Abstract
Over the last four decades, pharmaceutical companies’ expenditures on research and development have increased 51‐fold. During this same time, clinical success rates for new drugs have remained unchanged at about 10 percent, predominantly due to lack of efficacy and/or safety concerns. This persistent problem underscores the need to innovate across the entire drug development process, particularly in drug formulation, which is often deprioritized and under‐resourced.
Keywords: AI, drug development, drug formulation, machine learning, robotics
Over four decades, pharmaceutical R&D expenditures have risen 51‐fold, yet clinical success rates stagnate at 10% due to efficacy and safety issues. This article proposes AI and automation to revolutionize drug formulation, optimizing development, reducing costs, and enhancing patient‐centered outcomes, ultimately increasing the success rates of new treatments.
This perspective article examines the pivotal role of cutting‐edge technologies, specifically artificial intelligence (AI) and robotics, in revolutionizing drug formulation development. Integrating these technologies could enable the pharmaceutical industry to rapidly identify the optimal formulations of drugs, overcoming traditional time and resource constraints. The adoption of AI and robotics can streamline the drug formulation process, while prioritizing the creation of safer, more effective and patient‐centered drug products. The cost savings from these technological advancements can support the development of additional treatments, ultimately benefiting a broader patient population.
This article outlines critical recommendations for the accelerated integration of AI and robotics in drug formulation, emphasizing the role of automation in the innovation stage. By leveraging these technological advances, we aim to rapidly identify the optimal formulations of drugs as a means to mitigate the low success rates associated with clinical development. Our approach aims not just to streamline the development process but to fundamentally transform it, ensuring more drugs successfully reach those who need them most.
Drug delivery stands at a pivotal crossroads, facing a critical decision: embrace cutting‐edge technologies, such as artificial intelligence (AI) and robotics, or risk stagnation. This isn't just a choice, but a fundamental requirement to keep pace with the evolving pharmaceutical sector. At the same time, there's a significant opportunity to address the high failure rates in drug development. By leveraging these innovative technologies in drug formulation, we can potentially transform the efficiency and success rates of drug development processes. This strategic integration could mark a paradigm shift in the way new drugs are developed, leading to more effective and swift advancements in the pharmaceutical industry.
For decades, the success rate associated with clinical drug development has been reported to be as low as 10 percent – meaning that only 1 in 10 drugs that entered clinical development received regulatory approval.[ 1 ] Typically, each of these failures embodied years of research and development (R&D), incurring costs ranging from millions to billions of dollars, depending on the stage of failure. Furthermore, as illustrated in Figure 1 , the costs associated with drug development have sharply increased over the past few decades. It's important to note that each of the drugs that failed was intended for treatment of a specific patient population. And, in some cases, these were patients with life‐threatening diseases, in which a newly approved drug represented their final and most promising treatment option.
Figure 1.
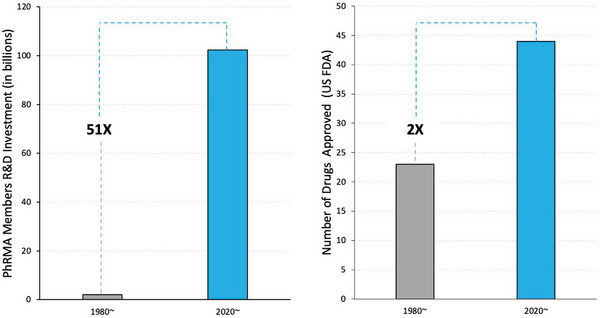
As reported by the Pharmaceutical Research and Manufacturers of America, capital invested in R&D increased 51‐fold from 1980 to 2020. Despite this massive increase in capital expenditure into R&D by the leading biopharmaceutical research companies in the United States, only twice as many drugs were approved by the U.S. FDA in 2020 compared to 1980.[ 2 , 3 , 4 ]
When a drug fails in preclinical or clinical development, it is often shelved, with much of the data associated with that drug remaining behind closed doors. If the drug is first‐in‐class, its failure can completely deter further investment in, and development of, that class of drugs.
Clinical failures can also lead to the termination of research programs, loss of jobs, closure of companies, loss of public trust, and delays in the advancement of science and medicine. As a result, the impact of failure in drug development is far‐reaching, extending beyond a single drug, company, indication or patient population. Centering the tremendous impact of drug failure leads us to ask whether we are doing everything we can to ensure that drug candidates entering clinical development are best positioned for success.
1. The Importance of Drug Delivery Cannot be Overstated
Mastering the science of drug delivery has the power to transform patient outcomes. Imagine precisely guiding life‐saving medications to the exact location they're needed within the body, releasing them at the ideal moment, and ensuring the dose is meticulously accurate. This isn't just a part of the drug development process. It's an often overlooked critical component.
Among the various principles of drug delivery, formulation is where science meets innovation – producing unique combinations of various chemical substances, including the active pharmaceutical ingredient (API) to forge a product that's not only safe and effective but also user‐friendly. However, the formulation doesn't just influence the drug's performance. It dictates its stability, shelf life, and useability. The development of a drug formulation is where rigorous science takes place, ensuring that each medicine is not just a compound, but also a carefully crafted solution to a complex health issue. Numerous pharmaceutical products owe their existence to advances in formulation and delivery technologies. Table 1 summarizes instances in which the formulation has successfully addressed intrinsic limitations of the API or has enabled performance enhancements that the API, alone, could not achieve.
