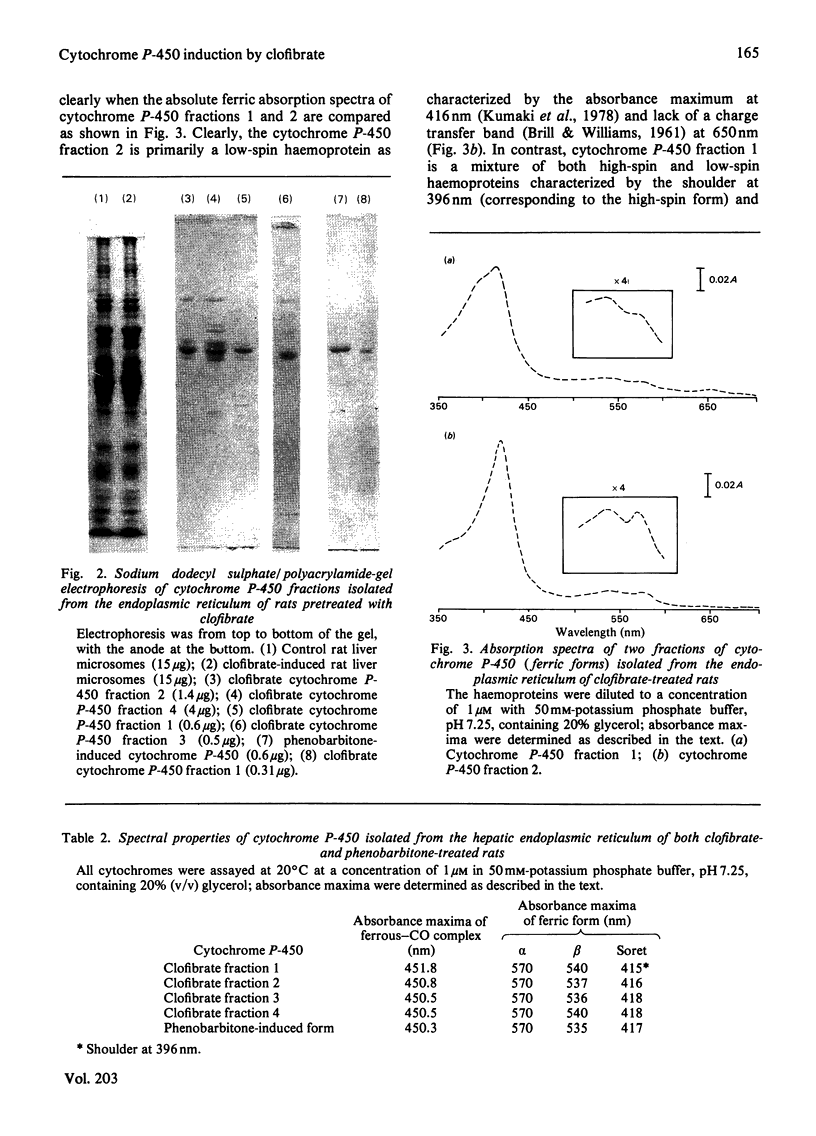
Abstract
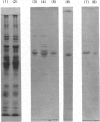
Hypolipidaemic drugs induce peroxisomal proliferation in the liver and many induce the formation of the hepatic endoplasmic reticulum in general and the formation of cytochrome P-450 in particular. We have induced the formation of rat liver microsomal cytochrome P-450 by the administration of the hypolipidaemic drug clofibrate, isolated the endoplasmic reticulum, solubilized the cytochrome P-450 from these membranes and subdivided the cytochrome P-450 into four fractions by the use of hydrophobic, anionic, cationic and adsorption chromatography. One of these fractions (cytochrome P-450 fraction 1) was highly purified to a specific content of 17nmol of cytochrome P-450/mg of protein and the protein was active in a reconstituted enzyme system towards the 12- and 11-hydroxylation of the fatty acid, dodecanoic (lauric) acid, with preferential activity towards the 12-hydroxy metabolite. This reconstituted activity was absolutely dependent on NADPH, NADPH-cytochrome P-450 reductase and cytochrome P-450, indicating the role of the mixed-function oxidase system in the metabolism of lauric acid. Another fraction of the haemoprotein (cytochrome P-450 fraction 2) preferentially formed 11-hydroxylauric acid, whereas a third fraction (cytochrome P-450 fraction 3) exhibited only trace laurate oxidase activity and was similar to the phenobarbitone form of the haemoprotein in that these last two cytochromes rapidly turned-over the drug benzphetamine. The molecular weights and spectral properties of these cytochrome P-450 fractions are reported, along with the phenobarbitone-induced form of the enzyme and the nature of the cytochrome(s) induced by clofibrate pretreatment are discussed in the terms of possible haemoprotein heterogeneity.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brill A. S., Williams R. J. The absorption spectra, magnetic moments and the binding of iron in some haemoproteins. Biochem J. 1961 Feb;78(2):246–253. doi: 10.1042/bj0780246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPER D. Y., LEVIN S., NARASIMHULU S., ROSENTHAL O. PHOTOCHEMICAL ACTION SPECTRUM OF THE TERMINAL OXIDASE OF MIXED FUNCTION OXIDASE SYSTEMS. Science. 1965 Jan 22;147(3656):400–402. doi: 10.1126/science.147.3656.400. [DOI] [PubMed] [Google Scholar]
- Conney A. H. Pharmacological implications of microsomal enzyme induction. Pharmacol Rev. 1967 Sep;19(3):317–366. [PubMed] [Google Scholar]
