Abstract
Recent studies are showing that some lights suitable for illuminating the urban fabric (i.e. that do not include the red, green and blue sets of primary colours) may halt biological colonisation on monuments, mainly that caused by phototrophic subaerial biofilms (SABs), which may exacerbate the biodeterioration of substrates. However, the light-triggered mechanisms that cause changes in the growth of the phototrophs remain unknown. Environmental proteomics could be used to provide information about the changes in the SAB metabolism under stress inflicted by nocturnal lighting. Here, laboratory-produced SABs, composed of Chlorophyta, Streptophyta and Cyanobacteriota, were subjected to three types of lighting used for monuments: cool white, warm white and amber + green (potentially with a biostatic effect). A control without light (i.e. darkness) was also included for comparison. The nocturnal lighting impaired the capacity of the SABs to decompose superoxide radicals and thus protect themselves from oxidative stress. Cool white and warm white light both strongly affected the proteomes of the SABs and reduced the total peptide content, with the extent of the reduction depending on the genera of the organisms involved. Analysis of the photo-damaging effect of amber + green light on the biofilm metabolism revealed a negative impact on photosystems I and II and production of photosystem antenna protein-like, as well as a triggering effect on protein metabolism (synthesis, folding and degradation). This research provides, for the first-time, a description of the proteomic changes induced by lighting on SABs colonising illuminated monuments in urban areas.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00248-024-02465-1.
Keywords: Lighting Treatment, Triggered Processes, Environmental Proteomics, Green Algae, Cultural Heritage, Biofilms
Introduction
Lighting of the urban fabric at night is a controversial issue. It is debated if increasing the number of points that are illuminated in cities makes transit safer and more secure for pedestrians [1]. Illumination can also make cities appear more modern and aesthetically pleasing (in the eyes of some). Lighting cultural and architectural assets of cities to enhance and valorize the cultural heritage has grown in popularity since the 1990s, especially since around 2010 [2]. Lighting should highlight specific features and the geometric shapes of monuments or generate visual contrasts while remaining respectful of the original aesthetics of the buildings [3]. Night-time tourism is now more common in cities than it was in the early 1990s, and the change has been attributed to increased illumination [4]. However, illumination of monuments is an emerging contribution to artificial light at night (ALAN) pollution—or more simply, light pollution—[5], which increased by 9.6% between 2011 and 2022 [6]. ALAN affects the survival, existence and well-being of people, microorganisms and plants and eventually disrupts the entire ecosystem [7]. However, appropriate ornamental lighting could help to reduce biodeterioration of monuments and protect biodiversity [5].
Light emitting diodes (LEDs) are increasingly used to illuminate monuments [8] because they use less energy, yield more light, last longer than other types of light technologies and are also mercury-free. However, illumination can cause biodeterioration of heritage monuments by promoting colonisation by phototrophic organisms [9], which use light as the main source of energy for metabolism (i.e. photosynthesis). Testing has been carried out to evaluate the use of different artificial monochromatic lights (blue, green and red) to shape the ecophysiological features of the algae and cyanobacteria to exert a biostatic (halting growth) effect on the organisms. Studies conducted in subterranean heritage such as caves, catacombs and mausoleums [9–11] and on outdoor monuments [12] have demonstrated inhibition of colonisation by phototrophs, especially of cyanobacteria rich in phycocyanin exposed to blue light and of algae exposed to green light. In the first study, carried out in the early 2000s in the Roman Catacombs of St. Callistus and Domitilla, Rome (Italy), the photosynthetic activity of the cyanobacterial and chemoorganotrophic bacterial biofilms was inhibited after 5 months under blue lighting [10], and the area occupied by phototrophic communities was reduced after 10 years [11]. Green lighting tested in on-site studies has been found to inhibit the growth of phototrophic biofilms, such as those predominated by the alga Chlorella sorokiniana in the Bats’ Cave, Zuheros, Spain [13], by the cyanobacterium Chroococcidiopsis sp. and red microalga Cyanidium sp. in the Nerja Cave, Malaga, Spain [14] and by cyanobacteria and heterotrophic species belonging to proteobacteria in two Mausoleums of the Southern Tang Dynasty, Nanjing, China [9]. Compared to a non-illuminated or white light control, illumination with red light inhibited growth of biofilms mainly predominated by the green alga Bracteacoccus minor (in this case in combination with a period of daylight) [15] and the green alga Chlamydomonas reinhardtii CC-1690 [16].
The aim of these lighting-based strategies is to prevent further biodeterioration on outdoor stone monuments (sometimes only aesthetic) by using narrow-band LEDs avoiding certain photosynthetic active radiation (PAR) spectral bands, between 350–400 nm and 700–750 nm [17, 18]. These strategies are much more difficult to apply to naturally lit outdoor monuments, where the use of monochromatic colours for lighting is not acceptable in urban planning for aesthetic reasons, and negative effects on humans and animals must be avoided [5]. However, exposure of monuments to the light generated by combining amber and green LEDs (CromaLux light, currently under trial), which generates warm tones, has shown an impact similar to an unlit area; with little to no impact on the insects of the surrounding area [19] and a slightly enhancing impact on bacterial and fungal microbiota of monument facades [20], and also a long-term biostatic effect on phototrophs, especially algae (unpublished data). The use of this light is proposed as a preventive conservation tool, using the existing ornamental lighting infrastructure, in order to achieve also reduce the cleaning campaigns of facades and structures. It is not a biocidal light so its role is not to replace the use of chemicals to remove biocolonisation already present at the field site.
Different spectral bands affect the various taxonomic groups that comprise SABs differently, and the specific changes exerted by the lighting in the underlying metabolic machinery remain unknown. Considering the heterogeneity of the SABs and the long times required to remove them from heritage surfaces (10 years in the study by Bruno et al. [11]), research should involve different taxonomic groups and different types of illumination. The data obtained could then be used as the basis for selecting lights to eliminate existing SABs or to prevent the appearance of SABs on particular monuments. Thanks to great technical advances achieved in recent years, environmental proteomics provides information on both the taxonomies and the functionalities from complex mixed microbial communities. We hypothesize that this approach could be useful to cover the gap in information on the molecular damage induced by the different types of lights, as it has been previously shown to elucidate the links between proteome alteration, metabolism and phenotype changes under environmental stresses [21].
In the present study, SABs [22, 23] were generated in the laboratory to further understand the molecular mechanism of phototrophic SABSs under nocturnal ornamental lighting using a culture obtained from natural phototrophic biofilms of granite monuments in Santiago de Compostela (UNESCO World Heritage City since 1985, NW Spain). The SABs were subsequently grown under a photoperiod comprised of 13 h of LED daylight simulating the solar illuminance in spring in the historical centre of Santiago de Compostela (see the “SAB formation and lighting set-up” section), followed by 6 h of different types of ornamental lighting and 5 h of darkness, to simulate outdoor conditions. The following types of ornamental lighting, considered suitable for the urban fabric, were tested: the amber + green light combination currently under trial, a warm white light (yellow tone), a cool white light (blue tone) and no light. Environmental proteomics analysis was used to study the effect of ornamental lighting on the physiology of the different species forming the mature SABs, with a particular focus on the photosynthetic machineries. To our knowledge, this is the first proteomics study of SABs colonizing illuminated monuments in urban areas.
Materials and Methods
SAB Formation and Lighting Set-up
Multi-species SABs, mainly composed of eukaryotic microalgae (hereinafter green algae) but also including prokaryotic microalgae (hereinafter cyanobacteria), were grown according to the membrane-supported biofilm protocol first described by Anderl et al. [24] and later implemented for phototrophs by Sanmartín et al. [25]. The species comprising the SABs were examined under light microscopy, with a Nikon Eclipse E600 equipped with an E-Plan 40 × objective (N.A. 0.65) and differential interference contrast (Nomarski) optics. Taxonomic determinations were based on the morphometry and reproduction of the species in the cultures. The main taxonomic references used for the identification of green algae were [26–28] and for cyanobacteria [29]. The results were used to select an adequate database to perform the proteomic data analyses. The biomass of each SAB was also measured gravimetrically by weighing the membrane before and after removal of the biofilm (by gentle scraping with a sterile loop) with a precision laboratory balance (Denver Instruments, USA, readability, 0.001 mg; uncertainty, i.e. repeatability, 0.002 g).
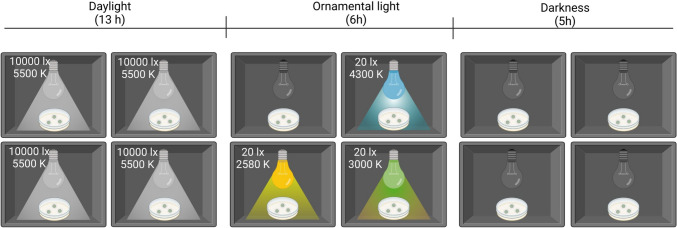
Aliquots of exponentially growing cells from a homogeneous suspension (50 µL, corresponding to 1.52 mg L−1 of dry mass) were used to inoculate sterile (by exposure to UV-C light) polycarbonate membrane discs (Nuclepore™ Track-Etch Membrane Filtration products, diameter 2.5 cm, area 4.9 cm2, pore diameter 0.2 µm, Whatman™). Twelve inoculated membrane discs were placed on Bold’s Basal Medium (BBM) [30]. The membranes were transferred, with the aid of sterile plastic forceps, to fresh agar plates, every 3 days. The SABs were cultured until they reached maturity (after 37 days) in controlled ad hoc customised cabinets (Fig. 1), with one compartment for each of the four LED light photoperiods. Each compartment held a plate with three membrane discs. After the growing period, the SABs were scraped from the membranes, to enable quantification of the biomass of each (values ranged from 65.90 ± 10.46 mg to 53.42 ± 10.77 mg) and were stored at − 80 °C before further processing.
Fig. 1.
