Abstract
The cocoa tree (Theobroma cacao L.) is a native crop of the tropical forests of Central America and the Amazon. It plays an important role in the economy of producing regions; however, the infrastructure remains incipient, leading to inadequate processing practices and affecting the quality of the cocoa beans. Therefore, this research aimed to evaluate the quality of 55 samples of Amazonian cocoa beans produced in five regions of state of Pará (Lower Tocantins River, Northeast, West, Southeast, and Trans-Amazon) through physical and physicochemical evaluations (proteins, lipids, moisture, ash, pH, acidity, and water activity), and a questionnaire with producers about fermentation and drying practices. This was followed by physical, physicochemical, antioxidant capacity, quantification of total polyphenols, and bioactive compound evaluations. According to the physical evaluation, the samples met the acceptable commercial standard, but the cut test results showed a lack of standardization in the pre-processing of cocoa beans according to the International Cocoa Organization (ICCO). Among the physicochemical parameters, moisture was within the limit tolerated by legislation, and only lipids showed significant differences. Regarding antioxidant capacity, total polyphenols, and bioactive compounds, only the bioactive compounds showed significant differences, with the Lower Tocantins River region exhibiting higher values for all studied compounds. Inadequate processing practices, such as poor fermentation and drying, may lead to quality deficiencies in cocoa beans. Therefore, this study emphasizes the need for characterization of commercial cocoa beans, as well as standardization in the pre-processing of these beans. It also highlights potential variations in the quality of the beans among producing regions, owing to the vast territorial expanse of Pará, diverse cultivation conditions, variety of cocoa trees, and different methods of cocoa bean pre-processing.
Keywords: Theobroma cacao, Amazonian region, Bioactive compounds, Antioxidant capacity, Flavonoids, Methylxanthines
1. Introduction
The cocoa tree (Theobroma cacao L.) is a dicotyledonous plant of the Malvaceae family, whose center of origin is in the regions of tropical forests of the Americas, particularly the Amazon, globally recognized for providing the raw material for chocolate, which are its fermented and dried cocoa beans [[1], [2], [3]]. According to the International Cocoa Organization (ICCO) [4], Brazil ranks sixth among the world's cocoa bean producers, with almost 270 thousand tons per year. The state of Pará achieved the highest Brazilian production in 2020 with more than 144 thousand tons, which corresponded to 52 % of national production and an average productivity of 964 kg/ha [5].
The cocoa production chain for chocolate production consists of stages where various chemical, biochemical, physical, and structural reactions involving microorganisms, phenolic compounds, and other secondary compounds occur, which allow for the reduction of the characteristic bitterness of the cocoa beans and the development of characteristic color and flavor [[6], [7], [8]]. One of these stages is the fermentation of the cocoa beans, which is influenced, among other things, by the cocoa variety, the microbial population, and the soil and climatic conditions characteristic of each producing region [9,10]. In addition to fermentation, drying emerges as an important stage. Its main function is to reduce residual moisture to a range of 7–8 % through a slow process that enables the conversion of phenolic compounds into quinones [11,12].
The ISO 2451 standard [13] specifies the requirements, classification, sampling, test methods, packaging, and labeling desirable for commercial purposes. In Brazil, Normative Instruction No. 38 of June 23, 2008 [14] defines the official standard for the importation of cocoa beans based on the maximum tolerance limits for defects. These standards govern the quality parameters of cocoa beans, but do not reflect their quality levels associated with the biological value of the cocoa beans, especially the concentrations of secondary compounds (flavonoids and methylxanthines) and antioxidant capacity [15].
In the state of Pará, in the Eastern Amazon, there are five regions that produce Amazonian cocoa: Lower Tocantins River, Northeast, West, Southeast, and Trans-Amazon. Given the vast territorial area of Pará (1,248,000 km2), the varied cultivation conditions, the diversity of cocoa trees, along with the different methods of pre-processing cocoa beans, significant variations in the quality of the beans among the producing regions are expected. Therefore, given the lack of characterization of the quality parameters of cocoa beans produced in the regions of the state of Pará, and especially the lack of scientific literature reporting on the profile of flavonoids and methylxanthines as well as antioxidant capacity of these cocoa beans, this manuscript aims to evaluate the quality of Amazonian cocoa beans from the main producing regions of Eastern Amazonia (Lower Tocantins River, Northeast, West, Southeast, and Trans-Amazon) according to the ISO 2451 [13] and Normative Instruction (NI) n° 38 [14] standards. In addition, the total phenolic compounds, the profile of flavonoids ((+) catechin, (−) epicatechin, and procyanidin B2) and methylxanthines (caffeine, theophylline, and theobromine) of the cocoa beans and their antioxidant capacity by the ORAC method will be evaluated.
2. Materials and methods
2.1. Sampling
Fifty-five samples of commercial cocoa beans (∼500 g each) from 17 municipalities in five regions of Amazonian cocoa bean production (Lower Tocantins River, Northeast, West, Southeast, and Trans-Amazon) were analyzed. At least three samples were collected from each region. Information on fermentation and drying time was provided by the producer during sample collection. The cocoa beans were manually shelled, and the cocoa nibs (cotyledons), cocoa shells, and embryos were separated. The cocoa nibs were ground, producing cocoa nibs powder (particle size <0.5 mm). This cocoa nibs powder was packed in high-density polyethylene bags and stored at −22 °C until the analyses were performed.
2.2. Physical evaluation of the cocoa beans
The evaluations of bean count, average weight, and cut test of cocoa beans were performed according to the procedure established by ISO 2451 [13] and NI n° 38 [14].
2.3. Physicochemical analyses of the cocoa nibs powder
The determinations of moisture (method 925.09), ash (method 923.03), protein (method 920.87), and lipids (method 920.85) were performed according to methodologies proposed by the Association of Official Analytical Chemists [16] and the results were expressed in grams per 100 g of cocoa nibs powder (g/100g). Total titratable acidity and pH were determined according to the standards of the Brazilian Adolfo Lutz Institute [17]. Water activity was determined at 25 °C using a water activity analyzer (AQUALAB model, Decagon Devices, USA). All samples were analyzed in triplicate.
