Abstract
Background
While COVID-19 vaccination has been shown to reduce the risk of severe illness, its impact on the occurrence of persistent symptoms in patients with mild Omicron infection remains uncertain. Our objective was to investigate whether COVID-19 vaccination reduces the occurrence of persistent COVID-19-related symptoms 3 months after mild Omicron infection.
Methods
Multicenter prospective cohort study was conducted in Brazil between January 2022 and June 2023 when Omicron was predominant. Participants ≥ 18 years seeking outpatient care for symptomatic SARS-CoV-2 infection were enrolled. Complete vaccination included individuals who received the full primary series and any booster dose, while incomplete vaccination included those with incomplete primary series or no vaccination. The primary outcome was any persistent symptoms at 3 months. Secondary outcomes were organ system-specific persistent symptoms and the EQ-5D-3L utility score. All outcomes were assessed by means of structured telephone interviews 3 months after enrollment.
Results
1,067 patients were enrolled (median age, 39 years), of which 914 (871 completely vaccinated and 43 unvaccinated or incompletely vaccinated). Among the vaccinated participants the median time since the last vaccine dose was 145 (interquartile range, 106–251) days. A total of 388/1067 (36.9 %) had a prior infection at the time of study entry. The occurrence of overall persistent COVID-19-related symptoms at 3 months was 41.6 % (n = 362) among completely vaccinated and 44.2 % (n = 19) among unvaccinated or incompletely vaccinated patients (adjusted risk ratio [aRR], 0.87; 95 % confidence interval [CI], 0.61–1.23; p = 0.43). Complete vaccination was associated with lower occurrence of mental health symptoms (aRR, 0.44; 95 % CI, 0.24–0.81; p = 0.01). No differences were found in the occurrence of persistent symptoms in other specific domains, nor in EQ-5D-3L utility scores.
Conclusions
This study was not able to identify a statistically significant protection of complete COVID-19 vaccination against any overall persistent symptoms at 3 months. Nevertheless, complete vaccination was associated with a lower occurrence of persistent mental health symptoms.
Keywords: COVID-19 vaccine, COVID-19 vaccine booster shot, Post-Acute COVID-19 Syndrome
1. Introduction
Following recovery from the acute phase of COVID-19, a significant proportion of patients continue to experience physical, cognitive, or psychological symptoms. This phenomenon has been termed long COVID or post COVID-19 condition [1], [2], [3]. However, there is no consensus on the precise definition of long COVID, and the methodological limitations of the existing studies contribute to this issue. Currently, long COVID is recognized as a major health concern, with projections suggesting that its effects might afflict over 100 million individuals worldwide [2].
The effectiveness of COVID-19 vaccines in reducing acute complications of SARS-CoV-2 infection, such as hospitalization and mortality, has been well established [4]. However, the impact of vaccination on persistent COVID-19-related symptoms is not fully understood. While some studies indicate a significant protective effect [5], others have reported varying outcomes [6], [7], including mixed results [8], [9], no effect, or even the possibility of increased risk [6], [7]. Most of the studies conducted to date are observational and have limitations, such as low sample size and an increased risk of systematic errors, such as selection bias and confounding. Additionally, the majority of these studies were conducted in contexts that may not necessarily be representative of the current scenario characterized by high vaccination coverage and the predominance of the Omicron variant as the main cause of COVID-19. Evidence of the impact of vaccination on persistent COVID-19-related symptoms among patients with mild SARS-CoV-2 infection is limited, especially in the aforementioned scenario [7]. Accordingly, this study was conducted when Omicron was a predominant variant, and it investigated whether COVID-19 vaccination is associated with decreased occurrence of persistent COVID-19-related symptoms 3 months after symptomatic mild SARS-CoV-2 infection.
2. Methods
2.1. Study design
This article describes an ancillary study of the Post COVID-19 Brazil II study (NCT05197647), a prospective multicenter cohort designed to assess quality of life and long-term disabilities among patients with symptomatic mild SARS-CoV-2 infection in Brazil. The study rationale and design have been published elsewhere [10]. Briefly, consecutive adult patients with symptomatic COVID-19 not requiring hospitalization were enrolled across 14 centers in Brazil (Supplementary e-Table 1 and e-Fig. 1), and they were followed up using structured telephone interviews. Vaccination status prior to COVID-19 diagnosis was assessed at the time of enrollment. The presence of persistent COVID-19 self-reported symptoms was assessed 3 months after enrollment, aligning with the World Health Organization (WHO) definition of Long COVID or Post-COVID Conditions, which specifies symptoms persisting or emerging 3 months after initial SARS-CoV-2 infection. The institutional review boards of all participating centers approved the study protocol. Consent for participation was obtained from all enrolled patients or their proxies.
2.2. Participants
The study included patients ≥ 18 years of age seeking outpatient care for symptomatic COVID-19, defined as the presence of at least one of the following symptoms: fever, cough, sneezing, dyspnea, low peripheral oxygen saturation (<95 % while breathing room air), dysosmia, ageusia, rhinorrhea, sore throat, myalgia, arthralgia, or diarrhea, in addition to a positive reverse transcription polymerase chain reaction (RT-PCR) or antigen test for SARS-CoV-2 infection. Confirmed positive test results were a strict eligibility criterion. Exclusion criteria were severe underlying illnesses with a life expectancy of less than 3 months, need for COVID-19-related hospitalization within 30 days following inclusion, lack of a responsible family member (for patients with communication difficulties such as aphasia, cognitive deficit, and non-Portuguese speakers), unavailability for telephone contact, withdrawal of consent, and previous inclusion in the study. Patients with unknown vaccination status were also excluded from the analysis.