Table 1.
Nonexhaustive list of drug products approved by the FDA that are enabled by formulation technology.
| API | Product | Challenge | Solution | Product sales US (2023) | Product |
|---|---|---|---|---|---|
| Semaglutide (Oral) | Rybelsus® | Poor absorption – Peptide drugs are generally not stable in the gastrointestinal tract and have low permeability across the intestinal epithelium. | Sodium N‐ (8‐ [2‐hydroxylbenzoyl] amino) caprylate in oral semaglutide increases local pH and reduces peptide degradation, enhancing its absorption and effectiveness in entering the bloodstream.[ 5 ] | $1601 M[ 6 ] |
|
| Insulin (Pulmonary) | Afrezza® | Inhalable insulin offers noninvasive, needle‐free administration, rapid action, and portability, aimed at improving patient compliance and comfort. | Dry powder inhalers stabilize insulin, normally unstable outside liquid, using Fumaryl diketopiperazine to form effective microspheres for inhalation.[ 7 ] | $55 M[ 8 ] |
|
| Cabotegravir | Apretude® | Releasing the drug at a consistent rate maintains therapeutic efficacy. | Apretude® is an injectable formulation that reduces the need for adherence and dosing frequency compared to daily oral pre‐exposure prophylaxis, demonstrating superior efficacy in preventing HIV‐1 in high‐risk populations.[ 9 ] | $185 M[ 10 ] |
|
| Olaparib | Lynparza® | Originally formulated as capsules, which had to be administered 16 times a day. | New tablet formulation (amorphous solid dispersion) enables the dosage regimen to be reduced to two tablets, twice a day.[ 11 ] | $1861 M[ 12 , 13 ] |
|
Despite the demonstrated impact of formulation on the success of pharmaceutical products, the formulation of a drug is commonly overlooked or is an exercise completed with minimal time and resources.[ 14 ] As a result, pharmaceutical scientists are not always advancing the absolute best formulation of a drug into the clinic. Rather the formulation that is advanced is the best that could be developed with the time and resources allowed.
The fact that the majority of drugs fail in clinical development due to a lack of drug efficacy (i.e., 50 percent) and safety (i.e., 30 percent) demonstrates that we may not be placing sufficient emphasis on the design of optimal formulations of drugs.[ 1 ] This is evidenced by the fact that there are many well‐known examples of drugs, wherein reformulation has resulted in significant improvements in their safety and/or efficacy. These include the reformulation of doxorubicin from a conventional formulation to encapsulation in liposomes such as Doxil. The original formulation of doxorubicin, a potent chemotherapy drug, was associated with severe cardiotoxicity, which limited its use. By encapsulating doxorubicin in liposomes (fat‐like particles), the reformulated version, Doxil, significantly reduces the risk of heart damage by altering the drug's distribution in the body, thereby enhancing its safety profile.[ 15 ]
The reformulation of monoclonal antibodies, such as Adalimumab, for subcutaneous administration (HUMIRA®) instead of intravenous infusion allows for patients to self‐administer at home.[ 16 ] These and similar reformulations reduce the need for hospital visits and improve patient quality of life through enhanced portability, convenience, and flexible dosing schedules. Another example is Nifedipine, a calcium channel blocker used to treat hypertension and angina, which was initially available in an immediate‐release form that was linked to an increased risk of mortality, possibly due to sudden changes in blood pressure.[ 17 ] The development of an extended‐release formulation mitigated these risks by providing a more controlled release of the medication, leading to steadier blood levels and improved patient outcomes.[ 18 ] These are just a few of many examples, which clearly demonstrate the power of formulation in improving the performance of a therapeutic.
Recent estimates suggest that between 70 percent to 90 percent of new chemical entities (NCEs) in development pipelines are poorly soluble. Not to mention that many of the NCEs, such as biologics, also have stability and absorption issues.[ 14 ] Therefore, given that formulation can help overcome these potential barriers to drug delivery and positively impact the majority of drug development projects, it raises the question: Why isn't more emphasis placed on formulation development?
For the most part, traditional formulation development is a time‐consuming and tedious process, often requiring multiple iterations to achieve a formulation with properties and performance sufficient to move forward into preclinical testing and then clinical development. Delays in formulation development can't be entertained, as they have a cascading effect on the overall drug development timeline, and are incompatible with the tremendous pressure on pharma and biotech companies to move new drug candidates forward into the clinic and through to regulatory approval. This pressure is driven by a combination of rising drug development costs,[ 19 ] patent exclusivity, patients needs and hopes, investor expectations, and the competitive pharmaceutical landscape. Increasing the speed and efficiency of formulation development at every stage of the drug development process could significantly boost the chances of clinical success. Moreover, it may enhance patient safety and the effectiveness of treatments. But, how can this be accomplished? A promising approach lies in the integration and strategic use of technological advancements in AI, machine learning (ML) and robotics to streamline workflows.