2.4. Evaluation of bioactive compounds
2.4.1. Extraction procedure
The extraction of bioactive compounds was carried out according to the methodology proposed by Counet and Collin [18] with modifications. In a screw-capped test tube, 1 g of cocoa nibs powder was weighed and 19 mL of extracting solution containing acetone, water, and acetic acid (70:29.5:0.5 v/v) was added. The tubes were vortexed for 1 min at room temperature. Then, 2 mL of the resulting extract was transferred to Eppendorf tubes and centrifuged at 8000 rpm for 20 min at 4 °C. The supernatant was then stored in Eppendorf tubes at −18 °C until the analyses were performed.
2.4.2. Determination of total phenolic compounds
The total phenolic compounds were estimated using the Folin-Ciocalteu method [19] with absorbance measured at 750 nm. The analyses were conducted in triplicate, and the results were expressed in milligrams of catechin equivalents per gram of dry cocoa nibs powder (mgCE/gDN).
2.4.3. Quantification of the main flavanol and methylxanthine compounds by ultra-high performance liquid chromatography - UHPLC
A UHPLC method was developed to quantify the major phenolic compounds ((+) catechin, (−) epicatechin, and procyanidin B2) and xanthine alkaloids (caffeine, theobromine, and theophylline). The UHPLC system (Thermo Scientific, USA) was equipped with a quaternary pump (LPG-3400 RS), an automatic injector (WPS-3000SL Analytical), a flow cell (Standard Analytical), a diode-array detector (DAD), and Chromeleon 7.1 SR2 software. A Kinetex C18 2.6 μm 100 × 4.6 mm column (Phenomenex, USA) was used in the analysis. The mobile phases consisted of ultrapure water (mobile phase A) and HPLC-grade acetonitrile (mobile phase B), both acidified with 2.5 % HPLC-grade glacial acetic acid, and a flow rate of 1 ml/min. The elution gradient started at 5 % B and increased to 25 % B over 9 min, then to 95 % B until 12.5 min, remaining constant until 15 min. Then, it returned to 5 % B until 18 min for the initial system conditions. Chromatograms were recorded between 200 and 800 nm, and the compounds were quantified at 280 nm, except for procyanidin B2, which was quantified at 233 nm. Standard compounds were used for calibration curve construction and comparison of elution and co-elution times for each compound. The cocoa nibs powder extracts were diluted in extraction solution, filtered through 0.22 μm PVDF filter units, and injected with 5 μL into the UHPLC system. All determinations were performed in triplicate, and the results were expressed in mg per gram of dry cocoa nibs powder (mg/g DN).
2.4.4. Oxygen radical absorbance capacity (ORAC) method
The oxygen radical absorbance capacity assay, using fluorescein as the fluorescent probe, was performed on a microplate fluorimeter. The procedure used was that described by Gomes et al. [20] and was an adaptation of the protocols proposed by Huang et al. [21]. The ORAC was expressed as μmol of trolox equivalent (TE) per g of DN. Each sample was measured in triplicate.
2.5. Principal component analysis (PCA)
Exploratory data analysis was performed using principal component analysis (PCA), testing different spectral ranges to visualize possible correlations between variables and groupings between samples. All data were mean-centered and model validation was performed using the full cross-validation method. PCAs were conducted using The Unscrambler® X version 10.2 software.
2.6. Statistical analysis
The results obtained from physical, physicochemical, colorimetric, and chromatographic analyses were analyzed using Analysis of Variance (ANOVA) and Tukey's test to determine the difference between means at a significance level of 95 % (p < 0.05), using STATISTIC 13.0 software.
3. Results and discussion
3.1. Evaluation of fermentation and drying practices reported by cocoa bean producers from the five production regions of the state of Pará
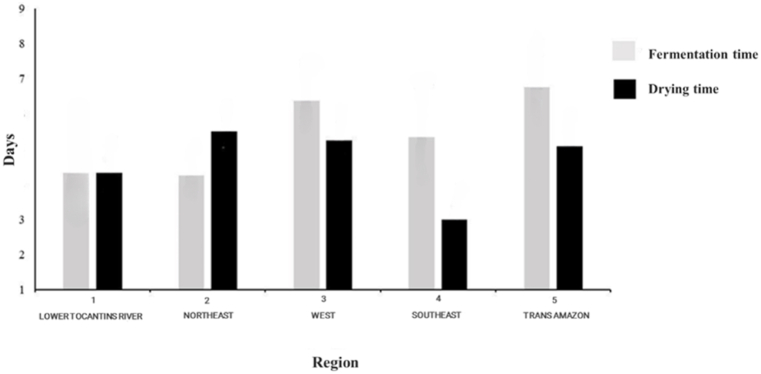
Fig. 1 shows the data on the fermentation and drying times of cocoa beans from the five cocoa-producing regions of the state of Pará, namely: the Lower Tocantins River region, Northeast region, West region, Southeast region, and Trans-Amazon region.
Fig. 1.
Fermentation and drying times of cocoa beans produced in the five producing regions of the state of Pará.
Overly extended fermentation processes can be a risk factor for the development of undesirable flavors, while shorter times do not allow for the development of components responsible for the characteristic aroma and flavor, potentially compromising product quality [10,22]. In the present study, the average fermentation time of the cocoa beans ranged from 4.1 to 6.8 days, with the Trans-Amazon region (6.8 days) differing from the Northeast (4.1 days) and Lower Tocantins River (4.2 days) regions. It should be noted that these values for fermentation and drying times of the cocoa beans were obtained from information provided by the producers themselves, values close to those found in the literature [6,23]. Generally, cocoa bean fermentations in the studied regions are carried out by farmers after harvesting and breaking the pods, in wooden boxes covered with banana leaves, followed by drying with direct sun exposure on plastic tarps or in barges with movable covers for rain protection.
According to the ICCO [4], ideal drying should ensure a reduction in moisture to values of up to 8 %. This value is considered the ideal range to avoid the proliferation of undesirable fungi and the cessation of enzymatic activities. Furthermore, the lower the moisture content, the more microbiologically stable the cocoa beans will be, thus facilitating transport and storage. In Fig. 1, it is observed that the variation of the average drying time of the cocoa beans ranged from 3.0 to 5.5 days. Several factors influence drying time, such as the method applied and the solar incidence in the region, thus drying time may vary from 3 to 5 days, and it is important to observe this rate, as too fast drying tends to favor the accumulation of acetic acid in the beans, while too slow rates tend to produce molds and fungi [6,24]. Do Carmo Brito et al. [15] report that fermentation and drying times affect the content of phenolic compounds in cocoa beans, causing a significant decrease due to the action of oxidative enzymes, mainly polyphenoloxidase (PPO).