Schematic diagram of the experimental set-up of the cabinets for the four LED light photoperiods
In all four compartments, the photoperiod comprised 13 h of LED daylight (SUN@HOME, Spot PAR16 40 GU10 TW, Ledvance, Germany) represented by high illuminance (10,000 lx, corresponding to 252.59 µmol s−1 m−2) and colour temperature of 5500 K, followed by 6 h of different test ornamental illuminations and 5 h of darkness. The diurnal illuminance was based on light measurements taken in spring 2022 on the facades of six granite-built heritage buildings in the historical centre of Santiago de Compostela (the Cathedral, the Monastery of San Martiño Pinario, the Palace of Xelmírez, the School of Medicine, the Cabildo House and the old town hall). The light was measured with a radiometer (DHD 2302.0, HERTER) on each (differently oriented) facade. In addition, the seasonal average daily insolation in the city, reported on the website of the Galician meteorological service (https://www.meteogalicia.gal/), was used to assess whether the cumulative solar intensity applied during the 13 h daylight period was consistent with the daily insolation in spring. The three (test) ornamental lighting set-ups were adjusted with a radiometer (DHD 2302.0, HERTER) to yield an illuminance (quantity) of 20 lx and different light quality (spectral composition) with an associated correlated colour temperature (CCT), both measured with a StellarNet Blue-Wave spectrometer (StellarNet, USA) (Fig. 2). The ornamental illuminance (20 lx) was based on urban planning directives of the city of Santiago de Compostela for the illumination of historical building facades, which included a reduction in nocturnal lighting to the minimum possible while being higher than the average illuminance on the street (10–15 lx) to make the facade more prominent. The unit of measurement for illuminance was selected as Lx (lumens m−2), as it is the preferred unit used by urban planners to mark the intensity of artificial light at night, regardless of the type of light used.
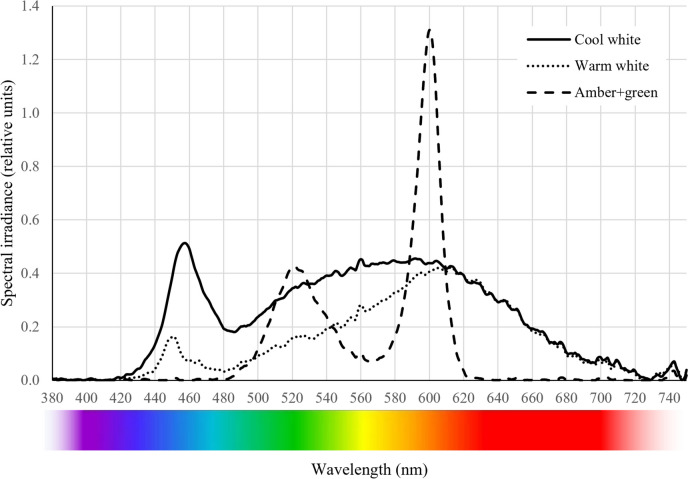
Fig. 2.
Light quality (spectra) in the PAR region yielded by the three ornamental lightings. The cool white spectrum was measured after dimming the light bulb with a slightly yellowish, heat-resistant paper film
Furthermore, it is used by Spanish legislation to indicate acceptable thresholds for night-time lighting [31]. Both types of white light (cool and warm) comprise the full range of visible light spectrum (around 380–700 nm). Cool white light (blueish tone, A5 GU10 9W, Aigostar, Spain) contains more blue light than warm white light (yellowish tone, cod. 671992, Televés, Spain). The spectrum of cool white light thus has a peak centred at 457 nm, while that of warm white lights has a less intense peak centred at 450 nm. For both types of white light, a wide band between 480 and 700 nm was observed in the spectra, with a main peak around 600 nm (amber). The cool white luminaire was shielded with a slightly yellowish, heat-resistant paper film to dim the light to an illuminance of 20 lx. The shading led to a change in the correlated colour temperature (CCT) from 6400 K (as stated by the manufacturer) to 4300 K yielding a photon flux density of 1.84 µmol s−1 m−2. The CCT of the warm white light is 2580 K and produces a photon flux density of 1.13 µmol s−1 m−2. The amber + green light (combination under trial, cod. 671990–1, Televés, Spain) yields a bimodal spectrum with two peaks, one at 528 nm (green) and a main peak at 593 nm (amber), producing a CCT of 3000 K with no emission of wavelengths in the blue and red parts of the spectrum (Fig. 2), resulting in an emission of 0.89 µmol s−1 m−2 of photon flux density. The photon flux density of the ornamental lights was obtained from the spectra measured over the samples, according to the photon energy equation.
Protein Extraction
A total of 12 SAB samples (three per ornamental lighting condition) were resuspended in 0.5 mL of EDTA (0.1 M) and centrifuged at 8000 rpm for 15 min at 4 °C to separate the extracellular matrix from the cellular material. The pellets were resuspended in extraction buffer (50 mM Tris Buffer, 1% SDS, pH = 7.5) and incubated at 90 °C for 20 min. The cells were then lysed by shaking with glass beads in a cell disruptor (Vortex Genie, Scientific Industries, USA), with three cycles of shaking, each lasting 4 min, alternated with incubation on ice for 1 min. The samples were then centrifuged for 20 min at 3300 rpm and 4 °C. The proteins were precipitated from the samples by two additions of cold acetone (at − 20 °C) to remove organic contaminants, salts and buffer residues. The acetone was carefully removed, and the protein pellets were dried at room temperature. The proteins thus obtained were resuspended in triethylammonium bicarbonate buffer (TEAB buffer, Merck, Germany) and quantified with the BCA Protein Assay Kit (Thermo Fisher Scientific, USA) and a Nanodrop spectrophotometer (Thermo Fisher Scientific, USA), with bovine serum albumin as the calibration curve standard.
SDS-PAGE Electrophoresis
To assess the quality and integrity of the protein samples, protein electrophoresis was performed in denaturing conditions [32]. Samples were denatured at 70 °C for 10 min and mixed with NuPAGE LDS Sample Buffer and NuPAGE Reducing Agent (Thermo Fisher Scientific), following the manufacturer instructions, and loading 20 µg of protein per gel lane. For the electrophoresis, NuPAGE Bis–Tris 4–12% gels (Thermo Fisher Scientific, USA) were used with 1X Running Buffer (Thermo Fisher Scientific, 20X Running Buffer containing 50 mM MES, 50 mM Tris Base, 0.1% SDS and 1 mM EDTA, pH = 7.3). Three microlitres of pre-stained protein markers (PageRuler™ Plus Prestained Ladder from 10 to 250 kDa, Thermo Fisher Scientific, USA) were loaded on each side of the gel. The electrophoresis was run at 200 V for 30 min, and the gel was stained with a standard Coomassie Blue staining protocol.
Shotgun Proteomic Analysis by Mass Spectrometry
A label-free approach was used to quantify the proteins in each sample [33]. The samples were first trypsin-digested, reduced-alkylated and finally desalted with ZipTip-μC18 (Merck, Germany). The peptide samples thus obtained were analysed by in-solution shotgun proteomics [33]. Peptide samples (0.3 µg of protein) were injected in a timsTOF Pro mass spectrometer (Bruker, Germany) equipped with a nanoelectrospray source (CaptiveSpray) and a tims-QTOF analyser. The chromatographic analysis was performed using a nanoELUTE chromatograph (Bruker, Germany) with an Aurora analytical column (C18, 250 × 0.075 mm, 1.6 µm, 120 Å, IonOpticks). The nHPLC was configured with binary mobile phases that included solvent A (0.1% formic acid in miliQ H2O) and solvent B (0.1% formic acid in acetonitrile). The analysis time was 105 min, during which the B/A solvent ratio was gradually increased. Blanks were injected between samples, to check for carry-over, and the analysis time was 60 min. For mass spectrometry (MS) acquisition, a collision-induced dissociation (CID) fragmentation and a nanoESI positive ionization mode were used. PASEF-MS/MS scan mode was established for an acquisition range of 100–1700 m/z [34]. Matches were filtered for 1% false discovery rate (FDR) at the peptide level. MS analyses were performed at the Mass Spectrometry and Proteomics Unit (Area of Infrastructures) of the University of Santiago de Compostela.
MS/MS spectra were processed with PEAKS Studio software (Bioinformatics Solutions, Canada) for protein identification and quantification based on the spectral counting method and the Spec value. The data were compared with a database containing all amino acid sequences available in the NCBI protein database for the phyla Streptophyta (previously Charophyta), Chlorophyta and Cyanobacteriota, in September 2023, selected according to prior taxonomic identification by morphology (see the “SAB formation and lighting set-up” section). The Label-Free module from PEAKS was used for protein quantification in Group Mode.
Proteomic Data Analyses
Proteomic taxonomic results were restricted to the genus level, and proteins identified with < 2 unique peptides were excluded from the study. Only the first identification from each protein group was considered. After applying these quality criteria, the relative contribution of peptides from each genus to the total in each sample was calculated. Those genera contributing < 1% to the total in the samples from all ornamental light tested were eliminated from the study. The relative contribution of each genus in each ornamental light condition tested against their contribution when no light was applied was calculated to evaluate the impact of each ornamental light. A genus was considered to have decreased or increased in abundance when the change in its relative contribution was > 1%. For functional analyses, Unipept 2.0 (https://unipept.ugent.be/) and DAVID (https://david.ncifcrf.gov/) were used. Data were processed with Sigma Plot, and all graphs were generated with GraphPad Prism 10. Data are available via ProteomeXchange with identifier PXD050424.
Results
Identification of SAB Species by Morphological Characters
The taxonomic identification of the sample SABs by morphological characters revealed the presence of 3 different groups: two groups of eukaryotes (Chlorophyta and Streptophyta) and one group of prokaryotes (Cyanobacteriota). The most diverse and abundant group was Chlorophyta with the following composition: Chlamydomonas sp., Chlorella vulgaris Beijerinck, Coelastrella terrestris (Reisigl) Hegewald & N. Hanagata, Ettlia sp., Monoraphidium obtusum (Korshikov) Komárková-Legnerová, Scenedesmus sp., and Tetradesmus obliquus (Turpin) M.J. Wynne. The following species of Chlorophyta were also present, although less common: Raphidocelis sp., Asterarcys sp., Polytomella sp., Tetraselmis sp., Astrephomene sp., Pleodorina sp. and Tetrabaena sp. Only one species of the Streptophyta group was detected: Klebsormidium flaccidum (Kützing) P.C. Silva, Mattox & W.H. Blackwell. Finally, two genera of Cyanobacteriota were detected: Pseudoanabaena sp. and Synechocystis sp.