2.3. Vaccination status
COVID-19 vaccination status was based on source documentation or plausible reporting by the patient or their proxies at the time of enrollment. The primary exposure variable was complete vaccination, which included (I) those with only the primary vaccination series (i.e., two doses of the CoronaVac, ChAdOx1 nCov-19, or BNT162b2 vaccines or the single-dose Ad26.Cov2.S vaccine) and (II) those with the primary vaccination series plus one or more booster doses. The reference group for the primary exposure consisted of participants who had not received any COVID-19 vaccine and those with an incomplete primary series (i.e., a single dose of the CoronaVac, ChAdOx1 nCov-19, or BNT162b2 vaccines) prior to enrollment. The secondary exposure variable was complete vaccination plus one or more booster doses (versus complete vaccination without a booster dose) (Supplementary e-Table 2).
2.4. Outcomes and follow-up
The primary outcome was any persistent COVID-19-related symptoms 3 months after study enrollment. To identify persistent symptoms, patients were questioned about COVID-19-related symptoms during the 3-month evaluation, considering the resolution or persistence of (I) symptoms initially linked to the COVID-19 diagnosis and (II) new symptoms emerging post-COVID. Symptoms were self-reported and classified according to the symptoms described in the literature on “long COVID” and “post COVID-19 condition” (Supplementary e-Table 3) [3], [11], [12]. Secondary outcomes were organ system-specific persistent symptoms (general, neurological, respiratory, and mental health) [13] (Supplementary e-Table 4) and a health-related quality-of-life utility score, measured at 3 months by the three-level version of the EuroQol five-dimension (EQ-5D-3L) [14] descriptive system (range, −0.17 to 1.0, where negative values are valued as worse than death, and 1.0 is the best health state possible). The estimated minimal clinically important difference in EQ-5D-3L score is 0.03 [15], and the mean value for the Brazilian population is 0.82 [16].
All outcomes were assessed by trained researchers not involved in patient care by means of structured telephone interviews 3 months after enrollment. Patients were classified as lost to follow-up after 10 unsuccessful attempts at telephone contact at different times of day on several days.
2.5. Power analysis
The actual sample size of 914 analyzed patients has a statistical power of 80 % to detect vaccine effectiveness ≥ 46 % for the primary outcome with an alpha of 0.05. This estimate takes into account the observed uneven group sizes (871 completely vaccinated and 43 unvaccinated or incompletely vaccinated). To calculate the power, logistic regression methodology was used for a binary predictor [17].
2.6. Statistical analysis
Continuous variables are expressed as median and interquartile range (IQR). Categorical variables are expressed as counts and percentages. Poisson regression with robust variance adjusted for age, sex, history of anxiety or depression, Charlson comorbidity index (CCI), and SARS-CoV-2 variant proxy period (periods in which different Omicron subvariants were predominant) was used to estimate the adjusted risk ratios (aRRs) of the primary outcome among exposed and non-exposed groups. The variables selected for the multivariate model were chosen based on existing evidence in the literature regarding long COVID. Vaccine effectiveness was estimated by the formula (1 – aRR) × 100. The EQ-5D-3L utility score was evaluated using a gaussian regression model adjusted for the same covariates used in the primary outcome analysis. Additional outcomes were assessed using the same model used for the primary outcome. To check for consistency and possible biases, we performed five sensitivity analyses for the primary outcome: (I) excluding individuals with a CCI > 0 to avoid the effect of preexisting comorbidities on persistent symptoms; (II) using only unvaccinated patients as the reference group for the primary exposure to avoid a potential effect of incomplete primary vaccination series on the primary outcome analysis; (III) adding to the primary statistical model additional baseline variables that were unbalanced across the two primary exposure groups (years of education being a healthcare worker and number of COVID symptoms at inclusion); (IV) adding to the primary statistical model the number of acute COVID-19 symptoms; and (V) excluding participants with a baseline history of anxiety or depression. All statistical analyses were performed in R version 4.3.0 [18]. All tests were two-tailed, and p values < 0.05 were considered statistically significant. The confidence interval (CI) widths or p values of secondary outcomes were not adjusted for multiple testing.
3. Results
3.1. Recruitment and follow-up
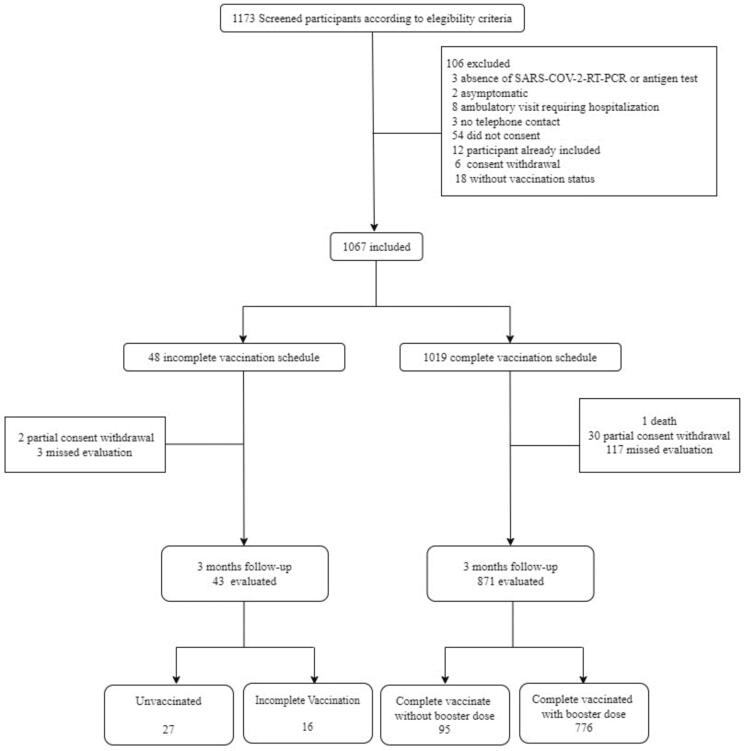
From January to November 2022, 1,173 patients were screened; of these, 1,067 were enrolled (Fig. 1). One patient died and 32 withdrew consent before outcome assessment, and 120 patients missed the 3-month follow-up assessment. Therefore, 914 patients were ultimately included in the primary outcome analysis (871 completely vaccinated and 43 unvaccinated or incompletely vaccinated). The last follow-up was performed in June 2023. Compared with patients included in the primary outcome analysis (Supplementary e-Table 4), the group of patients who died, withdrew consent, or missed the 3-month assessment had fewer years of education, a lower proportion of health care workers, and fewer individuals with a history of smoking and depression at baseline (Supplementary e-Table 6). Supplementary e-Table 7 shows the characteristics of participating centers, most of which were teaching hospitals designated as referral centers for outpatient COVID-19 management.