In some studies, shorter drying times have been reported as efficient for moisture removal. According to the study by Niikoi Kotey et al. [25], which investigates the effect of the duration of the traditional fermentation period and different drying methods on the quality of the grain of two cocoa varieties (hybrid cocoa and Amazonia) in Ghana, the results indicated that the optimal moisture content was recorded on the 3rd day of drying for both methods after 5 days of fermentation. It is important to highlight that the third day of drying is crucial to achieve the ideal moisture content in the cocoa beans and that the beans of the Amazonia variety have the fastest drying time. It is important to note that the information about the drying and fermentation times in this study was provided by the cocoa bean producers.
3.2. Evaluation of physical parameters of cocoa beans
Table 1 presents the results for the physical parameters of cutting test, bean count, and average weight of cocoa beans. The evaluation of bean count and average weight allows for comparing the sizes and masses of the cocoa beans. According to the Federation of Cocoa Commerce [26], there should be a maximum of 100 units per 100 g of cocoa beans, with an average weight of 1 g or more per bean. According to Table 1, only the Lower Tocantins River region showed averages higher than those recommended by the FCC. However, they did not differ significantly (p < 0.05) compared to the Northeast and Southeast regions.
Table 1.
Average results related to physical parameters used to evaluate the quality of cocoa beans from the five production regions in the state of Pará. ∗
| Regions | Bean count |
Individual Average Mass |
Brown |
Partially brown |
Violet |
White |
Compartmenta-lized cocoa beans |
Defects |
|---|---|---|---|---|---|---|---|---|
| (Number of cocoa beans/100g) | (g/bean) | (%) | (%) | (%) | (%) | (%) | (%) | |
| Lower Tocantins River | 103.78 ± 7.39a | 0.97 ± 0.08a | 25.17 ± 20.54a | 71.28 ± 20.52a | 1.22 ± 0.96a | 0.28 ± 0.53a | 86.50 ± 16.30a | 1.76 ± 0.09a |
| Northeast | 94.83 ± 6.32ab | 1.07 ± 0.08ab | 24.75 ± 17.81a | 70.67 ± 19.20ab | 1.00 ± 1.12a | 0.92 ± 0.92a | 91.42 ± 5.66a | 0.97 ± 0.21ab |
| West | 86.22 ± 7.4b | 1.18 ± 0.11b | 61.33 ± 20.92b | 36.00 ± 17.70b | 1.22 ± 2.12a | 0.78 ± 1.07a | 96.89 ± 2.83a | 0.59 ± 0.10ab |
| Southeast | 93.27 ± 7.31ab | 1.08 ± 0.10ab | 56.86 ± 19.92b | 41.63 ± 20.75b | 0.21 ± 0.59a | 0.35 ± 0.34a | 91.47 ± 10.26a | 0.17 ± 0.03b |
| Trans-Amazon | 87.57 ± 6.78b | 1.17 ± 0.09b | 50.63 ± 18.37b | 47.60 ± 18.42ab | 0.66 ± 2.75a | 0.55 ± 0.81a | 94.18 ± 8.73a | 0.32 ± 0.04b |
The cutting test allows for evaluating the physical parameters of color, compartmentalization, and defects of cocoa beans and relating them to the fermentation process. For the Forastero genetic group, a well-fermented bean should have a higher percentage of brown color, ideally above 65 %, while having a maximum of 15 % of violet cocoa beans and good compartmentalization [27]. Therefore, the cocoa beans from the Lower Tocantins River and Northeast regions showed significant differences in the Brown and Partially Brown parameters (Table 1) compared to the other regions, which may be related to the shorter fermentation time (4 days), as shown in Fig. 1, highlighting that these cooca beans also underwent other pre-processing steps in addition to fermentation, such as harvesting, breaking, and drying. the shorter fermentation time (4 days), as presented in Fig. 1. On the other hand, the parameter of compartmentalization also indicates good pre-processing, which leads to important biochemical reactions during fermentation and drying, allowing for the transformation of substances that were previously separated, such as phenolic compounds by enzymes [28]. According to the analyzed data, all regions showed good compartmentalization.
In Brazil, according to the Normative Instruction No. 38 of 2008 [14], cocoa beans are classified into types (1, 2, 3 and out of type), in relation to a tolerance limit for the quantity of defects. Therefore, all regions presented a low percentage of defects (Table 1), falling into Type 1 cocoa beans.
3.3. Physicochemical composition of amazonian cocoa beans
Table 2 shows the results for the physicochemical parameters of proteins, lipids, moisture, ash, pH, volatile acidity, and water activity of the cocoa beans from the five producing regions of the state of Pará (Brazil) studied.
Table 2.
Physicochemical composition of cocoa beans from the five producing regions of Pará state.
| Regions | Protein |
Lipid |
Moisture |
Ash |
pH |
Total acidity |
aW |
|---|---|---|---|---|---|---|---|
| (g/100g) | (g/100g) | (g/100g) | (g/100g) | (gAa/100g) | |||
| Lower Tocantins River | 15.57 ± 2.07a | 31.31 ± 9.09ab | 6.11 ± 0.62a | 3.11 ± 0.36ab | 5.66 ± 0.51a | 0.21 ± 0.08a | 0.62 ± 0.06a |
| Northeast | 18.42 ± 1.45a | 35.85 ± 10.99ab | 5.42 ± 0.37a | 2.51 ± 0.11b | 4.95 ± 0.06a | 0.33 ± 0.09ab | 0.59 ± 0.08a |
| West | 14.75 ± 1.86a | 36.39 ± 7.55ab | 5.47 ± 0.48a | 2.93 ± 0.36 ab | 4.57 ± 0.13a | 0.44 ± 0.13b | 0.59 ± 0.02a |
| Southeast | 15.08 ± 1.48a | 35.65 ± 7.99a | 5.77 ± 0.29a | 3.17 ± 0.36a | 4.89 ± 0.18a | 0.47 ± 0.25b | 0.62 ± 0.03a |
| Trans-Amazon | 14.48 ± 6.13a | 26.27 ± 6.44b | 6.05 ± 0.60a | 2.59 ± 0.47ab | 4.91 ± 0.22a | 0.44 ± 0.13b | 0.63 ± 0.04a |
Results are expressed as mean ± standard deviation. Means with the same letter in each column do not present significant difference (p < 0.05) by Tukey's test.