- Guengerich F. P. Separation and purification of multiple forms of microsomal cytochrome P-450. Partial characterization of three apparently homogeneous cytochromes P-450 prepared from livers of phenobarbital- and 3-methylcholanthrene-treated rats. J Biol Chem. 1978 Nov 10;253(21):7931–7939. [PubMed] [Google Scholar]
- Haugen D. A., van der Hoeven T. A., Coon M. J. Purified liver microsomal cytochrome P-450. Separation and characterization of multiple forms. J Biol Chem. 1975 May 10;250(9):3567–3570. [PubMed] [Google Scholar]
- Heady J. A. A cooperative trial on the primary prevention of ischaemic heart disease using clofibrate: design, methods, and progress. Bull World Health Organ. 1973;48(2):243–256. [PMC free article] [PubMed] [Google Scholar]
- Hess R., Stäubli W., Riess W. Nature of the hepatomegalic effect produced by ethyl-chlorophenoxy-isobutyrate in the rat. Nature. 1965 Nov 27;208(5013):856–858. doi: 10.1038/208856a0. [DOI] [PubMed] [Google Scholar]
- Kumaki K., Sato M., Kon H., Nebert D. W. Correlation of type I, type II, and reverse type I difference spectra with absolute changes in spin state of hepatic microsomal cytochrome P-450 iron from five mammalian species. J Biol Chem. 1978 Feb 25;253(4):1048–1058. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- NASH T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem J. 1953 Oct;55(3):416–421. doi: 10.1042/bj0550416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Reddy J. K., Azarnoff D. L., Hignite C. E. Hypolipidaemic hepatic peroxisome proliferators form a novel class of chemical carcinogens. Nature. 1980 Jan 24;283(5745):397–398. doi: 10.1038/283397a0. [DOI] [PubMed] [Google Scholar]
- Remmer H., Schenkman J., Estabrook R. W., Sasame H., Gillette J., Narasimhulu S., Cooper D. Y., Rosenthal O. Drug interaction with hepatic microsomal cytochrome. Mol Pharmacol. 1966 Mar;2(2):187–190. [PubMed] [Google Scholar]
- Salvador R. A., Haber S., Atkins C., Gommi B. W., Welch R. M. Effect of clofibrate and 1-methyl-4-piperidyl bis (p-chlorophenoxy) acetate (Sandox 42-348) on steroid and drug metabolism by rat liver microsomes. Life Sci. 1970 Apr 8;9(7):397–407. doi: 10.1016/0024-3205(70)90242-0. [DOI] [PubMed] [Google Scholar]
- Schenkman J. B., Remmer H., Estabrook R. W. Spectral studies of drug interaction with hepatic microsomal cytochrome. Mol Pharmacol. 1967 Mar;3(2):113–123. [PubMed] [Google Scholar]
- Yasukochi Y., Masters B. S. Some properties of a detergent-solubilized NADPH-cytochrome c(cytochrome P-450) reductase purified by biospecific affinity chromatography. J Biol Chem. 1976 Sep 10;251(17):5337–5344. [PubMed] [Google Scholar]