Effect of Different Types of Ornamental Lighting on the Taxonomic Composition of the SABs
Proteomics analysis of SABs under the four different ornamental lighting conditions yielded lists of the proteins expressed by the different coexisting genera (Tables S1–S4 of Supplementary Material). The numbers of identifications obtained for each sample are summarized in Fig. 3a. Relative to the control SABs (not exposed to light), the most significant decrease in the total number of peptides/identifications corresponded to the cool white light followed by the warm white light, while the amber + green light did not decrease the number.
Fig. 3.
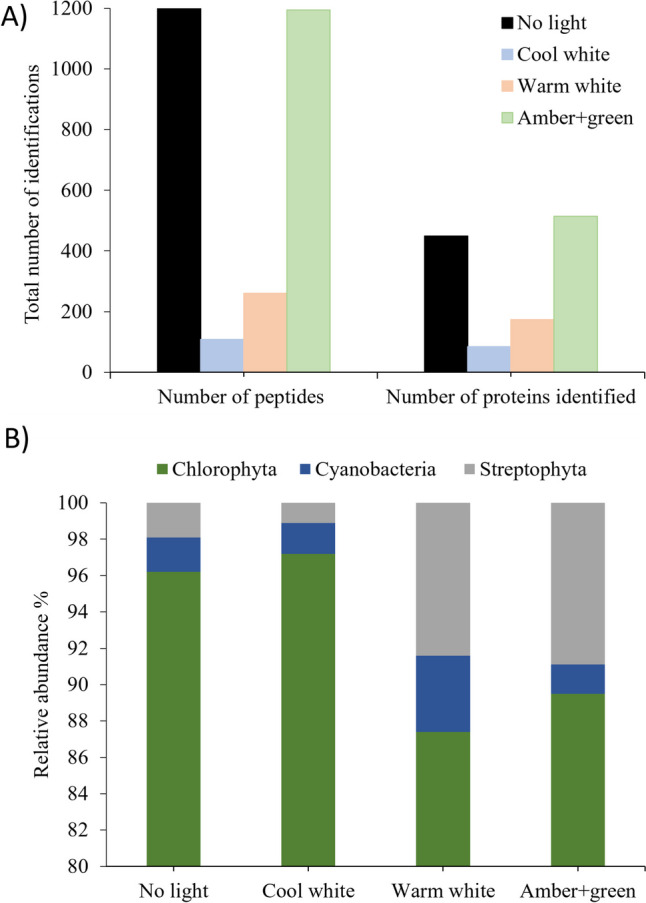
a Total number of identified peptides and proteins under the different light conditions studied. b Relative peptide abundances of the 3 phyla under the different ornamental lights
The proteome of all SABs was strongly dominated by green algae (phylum Chlorophyta) (87.2–96.17%) (Fig. 3b). Cyanobacteria (phylum Cyanobacteriota) were detected in very low abundance and represented by two genera. Specifically, Synechocystis was present in 1.57–2.67% of all samples, while Leptolyngbya was detected in 0.46–1.53% of the samples. Finally, only one genus (Klebsormidium) in the phylum Streptophyta (previously Charophyta) was detected and was found to be present in all samples. The relative abundance of the genus was < 2% in the control (no light) and cool white treatments, but it increased to 8.4% in the warm white and 8.8% in amber + green light treatments. The total number of genera was 53 in the control (no light), 36 in the cool white, 42 in the warm white, and 68 in the amber + green light treatments (Table S1).
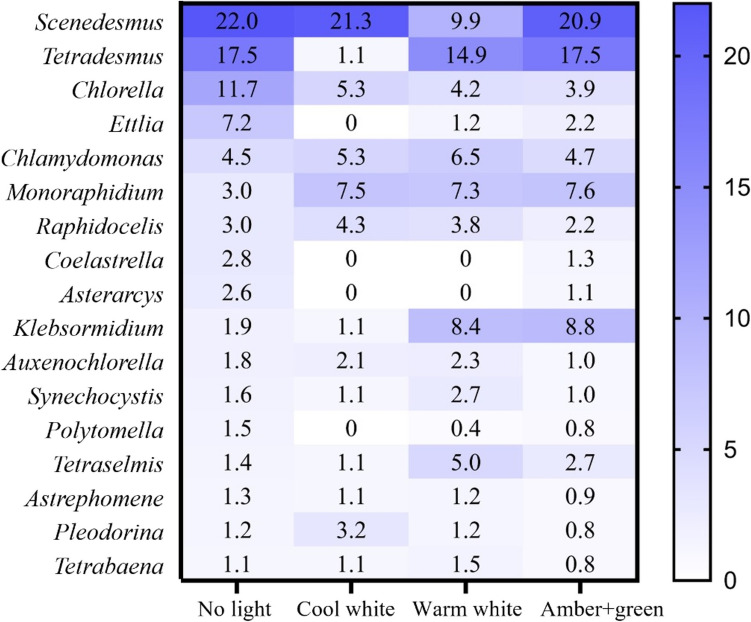
The relative peptide contributions of each genus to the total in each sample are shown in Fig. 4, with the complete data available in Tables S1 to S5 of Supplementary Material. The most abundant genera in the control (no light) treatment were three Chlorophyta genera (Scenedesmus, Tetradesmus and Chlorella) and the sum of these three constitutes 51.1% of the total peptides detected. Nevertheless, the impact of the lights on peptide abundance varies over the different genera detected (Fig. 4). Most of the genera were negatively affected (decreased > 1%) by the cool white light, except Scenedesmus, in which there was no significant difference, and in Chlamydomonas, Monoraphidium, Raphidocelis and Auxenochlorella, which increased in abundance.
Fig. 4.
Relative contribution of each genus to the total number of peptides detected in each sample. Only those taxa with > 1% of relative contribution on the non-light control were evaluated. The effect of the light was considered significant when the relative abundance changed by > 1% in one condition relative to the control (no light) treatment
All genera were negatively affected by the warm white light, except Chlamydomonas, Monoraphidium, Klebsomidium, Synechosysti and Tetraselmis, which increased in abundance. Raphidocellis, Auxenochlorella, Astrephomene, Pleodorina and Tetrabaena were not affected.
Regarding the amber + green light, Tetradesmus was not affected, while Scenedesumus decreased slightly and Chlorella and Ettlia decreased substantially in abundance. By contrast, this type of light positively affected Monoraphidum, Klebsomidium, Tetraselmis and Chlamydomonas (increased in abundance by > 1%).
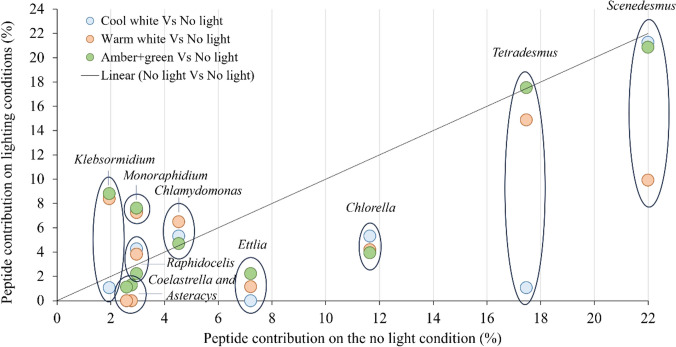
The ornamental lighting treatments had different impacts on the relative contributions to the proteomes of the predominant genera: Scenedesmus, Tetradesmus and Chlorella (Fig. 5). Scenedesmus was strongly affected (< 12.1%) by the warm white light and slightly affected by the amber + green (< 1.9%) and cool white lights (< 0.7%), while Tetradesmus was strongly affected by the cool white light (< 16.4%), slightly affected by the warm white light (< 2.6%) and not affected by the amber + green light. By contrast, Chlorella was affected to a similar extent by all three types of light (6.4–7.8%). All three of these genera belong to the same phylum (Chlorophyta), and Tetradesmus and Scenedesmus both belong to the family Scenedesmaceae, while Chlorella belongs to the family Chlorellaceae. Although Klebsormidium (Streptophyta) had a relatively low abundance of peptides, it was strongly and negatively affected by the cool white light, whereas the warm white and the amber + green lights increased the number of peptides.
Fig. 5.
Effect of the different ornamental lighting conditions on the relative contribution peptides from the 10 most abundant genera present in the SABs
Effect of Different Types of Illumination on the SAB Protein Expression
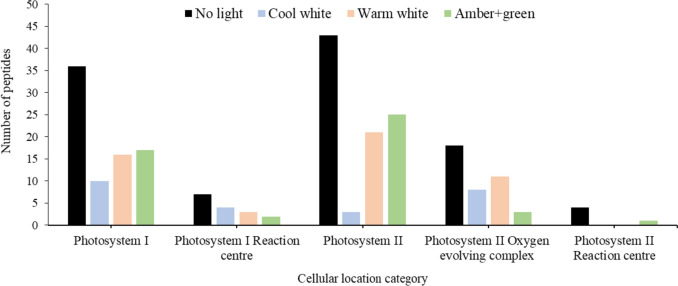
We categorized the proteins identified from the photosynthesis on each SAB by the Gene Ontology classification of cellular compartments (https://www.informatics.jax.org/vocab/gene_ontology/) focusing on the photosystems I and II in an initial attempt to understand the protein expression regarding the effect of the different ornamental lightings on the light-dependant reactions (Fig. 6). In all categories, no light yielded the highest peptides, followed by amber + green, warm white and cool white in the Photosystem I (GO:0009522) and Photosystem II (GO:0009523) categories. However, this pattern is broken in the subcategories of peptides found in the SAB. First, in the Photosystem I Reaction centre (GO:0009538), containing the primary electron donor of the PSI, the combination of amber + green light yielded 3 peptides in comparison with the 4, 5 and 7 of the warm white, cool white and no light, respectively. Under amber + green also was detected the lowest number of peptides for the Photosystem II Oxygen evolving complex (GO:0009654), and a general reduction in peptides was found under all three ornamental lightings for the Photosystem II Reaction centre (GO:0009539), with only 1 peptide under amber + green against the 4 of the no light condition.
Fig. 6.
Number of peptides detected in different cellular compartments found for the two photosystems (according to Gene Ontology categorization) under the different ornamental lights
Protein Expression that Decreased Due to the Ornamental Lighting
The proteins detected were classified according to InterPro active site classification to evaluate the impact of ornamental light on more specific SAB protein expression. The lack of a normal photoperiod (i.e. subjected to nocturnal illumination) generally reduced the number of peptides detected in most categories, relative to the no light treatment, and the effect was greater in the cool white light treatment than in the warm white light treatment (Table S6). The effect of amber + green light depended on the category, although the total number of peptides detected in this case was equal to the no light scenario.