According to D'Souza et al. [29], the protein concentration in cocoa beans tends to decrease during fermentation due to protein hydrolysis and complexation with phenolic compounds, causing gradual degradation of proteins into oligopeptides. When checking the protein content, no significant difference (p < 0.05) is observed between regions, and it is within the expected range of values, from 11 % to 16 % [30].
The lipid content in cocoa beans is essential for obtaining cocoa butter, one of the main raw materials for the chocolate industry. The Southeast and Trans-Amazon regions showed significant differences (p < 0.05) in lipid content. However, the average values found ranged from 26.27 % to 35.65 %, which are close to the range reported by Pimentel [31], who mentions the average lipid content in cocoa beans as 31 % and a range of 28.5 %–33.7 % for the cloned cocoa beans from the state of Bahia (Brazil).
Moisture and water activity analysis are fundamental instruments in quality control. Drying the cocoa beans to values below 8 % prevents fungal infestation during storage and allows some of the chemical changes that occurred during fermentation to continue and improve the development of flavor [3,32]. The analyzed samples had a moisture content ranging from 5.42 % to 6.11 % and aW of 0.59–0.63 (Table 2), with no significant difference between regions, indicating that the drying process used by the producers was efficient.
According to Efraim et al. [28], cocoa beans with little fermentation have higher ash content. For the analyzed samples, there was a variation from 2.51 to 3.17g/100g, in agreement with the range reported by Hashimoto et al. [33], which varied from 2.6 % to 4.2 %.
Due to the fermentation and drying processes, both the pH and acidity of the cocoa beans undergo changes. During bacterial fermentation, lactic and acetic acids are produced, decreasing the pH and increasing the total acidity, which can be an important quality attribute that can interfere with the flavor of cocoa beans and derived products, while during drying, there is an increase in pH that can reach 6.0 due to the volatilization of part of organic acids [9,34]. In this study, the pH and acidity between regions varied from 4.57 to 5.66 and from 0.21 to 0.44, respectively, which are similar to the data reported in the literature [8,34,35].
3.4. Quantification of the main bioactive compounds and antioxidant capacity in amazonian cocoa beans
The levels of the main bioactive compounds and antioxidant capacity present in fermented and dried Amazonian cocoa beans are presented in Table 3. The phenolic compounds epicatechin, catechin, and procyanidin B2 have been reported as the main flavan-3-ols in cocoa beans [36,37]. The cocoa cocoa nibs produced in the Lower Tocantins River region showed a higher concentration of epicatechin and procyanidin (p < 0.05) compared to other regions. The catechin content could not be quantified due to values falling below the limit of detection.
Table 3.
Evaluation of antioxidant capacity and quantification of bioactive compounds found in cocoa nibs from producing regions of the state of Pará (Brazil).
| Regions | Total polyphenols |
ORAC |
Epicatequin |
Procyanidin B2 |
Caffeine |
Theophylline |
Theobromine |
|---|---|---|---|---|---|---|---|
| (mgEC/gDN) | (μmolET/gDN) | (mg/gDN) | (mg/gDN) | (mg/gDN) | (mg/gDN) | (mg/gDN) | |
| Lower Tocantins River | 21.5 ± 13.3a | 730.0 ± 288.4a | 6.63 ± 4.22a | 2.28 ± 1.88a | 1.83 ± 0.37a | 0.16 ± 0.09ab | 20.88 ± 3.83a |
| Northeast | 16.2 ± 3.2a | 335.2 ± 113.6b | 2.00 ± 1.09b | 0.73 ± 0.27b | 1.96 ± 0.21a | 0.15 ± 0.06ab | 19.01 ± 1.75ab |
| West | 14.7 ± 6.0a | 334.0 ± 88.6b | 1.64 ± 0.22b | 0.65 ± 0.13b | 1.98 ± 0.24a | 0.18 ± 0.08ab | 17.02 ± 1.92ab |
| Southeast | 15.4 ± 5.5a | 358.5 ± 107.5b | 2.73 ± 1.46b | 0.98 ± 0.53b | 2.20 ± 0.54a | 0.18 ± 0.12a | 18.35 ± 2.15ab |
| Trans-Amazon | 15.8 ± 6.3a | 322.7 ± 145.2b | 1.84 ± 1.25b | 0.61 ± 0.41b | 1.96 ± 0.52a | 0.10 ± 0.04b | 15.99 ± 2.96b |
Results expressed as mean ± standard deviation. Total polyphenols: mgEC/gDN - milligrams of catechin equivalent per gram of dry cocoa nibs. ORAC value: μmolET/gDN - micromoles of trolox equivalent per gram of dry cocoa nibs. Values in the same column with the same letter do not differ significantly from each other (Tukey's test with a 5 % significance level).
Methylxanthines (caffeine, theobromine, and theophylline) represented more than 2 % of dry cocoa nibs, and the differences in levels between some regions may be due to the fermentation process and the complexation with peptides, as reported by Brunetto et al. [38]. Methylxanthines and phenolic compounds are associated with various functional properties of foods, such as reducing oxidative stress and beneficial effects in preventing cardiovascular diseases, metabolic disorders, and cancer [39,40].
Several methods are used to estimate the antioxidant capacity of a food or extract, and the most applied methods are related to the mechanisms of electron or proton transfer in oxidation-reduction reactions. The Folin-Ciocalteu and ORAC methods are the most used, respectively [36,41]. In this study, the nib extracts showed high antioxidant capacity, and the methodologies used showed good correlation (Table 3).
Cocoa farming in the Eastern Amazon, both organic and conventional, is predominantly conducted in three distinct production systems. The first involves full-sun monoculture, where cocoa trees prevail and are typically of clonal or hybrid origins. In contrast, Agroforestry Systems (AFS) are operated on soils of medium to high fertility, often by small-scale producers. Lastly, the Floodplain System is characterized by the influence of tidal fluctuations on soils. Except in the floodplain system, where plants are mainly of native origin, in the other systems, cocoa trees are predominantly of hybrid origins, originating from mixed accessions distributed by the Brazilian government to producers, or from varietal clones planted by farmers. This diversity of genetic origins and the spatial extent of each studied region results in commercial samples with high standard deviations, making it difficult to identify significant differences in many evaluated parameters.