Fig. 1.
Study flowchart.
3.2. Baseline characteristics
Table 1 and e-Fig S4 show the baseline characteristics of all enrolled participants. The median age was 39 years, and 69.2 % of patients were women. Most patients (84.4 %) had a CCI of zero, and 44.2 % were health care workers. A detailed description of COVID-19 vaccines administered is provided in e-Fig S3. Compared with completely vaccinated patients, the group of unvaccinated or incompletely vaccinated patients had fewer years of education, a lower median number of COVID-19-related acute symptoms, a lower proportion of health care workers, and fewer individuals with a history of anxiety at baseline. Among the vaccinated participants the median time since the last vaccine dose was 145 (interquartile range, 106–251) days. A total of 388/1067 (36.9 %) had a prior infection at the time of study entry.
Table 1.
Characteristics of participants enrolled.
| All cohort (n = 1,067) | Unvaccinated or incompletely vaccinated group (n = 48) | Completely vaccinated group (n = 1,019) | Completely vaccinated without a booster dose group (n = 132) | Completely vaccinated with a booster dose group (n = 887) | |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 738/1,067 (69.2) | 29/48 (60.4) | 709/1,019 (69.6) | 83/132 (62.8) | 626/887 (70.6) |
| Age, median (IQR) | 39.0 (30.8;50.0) | 35.0 (26.0;47.0) | 39.0 (31.0;50.5) | 37.0 (28.8;49.3) | 39.0 (31.0;51.0) |
| Age ≥ 60 years | 133/1.067 (12.0) | 8/48 (17.0) | 125/1,019 (12.0) | 10/132 (8.0) | 115/887 (13) |
| Self-identified race2 | |||||
| White | 794/1,066 (74.5) | 37/48 (77.1) | 757/1,018 (74.4) | 110/132 (83.3) | 647/886 (73.0) |
| No White | 272/1,066 (25.5) | 11/48 (22.9) | 261/1,018 (25.6) | 22/132 (16.7) | 239/886 (27.0) |
| Years of education | 15.0 (11.0;17.0) | 11.0 (10.0;15.5) | 15.0 (11.0;18.0) | 13.0 (10.0;16.0) | 15.0 (11.0;18.0) |
| Health care worker | 410/929 (44.1) | 9/39 (23.1) | 401/890 (45.1) | 20/111 (18.0) | 381/779 (48.9) |
| History of smoking | 108/1,061 (10.2) | 4/47 (8.5) | 104/1,014 (10.3) | 11/130 (8.5) | 93/884 (10.5) |
| Hazardous alcohol consumption1 | 88/1,067 (8.3) | 3/48 (6.3) | 85/1.019 (8.3) | 9/132 (6.8) | 76/887 (8.6) |
| BMI | 25.9 (23.3;29.4) | 25.7 (23.3;29.3) | 25.9 (23.3;29.4) | 26.5 (23.6;29.5) | 25.9 (23.2;29.4) |
| CCI < 1 | 900/1,067 (84.4) | 39/48 (81.3) | 861/1,019 (84.5) | 116/132 (87.9) | 745/887 (84.0) |
| History of anxiety | 194/1,061 (18.3) | 3/48 (6.3) | 191/1,013 (18.9) | 12/131 (9.2) | 179/882 (20.3) |
| History of depression | 86/1,060 (8.1) | 3/48 (6.3) | 83/1,012 (8.2) | 7/131 (5.3) | 76/881 (8.6) |
| Number of COVID symptoms at inclusion | 4.00 (3.0;6.0) | 3.00 (2.0;4.0) | 4.00 (3.0;7.0) | 3.00 (2.0;5.0) | 4.00 (3.0;7.0) |
| SARS-CoV-2 variants by proxy variant period | |||||
| BA.1 | 52/1,067 (4.9) | 2/48 (4.2) | 50/1,019 (4.9) | 16/132 (12.1) | 34/887 (3.8) |
| BA.2 | 319/1,061 (29.9) | 20/48 (41.7) | 299/1,019 (29.3) | 40/132 (30.3) | 259/887 (29.2) |
| BA.5 | 592/1,067 (55.5) | 21/48 (43.8) | 571/1,019 (56.0) | 68/132 (51.5) | 503/887 (56.7) |
| BQ1.1 | 104/1,067 (9.8) | 5/48 (10.4) | 99/1,019 (9.7) | 8/132 (6.1) | 91/887 (10.3) |
Data are presented as n/N (%) or median (interquartile range). BMI, body mass index; CCI, Charlson comorbidity index.
> 14 standard drinks per week for women and 21 standard drinks per week for men.
> According to IBGE (Brazilian Institute of Geography and Statistics), there are five main categories of racial self- identification in Brazil: White (descendants of Europeans), No White: Black (descendants of Africans), Brown, Yellow (descendants of Asians), and Indigenous.