The results showed a reduction in the expression of specific proteins involved in the energy metabolism attributed to the disruption of a normal photoperiod. Among the proteins most affected by the nocturnal illumination, some of those involved in carbon metabolism (including the Calvin cycle) are noteworthy. Glyceraldehyde dehydrogenase (IPR020831) and fructose-bisphosphate aldolase (IPR000741) protein families were only absent in the amber + green light treatment. Enolase (IPR000941) and glycosyl transferase (IPR001296), which are involved in the glycolytic and gluconeogenesis pathways, respectively, were only expressed in the SABs in the control (no light) treatment. The same applies to P-Type ATPases (IPR008250) and FAD/NAD(P)-binding domain superfamilies (IPR036188), indicating a decrease in the transportation flux of protons and other cations across the cellular membrane mediated by ATP hydrolysis [35].
In some cases, the negative effect depended on the light type. The NAD(P)-binding domain superfamily (IPR036291) was most strongly impacted by the cool white light, followed by the warm white light and the amber + green light. Expression of the chlorophyll A-B binding protein (IPR022796) was greatly reduced in the cool white and the amber + green light treatments, while the decrease in the warm white light treatment was not as evident.
The nocturnal illumination caused impaired defence against oxidative stress, as indicated by the significant decrease in peptides associated with manganese/iron superoxide dismutase (IPR001189) across all three ornamental lighting conditions, reflecting a profound impairment in the capacity of the SABs to decompose superoxide radicals.
Protein Expression that Increased Due to the Ornamental Lighting
Some protein increased their expression under ornamental lighting at night, except in the case of the cool white light, which always yielded a lower abundance of peptides as shown in Table S6 of Supplementary Material. This was the case of the ATP formation through phosphoglycerate kinase superfamily (IPR036043) and the oxygen transport, indicated by the increase in globin-like superfamily (IPR009050). The warm white light treatment increased the abundance of phycocyanin beta subunit (IPR006247) and phycobilisomes (IPR038719).
In particular, the amber + green light triggered the expression of specific proteins in the photosynthetic machinery. This effect deserves some attention, as amber + green light potentially has a biostatic effect on biological colonisation. For example, the photosystem antenna protein-like (IPR036001) binds chlorophyll and β-carotene pigments to the two photosystems, assisting in harnessing energy from wavelengths that PSI and PSII are unable to capture [36]. In addition, peptides related to both photosystems (D1/D2 superfamily of the PSII (IPR036854) and the PsA/PsB proteins of the PSI (IPR001280)) and the photosynthetic reaction centre L/M (IPR000484) in photosynthetic bacteria were found to increase under amber + green light. Surprisingly, photosystem II extrinsic protein O (PsbO) (IPR002628), which catalyses the splitting of water to O2 and 4H+, was not detected in the SABs in the amber + green light treatment (Table S6).
Protein metabolism, including synthesis, assembly and degradation, was also enhanced by the amber + green treatment. Thus, expression of the translation elongation factor EFTu-like, domain 2 (IPR004161) and translation elongation factor EFTu/EF1A, C-terminal (IPR004160), which are involved in protein synthesis, was increased. This was also true for the chaperonin Cpn60/GroEL/TCP-1 family (IPR002423), required for the correct folding of many proteins and for the ClpA/B family (IPR001270), involved in proteolysis. Additionally, Clp ATPase, ClpA/B and ClpB were absent in the SABs in the control (no light) treatment, but 12 peptides were detected in the SABs in the amber + green light treatment for all aforementioned Clp proteins (Table S6).
Histones H2A/H2B/H3 (IPR007125), which provide structural support for chromosomes and are involved in regulating gene expression, were also more abundant in the amber + green light treatment than in the other treatments. The same was observed for the helicase superfamily 1/2 (IPR014001), which catalyses the separation of double-stranded nucleic acids, and nucleotide excision repair and DNA damage recognition occurred during protein synthesis induced by the action of UVR domain proteins (IPR001943).
The P-loop NTPase (IPR027417) catalyses the hydrolysis of bound nucleoside triphosphate (NTP). Adenosine triphosphate AAA-Type ATPases (IPR003593), which catalyses the degradation of ATP in a variety of cellular processes, was the most abundant of that family. The F1/V1/A1 complex of the ATPase (IPR036121), which includes the catalytic core that synthesizes and hydrolyses ATP [37], was over expressed under the amber + green light.
Finally, tubulin, tryptophan synthase and the heat shock proteins Hsp70 (IPR013126) and Hsp90 (IPR001404) families also increased after exposure to nocturnal amber + green light (Table S6). These families are fundamental to proper protein folding and also play an important role in the management of thermal and oxidative stress.
Effect of Different Types of Illumination on the Protein Expression of Different Genera of Algae
As shown in Fig. 5 and discussed in the “Identification of SAB Species by Morphological Characters” section, each lighting condition altered the taxonomic composition of the biofilm. The use of gene ontology (GO) categories, included in Table S8 of Supplementary Material, enabled observation of the different metabolic responses of the most abundant and relevant genera.
The main proteins expressed in Scenedesmus under the control (no light) treatment are related to glycolytic processes and other carbohydrate metabolisms, proton export across the plasma membrane and PSII assembly (all with 2 peptides). Some of these proteins were promoted by warm white light (1 peptide detected for glycolytic process and 1 for PSII assembly) and cool white light (only 1 peptide for glycolytic process). However, the amber + green light treatment did not promote these protein expressions, which stimulated protein metabolism expression (biosynthesis of amino acids, translation, and protein refolding, all with 1 peptide).
For Tetradesmus, translation was the process for which more peptides were detected under no light (5 peptides), followed by glutamine biosynthesis and photosynthesis (2 peptides each). A similar response was yielded by the cool white treatment, with the only 1 peptide detected falling in the same categories. Warm white stimulated glycolysis and PSII (1 peptide each), while amber + green light enhanced protein metabolism (as in Scenedesmus) and glutamine biosynthesis (2 peptides). The last of the three main Chlorophyta species, Chlorella, was most negatively affected by warm white light, as no peptides were detected for any major category. No light and cool white light promoted the production of peptides related to photosynthesis (5 and 1 peptides, respectively), whereas amber + green light promoted carbohydrate metabolism (2 peptides).
The increase in peptide detection under warm white light and amber + green light that Klebsormidium suffered relative to the no light treatment is worth noting. Warm white light promoted the production pf peptides related to photosynthesis, photosynthetic electron transport and response to light stimulus (all with 2 peptides each) and amber + green triggered ATP synthesis (3 peptides), photosynthetic electron transport (1 peptide), photorespiration (1 peptide) and the Calvin cycle (1 peptide).
Discussion
In the SAB samples, the predominant taxa identified by morphological taxonomy were Chlorophyta (green algae), accounting for 81% of all taxa, and Cyanobacteriota and Streptophyta, accounting for about 12% and 6%, respectively, of the total. Although the SABs were grown in the laboratory, the planktonic stock culture of the inoculum was generated from phototrophic biofilms growing naturally on granitic facades of historic buildings in Santiago de Compostela (NW Spain), where algae were already relatively much more abundant than cyanobacteria. The predominance of algal biofilms is typical of Atlantic European regions with high rainfall and mild temperatures [38], while occasional rainfall and high temperatures, typical of e.g. Latin America, favour the dominance of cyanobacteria [39].
The most common algal genera identified by proteomics analysis were Scenedesmus, Tetradesmus and to a lesser extent Chlorella, which is consistent with the initial morphological identification. Scenedesmus is one of the most common freshwater algae genera but also commonly occurs as an epilithic alga on rocky substrates and building facades and monuments [40, 41]. According to the review by Macedo et al. [41], species of Scenedesmus have been found colonising stone monuments, like Scenedesmus ecornis on marble and limestone substratum. In addition, Ortega-Calvo et al. [42] isolated Scenedesmus quadricauda from the walls made from calcarenite (from the Puerto de Santa Maria quarry, Spain) in the Cathedral of Seville (SW Spain). Tetradesmus was previously considered a subgenus of Scenedesmus [43], and several species of Tetradesmus were previously classified as Scenedesmus species [44], which may explain the lack of specific scientific literature on the presence of Tetradesmus as a colonising taxa of cultural heritage structures and the complexity of the discussion regarding the presence of those genera. For example, Rifón-Lastra and Noguerol-Seoane [45] found Scenedesmus acutus, which is now regarded as a synonym of Tetradesmus obliquus [46], on the granite walls of two monuments in Lugo (Galicia, NW Spain): the Torre del Homenaje in Monforte de Lemos and the Church of Santa María in Meira. The genus Chlorella is, along with Apatococcus, Klebsormidium and Trentepohlia, the most frequent algal coloniser in the terrestrial environment [47] and is commonly found on monuments located in Europe, America, and Asia [41, 48]. However, no correlation has been established between these three algal genera and any particular substratum or climate.
When assessing the effects of the lights on the populations of the different phyla, the greatest changes in terms of relative contributions were detected after the warm white and amber + green light treatments. The spectra and colour temperatures of these types of light are furthest from the spectrum of normal sunlight, while cool white light is the most similar. The SAB communities analysed in the present study underwent an increase in cyanobacterial presence under the warm white light treatment (which has a higher proportion of red long wavelengths relative to blue short wavelengths). These findings are consistent with those obtained in benthic mesocosm experiments, in which algal communities shifted to dominance of cyanobacteria with an extended light period including long (red) wavelengths [49]. In addition, in a study of cave lampenflora, cyanobacteria were found to be more abundant in areas illuminated by warm light than by cold light, in contrast to chlorophytes, which were more abundant in areas lit by cool white light [50]. In fact, cyanobacterium absorbs blue light much less efficiently than green algae [51], thus increasing its presence with the reduction of the blue part of the spectrum of the warm white light. A relative increase in Klebsormidium occurred in response to illumination by warm white and amber + green lights, but to the best of our knowledge, the relative increase in the Klebsormidium genus under warm white and amber + green light has not been explained. The only reference found in relation to this genus and sensitivity to light spectra is the detection of a specific channel-rhodopsine photoreceptor in Klebsormidium nitens, which displays a maximum spectrum of action at 450 nm [52]; however, the relationship between this photoreceptor and its impact in photo-stress to changes in abundance due to nocturnal light remains unknown.