In fact, the antioxidant capacity present in cocoa extracts is associated with its content of phenolic compounds and the presence of other methanol-soluble compounds such as methylxanthines [39,42]. The total phenolic content ranged from 14.73 to 21.48 mgEC/gNS, with no significant difference between regions (Table 3). Chagas Junior et al. [43] found a value of 26.35 mgECE/g in fermented and dried cocoa beans in Tomé-Açú (Northeast). From a sensory standpoint, this reduction is interesting, as an excessive presence of these compounds causes bitterness and astringency in the various food products obtained from the cocoa beans, such as chocolate bars and cocoa powder. Nevertheless, cocoa still has a greater antioxidant capacity than various products recognized for these properties, such as tea and red wine [44].
According to Table 3, the reported range for ORAC value ranged from 322.67 to 730.05 μmolET/gNS, with only Lower Tocantins River differing significantly (p < 0.05) from the other regions. Carrillo et al. [45] in their studies found similar values (387.29 ± 10.84 to 639.51 ± 73.84 μmolET/gNS) using the same method. The regions presented good antioxidant capacity, which makes it interesting to use these cocoa beans for the production of functional chocolates, paying attention to the Lower Tocantins River region, which has significantly higher values than the others.
3.5. Principal component analysis (PCA)
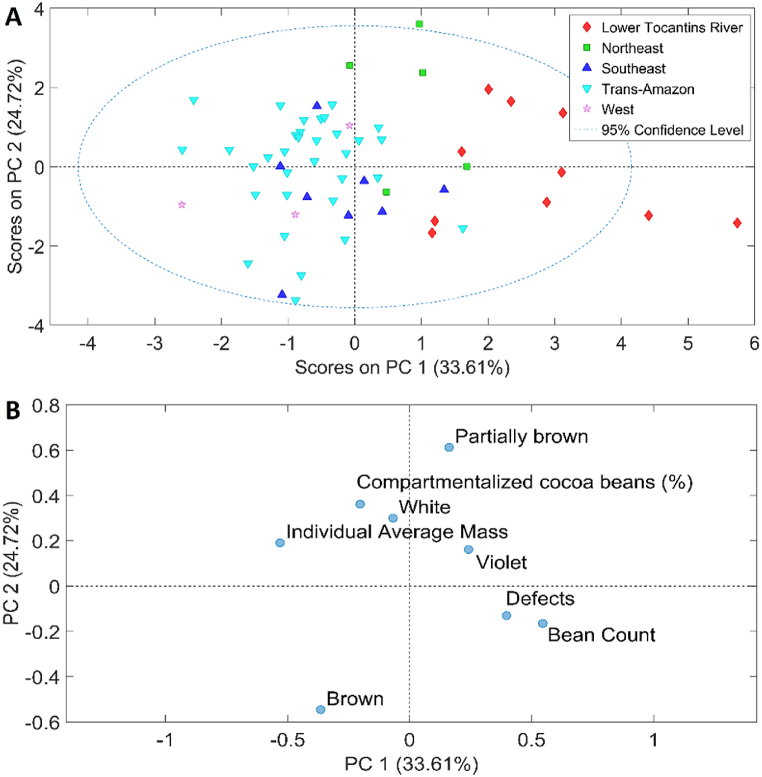
PCA was applied to the physical parameters and physicochemical analyses. Fig. 2, Fig. 3 show the score plots, while Fig. 2, Fig. 3 show the loading plots, respectively. A general trend is observed in PC1, both in the data of physical parameters and in physicochemical analyses. This trend reflects the variability between samples from the Lower Tocantins River region (positive side of PC1) and the Trans-Amazon region (negative side of PC1).
Fig. 2.
Score plot (a) of PC1 versus PC2 for cocoa beans and loading plot (b) of the physical parameters for cocoa beans.
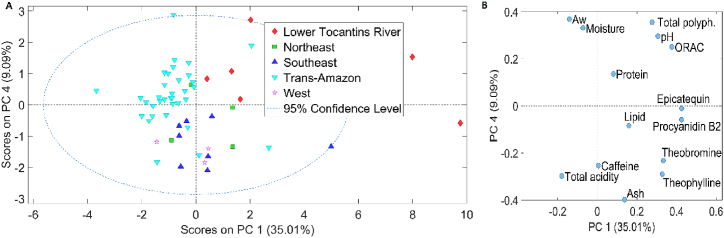
Fig. 3.
Score plot (a) PC1 versus PC4 in cocoa beans and Loading plot (b) of the physicochemical analyses for cocoa beans.
Specifically for the physical parameters (Fig. 2), the samples from the Lower Tocantins River region exhibit a higher incidence of bean count, defects, violet hues, and partially brown colors. These attributes indicate particular characteristics of the samples from this region, possibly related to environmental factors, management practices, and specific cultivation conditions. Conversely, the samples from the Trans-Amazon region are characterized by a greater prominence of brown color, individual average mass, and compartmentalized cocoa beans. The predominance of these attributes in the Trans-Amazon samples suggests significant differences in terms of composition and quality, which may be associated with different pre-processing and agronomic conditions.
These observations are essential for understanding the diversity and quality of samples from these two regions. The analysis of PC1 reveals a clear distinction between the characteristics of samples from both regions, which can have important implications for sample selection, management strategies, and market potential. This compartmentalization of characteristics highlights the importance of considering regional factors in the evaluation and processing of samples to optimize the quality and performance of derived products.
This great dispersion of samples from Lower Tocantins River may be because this region has a greater number of producers and a significant difference in cocoa processing methods among different farms. Thus, it is necessary to provide training for the producers, emphasizing the importance of establishing quality standards for cocoa, as the processing steps directly impact the quality of chocolate.
Vázquez-Ovanto et al. [46] reported different results when applying this analysis to fermented Criollo cocoa beans obtained from different cities in Mexico, as these authors were able to classify the samples into well-homogeneous groups and define the real influence of each physical-chemical component on the quality of the fermented cocoa beans.