3.3. Overall persistent symptoms
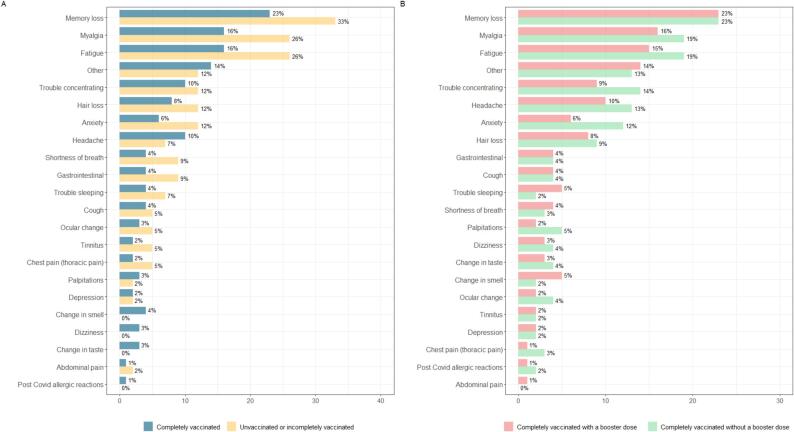
At 3 months, persistent COVID-19-related symptoms were found in 381 of 914 (41.6 %) participants. The most commonly reported symptoms were memory loss in 211 participants (23 %), myalgia in 150 (16 %), fatigue in 149 (16 %), headache in 92 (10 %), concentration problems in 90 (10 %), hair loss in 75 (8 %), anxiety in 59 (6 %), and shortness of breath in 40 (4 %). The frequency of persistent COVID-19-related symptoms stratified by the organ system is shown in e-Fig S5 and e-Fig S6. Of the 381 participants who reported overall persistent symptoms, 275 (72.2 %) reported general symptoms, 278 (73.0 %) reported neurological symptoms, 93 (24.4 %) reported mental health symptoms, and 73 (19.2 %) reported respiratory symptoms. Among the participants with persistent COVID-19-related symptoms, 19 (5.0 %) reported symptoms affecting all domains (general, neurological, respiratory, and mental health), whereas 204 (53.5 %) had persistent symptoms in two or more domains (e-Fig S5).
3.4. Outcomes
Results for the primary and secondary study outcomes are summarized in Table 2 and Table 3. There was no significant difference in the primary outcome between the two primary exposure groups: the occurrence of overall persistent COVID-19-related symptoms at 3 months was 41.6 % (n = 362) among completely vaccinated patients and 44.2 % (n = 19) among unvaccinated or incompletely vaccinated patients (aRR, 0.87; 95 % CI, 0.61–1.23; p = 0.43). The results of sensitivity analyses for the primary outcome were similar to those of the main analysis (Supplementary e-Table 8). Completely vaccinated patients had a significantly lower occurrence of mental health symptoms than those with incomplete or no vaccination (9.6 % vs 20.9 %; aRR, 0.44; 95 % CI, 0.24–0.81; p = 0.01). However, no significant differences were found between the two primary exposure groups in the occurrence of persistent symptoms in the general, neurological, or respiratory domains, nor in the mean number of organ systems affected or EQ-5D-3L utility scores, as shown in Table 2.
Table 2.
Comparison of outcomes between completely vaccinated and unvaccinated or incompletely vaccinated patients.
| Unvaccinated or incompletely vaccinated |
Completely vaccinated |
Crude model |
Adjusted model** |
|||||
|---|---|---|---|---|---|---|---|---|
| (n = 43) | (n = 871) | Effect size1 (95 % CI) | VE (95 % CI) | p | Effect size1 (95 % CI) | VE (95 % CI) | p | |
| Any persistent COVID-19-related symptoms | 19/43 (44.2) | 362/871 (41.6) | 0.94 (0.67 to 1.33) | 5.94 % (–32.8 % to 33.4 %) | 0.73 | 0.87 (0.61 to 1.23) | 13.0 % (–23.1 % to 38.5 %) | 0.43 |
| Organ system-specific persistent COVID-19-related symptoms | ||||||||
| Neurological | 15/43 (34.9) | 263/871 (30.2) | 0.87 (0.57 to 1.32) | 13.4 % (−31.8 % to 43.2 %) | 0.50 | 0.80 (0.52 to 1.23) | 19.9 % (–22.7 % to 47.7 %) | 0.31 |
| Mental health | 9/43 (20.9) | 84/871 (9.6) | 0.46 (0.25 to 0.85) | 53.9 % (14.7 % to 75.1 %) | 0.01 | 0.44 (0.24 to 0.81) | 56.1 % (19.2 % to 76.1 %) | 0.01 |
| Respiratory | 6/43 (14.0) | 70/871 (8.0) | 0.58 (0.27 to 1.25) | 42.4 % (−25.1 % to 73.5 %) | 0.16 | 0.54 (0.25 to 1.16) | 46.3 % (−16.2 % to 75.2 %) | 0.11 |
| General | 14/43 (32.6) | 261/871 (30.0) | 0.92 (0.59 to 1.43) | 7.9 % (−43.2 % to 40.8 %) | 0.71 | 0.83 (0.54 to 1.27) | 17.0 % (−27.3 % to 45.9 %) | 0.39 |
| EQ-5D-3L | ||||||||
| n assessed | 41 | 851 | ||||||
| Mean (SD) | 0.83 (0.19) | 0.84 (0.15) | 0.001 (−0.04 to 0.06) | N/A | 0.74 | 0.02 (−0.03 to 0.07) | N/A | 0.41 |
| Median (IQR) | 0.80 (0.74, 1.00) | 0.80 (0.74, 1.00) | ||||||
Data are presented as n/N (%) unless otherwise specified. CI, confidence interval; SD, standard deviation; VE, vaccine effectiveness; EQ-5D-3L, EuroQol 5-dimension 3-level questionnaire.
**An effectiveness assessment of vaccination status was adjusted for the following confounding variables: age, sex, history of anxiety or depression, Charlson comorbidity index, and the proxy period for SARS-CoV-2 variants.
Risk ratio for persistent COVID-19, classified into domains, EQ-5D-3L below the Brazilian mean and difference in means for EQ-5D-3L.
Table 3.