The absence of a normal photoperiod due to ornamental illumination produced substantial changes in the biofilm proteome composition depending on the light quality received. The number of peptides and proteins detected in both white light treatments was significantly lower than in the no light and the amber + green light treatments, limiting comparisons with other treatments. The wet weight of SABs used in the study (53.42 ± 10.77 to 65.90 ± 10.46 mg) and the protein concentration determined in the samples after the extraction (1.78–2.07 µg protein. µL−1) were similar and thus, the low count can possibly be attributed to protein degradation due to the damaging effects of the blue wavelengths of the white lights over the proteins. We tentatively attribute this decrease to a damaging effect of specific wavelengths (blue) on the protein structures. In a similar manner as UV light deteriorates protein structures [53], this may have been the case with the lights being tested. This damaging effect is mainly expected to occur in cells located on the surface of the SABs, with those in underlying layers being protected by the EPS and therefore being less damaged. The predicted alteration of protein structures may have prevented tryptic digestion or correct ionization of the peptides in the MS device. Such changes could explain the overall growth of the biofilms even when exposed to the lights and, at the same time, the lower number of peptides detected in the cool white total proteome sample and, to a lesser extent, in the warm white sample. Blue light exposure has been shown to increase target protein degradation due to activation of a photosensitive degradation-inducing sequence (degron) [54], which undergoes conformational changes making the degron moiety accessible for proteasomal recognition followed by degradation [55], and which is found in algae [56]. More blue light is emitted by the cool white light than by the warm white light and is absent from amber + green light, and this distribution is inversely proportional to the number of peptides detected in the algal species conforming the SABs, which could be part of the reason of the differences between the tested lights.
Nevertheless, both types of white light were compared with the amber + green light to assess the underlying mechanisms that could explain its biostatic effect. The results indicate that night-time lighting affects the proteome of organisms by altering the structure of the proteins; however, no specific signal was detected that might indicate that organisms were undergoing a stress process (considering the possible masking due to detection of small numbers of peptides). By contrast, although amber + green light produced a similar number of proteins to the control (no light) treatment, the data reflect a greater change in the distribution of proteins, with overexpression of proteins related to the photosynthetic machinery and stress management, as well as a reduction of certain proteins also found under the other lights studied.
InterPRO uses predictive models based on different databases of protein signatures to provide functional analysis of proteins by categorising them into families. This approach enables examination of proteins that are over- or underexpressed due to nocturnal illumination, starting with the identification of light stress indicators/signs. Superoxide dismutase (SOD), which catalyses the disproportionation of O2•- to O2 and H2O2 under ROS stress, is known to vary significantly under different light regimes, exhibiting circadian oscillations with wavelength, intensity and photoperiod [57]. Asano [58] showed that manganese-SOD isolated from the microalga Gonyaulax polyedra (currently known as Lingulaulax polyedra [59]) follows a circadian rhythm, being more active during the day. However, the results of the present study are not consistent with this statement or with those of the studies carried out by Chen et al. [60] with four Chlorella species, in which SOD activity was higher in the longer light period. Here, we observed a reduction in Mn/Fe-SOD in response to all three illumination treatments, relative to the control (no light). From the data obtained, it is not possible to distinguish whether the reduction was due to a fluctuation in SOD during different photoperiods or whether light stress due to night illumination affected the capacity of the organisms to overcome oxidative stress.
A stress response, however, was detected via overexpression of Clp and UV responsive (UVR) proteins, particularly under amber + green light, with the ClpA/B family and UVR being significantly overexpressed relative to the levels in the other treatments. On the one hand, UVR proteins are involved in excision repair and DNA damage recognition [61]. On the other hand, ClpA (among other types of Clps) forms associations with ClpP, resulting in a Clp proteolytic complex that specifically targets damaged or misfolded proteins for translocation and degradation [62]. Nevertheless, ClpP was not overexpressed under any nocturnal light conditions tested, which was essential for chloroplast function, as its deletion prevents phototrophic growth [63]. Regardless of whether their expression is stress response-dependent or not, ATPases associated with diverse cell activities (AAA +), of which Clp A/B form a part, are related to the maintenance of proteostasis in algae and cyanobacteria, playing a role in cellular proteolytic systems [64]. To manage the oxidative stress, organisms also produce heat shock proteins (HSPs), which play important roles in cell repair and exert protective mechanisms. The results of this study indicate that the sublethal oxidative stress produced by amber + green light is sufficient to induce expression of Hsps. Moreover, the greater increase in globin-like proteins in the three ornamental lighting treatments (especially warm white and amber + green light) may also indicate some degree of oxidative stress, as globins in green algae (and a cyanoglobin in cyanobacteria) have been linked to regulation of photosynthesis and/or mitigation of oxidative damage [65].
In organisms subjected to nocturnal illumination, stress emerges in the form of photo-oxidative damage due to an imbalance between the light energy received and that which can be utilized in photosynthesis at a time when darkness is needed. The presence of antenna pigments in light-harvesting complexes (LHCs) superfamily generally has the dual function of capturing and transferring energy for photosynthesis and dissipating the excess light that cannot be used [66] via non-photochemical quenching (NPQ). This process prevents oxidative stress, which in the case of amber + green light is due to low absorption by algal pigments (chlorophylls and carotenoids). Expression of LHC superfamily proteins and photosystem antenna protein-like is altered by ornamental illumination, indicating regulation of the night-time light to which the organisms are subjected. Chlorophyll a-b binding protein was downregulated and its abundance decreased under all of the ornamental lights tested, which could signal stress in the SABs due to the interruption of the normal photoperiod. Under changing light conditions, this protein balances the excitation energy between the two photosystems [67], which regulates light harvesting and is also involved in various types of abiotic stress. This has been demonstrated in studies of Triticum aestivum, in which expression of the gene coding for the light-harvesting chlorophyll a/b binding protein (TaLhc2) is downregulated by multiple types of stress [68], and of the microalgae Tisochrysis lutea, in which the gene lhcx2 that encodes for a protein that binds chlorophyll a and c was only expressed at night [69].
Cellular component analysis suggested changes in the PSII and PSI dependent on the type of nocturnal light that SAB organisms are exposed to. The decrease in the peptides of the PSII protein complex under amber + green light, and specifically the absence of the PsbO subunit, are particularly noteworthy. Ohnishi et al. [70] suggested that blue and green light can inactivate the water-oxidizing complex to a lesser extent than UV light, followed by red light-driven inactivation of the PSII reaction centre. The combination of amber + green LED light decreased the water-evolving complex by under-expressing proteins of the PsbO and PsbQ subunits of the PSII, but the data do not show any signs of inactivation of the PSII reaction centre, as the analyses performed only determine the number of peptides detected.
The D1 protein of the PSII is generally the primary target of photodamaging stress [71], in the form of ROS that the NPQ alternative electron pathways must manage to prevent excess damage [72, 73] and a consequent reduction in photosynthetic efficiency. The findings reported by Yokthongwattana et al. [74] strongly correlate the amount of photodamaged D1 and the HSP70B protein pool size in the alga Dunaliella salina, confirming the involvement of HSP70B in the PSII repair process. In the present study, the biofilm response to amber + green illumination is consistent with the response of D. salina, indicating the potential of the amber + green light to cause damage in phototrophic colonisation of monuments due to the increment in Hsp70 protein family. The increment in peptides D1/D2 proteins found under the exposure to warm white light and amber + green light could be attributed to the “D1 repair cycle”, as this subunit is constantly replaced when exposed to light stress. This replacement requires the assistance of several auxiliary proteins, e.g. translation factors, for accurate translation during protein synthesis, or of chaperonins for correct folding of the newly formed D1 protein. In the present study, those two scenarios were clearly affected by amber + green light, as this type of light promotes the synthesis of these proteins as a repair response to the photodamage caused by the light. Light-inducible expression of the translation factor EF-Tu is a possible mechanism regulating the repair of PSII in plants and other photosynthetic organisms [75]. In fact, overexpression of EF-Tu in the cyanobacteria Synechocystis sp. has been shown to enhance protein synthesis and PSII repair under strong light exposure [76].
Phycocyanin beta subunit and phycobilisomes increased in abundance in the warm white light treatment, consistent with the increase in the proportion of cyanobacteria. The abundance of this photosystem antenna protein-like also increased under amber + green light, although the proportion of cyanobacterial species did not increase accordingly. This could be attributed to a mechanism of photo-oxidative stress avoidance similar to the increase in antenna pigments of green algae, as bulk accumulation of secondary carotenoids in microalgae are mostly induced by oxidative stress of cells [77]. In fact, the cyanobacterium Spirulina was found to produce more phycocyanin when cultivated under yellow and red light than under white, blue or green light [78].
The signs of photo-oxidative stress and the consequent alteration in the photosynthetic proteome indicate the survival strategies of the organisms, and there are no clear signs that the energy is used to obtain energy. The protein enolase (2-phospho-D-glycerate dehydroxylase), which catalyses the conversion of 2-phosphoglycerate (2-PG) to phosphoenolpyruvate (PEP) in glycolysis, decreased under all light conditions relative to the control (no light). Other stressors, e.g. hyperosmotic salt stress, have been found to decrease enolase production, while heat-shock treatment induces the production of the enzyme in the green alga Dunaliella salina [79]. In another study, the production of enzymes related to glycolysis in Haematococcus pluvialis (currently known as Haematococcus lacustris [80]) under oxidative stress was either upregulated or downregulated depending on the enzyme [81]. In the present study, the phosphoglycerate kinase (PGK) superfamily, which catalyses the formation of ATP to ADP and vice versa and is involved in glycolysis, was particularly strongly induced under warm white and amber + green light, unlike enolase. Liao et al. [82] showed that exposing the red alga Pyropia haitanensis to high light stress resulted in upregulation of one of the two isoforms of the PGK gene, which further strengthens the hypothesis that night-time illumination stresses the organisms that form SABs. This different response seems to indicate that expression of glycolysis proteins is species- and stress-dependent and that nocturnal light stress seems to specifically downregulate enolase production in algal SABs.