2. AI and Robotics Continue to Transform Industry Sectors
AI and robotics have begun to transform the landscape of drug development, with further integration having the potential to create a more efficient, accurate and cost‐effective process. In fact, a recent report, which reviewed “publicly available data from early AI programs,” showed that “AI‐driven R&D efforts from discovery up to preclinical could deliver time and cost savings of at least 25 – 50 percent.”[ 20 ] Given the substantial investment in pharmaceutical R&D today (Figure 1), adopting these technologies could lead to considerable cost savings. These savings could then be utilized to develop a wider array of treatment options for patients in need. Clinical evaluation of these AI‐discovered drugs will be a critical test of their success, as there is keen interest in determining whether these drugs are associated with a higher probability of success in the clinic and improved patient outcomes. Indeed, a recent report by BCG provides an early assessment of the clinical success rate associated with AI‐discovered drugs.[ 21 ]
AI is also playing a key role in transforming clinical drug development with its use in trial design, patient stratification, and patient adherence and retention, as well as data collection, management, analysis and more.[ 22 ] It was reported that 300 applications received by the U.S. Food and Drug Administration (FDA) between 2016 and 2022 incorporated AI or ML, with the majority focused on the application of AI and ML in clinical development.[ 23 ] However, the application of these technologies in drug formulation – a critical step in drug development – has been slower to gain traction. Despite some recent applications of modern computational science to drug delivery,[ 24 , 25 , 26 , 27 ] outlined in greater detail in the following section, the limited progress in this area is reflected in a recent discussion paper put forward by the FDA on the use of AI and ML in drug development, which covers drug discovery, various aspects of preclinical research, clinical development and pharmaceutical manufacturing, but features no mention of drug formulation or delivery.[ 28 ] We propose that many of the advances shown in Table 1 could have been achieved much more rapidly and efficiently by leveraging AI and automation in formulation development.
3. The Current State of Automation and AI Research in Formulation Development
Supervised machine learning encompasses predictive algorithms that are trained on labelled datasets in order to learn a mapping between input features and labels. In the context of formulation development, inputs could be the composition of the formulation, and outputs could be any measurable formulation figure of merit, such as API solubility, particle size, or oral bioavailability. A plethora of studies have been published that examine the performance of supervised learning algorithms modelling formulation figures of merit for specific formulation types,[ 26 , 27 ] such as long‐acting injectable systems,[ 29 , 30 , 31 , 32 , 33 ] self‐emulsifying drug delivery systems,[ 34 , 35 ] amorphous solid dispersions (ASDs),[ 36 , 37 ] nanomedicines,[ 38 , 39 , 40 , 41 ] and 3D printed oral formulations.[ 42 , 43 , 44 ] Typically, these authors procure one or more formulation datasets, divide into train, test, and validation segments, train a panel of ML models, and compare each model's performance on held out portions of the dataset using one or more classification or regression performance metrics, depending on the type of supervised learning task. For example, Schmitt et al. constructed a dataset of 680 ASDs and found that the best performing model for predicting ASD particle size was an ensemble model consisting of a support vector regressor, neural network and partial least squares. This ensemble model produced a root‐mean squared error of 6 µm (particle sizes in the dataset ranged from 8 to 104 µm).[ 37 ]
One of the limitations of using supervised learning is the need for datasets which span the formulation space of interest and also include high‐quality, standardized measurements of the formulation property or properties of interest.[ 26 ] Furthermore, well‐known generalizability problems of supervised learning algorithms often limit the scope of formulation and API types for which accurate predictions are available to the (often narrow) training set chemical space.[ 45 ] Active learning (AL) helps address the challenge of out‐of‐distribution (OOD) prediction by dynamically exploring and requesting measurements for OOD formulation instances.[ 46 ] AL autonomously refines its understanding of the design space and suggests the next instances to evaluate. Bayesian optimization (BO)[ 47 ] is a key AL strategy that uses a surrogate model, such as a Gaussian process (GP),[ 48 ] to approximate formulation parameter‐property relationships and an acquisition function to determine the next instances to investigate. BO is considered the gold standard for noisy black‐box optimization, making it well‐suited for drug formulation where complex relationships between manufacturing parameters and the properties of formulations may not be intuitive.
To further minimize materials required, and to accelerate formulation development, automated workflows can be integrated with AI‐guided experiment planning. For example, self‐driving labs (SDLs) seek to automatize the scientific method of experimentation by preparing and testing machine‐learned hypotheses conditioned on observations using computerized laboratory equipment.[ 49 , 50 , 51 , 52 , 53 , 54 ] Touted as a potential next‐generation component of the fourth industrial revolution,[ 55 ] SDLs promise to increase experimental and productional throughput, enhance sample efficiency and repeatability, and alleviate human researchers from tedious, labor‐intensive tasks. SDL frameworks for expedited nanomedicine[ 56 ] and biologic formulation design,[ 57 ] among others,[ 58 , 59 ] have been outlined. Cao et al. reported a closed‐loop robotic platform, informed by Thompson sampling to design a formulated liquid product of commercial interest, without the need for physical models derived from pre‐existing empirical data. Their iterative approach yielded satisfactory formulations within only 15 working days of operation, showcasing the time and resource efficiency promised by implementation of AI‐driven autonomous research platforms.