Comparison of outcomes between completely vaccinated patients without a booster dose and completely vaccinated patients who received at least one booster dose.
| Completely vaccinated without a booster dose |
Completely vaccinated with a booster dose |
Crude model |
Adjusted model* |
|||||
|---|---|---|---|---|---|---|---|---|
| (n = 95) | (n = 776) | Effect size1 (95 % CI) | VE (95 % CI) | P | Effect size1 (95 % CI) | VE (95 % CI) | p | |
| Any persistent COVID-19-related symptoms | 43/95 (45.3) | 319/776 (41.1) | 0.91 (0.72 to 1.15) | 9.18 (−15.1 % to 28.3 %) | 0.43 | 0.81 (0.64 to 1.02) | 19.5 % (−1.7 % to 36.3 %) | 0.07 |
| Organ system-specific persistent COVID-19-related symptoms | ||||||||
| Neurological | 31/95 (32.6) | 232/776 (29.9) | 0.92 (0.67 to 1.25) | 8.38 (−24.7 % to 32.7 %) | 0.58 | 0.78 (0.57 to 1.07) | 21.8 % (−6.8 % to 42.8 %) | 0.12 |
| Mental health | 13/95 (13.7) | 71/776 (9.1) | 0.67 (0.39 to 1.16) | 33.14 (−16.1 % to 61.5 %) | 0.15 | 0.57 (0.33 to 0.98) | 43.3 % (1.9 % to 67.2 %) | 0.04 |
| Respiratory | 8/95 (8.4) | 62/776 (8.0) | 0.95 (0.47 to 1.92) | 5.12 (−912.0 % to 53.1 %) | 0.88 | 0.83 (0.41 to 1.68) | 17.2 % (−68.4 % to 59.3 %) | 0.60 |
| General | 32/95 (33.7) | 229/776 (29.5) | 0.88 (0.65 to 1.19) | 12.39 (−18.5 % to 35.3 %) | 0.39 | 0.75 (0.56 to 1.01) | 24.7 % (−0.9 % to 43.9 %) | 0.06 |
| EQ-5D-3L | ||||||||
| n assessed | 90 | 761 | ||||||
| Mean (SD) | 0.85 (0.15) | 0.84 (0.15) | −0.01 (−0.05 to 0.02) | N/A | 0.52 | 0.01 (−0.02 to 0.05) | N/A | 0.48 |
| Median (IQR) | 0.80 (0.74,1.00) | 0.80 (0.74,1.00) | ||||||
Data are presented as n/N (%) unless otherwise specified. CI, confidence interval; SD, standard deviation; VE, vaccine effectiveness; EQ-5D-3L, EuroQol 5-dimension 3-level questionnaire.
**An effectiveness assessment of vaccination status was adjusted for the following confounding variables: age, sex, history of anxiety or depression, Charlson comorbidity index, and the proxy period for SARS-CoV-2 variants.
Risk ratio for persistent COVID-19, classified into domains, EQ-5D-3L below the Brazilian mean and difference in means for EQ-5D-3L.
There was no significant difference between completely vaccinated patients who received a booster dose and those who did not receive a booster dose regarding the occurrence of any overall persistent symptoms, neurological, respiratory or general persistent COVID-19-related symptoms, nor in mean EQ-5D-3L utility scores at 3 months, as shown in Table 3. Nevertheless, completely vaccinated patients who received a booster dose had a significantly lower occurrence of mental health symptoms (9.1 % vs 13.7 %; aRR, 0.57; 95 % CI, 0.33–0.98; p = 0.04) and a higher mean number of organ systems affected (0.64 vs 0.67; p = 0.03) than completely vaccinated patients without a booster dose. The comparison of individual persistent COVID-19-related symptoms across exposure variables is shown in Fig. 2. There was no statistically significant difference in any of the overall persistent symptoms when compared across the different vaccine groups.
Fig. 2.
Proportion of occurrence of symptoms reported at 3-month follow-up1.
4. Discussion
In this multicenter prospective cohort of relatively young healthy patients with symptomatic mild Omicron infection, we found that, at 3-month follow-up, the overall occurrence of persistent COVID-19-related symptoms was 41.6 %.The occurrence of persistent COVID-19-related symptoms at 3 months did not differ significantly between completely vaccinated and unvaccinated or incompletely vaccinated patients. However, the occurrence of persistent mental health symptoms was significantly lower in completely vaccinated than in unvaccinated or incompletely vaccinated patients, and also lower for those completely vaccinated with a booster dose vs those with a complete primary vaccination series but no booster dose.
The findings of this study regarding the occurrence of persistent COVID-19-related symptoms at 3 months are distinct from those observed in previous studies. Patients have reported severe problems in different domains – including the general health domain of the 36-Item Short Form Health Survey, several subdomains of the Nijmegen Clinical Screening Instrument, and the fatigue domains of both instruments – 3 months after recovery from mild acute COVID-19 [19]. Our estimate of the occurrence of persistent COVID-19-related symptoms at 3 months was lower than that of previous studies conducted with different populations. Goërtz et al., assessing a completely unvaccinated population, found that a large percentage of patients still experienced respiratory symptoms after 3 months: 71 % reported shortness of breath, 29 % had a cough, and 24 % had pain in their lungs [20]. Dennis et al. reported that, of 201 individuals assessed, 70 % had impairment in at least one organ system and 29 % had multiorgan impairment, with overlap across multiple organs [3]. In contrast, our estimate of the occurrence of persistent COVID-19-related symptoms at 3 months was higher than that of the Netherlands Cohort study, which reported that, in 12.7 % of patients with COVID-19, increased core symptoms with moderate severity at 3 months after COVID-19 could be attributed to SARS-CoV-2 infection [21]. A recently published study with a majority of Omicron cases showed a lower risk of long-term symptoms (only 13.6 %) compared with other variants. Vaccination did not appear to significantly reduce this risk [22]. Differences in the findings among the cited studies are mainly due to variations in the severity of the populations, the lack of standardized diagnostic criteria for COVID-19, and varying methods for collecting persistent symptoms. Long-term post-COVID-19 symptoms significantly impact quality of life in a substantial number of individuals [23]. Nevertheless, the majority of the initial studies assessing persistent symptoms and their influence on quality of life were conducted in populations with severe disease who required hospitalization [24], [25].