Considering the GO categories by genus and the wider biological processes involving these peptides, the ornamental lights affected each genus differently. Protein metabolism was promoted under amber + green light in the case of the two most abundant genera (Scenedesmus and Tetradesmus), whereas under the other types of light, the most important categories involved photosynthesis and the glycolytic process. In an experiment with Scenedesmus obliquus (currently known as Tetradesmus obliquus [83]), light stress due to both high and low light intensities resulted in less protein and carotenoid than optimal illumination [84]. Moreover, other forms of stress are found to modify the proteome profile of Tetradesmus. For example, thermal stress due to growth at 34 °C caused downregulation of photosynthesis light-harvesting and an increase in the biosynthesis of polyamine (derived from aminoacid metabolism), accompanied by HSP production [85], or stress due to lack of nitrogen in media that caused downregulation of proteins related to photosynthesis and the photosynthetic machinery in Scenedesmus acuminatus [86] (currently known as Tetradesmus lagerheimii [87]).
The findings for the GO categories and Chlorella presented here also support the reduction in the importance of the photosynthetic protein category relative to no light and cold white light, which are consistent with the findings reported by Cecchin et al. [88]. Chlorella downregulated the photosynthetic apparatus (both the PSI and PSII) after acclimatation to high light stress, but no changes in carbon fixation were observed. Interestingly, the peptides for Chlorella identified under amber + green light fall in the GO categories for glucose and carbohydrate metabolic processes.
Conclusions
For the first time and to the best of our knowledge, proteomic changes triggered on the SABs that colonize illuminated monuments in urban areas have been reported. The taxonomic composition of the SABs examined in this study is representative of the biofilms that affect granitic monuments in NW Spain, and the proteomics analysis clearly showed that nocturnal illumination affected the proteomes of those organisms.
Impairment of the response to oxidative stress, increased production of photosystem antenna protein-like and increased protein metabolism were generally detected and indicative of an overall stress on the cells transferring their energies to different metabolic processes required to survive luminic stress. These responses were much more pronounced for amber + green light than for the other two types of ornamental lighting (cool white and warm white). Despite the lower number of protein identifications in these two treatments, we could identify a stress response derived from deregulation of the energy metabolism, PGK and Glbs. In addition, warm white light promoted the growth of cyanobacteria.
Exposure of SABs to amber + green lighting at night induces oxidative stress due to the low energy use efficiency, leading to a stress response (via HSPs and Clps, among others) and an increase in LHC superfamily to dissipate excess energy via NPQ. In turn, overexpression of LHC superfamily proteins forces organisms to maintain proteostasis through constant protein synthesis, repair and degradation, in a classic response to photooxidative stress. Energy was not effectively used for photosynthesis due to the lack of stimulation of the Calvin cycle (despite the clear stimulation of the LHCs superfamily), the lack of detection of the PsbO subunit (oxygen-evolving complex) of the PSII and the lack of overexpression of ClpP.
The study findings are of interest for designing ornamental lighting in stone heritage affected by biological colonisation and further reinforce the biostatic effect of the combinatory use of amber and green monochromatic light within preventative conservation strategies, thus reducing cleaning operations, while providing an in-depth explanation of the underlying biochemical mechanisms. Further studies with pure cultures (of Scenedesmus, Tetradesmus and Klebsormidium, most affected in the present study, and cyanobacteria) and mutant strains would be interesting because evaluation of strains and communities with different taxonomic compositions is necessary in order to establish patterns, as the response to the different types of lights differed among the taxa comprising the SABs. It should also be noted that this was a laboratory approach, so the conclusions drawn should be correlated with future experiments on architectural heritage illuminated with amber + green light.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This study was developed within the framework of the CROMALUX project: Third SMARTIAGO Challenge – Smart lighting system for Heritage Conservation. The authors thank Rafael Carballeira for help with morphological identification of the taxa comprising the biofilms. A. Méndez acknowledges receipt of a grant in the Programa de Doutoramento Industrial (04_IN606D_2021_2598528) financed by the Xunta de Galicia. P. Sanmartín acknowledges receipt of a Ramón y Cajal contract (RYC2020-029987-I) financed by the Spanish State Research Agency (AEI) of the Ministry of Science and Innovation (MICIN). The authors are also grateful to the Xunta de Galicia for concession of the FONTES project (ED431F 2022/14) and the Competitive Reference Group (GRC) grants ED431C 2022/09 (Gemap) and ED431C-2021/37 (Biogroup).
Author Contribution
Conceptualization, A.M., P.S., S.B., A.T.-S. Data curation, A.M., S.B., A.T.-S. Formal analysis, A.M., A.T.-S. Investigation, A.M., A.T.-S. Writing—original draft, A.M., P.S., A.T.-S. Writing—review and editing, A.M., P.S., S.B., A.T.-S. Funding acquisition, P.S. Project administration, P.S. Supervision. P.S., A.T.S. All authors have read and approved the final manuscript.
Data Availability
Data are available via ProteomeXchange with identifier PXD050424. During the revision process, the data can be accessed using the following username: reviewer_pxd050424@ebi.ac.uk and password: XSSxVmrD.
Declarations
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Marchant P, Hale JD, Sadler JP (2020) Does changing to brighter road lighting improve road safety? Multilevel longitudinal analysis of road traffic collision frequency during the relighting of a UK city. J Epidemiol Community Health 74(5):467–472. 10.1136/jech-2019-212208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tural M, Yener C (2006) Lighting monuments: reflections on outdoor lighting and environmental appraisal. Build Environ 41:775–782. 10.1016/j.buildenv.2005.03.014 [Google Scholar]
- 3.Deme L (2013) Budapest illuminated. Hung Rev 4(2):112–127 [Google Scholar]
- 4.Carleo D, Gargiulo M, Scorpio M, Ciampi G, Corniello L, Spanodimitriou Y, Sibilio S, Chìas P, (2021) Lighting solutions to improve the valorisation and fruition of the Parque del Retiro in Madrid. IOP Conf Ser: Mater Sci Eng 1203:022083. https://iopscience.iop.org/article/10.1088/1757-899X/1203/2/022083 Accessed 23 Oct 2024
- 5.Méndez A, Prieto B, Aguirre i Font JM, Sanmartín P (2024) Better, not more, lighting: policies in urban areas towards environmentally-sound illumination of historical stone buildings that also halts biological colonisation. Sci Total Environ 906:167560. 10.1016/j.scitotenv.2023.167560 [DOI] [PubMed] [Google Scholar]
- 6.Kyba CCM, Altıntaş YO, Walker CE, Newhouse M (2023) Citizen scientists report global rapid reductions in the visibility of stars from 2011 to 2022. Science 379:265–268. https://www.science.org/doi/10.1126/science.abq7781 Accessed 23 Oct 2024 [DOI] [PubMed]
- 7.Hölker F, Bolliger J, Davies T, Giavi S, Jechow A, Kalinkat G, Longcore T, Spoelstra K, Tidau S, Visser M, Knop E (2021) 11 pressing research questions on how light pollution affects biodiversity. Front Ecol Evol 9:767177. 10.3389/fevo.2021.767177 [Google Scholar]
- 8.Salata F, Golasi I, Falanga G, Allegri M, de Lieto VE, Nardecchia F, Pagliaro F, Gugliermetti F, de Lieto VA (2015) Maintenance and energy optimization of lighting systems for the improvement of historic buildings: a case study. Sustainability 7(8):10770–10788. 10.3390/su70810770 [Google Scholar]
- 9.Bao Y, Ma Y, Liu W, Li X, Li Y, Zhou P, Feng Y, Delgado-Baquerizo M (2023) Innovative strategy for the conservation of a millennial mausoleum from biodeterioration through artificial light management. NPJ Biofilms Microbiomes 9:69. 10.1038/s41522-023-00438-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albertano P, Bisconti F, Gallon JR, Giuliani R, Graziottin F, Groth I, Mattila-Sandholm T, Moscone D, Palleschi G, Hermosín Campos G, Hernández-Mariné M, Saarela M, Saiz-Jimenez C, Sánchez-Moral S, Shroeckh V, Urzì C (2003) Cyanobacteria attack rocks (CATS): control and preventive strategies to avoid damage caused by cyanobacteria and associated microorganisms in Roman hypogean monuments. In: Saiz-Jimenez C (ed) Molecular biology and cultural heritage, Swets & Zeitlinger BV, Lisse, pp 151–162
- 11.Bruno L, Belleza S, Urzì C, De Leo F (2014) A study for monitoring and conservation in the Roman Catacombs of St. Callistus and Domitilla, Rome (Italy). In: Saiz-Jimenez C (ed) The conservation of subterranean cultural heritage, CRC Press/Bakelma/Taylor & Francis Group, Leiden, pp 37–44
- 12.Sanmartín P (2021) New perspectives against biodeterioration through public lighting. In: Joseph E (ed) Microorganisms in the deterioration and preservation of cultural heritage. Springer International Publishing, New York, pp 155–171 [Google Scholar]