For the physicochemical analyses (Fig. 3), total acidity and water activity are more prominent in samples from the Trans-Amazon region. In contrast, the samples from the Lower Tocantins River region exhibit higher concentrations of epicatechin, procyanidin B2, ORAC, theobromine, theophylline, pH, and total polyphenols. These differences indicate significant variation in the physicochemical components of the samples from each region, possibly reflecting different environmental conditions, management practices, and cultivation processes.
Furthermore, a specific pattern was observed in the physicochemical analysis data when analyzing principal component 4 (PC4), as illustrated in Fig. 3a. In this analysis, the samples from the Northeast, Southeast, and West regions appear at the lower part of the graph, more separated from the samples from the Lower Tocantins River and Trans-Amazon regions. The main variables responsible for this separation include ash, theophylline, total acidity, caffeine, and theobromine.
These observations are crucial for understanding the diversity and specific characteristics of samples from different regions. Principal component analysis reveals clear distinctions in physicochemical characteristics, which can have important implications for sample selection and processing, as well as for commercialization strategies and optimizing product quality. Considering these regional variables is essential for detailed evaluation and maximizing the unique properties of each region.
This plot also shows that cocoa beans from the Trans-Amazon region presented less dispersion, while the Northeast and Lower Tocantins River regions showed much greater dispersion than the others, occupying almost all quadrants of the plot, making it difficult to verify patterns of separation of cocoa beans by region.
3.6. Technical solutions
Based on the collected data and obtained results, it is suggested that the quality of cocoa beans from the Lower Tocantins region could be improved by extending the fermentation duration. This extension could lead to a higher proportion of fermented beans, thereby enhancing the overall quality of the cocoa beans. The exact duration of fermentation would need to be determined through additional experimentation, but preliminary findings suggest that an extension could be beneficial.
Furthermore, the authors propose that improvements could also be made in the drying practices. Given that the drying time significantly affects the quality of the cocoa beans, optimizing this process could result in better quality beans. For instance, the use of mechanical dryers could help achieve the optimal moisture content more consistently. Additionally, training programs for producers on best practices for fermentation and drying could also help improve the quality of the cocoa beans. These technical solutions, combined with the findings of this study, could help enhance the quality of cocoa beans produced in the regions.
4. Conclusions
The physical evaluation of cocoa beans by the cutting test allowed us to conclude that the samples from the five studied regions presented a satisfactory commercial standard. However, it highlighted the lack of standardization in the bean processing step, presenting a significant percentage of partially brown cocoa beans. The physicochemical characterization of the cocoa beans allowed observing significant differences in lipids, ash, acidity, antioxidant capacity, epicatechin, procyanidin B2, theophylline, and theobromine among samples from different regions of the state. The differences can directly contribute to the quality and technological performance of derived products. Overall, the evaluation of the quality of Amazonian cocoa beans provided important information not available in the literature. In conclusion, the results suggest that cocoa from the Lower Tocantins River region has great potential for use in the production of functional chocolates with high antioxidant capacity, but further studies are needed to confirm these findings.
Funding sources
This work was funded by the National Council for Scientific and Technological Development (CNPq), Brazil; Coordination for the Improvement of Higher Education Personnel (CAPES), Brazil; Pará Cocoa Development Fund (Funcacau), SEDAP, Government of the State of Pará, Brazil; Pro-rectorate for Research and Graduate Studies-UFPA (PROPESP-UFPA), and Federal University of Pará (UFPA), Brazil.
CRediT authorship contribution statement
Niara Maria de Jesus Silva: Writing – review & editing, Writing – original draft, Validation, Methodology, Investigation, Formal analysis, Conceptualization. Consuelo Lúcia Sousa de Lima: Writing – review & editing, Validation, Supervision, Resources, Methodology, Conceptualization. Renato Meireles dos Santos: Investigation, Formal analysis, Conceptualization. Hervé Rogez: Writing – review & editing, Writing – original draft, Validation, Resources, Conceptualization. Jesus Nazareno Silva de Souza: Writing – review & editing, Writing – original draft, Validation, Supervision, Resources, Project administration, Methodology, Funding acquisition, Data curation, Conceptualization.
Declaration of Competing interest
All co-authors have seen and agree with the contents of the manuscript and there is no financial interest to report. The manuscript has not been previously published, is not currently submitted for review to any journal, and will not be submitted elsewhere before a decision is made by this journal.
References