The impact of different COVID-19 vaccination protocols on COVID-19 symptom persistence remains unknown. A study using big data techniques found a protective effect against long-term symptoms even for single COVID-19 vaccine doses [5]. However, two systematic reviews suggest that the findings are inconclusive and additional studies are required [6], [7]. More recently, a study of 441,583 veterans with SARS-CoV-2 found that the incidence of post-acute sequelae (PASC) decreased from 10.42 per 100 in the pre-delta era to 7.76 in the omicron era among unvaccinated individuals, with vaccinated individuals experiencing even lower rates (5.34 and 3.50 events per 100 persons). The decline was mainly due to vaccination (71.89 %) and virus changes (28.11 %), but the risk of PASC remained significant among vaccinated individuals during the omicron phase [26].
We considered several potential explanations for the lack of a preventive effect of complete vaccination against the occurrence of persistent COVID-19-related symptoms in our study. First, this study had a limited power to detect small differences in the primary outcome across study groups; nevertheless, the study had an appropriate sample size to detect meaningful differences in vaccine effectiveness that would support its use for preventing persistent COVID-19-related symptoms. Second, the highly heterogeneous definition of symptoms, which includes different degrees of severity and self-reported symptoms without an established medical diagnosis of long COVID, is a limitation. Third, COVID-19 vaccines may not be effective in reducing long COVID-19, as more than 35 % of participants from a mostly vaccinated population with booster doses reported the occurrence of overall persistent symptoms.
When assessing persistent symptoms by organ system-specific domains, we found no differences in general, neurological, or respiratory symptoms according to vaccination status. However, complete vaccination was associated with a lower prevalence of mental health-related persistent symptoms. Participants who received more vaccine doses had less anxiety and depression than unvaccinated participants or those with an incomplete vaccination schedule, suggesting a possible relationship between vaccination and improved mental health. Our study does not explore the mechanisms whereby full vaccination might promote a better mental health; however, some direct and indirect effects of vaccination on mental health may be addressed. Neurological disorders due to a direct effect of SARS-CoV-2, as the virus can cross the blood–brain barrier and cause neuroinflammation, could be linked to poor mental health after the acute phase of the illness, due to the limited viremia associated with several vaccines [27]. This finding may be consistent with the hypothesis that a vaccine might accelerate clearance of the remaining SARS-CoV-2 virus from specific body compartments or partially blunt the host immune response, which has been implicated in the development of long COVID [5], [28]. Also, the lower rate of severe COVID-19 cases among vaccinated individuals may have led to a sense of safety, resulting in improved long-term mental health. A recent observational study of self-reported symptoms goes further, concluding that vaccination may also reduce the likelihood and intensity of long COVID [9].
Prior studies evaluating symptom persistence according to vaccination status share limitations, such as populations with more severe COVID-19 cases (participants with comorbidities or requiring hospitalization for COVID-19) and retrospective designs. Notably, two recent systematic reviews included studies with significant limitations: the diagnosis of long COVID was based on participants’ self-reported symptom duration or data from electronic health records and ICD-10 codes, rather than on diagnoses made by health professionals, as anticipated in most of the included studies; and the cutoff time frames used to define long COVID were heterogeneous, with the shortest being 28 days [6], [8]. While several studies did observe changes in symptoms post-vaccination, they were primarily cross-sectional in design, making it challenging to establish definitive causality. Therefore, we excluded these studies from our analysis. Additionally, it bears noting that the characteristics and symptoms of long COVID are only now becoming well-established through global data collection efforts.
Strengths of the present study include its nationwide, multicenter design, prospective data collection, and enrollment of only patients symptomatic with laboratory-confirmed COVID-19. Finally, most studies assessing the impact of vaccination on long COVID have been conducted in European, U.S., and Asian contexts; this is one of the few studies that have evaluated this research question in South America.
4.1. Limitations
This study has limitations. First, it is potentially affected by two major sources of bias: recall bias regarding the exposure factor (to mitigate this, we implemented established strategies, such as using standardized questionnaires and minimizing recall periods) and the Hawthorne effect (to mitigate this, we stressed participant confidentiality and anonymity during data collection). Second, although the study was adequately powered to detect vaccine effectiveness values commonly used to recommend vaccine adoption in practice (>50 % [29]), the ancillary nature of our analyses might have hindered the detection of small of small yet potentially meaningful differences. Accordingly, the numerical imbalance between the groups contributed significantly to the reduced statistical power (yet inevitable in a real-world setting). Moreover, the current sample size does not allow ruling out a potentially meaningful vaccine effect of 38 % (upper limit of the 95 % CI) for the primary outcome as well as a clinically relevant effect of vaccination on individual persistent COVID-19-related symptoms. Third, there was a significant imbalance between the exposed and unexposed groups due to high vaccination rates during the recruitment phase, which may have confounded the association between vaccination and outcomes. However, we conducted sensitivity analyses to adjust for imbalanced variables, and the results obtained were consistent with the primary analysis. Despite efforts to adjust for covariates, unmeasured confounding variables might still be present and impact the observed associations. Fourth, our study was deliberately designed to include only individuals with symptomatic mild COVID-19; the effects of vaccination on symptom persistence may be different in patients with more severe disease. Fifth, the criterion used to define mild COVID was the absence of hospitalization. Sixth, asymptomatic individuals were not included in the study. However, it cannot be ruled out that COVID was a significant health event for these participants, given that the symptoms were severe enough to prompt seeking medical care. Finally, it is essential to recognize that, besides the potential for the vaccine to reduce persistent COVID-19-related symptoms among patients infected with SARS-CoV-2, it may also decrease the probability of persistent COVID-19-related symptoms by preventing infection or causing infections to be asymptomatic.
5. Conclusion
In this study conducted with a relatively young and healthy population with mild Omicron infection, we were not able to identify a statistically significant protection of complete COVID-19 vaccination against any overall persistent symptoms at 3 months. Nevertheless, complete vaccination was associated with a lower occurrence of persistent mental health symptoms.
Data Sharing
R codes used for statistical analyses are available at https://gitlab.com/mariana.dias3/persistent-covid-19-related-symptoms-3-months.