Liquid handling robotics (LHR) are essential for automating repetitive and time‐consuming liquid transfer tasks in preclinical formulation development. Commercial LHR platforms such as Chemspeed,[ 60 ] Hamilton,[ 61 ] and Tecan[ 62 ] offer versatile solutions. Many modern LHR platforms, such as those offered by Opentrons,[ 63 ] ship with accessible application programming interfaces, which enable small‐scale R&D teams to quickly automate protocols and integrate liquid formulation preparation into larger, integrated systems complete with processing, assay preparation, and characterization modules. For example, Bao et al. utilized an Opentrons OT‐2 LHR to screen for in vitro properties of solid lipid nanoparticles (SLNs) that encapsulated a hydrophobic drug.[ 64 ] The authors used the OT‐2 to prepare 128 unique SLN compositions which were then characterized. High‐performing formulations enabled up to a 3000‐fold solubility enhancement of the drug, and rivaled the in vivo oral bioavailability of the FDA approved product.
4. Using Automation During the Innovation Stage of Formulation Development
Automation and robotics have, indeed, been utilized in the drug development pipeline for decades.[ 65 , 66 ] More granular evaluation of these applications, however, reveals that the majority of uses of automation and other technologies are focused on streamlining specific processes that are well‐known and rigid in nature. For example, pharmaceutical companies rely heavily on automation and computerized technology for compound profiling/management, high‐throughput screening/assay development, quality control, manufacturing, and packaging or labelling.[ 67 , 68 , 69 ] Such tasks can be characterized by a high degree of predefinition. For example, automation and control systems are responsible for performing standardized, repetitive operations in a consistent and robust manner to increase operational efficiency, reduce costs, and ensure uniform quality.[ 70 ]
Comparatively, the innovation stages of drug development, including designing tailored formulations for new or existing APIs, have seen less exposure to such technologies. Clearly, tasks in which the goal is to produce a novel product or invention are routinely draped in failure and uncertainty.[ 71 , 72 ] Automating processes that are unpredictable in nature is significantly more difficult from an engineering perspective compared to the aforementioned applications, as the space of potential sources of error greatly expands as previously untested experiments and/or operations are added for consideration.[ 49 , 73 ]
Despite this challenge, we argue that the time to bring automation to the innovation stage of formulation development is now. Technologies that have enabled the fourth and fifth industrial revolutions – such as AI, the internet of things, and collaborative robotics – constitute building blocks from which sophisticated cyber‐physical systems can be constructed. Compared to the rigid automation protocols employed in, for example, manufacturing, these systems could be designed with the flexibility and modularity necessary to navigate the “messy” and unpredictable terrain of innovation‐stage experimental R&D. In Table 2 , we contrast “industrial automation” and “innovation automation.”
Table 2.
Contrast between the objectives, approach, and performance metrics in industrial and innovation automation.
| Industrial automation | Innovation automation | |
|---|---|---|
| Objective | Streamline processes, reduce costs, increase efficiency, ensure consistent quality, quality control | Innovation, novelty, experimentation, discovery. Develop innovative solutions to problems that can lead to new products |
| Approach | Implementation of robots needs to be highly standardized and optimized for a relatively narrow scope of specific tasks. Performing predefined actions efficiently and reliably (precision and speed) | Flexible, modular and exploratory approach. AI and automation used to assist researchers in conducting experiments, analyzing data, and generating hypotheses |
| Performance Metrics | Metrics can be gathered systematically. These could be statistics such as production rate, defect rate, cost, consistency, uptime, etc. | Metrics are more difficult to define and measure. Focused on breakthroughs, inventions, and intellectual property generation. Push boundaries of knowledge. Efficiency and reliability are still important, but emphasis should be placed on fostering creativity |
| Risk Tolerance | Generally low risk tolerance, as production disruptions have serious financial implications. The space of all sources of error is relatively small and, therefore, less “intelligent” control systems are still able to properly triage and troubleshoot problems | Often higher risk tolerance, as failure is often a necessary precursor for innovation. Advanced technologies used in settings in which the experimental outcomes (i.e., measured figures of merit) and the sources of error are less predictable. Therefore, more intelligent control systems must be implemented to effectively triage and troubleshoot – and even identify – problems |
Unlike industrial automation, quantifying the benefits of automating innovation, compared to traditional methods, is significantly more challenging. Despite this, the potential upside of turbo‐charging the rate of R&D breakthroughs, creating novel inventions, and generating intellectual property could be significantly more valuable to a business or society at large than streamlining an existing, repetitive process. It would be beneficial for our community to collaborate on developing a metric, such as the “discovery acceleration factor,” to quantify the benefits of AI and automation technologies in the realm of pharmaceutical formulation R&D. In section 5, we provide an example illustrating the application of innovation automation in the design of lipid nanoparticles for the delivery of a poorly soluble drug candidate with a goal toward improving its oral bioavailability.