- 13.Roldán M, Oliva F, Gónzales del Valle MA, Saiz-Jimenez C, Hernández-Mariné M (2006) Does green light influence the fluorescence properties and structure of phototrophic biofilms? Appl Environ Microbiol 72:3026–3303. 10.1128/AEM.72.4.3026-3031.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Rosal Y, Muñoz-Fernández J, Celis-Plá PSM, Hernández-Mariné M, Álvarez-Gómez F, Merino S, Figueroa FL (2022) Monitoring photosynthetic activity using in vivo chlorophylla fluorescence in microalgae and cyanobacteria biofilms in the Nerja Cave (Malaga, Spain). Int J Speleol 51(1):29–42. 10.5038/1827-806X.51.1.2404 [Google Scholar]
- 15.Sanmartín P, Méndez A, Carballeira R, López E (2021) New insights into the growth and diversity of subaerial biofilms colonizing granite-built heritage exposed to UV-A or UV-B radiation plus red LED light. Int Biodeterior Biodegradation 161:105225. 10.1016/j.ibiod.2021.105225 [Google Scholar]
- 16.de Mooij T, de Vries G, Latsos C, Wijffels RH, Janssen M (2016) Impact of light colour on photobioreactor productivity. Algal Res 15:32–42. 10.1016/j.algal.2016.01.015 [Google Scholar]
- 17.Liu XB, Koestler RJ, Warscheid T, Katayama Y, Gu JD (2020) Microbial deterioration and sustainable conservation of stone monuments and buildings. Nat Sustain 3:991–1004. 10.1038/s41893-020-00602-5 [Google Scholar]
- 18.Muñoz-Fernández J, Del Rosal Y, Álvarez-Gómez F, Hernández-Mariné M, Guzmán-Sepúlveda R, Korbee N, Figueroa FL (2021) Selection of LED lighting systems for the reduction of the biodeterioration of speleothems induced by photosynthetic biofilms in the Nerja Cave (Malaga, Spain). J Photochem Photobiol B 217:112155. 10.1016/j.jphotobiol.2021.112155 [DOI] [PubMed] [Google Scholar]
- 19.Méndez A, Martín L, Arines J, Carballeira R, Sanmartín P (2022) Attraction of insects to ornamental lighting used on cultural heritage buildings: a case study in an urban area. Insects 13(12):1153. 10.3390/insects13121153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Méndez A, Maisto F, Pavlović J, Rusková M, Pangallo D, Sanmartín P (2024) Microbiome shifts elicited by ornamental lighting of granite facades identified by MinION sequencing. J Photochem Photobiol B Biol 261:113065. 10.1016/j.jphotobiol.2024.113065 [DOI] [PubMed]
- 21.Guo H, Wang L, Deng Y, Ye J (2021) Novel perspectives of environmental proteomics. Sci Total Environ 788:147588. 10.1016/j.scitotenv.2021.147588 [DOI] [PubMed] [Google Scholar]
- 22.Gorbushina AA (2007) Life on the rocks. Environ Microbiol 9:1613–1631. 10.1111/j.1462-2920.2007.01301.x [DOI] [PubMed] [Google Scholar]
- 23.Villa F, Ludwig N, Mazzini S, Scaglioni L, Fuchs AL, Tripet B, Copié V, Stewart PS, Cappitelli F (2023) A desiccated dual-species subaerial biofilm reprograms its metabolism and affects water dynamics in limestone. Sci Total Environ 868:161666. 10.1016/j.scitotenv.2023.161666 [DOI] [PubMed] [Google Scholar]
- 24.Anderl JN, Franklin MJ, Stewart PS (2000) Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob Agents Chemother 44:1818–1824. 10.1128/aac.44.7.1818-1824.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanmartín P, Villa F, Silva B, Cappitelli F, Prieto B (2011) Colour measurements as a reliable method for estimating chlorophyll degradation to phaeopigments. Biodegradation 22:763–771. 10.1007/s10532-010-9402-8 [DOI] [PubMed] [Google Scholar]
- 26.Ettl H, Gärtner G (1995) Syllabus der Boden-, Luft-und Flechtenalgen. Urban and Fischer, New York
- 27.Khaybullina LS, Gaysina LA, Johansen JR, Krautov AM (2010) Examination of the terrestrial algae of the Great Smoky Mountains National Park, USA. Fottea 10:201–215. 10.5507/fot.2010.011 [Google Scholar]
- 28.Fučíková K, Flechtner VR, Lewis LA (2012) Revision of the genus Bracteacoccustereg (Chlorophyceae, Chlorophyta) based on a phylogenetic approach. Nova Hedwigia 96:15–59. 10.1127/0029-5035/2012/0067 [Google Scholar]
- 29.Komárek J (2013) Süsswasserflora von Mitteleuropa. Cyanoprokaryota: 3rd part: Heterocystous genera. Heidelberg, Springer Spektrum, Germany
- 30.Bischoff HW, Bold HC (1963) Phycological studies IV. Some soil algae from enchanted rock and related algal species. Univ Texas Publ 6318:195 [Google Scholar]
- 31.Ministry of Industry, Trade and Tourism and the Ministry for Ecological Transition and the Demographic Challenge (2021) Proyecto de Real Decreto por el que se aprueba el reglamento de eficiencia energética en instalaciones de alumbrado exterior y sus instrucciones técnicas complementarias EA-01 a EA-08. Agencia Estatal Boletín Oficial del Estado. https://www.boe.es/buscar/doc.php?id=BOE-A-2008-18634. Accessed 25 June 2024
- 32.Guzmán-Fierro V, Dieguez-Seoane A, Roeckel M, Lema JM, Trueba-Santiso T (2024) Environmental proteomics as a useful methodology for early-stage detection of stress in anammox engineered systems. Sci Total Environ 912:169349. 10.1016/j.scitotenv.2023.169349 [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Fonslow BR, Shan B, Baek MC, Yates JR (2013) Protein analysis by shotgun/bottom-up proteomics. Chem Rev 113:2343–2394. 10.1021/CR3003533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quiton-Tapia S, Trueba-Santiso A, Garrido JM, Suárez S, Omil F (2023) Metalloenzymes play major roles to achieve high-rate nitrogen removal in N-damo communities: lessons from metaproteomics. Bioresour Technol 129476:0960–8524. 10.1016/j.biortech.2023.129476 [DOI] [PubMed] [Google Scholar]
- 35.Axelsen K, Palmgren M (1998) Evolution of substrate specificitiest in the P-type ATPase superfamily. J Mol Evol 46:84–101. 10.1007/PL00006286 [DOI] [PubMed] [Google Scholar]
- 36.Barber J (2002) Photosystem II: a multisubunit membrane protein that oxidises water. Curr Opin Struct Biol 12(4):523–530. 10.1016/S0959-440X(02)00357-3 [DOI] [PubMed] [Google Scholar]
- 37.Wilkens S, Zhang Z, Zheng Y (2005) A structural model of the vacuolar ATPase from transmission electron microscopy. Micron 36(2):109–126. 10.1016/j.micron.2004.10.002 [DOI] [PubMed] [Google Scholar]
- 38.Barberousse H, Tell G, Yéprémian C, Couté A (2006) Diversity of algae and cyanobacteria growing on buildings facades in France. Algol Stud 120:81–105. 10.1127/1864-1318/2006/0120-0081 [Google Scholar]
- 39.Gaylarde CC, Gaylarde PM (2005) A comparative study of the major microbial biomass of biofilms on exteriors of buildings in Europe and Latin America. Int Biodeterior Biodegrad 55:31–139. 10.1016/j.ibiod.2004.10.001 [Google Scholar]
- 40.Samad L, Adhikary S (2008) Diversity of micro-algae and cyanobacteria on building facades and monuments in India. Algae 23(2):91–114. 10.4490/ALGAE.2008.23.2.091 [Google Scholar]
- 41.Macedo MF, Miller AZ, Dionísio A, Saiz-Jimenez C (2009) Biodiversity of cyanobacteria and green algae on monuments in the Mediterranean Basin: an overview. Microbiology 155:3476–3490. 10.1099/mic.0.032508-0 [DOI] [PubMed] [Google Scholar]
- 42.Ortega-Calvo JJ, Hernandez-Marine M, Saiz-Jimenez C (1991) Biodeterioration of building materials by cyanobacteria and algae. Int Biodeterior Biodegradation 28(1–4):165–185. 10.1016/0265-3036(91)90041-O [Google Scholar]
- 43.Cho HS, Lee J (2024) Taxonomic reinvestigation of the genus Tetradesmus (Scenedesmaceae; Sphaeropleales) based on morphological characteristics and chloroplast genomes. Front Plant Sci 15:1303175. 10.3389/fpls.2024.1303175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turiel S, Garrido-Cardenas JA, Gómez-Serrano C, Acién FG, Carretero-Paulet L, Blanco S (2021) A polyphasic characterisation of Tetradesmusalmeriensis sp. nov. (Chlorophyta: Scenedesmaceae). Processes 9:2006. 10.3390/pr9112006 [Google Scholar]
- 45.Rifón-Lastra A, Noguerol-Seoane A (2001) Green algae associated with granite walls of monuments in Galicia (NW Spain). Cryptogam Algol 22:305–326. 10.1016/S0181-1568(01)01069-8 [Google Scholar]
- 46.Guiry MD, Guiry, GM (2024). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway - Scenedesmus acutus Meyen 1829. https://www.algaebase.org/search/species/detail/?species_id=27860. Accessed 23 Oct 2024
- 47.Nowicka-Krawczyk P, Komar M, Gutarowska B (2022) Towards understanding the link between the deterioration of building materials and the nature of aerophytic green algae. Sci Total Environ 802:149856. 10.1016/j.scitotenv.2021.149856 [DOI] [PubMed] [Google Scholar]
- 48.Ortega-Calvo JJ, Ariño X, Hernandez-Marine M, Saiz-Jimenez C (1995) Factors affecting the weathering and colonisation of monuments by phototrophic microorganisms. Sci Total Environ 167:329–341. 10.1016/0048-9697(95)04593-P [Google Scholar]
- 49.Stockenreiter M, Isanta Navarro J, Buchberger F, Stibor H (2021) Community shifts from eukaryote to cyanobacteria dominated phytoplankton: the role of mixing depth and light quality. Freshw Biol 66(11):2145–2157. 10.1111/fwb.13822 [Google Scholar]
- 50.Popović S, Pećić M, Subakov Simić G (2022) Exploring lampenflora of Resavska cave. Serbia Biol Life Sci Forum 15(1):33. 10.3390/IECD2022-12425 [Google Scholar]
- 51.Zittelli GC, Mugnai G, Milia M, Cicchi B, Benavides AS, Angioni A, Adis P, Torzillo G (2022) Effects of blue, orange and white lights on growth, chlorophyll fluorescence, and phycocyanin production of Arthrospiraplatensis cultures. Algal Res 61:102583. 10.1016/j.algal.2021.102583 [Google Scholar]