- 1.Zarrillo S., Gaikwad N., Lanaud C., Powis T., Viot C., Lesur I., Fouet O., Argout X., Guichoux E., Salin F., Solorzano R.L., Bouchez O., Vignes H., Severts P., Hurtado J., Yepez A., Grivetti L., Blake M., Valdez F. Uso e domesticação de Theobroma cacao durante o Holoceno médio no alto Amazonas. Nature Ecology & Evolution. 2018;2:1879–1888. doi: 10.1038/s41559-018-0697-x. [DOI] [PubMed] [Google Scholar]
- 2.de Souza P.A., Moreira L.F., Sarmento D.H.A., da Costa F.B. Cacao—theobroma cacao, exotic fruits. 2018. 69–76. [DOI]
- 3.Kongor J.E., Hinneh M., de Walle D.V., Afoakwa E.O., Boeckx P., Dewettinck K. Factors influencing quality variation in cocoa (Theobroma cacao) bean flavour profile—a review. Food Res. Int. 2016;82:44–52. doi: 10.1016/j.foodres.2016.01.012. [DOI] [Google Scholar]
- 4.ICCO – International Cocoa Organization Cocoa market report may 2022. 2022. https://www.icco.org/cocoa-market-report-for-may-2022/
- 5.IBGE – Instituto Brasileiro de Geografia e Estatística. 2021. https://www.ibge.gov.br/busca.html?searchword=CACAU&searchphrase=all&I
- 6.Saltini R., Akkerman R., Frosch S. Optimizing chocolate production through traceability: a review of the influence of farming practices on cocoa bean quality. Food Control. 2013;29(1):167–187. doi: 10.1016/j.foodcont.2012.05.054. [DOI] [Google Scholar]
- 7.Herman C., Spreutels L., Turomzsa N., Konagano E.M., Haut B. Convective drying of fermented Amazonian cocoa beans (Theobroma cacao var. Forasteiro). Experiments and mathematical modeling. Food Bioprod. Process. 2018;108:81–94. doi: 10.1016/j.fbp.2018.01.002. [DOI] [Google Scholar]
- 8.dos Santos R.M., Silva N.M. de J., Moura F.G., Lourenço L. de F.H., de Souza J.N.S., Sousa de Lima C.L. Analysis of the sensory profile and physical and physicochemical characteristics of amazonian cocoa (Theobroma cacao L.) beans produced in different regions. Foods. 2024;13(14):2171. doi: 10.3390/foods13142171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Afoakwa E.O., Paterson A., Fowler M., Ryan A. Flavor Formation and character in cocoa and chocolate: a critical review. Crit. Rev. Food Sci. Nutr. 2008;48(9):840–857. doi: 10.1080/10408390701719272. [DOI] [PubMed] [Google Scholar]
- 10.Fang Y., Li R., Chu Z., Zhu K., Gu F., Zhang Y. Chemical and flavor profile changes of cocoa beans (Theobroma cacao L.) during primary fermentation. Food Sci. Nutr. 2020;8(8):4121–4133. doi: 10.1002/fsn3.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hii C.L., Law C.L., Cloke M., Suzannah S. Thin layer drying kinetics of cocoa and dried product quality. Biosyst. Eng. 2009;102(2):153–161. doi: 10.1016/j.biosystemseng.2008.10.007. [DOI] [Google Scholar]
- 12.Chee Leong B.C., Sarjadi M.S., Shah R.M., Majid M.H.A. The effect of processing operations on the polyphenol content of cocoa beans: a review. Malaysian Journal of Chemistry. 2024;26(2):46–66. doi: 10.55373/mjchem.v26i2.46. [DOI] [Google Scholar]
- 13.ISO . International Organization for Standardization; Geneva: 2014. Cocoa Beans - Specification (ISO 2451) [Google Scholar]
- 14.MAPA, Ministério da Agricultura. Abastecimento Pecuária e. Instrução Normativa N°. 2008. Regulamento Técnico da Amêndoa de Cacau; p. 38.https://sistemasweb.agricultura.gov.br/sislegis/action/detalhaAto.do?method=visualizarAtoPortalMapa&chave=250964455 [Google Scholar]
- 15.Do Carmo Brito B.N., Chisté R.C., Pena R., da S., Gloria M.B.A., Lopes A.S. Food Chem. 2017. Bioactive amines and phenolic compounds in cocoa beans are affected by fermentation; pp. 484–490. [DOI] [PubMed] [Google Scholar]
- 16.AOAC . eighteenth ed. Association of Official Analytical Chemists; Rockville, MA, USA: 2005. Official Methods of Analysis. [Google Scholar]
- 17.IAL (Instituto Adolfo Lutz) third ed., v. 1. IMESP; São Paulo: 2008. Normas Analíticas do Instituto Adolfo Lutz: Métodos químicos e físicos para análise de alimentos. [Google Scholar]
- 18.Counet C., Collin S. Effect of the number of flavanol units on the antioxidant activity of procyanidin fractions isolated from chocolate. J. Agric. Food Chem. 2003;51(23):6816–6822. doi: 10.1021/jf030349g. [DOI] [PubMed] [Google Scholar]
- 19.Singleton V.L., Orthofer R., Lamuela-Raventós R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- 20.Gomes M., Rocha M.S.R.S., Barbosa K.L., Abreu Í.B.S., Pimentel W.R.O., Silva C.E.F., Almeida R.M.R.G., Albuquerque E.C.M.C., Vieira R.C. Agricultural coconut cultivation wastes as feedstock for lignocellulosic ethanol production by kluyveromyces marxianus. Waste Biomass Valorization. 2021;12(9):4943–4951. doi: 10.1007/s12649-021-01345-w. 2021. [DOI] [Google Scholar]
- 21.Huang D., Ou B., Hampsch-Woodill M., Flanagan J.A., Prior R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002;50(16):4437–4444. doi: 10.1021/jf0201529. [DOI] [PubMed] [Google Scholar]
- 22.Gaspar D.P., Chagas Junior G.C.A., de Aguiar Andrade E.H., do Nascimento L.D., Chisté R.C., Ferreira N.R., Martins L.H. da S., Lopes A.S. How climatic seasons of the Amazon biome affect the aromatic and bioactive profiles of fermented and dried cocoa beans. Molecules. 2021;26(13):3759. doi: 10.3390/molecules26133759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Vuyst L., Weckx S. The cocoa bean fermentation process: from ecosystem analysis to starter culture development. J. Appl. Microbiol. 2016;121(1):5–17. doi: 10.1111/jam.13045. [DOI] [PubMed] [Google Scholar]
- 24.Brachène Y.C. Targeting Optimization and Standardization. Université Catholique de Louvain; Ottignies-Louvain-la-Neuve, Belgique: 2015. Assessment of qualitative parameters including polyphenol oxidase activity for different drying methods in Amazonian cocoa processing. Master's Thesis. [Google Scholar]
- 25.Niikoi Kotey R., Asomaning Odoom D., Kumah P., Oppong Akowuah J., Fobi Donkor E., Kwatei Quartey E., Kofi Sam E., Owusu-Kwarteng J., Gyasi Santo K., Kwami-Adala F., Owusu Boateng D. Effects of fermentation periods and drying methods on postharvest quality of cocoa (theobroma cacao) beans in Ghana. J. Food Qual. 2022;2022 doi: 10.1155/2022/7871543. [DOI] [Google Scholar]