5. An Illustrative Example of How Innovation Automation can be Applied to Develop New Drug Formulations
5.1. Task
Optimization of a lipid nanoparticle formulation of a poorly soluble drug to improve its oral bioavailability.
5.2. Framework Setup
We might establish an active learning framework aimed at streamlining the optimization of lipid nanoparticle formulations. This approach begins with the creation of an initial small set of experimental formulations, designed to span a broad, yet strategic, range of excipients and process parameters. The primary goal is to encapsulate a poorly soluble drug candidate in lipid nanoparticles to generate a formulation with high drug loading, good stability and small particle size.
5.3. Implementing Real‐Time Sensing Technologies
The formulation optimization process is fraught with challenges and uncertainties that necessitate advanced experimentation planning and coordination, including – but not limited to – precipitation risk, phase separation, stability challenges, and scale‐up considerations. Certain combinations of excipients and other parameters contained in the overall design space may lead to a failed formulation that does not adequately solubilize drug crystals or nanoparticle aggregates, which may render measurements, such as dynamic light scattering, impractical for accurate analysis of particle size and uniformity. In this case, a technological system must be capable of recognizing precipitation (ideally as early as possible), which could be done using computer vision or sensing.[ 74 ]
5.4. Active Experimentation Cycle
As experiments are conducted, data on formulation outcomes, including stability, precipitation incidents, and nanoparticle characteristics, are immediately captured and analyzed. This initial dataset serves as the foundation for training a preliminary optimization algorithm. Unlike traditional methods, this algorithm actively directs the selection of subsequent experiments, focusing on reducing uncertainty and improving formulation performance based on learned insights. This active learning mechanism leverages real‐time data to mitigate uncertainties and enhance formulation efficacy through strategically directed experimentation.
5.5. Autonomous Preparation and Characterization
Formulations proposed by the algorithm are not only autonomously devised but also prepared and characterized without manual intervention. This level of automation extends from the mixing of excipients and active ingredients to the real‐time assessment of nanoparticle formation and stability. The advanced vision and sensing system plays a pivotal role here, rapidly assessing each experiment's viability and ensuring that resources are allocated to promising formulations.
5.6. Adaptive Algorithm Enhancement with Multi‐Objective Optimization
The optimization algorithm, potentially leveraging advanced machine learning techniques, such as Bayesian optimization, is enhanced to perform multi‐objective optimization, allowing it to continuously refine its predictive accuracy with each new data point. This iterative process enables the algorithm to identify and prioritize formulation parameters that balance multiple objectives, such as maximizing drug solubility and formulation stability, while minimizing nanoparticle size and unit cost. By considering a broader set of desired outcomes, the algorithm effectively navigates the complex formulation space. It specifically seeks out parameter combinations that not only mitigate common challenges, such as precipitation and phase separation, but also align with other critical formulation goals.
5.7. Feedback Loop for Ongoing Improvement
As the continuous cycle of experimentation progresses, each experiment not only informs the immediate next steps, but also contributes to a growing knowledge base. This repository of experimental results and learned correlations becomes an invaluable asset for further refining the formulation strategy. Real‐time adjustments and predictions become more accurate over time, ensuring that the formulation process remains agile and responsive to new insights, leading to the efficient optimization of lipid nanoparticle formulations for drug delivery.
6. Limitations and Challenges Associated with AI for Formulation Development
One of the critical challenges with ML models, particularly those used in pharmaceutical applications, is the issue of model interpretability.[ 75 , 76 , 77 ] Lack of transparency in “black box” models can be problematic, especially in a field where understanding the underlying mechanisms of drug interactions and effects is crucial. Opaque models can induce mistrust among stakeholders and regulatory bodies, as the decision‐making process of these models cannot be easily scrutinized or validated. Approaches such as model‐agnostic interpretation techniques, which provide insights into model predictions, and the development of inherently interpretable models, are essential for enhancing transparency and trust in AI‐driven drug formulation processes.
While many ML strategies, such as deep learning, require large datasets to achieve high performance, there are techniques like active learning (AL) that can be effective with smaller amounts of data. AL iteratively selects the most informative data points to train the model, thereby reducing the overall data requirement. However, for models that do require extensive data, the challenge remains significant. In the pharmaceutical industry, obtaining large, high‐quality datasets can be particularly difficult due to the sensitive and proprietary nature of the data.[ 26 ] This challenge is exacerbated by inconsistent or incomplete data reporting, leading to difficulties in reproducing results and integrating data from multiple sources, not to mention ML models whose aptitude is strongly biased toward positive data[ 71 ] and/or narrow patient populations.[ 78 , 79 ]
Data from scientific experimentation is highly heterogeneous, including contributions from tabular data, images, videos, and time series. Given the diverse types of data generated in pharmaceutical research, advanced data storage technologies such as multi‐modal databases,[ 80 , 81 , 82 ] will become indispensable in the era of pharmaceutical foundation models and autonomous experimentation. Today, most databases are structured around a single data model that governs how its content can be stored, retrieved and manipulated. With the advent of foundation models as a general‐purpose AI technology that are broadly trained to be applicable to a wide range of use cases,[ 83 ] we must provide rich and diverse data to ensure the robustness and versatility of future drug formulation foundation models.
