Abstract
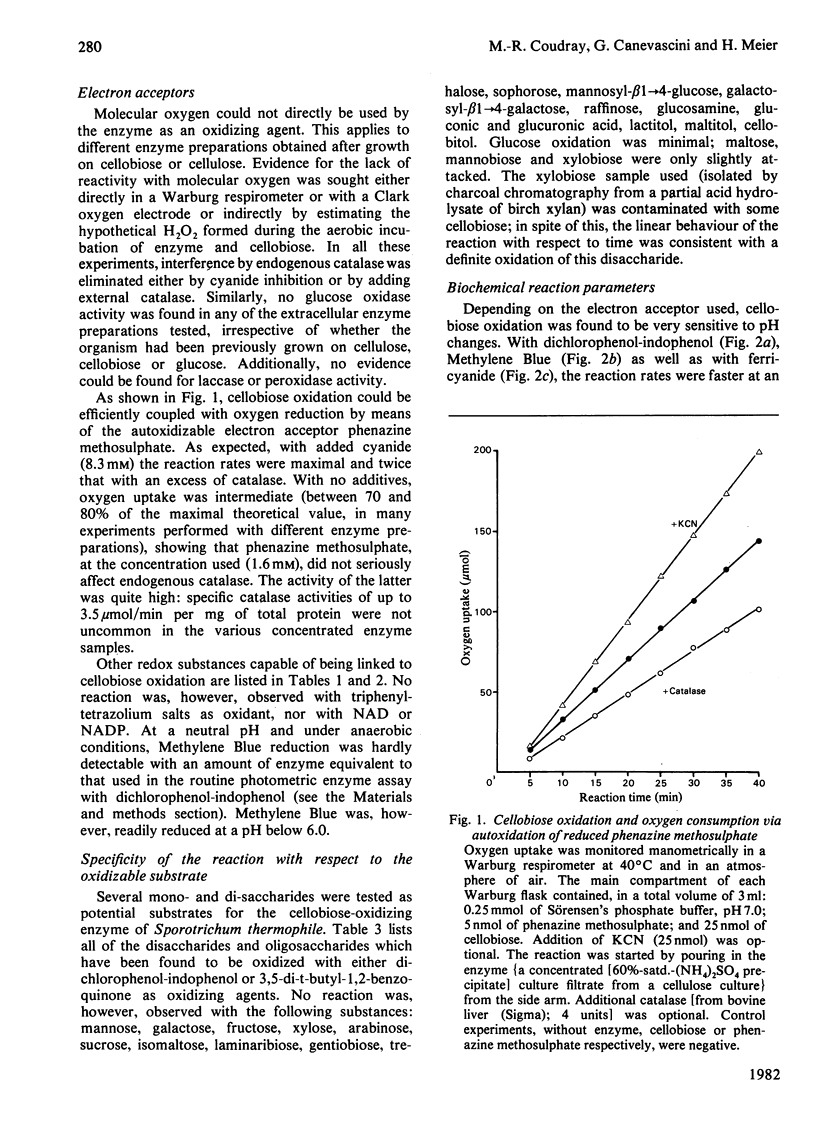
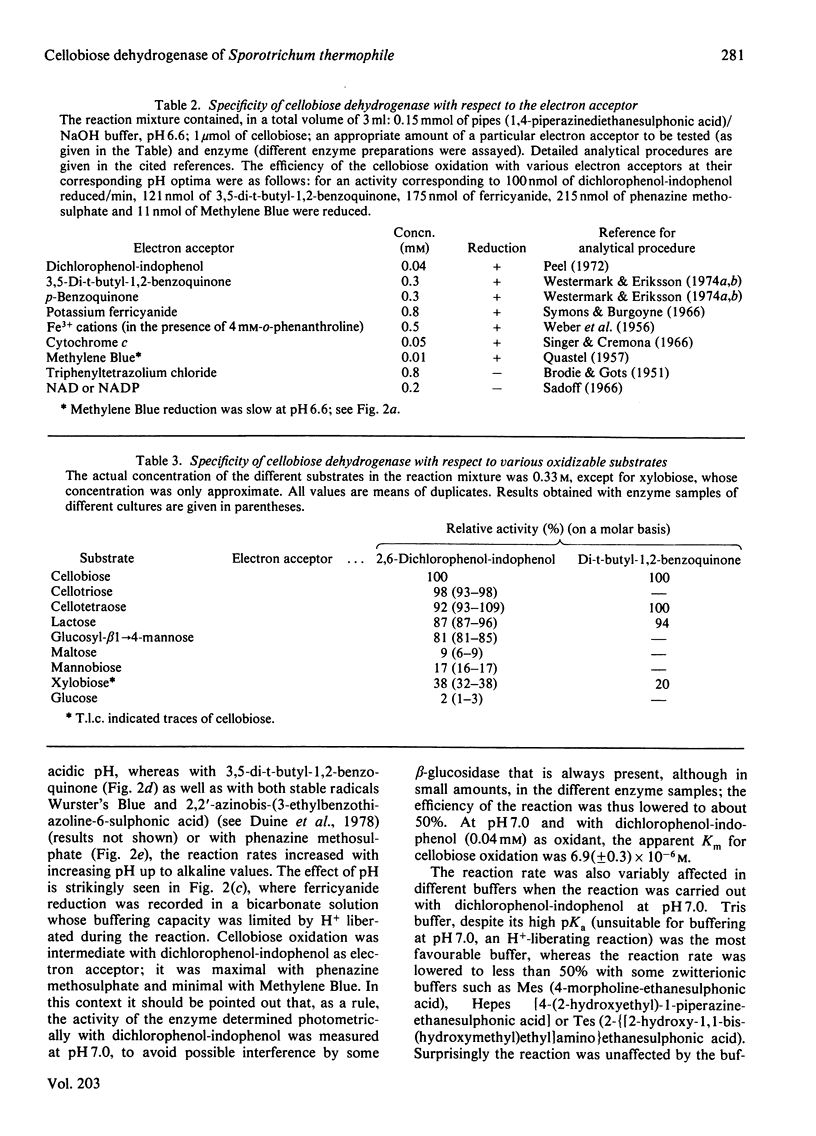
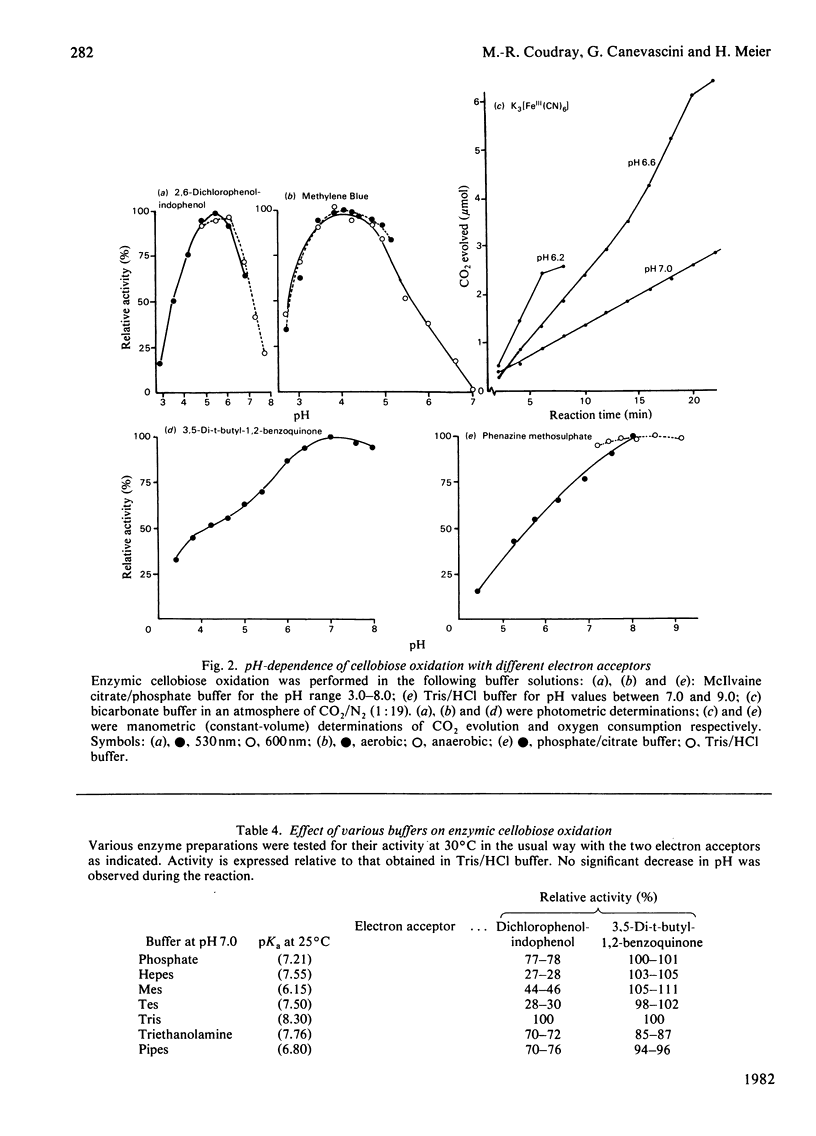
An extracellular enzyme from culture filtrates of Sporotrichum (Chrysosporium) thermophile (A.T.C.C. 42 464) after growth on cellulose or cellobiose was shown to oxidize cellobiose to cellobionic acid in vitro. Lactose and cellodextrins were also efficiently oxidized, but the enzyme was not active against most mono- and di-saccharides. Several redox substances could act as electron acceptors, but molecular oxygen, tetrazolium salts and NAD(P) were not reduced. Activity was stimulated up to 2-fold in the presence of 0.05 M-Mg2+. The pH optimum of the enzymic reaction was acidic when the activity was tested with dichlorophenol-indophenol or Methylene Blue, but was neutral to alkaline for 3,5-di-t-butyl-1,2-benzoquinone or phenazine methosulphate as electron acceptors. As the enzyme was formed inductively in parallel with the endocellulase, its possible function in relation to cellulolysis is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayers A. R., Ayers S. B., Eriksson K. E. Cellobiose oxidase, purification and partial characterization of a hemoprotein from Sporotrichum pulverulentum. Eur J Biochem. 1978 Sep 15;90(1):171–181. doi: 10.1111/j.1432-1033.1978.tb12588.x. [DOI] [PubMed] [Google Scholar]