- 26.FCC - Federation of Cocoa Commerce Ltd Quality Rules. 2012 https://www.icco.org/faq/59-fermentation-a-drying/108-how-is-the-quality-of-cocoa-checked-by-hand-by-machine.html [Google Scholar]
- 27.Ferreira A.C.R. 2017. Indicação Geográfica Sul da Bahia – Manual de Controle de Qualidade do Cacau Sul da Bahia, PTCSB, Ilhéus-BA. [Google Scholar]
- 28.Efraim P., Pezoa-García N.H., Jardim D.C.P., Nishikawa A., Haddad R., Eberlin M.N. Influência da fermentação e secagem de amêndoas de cacau no teor de compostos fenólicos e na aceitação sensorial. Ciência Tecnol. Aliment. 2010;30:142–150. doi: 10.1590/S0101-20612010000500022. [DOI] [Google Scholar]
- 29.D'Souza R.N., Grimbs S., Behrends B., Bernaert H., Ullrich M.S., Kuhnert N. Origin-based polyphenolic fingerprinting of Theobroma cacao in unfermented and fermented beans. Food Res. Int. 2017;99:550–559. doi: 10.1016/j.foodres.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 30.Beckett S.T., editor. Industrial Chocolate Manufacture and Use. fourth ed. Wiley-Blackwell; 2009. [Google Scholar]
- 31.Pimentel A.A. Universidade Estadual do Sudoeste da Bahia; Bahia: 2016. Avaliação da capacidade antioxidante e quantificação de constituintes fenólicos de cocoa nibs e chocolate de variedades clonais de cacaueiro, Dissertação de Mestrado. [Google Scholar]
- 32.Barrientos L.D.P., Oquendo J.D.T., Garzón M.A.G., Álvarez O.L.M. Effect of the solar drying process on the sensory and chemical quality of cocoa (Theobroma cacao L.) cultivated in Antioquia, Colombia. Food Res. Int. 2019;115:259–267. doi: 10.1016/j.foodres.2018.08.084. [DOI] [PubMed] [Google Scholar]
- 33.Hashimoto J.C., Lima J.C., Celeghini R.M.S., Nogueira A.B., Efraim P., Poppi R.J., Pallone J.A.L. Quality control of commercial cocoa beans (theobroma cacao L.) by near-infrared spectroscopy. Food Anal. Methods. 2018;11(5):1510–1517. doi: 10.1007/s12161-017-1137-2. [DOI] [Google Scholar]
- 34.Krähmer A., Engel A., Kadow D., Ali N., Umaharan P., Kroh L.W., Schulz H. Fast and neat – determination of biochemical quality parameters in cocoa using near infrared spectroscopy. Food Chem. 2015;181:152–159. doi: 10.1016/j.foodchem.2015.02.084. [DOI] [PubMed] [Google Scholar]
- 35.Nuñez J.M., Bahamón-Monje A.F., García-Rincón P.A. Características fisicoquímicas y sensoriales de almendras fermentadas de cacao nacional (Theobroma Cacao L.) con adición de probióticos en el centro de investigaciones amazónicas, Cimaz Macagual (Caquetá, Colombia) Ingeniería Y Competitividad. 2021;23(2) doi: 10.25100/iyc.v23i2.10885. [DOI] [Google Scholar]
- 36.Oracz J., Zyzelewicz D., Nebesny E. The content of polyphenolic compounds in cocoa beans (Theobroma cacao L.), depending on variety, growing region, and processing operations: a review. Crit. Rev. Food Sci. Nutr. 2015;55(9):1176–1192. doi: 10.1080/10408398.2012.686934. [DOI] [PubMed] [Google Scholar]
- 37.Quiroz-Reyes C.N., Fogliano V. Design cocoa processing towards healthy cocoa products: the role of phenolics and melanoidins. J. Funct.Foods. 2018;45:480–490. doi: 10.1016/j.jff.2018.04.031. [DOI] [Google Scholar]
- 38.Brunetto M. del R., Gallignani M., Orozco W., Clavijo S., Delgado Y., Ayala C., Zambrano A. The effect of fermentation and roasting on free amino acids profile in Criollo cocoa (Theobroma cacao L.) grown in Venezuela. Braz. J. Food Technol. 2020;23 doi: 10.1590/1981-6723.15019. [DOI] [Google Scholar]
- 39.Andújar I., Recio M.C., Giner R.M., Ríos J.L. Cocoa polyphenols and their potential benefits for human health. Oxid. Med. Cell. Longev. 2012;2012(1):1–23. doi: 10.1155/2012/906252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peres L.G., Brandão V.B., de Rezende A.J. Teobromina, substância encontrada no cacau. Revista JRG de Estudos Acadêmicos. 2018;1(3):48–55. doi: 10.5281/ZENODO.4450841. [DOI] [Google Scholar]
- 41.Carneiro N.S.P., de Souza F.D.P., de Souza J.N.S., da Costa H.S.C. In: Avanços em Ciência e Tecnologia de Alimentos—Volume 2, first ed., Científica Digital. Verruck S., editor. 2020. Avaliação da enzima peroxidase e dos compostos fenólicos durante etapas de fermentação de Theobroma cacao; pp. 44–54. [DOI] [Google Scholar]
- 42.Febrianto N.A., Zhu F. Composition of methylxanthines, polyphenols, key odorant volatiles and minerals in 22 cocoa beans obtained from different geographic origins. LWT. 2022;153 doi: 10.1016/j.lwt.2021.112395. [DOI] [Google Scholar]
- 43.Chagas Junior G.C.A., Ferreira N.R., Gloria M.B.A., Martins L.H. da S., Lopes A.S. Chemical implications and time reduction of on-farm cocoa fermentation by Saccharomyces cerevisiae and Pichia kudriavzevii. Food Chem. 2021;338 doi: 10.1016/j.foodchem.2020.127834. [DOI] [PubMed] [Google Scholar]
- 44.Lima L.J.R., Almeida M.H., Nout M.J.R., Zwietering M.H., Theobroma cacao L. “The food of the gods”: quality determinants of commercial cocoa beans, with particular reference to the impact of fermentation. Crit. Rev. Food Sci. Nutr. 2011;51(8):731–761. doi: 10.1080/10408391003799913. [DOI] [PubMed] [Google Scholar]
- 45.Carrillo L.C., Londoño-Londoño J., Gil A. Comparison of polyphenol, methylxanthines and antioxidant activity in Theobroma cacao beans from different cocoa-growing areas in Colombia. Food Res. Int. 2014;60:273–280. doi: 10.1016/j.foodres.2013.06.019. [DOI] [Google Scholar]
- 46.Vázquez-Ovando A., Chacón-Martínez L., Betancur-Ancona D., Escalona-Buendía H., Salvador-Figueroa M. Sensory descriptors of cocoa beans from cultivated trees of Soconusco, Chiapas, Mexico. Food Science and Technology (Campinas) 2015;35(2):285–290. doi: 10.1590/1678-457X.6552. [DOI] [Google Scholar]