- 52.Tashiro R, Sushmita K, Hososhima S, Sharma S, Kateriya S, Kandori H, Tsunoda SP (2021) Specific residues in the cytoplasmic domain modulate photocurrent kinetics of channel rhodopsin from Klebsormidiumnitens. Commun Biol 4(1):235. 10.1038/s42003-021-01755-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neves-Petersen MT, Gajula GP, Petersen BS (2012) UV light effects on proteins: from photochemistry to nanomedicine. In: Saha S (ed) Molecular photochemistry – various aspects. IntechOpen, London, pp 125–158 [Google Scholar]
- 54.Lutz AP, Renicke C, Taxis C (2016) Controlling protein activity and degradation using blue light. In: Kianianmomeni A (ed) Optogenetics: methods and protocols. Humana Press, New Jeresy, pp 67–78 [DOI] [PubMed] [Google Scholar]
- 55.Wu P, Mann D (2020) Optochemical control of protein degradation. ChemBioChem 21(16):2250–2252. 10.1002/cbic.202000113 [DOI] [PubMed] [Google Scholar]
- 56.Losi A, Gärtner W (2008) Shedding (blue) light on algal gene expression. Proc Natl Acad Sci USA 105(1):7–8. 10.1073/pnas.0710523105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barros MP, Pinto E, Sigaud-Kutner TC, Cardozo KH, Colepicolo P (2005) Rhythmicity and oxidative/nitrosative stress in algae. Biol Rhythm Res 36(1–2):67–82. 10.1080/09291010400028666 [Google Scholar]
- 58.Asano CS (1998) Mecanismos controladores do ritmo endógeno diário da atividade da superóxido dismutase na alga unicelular marinha Gonyaulaxpolyedra stein (Tese Doutorado). Universidade de São Paulo, São Paulo [Google Scholar]
- 59.Guiry MD, Guiry, GM (2024) AlgaeBase. World-wide electronic publication, National University of Ireland, Galway - Gonyaulax polyedra F.Stein 1883. https://www.algaebase.org/search/species/detail/?species_id=43853. Accessed 23 Oct 2024
- 60.Chen W, Liu J, Chu G, Wang Q, Zhang Y, Gao C, Gao M (2023) Comparative evaluation of four Chlorella species treating mariculture wastewater under different photoperiods: nitrogen removal performance, enzyme activity, and antioxidant response. Bioresour Technol 386:129511. 10.1016/j.biortech.2023.129511 [DOI] [PubMed] [Google Scholar]
- 61.Van Houten B, Snowden A (1993) Mechanism of action of the Escherichiacoli UvrABC nuclease: clues to the damage recognition problem. BioEssays 15(1):51–59. 10.1002/bies.950150108 [DOI] [PubMed] [Google Scholar]
- 62.Capestany CA, Tribble GD, Maeda K, Demuth DR, Lamont RJ (2008) Role of the Clp system in stress tolerance, biofilm formation, and intracellular invasion in Porphyromonas gingivalis. J Bacteriol 190(4):1436–1446. 10.1128/jb.01632-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Porankiewicz J, Wang J, Clarke AK (1999) New insights into the ATP-dependent Clp protease: Escherichia coli and beyond. Mol Microbiol 32(3):449–458. 10.1046/j.1365-2958.1999.01357.x [DOI] [PubMed] [Google Scholar]
- 64.Bouchnak I, van Wijk KJ (2021) Structure, function, and substrates of Clp AAA+ protease systems in cyanobacteria, plastids, and apicoplasts: a comparative analysis. J Biol Chem 296:100338. 10.1016/j.jbc.2021.100338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Becana M, Yruela I, Sarath G, Catalan P, Hargrove MS (2020) Plant hemoglobins: a journey from unicellular green algae to vascular plants. New Phytol 227(6):1618–1635. 10.1111/nph.16444 [DOI] [PubMed] [Google Scholar]
- 66.Schiphorst C, Bassi R (2020) Chlorophyll-xanthophyll antenna complexes: in between light harvesting and energy dissipation. In: Larkum AWD, Grossman AR, Raven JA (eds) Photosynthesis in algae: biochemical and physiological mechanisms. Springer, Berlin, pp 27–55 [Google Scholar]
- 67.Liu XD, Shen YG (2004) NaCl-induced phosphorylation of light harvesting chlorophyll a/b proteins in thylakoid membranes from the halotolerant green alga. Dunaliella salina FEBS letters 569(1–3):337–340. 10.1016/j.febslet.2004.05.065 [DOI] [PubMed] [Google Scholar]
- 68.Han X, Han S, Li Y, Li K, Yang L, Ma D, Fang Z, Yin J, Zhu Y, Gong S (2023) Double roles of light-harvesting chlorophyll a/b binding protein TaLhc2 in wheat stress tolerance and photosynthesis. Int J Biol Macromol 253:127215. 10.1016/j.ijbiomac.2023.127215 [DOI] [PubMed] [Google Scholar]
- 69.Pajot A, Lavaud J, Carrier G, Garnier M, Saint-Jean B, Marchal L, Nicolau E (2022) The fucoxanthin chlorophyll a/c-binding protein in Tisochrysislutea: influence of nitrogen and light on fucoxanthin and chlorophyll a/c-binding protein gene expression and fucoxanthin synthesis. Front Plant Sci 13:830069. 10.3389/fpls.2022.830069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ohnishi N, Allakhverdiev SI, Takahashi S, Higashi S, Watanabe M, Nishiyama Y, Murata N (2005) Two-step mechanism of photodamage to photosystem II: step 1 occurs at the oxygen-evolving complex and step 2 occurs at the photochemical reaction center. Biochem 44(23):8494–8499. 10.1021/bi047518q [DOI] [PubMed] [Google Scholar]
- 71.Mulo P, Sakurai I (1817) Aro EM (2012) Strategies for psbA gene expression in cyanobacteria, green algae and higher plants: from transcription to PSII repair. Biochim Biophys Acta Rev Bioenerg 1:247–257. 10.1016/j.bbabio.2011.04.011 [DOI] [PubMed] [Google Scholar]
- 72.Roach T (2020) LHCSR3-Type NPQ Prevents photoinhibition and slowed growth under fluctuating light in Chlamydomonasreinhardtii. Plants 9(11):1604. 10.3390/plants9111604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saccon F, Wilson S, Morey-Burrows FS, Ruban AV (2022) Quantifying the long-term interplay between photoprotection and repair mechanisms sustaining photosystem II activity. Biochem J 479(5):701–717. 10.1042/BCJ20220031 [DOI] [PubMed] [Google Scholar]
- 74.Yokthongwattana K, Chrost B, Behrman S, Casper-Lindley C, Melis A (2001) Photosystem II damage and repair cycle in the green alga Dunaliellasalina: involvement of a chloroplast-localized HSP70. Plant Cell Physiol 42(12):1389–1397. 10.1093/pcp/pce179 [DOI] [PubMed] [Google Scholar]
- 75.Jimbo H, Yutthanasirikul R, Nagano T, Hisabori T, Hihara Y, Nishiyama Y (2018) Oxidation of translation factor EF-Tu inhibits the repair of photosystem II. Plant Physiol 176(4):2691–2699. 10.1104/pp.18.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jimbo H, Izuhara T, Hihara Y, Hisabori T, Nishiyama Y (2019) Light-inducible expression of translation factor EF-Tu during acclimation to strong light enhances the repair of photosystem II. Proc Natl Acad Sci USA 116(42):21268–21273. 10.1073/pnas.1909520116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bouzidi NE, Grama SB, Khelef AE, Yang D, Li J (2022) Inhibition of antioxidant enzyme activities enhances carotenogenesis in microalga Dactylococcusdissociatus MT1. Front Bioeng Biotechnol 10:1014604. 10.3389/fbioe.2022.1014604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bachchhav MB, Kulkarni MV, Ingale AG (2017) Enhanced phycocyanin production from Spirulina platensis using light emitting diode. J Inst Eng (India) E 98:41–45.
- 79.Ruan K, Duan J, Bai F, Lemaire M, Ma X, Bai L (2009) Function of Dunaliellasalina (Dunaliellaceae) enolase and its expression during stress. Eur J Phycol 44(2):207–214. 10.1080/09670260802573105 [Google Scholar]
- 80.Guiry MD, Guiry, GM (2024). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway - Haematococcus pluvialis Flotow 1844. https://www.algaebase.org/search/species/detail/?species_id=27370. Accessed 23 Oct 2024
- 81.Wang SB, Chen F, Sommerfeld M, Hu Q (2004) Proteomic analysis of molecular response to oxidative stress by the green alga Haematococcuspluvialis (Chlorophyceae). Planta 220:17–29. 10.1007/s00425-004-1323-5 [DOI] [PubMed] [Google Scholar]
- 82.Liao Y, Ji D, Xu Y, Xu K, Chen C, Wang W, Xie C (2023) Cloning and functional analysis of a phosphoglycerate kinase (PhPGK) from Pyropiahaitanensis. J Appl Phycol 35(4):1933–1943. 10.1007/s10811-023-03013-z [Google Scholar]
- 83.Guiry MD, Guiry, GM (2024). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway - Scenedesmus obliquus (Turpin) Kützing 1833. https://www.algaebase.org/search/species/detail/?species_id=27885. Accessed 23 Oct 2024
- 84.Zapata LM, Jimenez Veuthey M, Zampedri PA, Flores A, Zampedri CA, Chabrillón, G (2020) Effect of light stress and concentrations of nitrogen and carbon in the production of phytonutrients in the microalga Scenedesmus obliquus (Chlorophyceae, Chlorococcales). J Algal Biomass Util 11(1):9–22
- 85.Hidehiko K (2022) Effect of temperature on growth and gene expression of Tetradesmus sp. for application in wastewater treatment (Master's thesis). Nord Universitet, Bodø
- 86.Zhang Y, Wu H, Sun M, Peng Q, Li A (2018) Photosynthetic physiological performance and proteomic profiling of the oleaginous algae Scenedesmusacuminatus reveal the mechanism of lipid accumulation under low and high nitrogen supplies. Photosynth Res 138:73–102. 10.1007/s11120-018-0549-1 [DOI] [PubMed] [Google Scholar]
- 87.Guiry MD, Guiry, GM (2024) AlgaeBase. World-wide electronic publication, National University of Ireland, Galway - Scenedesmus acuminatus (Lagerheim) Chodat 1902. https://www.algaebase.org/search/species/detail/?species_id=27857. Accessed 23 Oct 2024
- 88.Cecchin M, Simicevic J, Chaput L, Hernandez Gil M, Girolomoni L, Cazzaniga S, Remacle C, Hoeng J, Ivanov NI, Titz B, Ballottari M (2023) Acclimation strategies of the green alga Chlorellavulgaris to different light regimes revealed by physiologic and comparative proteomic analyses. J Exp Bot 74(15):4540–4558. 10.1093/jxb/erad170 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available via ProteomeXchange with identifier PXD050424. During the revision process, the data can be accessed using the following username: reviewer_pxd050424@ebi.ac.uk and password: XSSxVmrD.