7. Recommendations
To accelerate the adoption of these advanced technologies and enhance the outcomes of drug formulation development – and, thereby, the overall drug development process – we propose the following recommendations:
-
Create interdisciplinary research teams: Interdisciplinary teams of robotic engineers, formulation scientists and manufacturing engineers and AI/ML scientists are best positioned to accelerate and foster innovation in drug formulation development. Robotics experts can automate and refine formulation characterization and manufacturing processes. Formulation scientists and manufacturing engineers can provide complementary expertise and ensure the manufacturing and scalability of the drug products. And AI/ML scientists can develop new ML models, optimize existing models, and provide insights into data analysis and pattern recognition. In fact, the rapid pace of technological advancements underscores the critical role of AI/ML scientists. They are pivotal in developing and optimizing new ML models, offering deep insights into data analysis and pattern recognition, and ensuring that teams use the latest state‐of‐the‐art tools and resources effectively. Despite the availability of open‐source software libraries for model development, the intricacies of AI and ML technologies demand the specialized knowledge and experience of domain experts. Their ability to adeptly navigate and tailor these tools is essential for creating customized, finely tuned, and innovative solutions in this dynamic field.
Fundamental changes to scientific education are paramount to ensuring interdisciplinary pharmaceutical research can thrive in the future. Increasing integration of software and automation components into modern laboratories necessitates the following skills be developed in the classroom.[ 84 ]- Students must be exposed to technical topics in modern scientific computing from a young age, regardless of educational trajectory.
- A strong focus must be place on evaluating students’ creativity and critical problem solving skills, rather than their ability to regurgitate information and consistently execute rigid protocols. In a rapidly changing world, the ability to effectively memorize existing information and execute repetitive operating procedures with accuracy and precision is becoming less of an asset for young scientists. Advanced search engines, many infused with LLMs, are able to retrieve, summarize, explain, and write scientific documents with increasing aptitude.[ 85 ] Additionally, the automation of tasks such as pipetting and analytical method development is rapidly advancing, which may reduce the reliance on traditional manual methods. As a result, it is important to ensure that students are equipped with skills that align with future employment needs in an increasingly automated industry.[ 86 ] As much emphasis as possible, starting from a young age, should be shifted to a students’ ability to devise creative solutions to larger, more consequential problems.
- Teamwork should be emphasized. Effective multidisciplinary teams consist of talented individual contributors working together to solve difficult problems. Projects and class‐based assignments should incorporate elements of collaboration between individuals with different backgrounds and skill sets. For example, Vargas et al. reported a team‐based learning platform for scientific computing and automated experimentation, where teams of undergraduate students were introduced to modular collaboration in the context of automated chemical laboratories.[ 84 ]
Embrace advances in AI and automation: Embracing advances in AI within drug formulation development opens up transformative possibilities for the field. By leveraging the latest advancements in computer hardware, we can vastly improve our data processing capabilities, allowing for more sophisticated analyses and interpretations. Adopting best practices for data organization and storage further enhances this efficiency, ensuring that invaluable insights can be extracted more rapidly and accurately. Advancements in ML offer powerful tools for planning experiments, guiding decision‐making in experimental workflows, and developing predictive models that can foresee outcomes with good precision.[ 87 ] Additionally, the integration of computer vision technologies presents an opportunity to automate and refine the measurement of parameters traditionally assessed by the human eye, thereby increasing accuracy and consistency. Large language models also play a pivotal role – with their ability to summarize extensive bodies of literature and generate innovative research directions – propelling the field towards new discoveries and innovations. Together, these AI‐driven approaches not only streamline existing processes, but also unlock new potential in drug formulation development.[ 88 ]The use of robotics in drug formulation can afford faster, unremitting, consistent, miniaturized R&D and manufacturing workflows. Miniaturization of drug product screening experiments at the R&D stage reduces the quantity of solvents, chemicals and drug products required for formulation, resulting in a decrease in the carbon footprint associated with drug development. Furthermore, the ability of miniaturized development processes to deduce effective formulations using smaller quantities of drug may be advantageous in the early stages of drug development when only small amounts of some high‐value assets may be available.
-
Place focus on regulatory hurdles, stakeholder involvement, and economic impact: Regulatory bodies such as the FDA and EMA are increasingly recognizing the impact of AI and robotics in the pharmaceutical industry. The FDA has released several guidance documents, e.g.,[ 89 , 90 , 91 ] aimed at providing a framework for evaluating AI‐driven systems.
Enhancing AI model interpretability, especially for deep learning architectures, is important.[ 98 ] Transparent AI systems help build trust among stakeholders, including regulatory agencies, clinicians and patients. Model interpretability/explainability, along with the development of AI systems that can accurately communicate their confidence levels in predictions[ 99 ] are key aspects to address as the pharmaceutical sector ushers in new regulatory guidance and standards.[ 100 ]
Another concern is dataset quality and bias. Training datasets have paramount influence on the predictions and decision‐making policies of AI systems. Therefore, if datasets used by drug developers are not representative or are biased (often are highly biased toward positive results,[ 26 ] and/or toward specific patient populations[ 78 , 79 ]), it could lead to drug products which are unsafe or less effective for certain individuals. Scientists should work with regulatory bodies to assure safeguards against AI‐assisted drug products based on unreliable, biased data.