Financial/nonfinacial disclosures
The authors MMR, FLS, GT, MMDS, DS, RRMS, RFCS, ESR, GPE, GSR, DM, JMBS, APM, CRI, GNS, JMN, HJMF, CVPS, ASS, CMD, CMS, IFS, and LAN work at Hospital Moinhos de Vento, which received a research grant from the Brazilian Ministry of Health for the conduction of this study. RGR and MF had received research grants from the Brazilian Ministry of Health, Pfizer and MSD. All other authors declare that they have no conflict of interest.
Authors’ contributions
RGR, MMR, FLS, GT, MMDS, DS and RRMS contributed to study conception and design. DS, RRMS, RFCS, ESR, GSR, DM, JMBS, APM, CRI, GNS, JMN, HJMF, CVPS, ASS, CMD, CMS, JVG, IFS, SMC, VMI, OMS, KCMB, PDMMN, FAMJ, JCS, TPS, ELF, BALF, SLZ, MAP, LMF and OCEG contributed to data acquisition. RGR, MMR, GT, MMDS and GPE contributed to data analysis. RGR, MMR, FLS, GT, MMRDS and AB contributed to the drafting of the manuscript. RGR, MMR, FLS, AB, CAP, MF, CCR, BBB, PRS, ACPA, MSM, and LAN contributed to manuscript revision for important intellectual content. All authors have read and approved the final version of the manuscript.
Role of the Funder/Sponsor
Representatives of Brazilian Ministry of Health were not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript. The decision to submit the manuscript for publication was made by the authors, and the sponsor had no veto right to publish or to control the decision to which journal to submit.
Funding information
The present study was funded by the Brazilian Ministry of Health through the Brazilian Unified Health System Institutional Development Program (PROADI-SUS).
CRediT authorship contribution statement
Marciane Maria Rover: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Methodology, Investigation, Formal analysis, Conceptualization. Fernando Luis Scolari: Writing – review & editing, Writing – original draft, Supervision, Methodology, Formal analysis, Conceptualization. Geraldine Trott: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Methodology, Formal analysis, Conceptualization. Mariana Motta Dias da Silva: Writing – original draft, Validation, Supervision, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Denise de Souza: Writing – original draft, Methodology, Data curation, Conceptualization. Rosa da Rosa Minho dos Santos: Validation, Supervision, Project administration, Methodology, Investigation. Raíne Fogliati De Carli Schardosim: Validation, Supervision, Investigation, Data curation. Emelyn de Souza Roldão: Validation, Supervision, Project administration, Data curation. Gabriel Pozza Estivalete: Validation, Software, Formal analysis. Gabriela Soares Rech: Validation, Project administration, Methodology, Data curation. Duane Mocellin: Validation, Investigation, Data curation. Jennifer Menna Barreto de Souza: Validation, Supervision, Investigation, Data curation. Aline Paula Miozzo: Validation, Supervision, Investigation, Data curation. Carolina Rothmann Itaqui: Investigation, Data curation. Gabrielle Nunes da Silva: Validation, Investigation, Data curation. Juliana de Mesquita Neto: Investigation, Data curation. Hellen Jordan Martins Freitas: Investigation, Data curation. Catherine Vitória Pereira dos Santos: Investigation, Data curation. Alanys Santos da Silveira: Investigation, Data curation. Carla Moura D’Ávila: Investigation, Data curation. Christian Morais Soares: Data curation. João Vítor Gozzi: Data curation. Ingrid Flor dos Santos: Data curation. Sidiclei Machado Carvalho: Investigation, Data curation. Vivian Menezes Irineu: Data curation. Odilson Marcos Silvestre: Writing – review & editing, Data curation. Kênia do Carmo Marinho Borges: Writing – review & editing, Data curation. Precil Diego Miranda de Menezes Neves: Writing – review & editing, Data curation. Fernando Azevedo Medrado Junior: Writing – review & editing, Data curation. Juliana Carvalho Schleder: Writing – review & editing, Data curation. Thiago Pelissari dos Santos: Data curation. Estêvão Lanna Figueiredo: Writing – review & editing, Data curation. Benedito Antonio Lopes da Fonseca: Writing – review & editing, Data curation. Sérgio Luiz Zimmermann: Writing – review & editing, Data curation. Mauricio Antonio Pompilho: Writing – review & editing, Data curation. Luciane Maria Facchi: Data curation. Otavio Celso Eluf Gebara: Writing – review & editing, Data curation. Milena Soriano Marcolino: Writing – review & editing. Ana Carolina Peçanha Antonio: Writing – review & editing. Paulo R Schvartzman: Writing – review & editing. Bruna Brandao Barreto: Writing – review & editing. Caroline Cabral Robinson: Writing – review & editing. Maicon Falavigna: Writing – review & editing. Luiz Antônio Nasi: Writing – review & editing. Carisi Anne Polanczyk: Writing – review & editing. Andreia Biolo: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Methodology, Conceptualization. Regis Goulart Rosa: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The authors MMR, FLS, GT, MMDS, DS, RRMS, RFCS, ESR, GPE, GSR, DM, JMBS, APM, CRI, GNS, JMN, HJMF, CVPS, ASS, CMD, CMS, JVP and IFS work at Hospital Moinhos de Vento, which received a research grant from the Brazilian Ministry of Health for the conduction of this study. RGR and MF had received research grants from the Brazilian Ministry of Health, Pfizer and MSD. All other authors declare that they have no conflict of interest. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Guarantor statement: MMR and RGR had full access to all the data in the study and take responsibility for the integrity of data and accuracy of data analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jvacx.2024.100579.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
The data that has been used is confidential.
References
- 1.World Health Organization. A clinical case definition of post COVID-19 condition by a Delphi consensus, 6 October 2021. apps.who.int [Internet]. 2021. Available from: https://apps.who.int/iris/handle/10665/345824.
- 2.WHO. Coronavirus disease (COVID-19): Post COVID-19 condition. www.who.int [Internet]. 2021. Available from: https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-(covid-19)-post-covid-19-condition.