- BRODIE A. F., GOTS J. S. Effects of an isolated dehydrogenase enzyme and flavoprotein on the reduction of triphenyltetrazolium chloride. Science. 1951 Jul 13;114(2950):40–41. doi: 10.1126/science.114.2950.40. [DOI] [PubMed] [Google Scholar]
- Duine J. A., Frank J., Westerling J. Purification and properties of methanol dehydrogenase from Hyphomicrobium x. Biochim Biophys Acta. 1978 Jun 9;524(2):277–287. doi: 10.1016/0005-2744(78)90164-x. [DOI] [PubMed] [Google Scholar]
- Eriksson K. E., Pettersson B., Westermark U. Oxidation: an important enzyme reaction in fungal degradation of cellulose. FEBS Lett. 1974 Dec 15;49(2):282–285. doi: 10.1016/0014-5793(74)80531-4. [DOI] [PubMed] [Google Scholar]
- HAUGE J. G. Glucose dehydrogenation in bacteria: a comparative study. J Bacteriol. 1961 Oct;82:609–614. doi: 10.1128/jb.82.4.609-614.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highley T. L. Cellulose degradation by cellulose-clearing and non-cellulose-clearing brown-rot fungi. Appl Environ Microbiol. 1980 Dec;40(6):1145–1147. doi: 10.1128/aem.40.6.1145-1147.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Petroski R. J., Peczynska-Czoch W., Rosazza J. P. Analysis, Production, and Isolation of an Extracellular Laccase from Polyporus anceps. Appl Environ Microbiol. 