-
Consider the ethical ramifications associated with rapid adoption of next‐generation technologies: While we maintain that the adoption of new technologies in pharmaceutical science is an extremely promising direction, it is not without potential for challenges and negative consequences. Just as the Industrial Revolution saw the rapid adoption of mechanized manufacturing processes, steam power, and advancements in textile production, the advent of modern automation and IT advancements threaten to produce a digital divide, wherein many jobs could be displaced and social inequalities could be exacerbated.[ 92 , 93 , 94 ]
It will be important to equip the pharmaceutical workforce with the necessary skills to adapt to new technologies and ensure inclusive and fair access to digital advancements through reskilling programs, a continuous learning culture in industry, and inclusive access to training. Scientists should actively engage with policymakers to develop public policies that aim toward equitable distribution of technological advancements and support affected workers through transition programs. Academic researchers and pharmaceutical companies should adhere strictly to data governance frameworks like GDPR[ 95 ] and HIPAA[ 96 ] when using AI models to process patient data. Ethical AI practices should be upheld that prioritize patient privacy and data security, with regular reviews of AI models and data processing pipelines to make sure they have not compromised patient confidentiality.
As advanced software becomes a commonplace tool in virtually every scientific research discipline, the concept of open source software becomes increasingly important. Open source software enhances transparency and trust, fosters collaboration and innovation from community contributions, and provides a powerful learning resource for young scientists.[ 97 , 98 ] In addition to emphasis on open‐source software tools, the democratization of automated laboratory equipment for formulation science will aid in fostering inclusivity and widening access to state‐of‐the‐art technologies for scientists with limited resources. Formulation scientists should draw inspiration from the work of, for example Keesey et al.,[ 99 ] who created affordable 3D‐printed syringe pumps, allowing for the automation of liquid handling tasks. Several similar efforts toward democratized laboratory automation have also been undertaken.[ 100 , 101 ]
8. Conclusion
In conclusion, the critical and complex role of drug formulation in the pharmaceutical industry profoundly influences every stage of drug development. Despite its pivotal importance, formulation often remains underemphasized in the early stages, leading to substantial implications for efficacy and safety. Today, the industry stands at a crucial juncture: integrating advanced technologies – such as AI, ML, and robotics – represents a potential revolutionary leap in drug delivery and development. We recognize, however, that adopting these technologies involves significant upfront costs and challenges.
A pragmatic first step towards embracing these changes could be through strategic collaborations with academic laboratories or start‐ups that specialize in these areas. Such partnerships can provide a more accessible entry point into the advanced technological realm, mitigating initial costs and learning curves.
This transformative era in drug formulation and delivery is poised to significantly enhance drug efficacy, safety, and usability. By acknowledging the challenges, embracing these technological advancements, and leveraging collaborative opportunities, the pharmaceutical industry can not only improve the success rates and speed of drug development but also – most importantly – elevate patient outcomes. The recommendations provided here are designed to support this transition, ensuring that the industry doesn't just keep up with technological progress, but also leads in its innovative application, ultimately advancing healthcare and medicine.
Conflict of Interest
We are co‐founders of a start‐up that works in the area of AI and robotics for drug formulation development.
Acknowledgements
The authors thank Rav Kumar for his valuable input. The authors acknowledge an NSERC Discovery grant (RGPIN‐2022‐04910) to C.A. and funding from the Canada First Research Excellence Fund CFREF‐2022‐00042 to the University of Toronto's Acceleration Consortium.
Biographies
Pauric Bannigan Pauric has extensive experience leading the design and development of innovative drug delivery platforms. He was most recently the Director of Research and Partnerships for Christine Allen's research group. Pauric has worked as a senior formulation scientist in the biotech industry, authored dozens of research articles, and is an inventor on several formulation technology patents.

Riley Hickman Riley completed his doctoral research in Alán Aspuru‐Guzik's research group at the University of Toronto. He is an expert in applied mathematics, software development, machine learning and robotics. Riley has developed autonomous experimentation systems to rapidly identify material technologies to combat major societal problems.

Alán Aspuru‐Guzik Alán is internationally recognized for his contributions at the interface of quantum information, machine learning and chemistry. He is a full professor at the University of Toronto and is also the Canada 150 Research Chair in Theoretical Chemistry and a CIFAR AI Chair at the Vector Institute.

Christine Allen Christine is an internationally renowned scientist, entrepreneur and leader in drug formulation development. She is a full professor at the University of Toronto, has received numerous career awards and is a fellow of the American Institute for Medical and Biological Engineering, Canadian Academy of Health Sciences, Controlled Release Society, and the Canadian Society for Pharmaceutical Sciences.