- 3.Dennis A., Wamil M., Alberts J., et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. 2021;11(3):e048391. doi: 10.1136/bmjopen-2020-048391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahmani K., Shavaleh R., Forouhi M., et al. The effectiveness of COVID-19 vaccines in reducing the incidence, hospitalization, and mortality from COVID-19: A systematic review and meta-analysis. Front Public Health. 2022;10 doi: 10.3389/fpubh.2022.873596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simon M.A., Luginbuhl R.D., Parker R. Reduced incidence of long-COVID symptoms related to administration of COVID-19 vaccines both before COVID-19 diagnosis and up to 12 weeks after. Medrxiv. 2021 Nov 18 doi: 10.1101/2021.11.17.21263608. [DOI] [Google Scholar]
- 6.Notarte K.I., Catahay J.A., Velasco J.V., et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: a systematic review. eClinicalMedicine. 2022;53 doi: 10.1016/j.eclinm.2022.101624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byambasuren O., Stehlik P., Clark J., et al. Effect of covid-19 vaccination on long covid: systematic review. BMJ Med. 2023;2(1) doi: 10.1136/bmjmed-2022-000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antonelli M., Penfold R.S., Merino J., et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID symptom study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis. 2021;22(1):43–55. doi: 10.1016/S1473-3099(21)00460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azzolini E., Levi R., Sarti R., et al. Association between BNT162b2 vaccination and long COVID after infections not requiring hospitalization in health care workers. JAMA. 2022;328(7):676–678. doi: 10.1001/jama.2022.11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rover M.M., Trott G., Scolari F.L., et al. Health-related quality of life and long-term outcomes after mildly symptomatic COVID-19: The post-COVID Brazil study 2 protocol. Arq Bras Cardiol. 2023;120(9):e20220835. doi: 10.36660/abc.20220835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Kessel S.A.M., Olde Hartman T.C., Lucassen P.L.B.J., van Jaarsveld C.H.M. Post-acute and long-COVID-19 symptoms in patients with mild diseases: a systematic review. Fam Pract. 2022;39(1):159–167. doi: 10.1093/fampra/cmab076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raman B., Bluemke D.A., Lüscher T.F., Neubauer S. Long COVID: post-acute sequelae of COVID-19 with a cardiovascular focus. Eur Heart J. 2022;43(11):1157–1172. doi: 10.1093/eurheartj/ehac031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groff D., Sun A., Ssentongo A.E., et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: A systematic review. JAMA Netw Open. 2021;4(10):e2128568. doi: 10.1001/jamanetworkopen.2021.28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santos M., Cintra M.A.C.T., Monteiro A.L., et al. Brazilian Valuation of EQ-5D-3L Health States. Med Decis Making. 2016;36(2):253–263. doi: 10.1177/0272989X15613521. [DOI] [PubMed] [Google Scholar]
- 15.Coretti S., Ruggeri M., McNamee P. The minimum clinically important difference for EQ-5D index: a critical review. Expert Rev Pharmacoecon Outcomes Res. 2014;14(2):221–233. doi: 10.1586/14737167.2014.894462. [DOI] [PubMed] [Google Scholar]
- 16.Santos M., Monteiro A.L., Santos B. EQ-5D Brazilian population norms. Health Qual Life Outcomes. 2021;19(1):162. doi: 10.1186/s12955-021-01671-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsieh F.Y., Bloch D.A., Larsen M.D. A simple method of sample size calculation for linear and logistic regression. Stat Med. 1998 Jul 30;17(14):1623–1634. doi: 10.1002/(sici)1097-0258(19980730)17:14<1623::aid-sim871>3.0.co;2-s. PMID: 9699234. [DOI] [PubMed] [Google Scholar]
- 18.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria, 2018. Available from: https://www.R-project.org/.
- 19.van den Borst B., Peters J.B., Brink M., et al. Comprehensive health assessment 3 months after recovery from acute coronavirus disease 2019 (COVID-19) Clin Infect Dis. 2021;73(5):e1089–e1098. doi: 10.1093/cid/ciaa1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goërtz Y.M.J., Herck M.V., Delbressine J.M., et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res. 2020;6(4):00542–02020. doi: 10.1183/23120541.00542-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ballering A.V., van Zon S.K.R., Olde Hartman T.C., Rosmalen J.G.M. Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet. 2022;400(10350):452–461. doi: 10.1016/S0140-6736(22)01214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diexer S., Klee B., Gottschick C., Chao Xu., et al. Association between virus variants, vaccination, previous infections, and post-COVID-19 risk. Int J Infect Dis. 2023;136:14–21. doi: 10.1016/j.ijid.2023.08.019. [DOI] [PubMed] [Google Scholar]
- 23.Garrigues E., Janvier P., Kherabi Y., et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020;81(6):e4–e6. doi: 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nandasena H.M.R.K.G., Pathirathna M.L., Atapattu A.M.M.P., Prasanga P.T.S. Quality of life of COVID 19 patients after discharge: systematic review. PLoS One. 2022;17(2) doi: 10.1371/journal.pone.0263941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosa R.G., Cavalcanti A.B., Azevedo L.C.P., et al. Association between acute disease severity and one-year quality of life among post-hospitalisation COVID-19 patients: coalition VII prospective cohort study. Intensive Care Med. 2023;49(2):166–177. doi: 10.1007/s00134-022-06953-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie Y., Choi T., Al-Aly Z. Postacute sequelae of SARS-CoV-2 infection in the pre-delta, delta, and omicron eras. N Engl J Med. 2024;391(6):515–525. doi: 10.1056/NEJMoa2403211. Epub 2024 Jul 17 PMID: 39018527. [DOI] [PubMed] [Google Scholar]
- 27.Kubota T., Kuroda N., Sone D. Neuropsychiatric aspects of long COVID: a comprehensive review. Psychiatry Clin Neurosci. 2023;77(2):84–93. doi: 10.1111/pcn.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katella K. Why vaccines may be helping some with long COVID. Yale Medicine [Internet]. 2021 Apr 12. Available from: https://www.yalemedicine.org/news/vaccines-long-covid.
- 29.Https://www.who.int/news-room/feature-stories/detail/vaccine-efficacy-effectiveness-and-protection.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that has been used is confidential.