1980 Dec;40(6):1003–1006. doi: 10.1128/aem.40.6.1003-1006.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGER T. P., KEARNEY E. B. Determination of succinic dehydrogenase activity. Methods Biochem Anal. 1957;4:307–333. doi: 10.1002/9780470110201.ch9. [DOI] [PubMed] [Google Scholar]
- Sternberg D., Vijayakumar P., Reese E. T. beta-Glucosidase: microbial production and effect on enzymatic hydrolysis of cellulose. Can J Microbiol. 1977 Feb;23(2):139–147. doi: 10.1139/m77-020. [DOI] [PubMed] [Google Scholar]
- WEBER M. M., LENHOFF H. M., KAPLAN N. O. The reduction of inorganic iron and cytochrome c by flavin enzymes. J Biol Chem. 1956 May;220(1):93–104. [PubMed] [Google Scholar]
- Westermark U., Eriksson K. E. Purification and properties of cellobiose: quinone oxidoreductase from Sporotrichum pulverulentum. Acta Chem Scand B. 1975;29(4):419–424. [PubMed] [Google Scholar]
- von Klopotek A. Revision der thermophilen Sporotrichum-Arten: Chrysosporium thermophilum (Apinis) comb. nov und Chrysosporium fergusii spec. nov. equal status conidialis von Corynascus thermophilus (Fergus und Sinden) comb. nov. Arch Microbiol. 1974 Jul 22;98(4):365–369. doi: 10.1007/BF00425296. [DOI] [PubMed] [Google Scholar]