Bannigan P., Hickman R. J., Aspuru‐Guzik A., Allen C., The Dawn of a New Pharmaceutical Epoch: Can AI and Robotics Reshape Drug Formulation?. Adv. Healthcare Mater. 2024, 13, 2401312. 10.1002/adhm.202401312
References
- 1. Sun D., Gao W., Hu H., Zhou S., Acta Pharm. Sin. B 2022, 12, 3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Research and Development in the Pharmaceutical Industry | Congressional Budget Office, https://www.cbo.gov/publication/57025, 2021.
- 3. Doughman E., Pharm. Process. World 2019. [Google Scholar]
- 4. PhRMA member companies’ R&D investments reach record high of $102.3 billion in 2021, https://phrma.org/Blog/phrma‐member‐companies‐rd‐investments‐reach‐record‐high‐of‐1023‐billion‐in‐2021, 2021.
- 5. Isaacs D. M., Kruger D. F., Spollett G. R., Diab. Spectr. Publ. Am. Diab. Assoc. 2021, 34, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Novo Nordisk Ann. Rep. 2023. [Google Scholar]
- 7. Xia Y., Su Y., Wang Q., Yang C., Tang B., Zhang Y., Tu J., Shen Y., Drug Deliv. 2019, 26, 650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. MannKind Corporation Reports 2023 Fourth Quarter and Full Year Financial Results: Provides Clinical Development Update | MannKind Corporation, https://investors.mannkindcorp.com/news‐releases/news‐release‐details/mannkind‐corporation‐reports‐2023‐fourth‐quarter‐and‐full‐year, 2023.
- 9. Blair H. A., Drugs 2022, 82, 1489. [DOI] [PubMed] [Google Scholar]
- 10. Quarterly Results, GlaxoSmithKline, 2024 Second Quarter, https://www.gsk.com/en‐gb/investors/quarterly‐results/.
- 11. Moore K. N., Birrer M. J., Oncologist 2018, 23, 697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Annual report 2023, https://www.astrazeneca.com/investor‐relations/annual‐reports/annual‐report‐2023.html, 2023.
- 13. Merck Announces Fourth‐Quarter and Full‐Year 2023 Financial Results – Merck.com, https://www.merck.com/news/merck‐announces‐fourth‐quarter‐and‐full‐year‐2023‐financial‐results/, 2023.
- 14. Thomas F., Pharm. Technol. 2021, 45, 16. [Google Scholar]
- 15. O'Brien M. E. R., Wigler N., Inbar M., Rosso R., Grischke E., Santoro A., Catane R., Kieback D. G., Tomczak P., Ackland S. P., Orlandi F., Mellars L., Alland L., Tendler C., Ann. Oncol. 2004, 15, 440. [DOI] [PubMed] [Google Scholar]
- 16. Kivitz A., Segurado O. G., Expert Rev. Med. Devices 2007, 4, 109. [DOI] [PubMed] [Google Scholar]
- 17. Grossman E., Messerli F. H., Grodzicki T., Kowey P., JAMA, J. Am. Med. Assoc. 1996, 276, 1328. [PubMed] [Google Scholar]
- 18. Means L., Benken S. T., Tesoro E. P., J. Cardiovasc. Pharmacol. 2016, 68, 395. [DOI] [PubMed] [Google Scholar]
- 19. Accelerating biopharmaceutical development while reducing costs | McKinsey, https://www.mckinsey.com/industries/life‐sciences/our‐insights/the‐pursuit‐of‐excellence‐in‐new‐drug‐development.
- 20. Unlocking the potential of AI in drug discovery | Reports | Wellcome, https://wellcome.org/reports/unlocking‐potential‐ai‐drug‐discovery.
- 21. KP Jayatunga M., Ayers M., Bruens L., Jayanth D., Meier C., Drug Discov. Today 2024, 29, 104009. [DOI] [PubMed] [Google Scholar]
- 22. Niazi S. K., Drug Des. Devel. Ther. 2023, 17, 2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Insight: Big Pharma bets on AI to speed up clinical trials | Reuters, https://www.reuters.com/technology/big‐pharma‐bets‐ai‐speed‐up‐clinical‐trials‐2023‐09‐22/.
- 24. Wang W., Ye Z., Gao H., Ouyang D., J. Control Release 2021, 338, 119. [DOI] [PubMed] [Google Scholar]
- 25. Pham N. T., Phan L. T., Seo J., Kim Y., Song M., Lee S., Jeon Y.‐J., Manavalan B., Brief Bioinform. 2024, 25, bbad433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bannigan P., Aldeghi M., Bao Z., Häse F., Aspuru‐Guzik A., Allen C., Adv. Drug Deliv. Rev. 2021, 175, 113806. [DOI] [PubMed] [Google Scholar]
- 27. Bao Z., Bufton J., Hickman R. J., Aspuru‐Guzik A., Bannigan P., Allen C., Adv. Drug Deliv. Rev. 2023, 202, 115108. [DOI] [PubMed] [Google Scholar]
- 28. Fed. Regist. https://www.federalregister.gov/documents/2023/05/11/2023‐09985/using‐artificial‐intelligence‐and‐machine‐learning‐in‐the‐development‐of‐drug‐and‐biological. [Google Scholar]
- 29. Bannigan P., Bao Z., Hickman R. J., Aldeghi M., Häse F., Aspuru‐Guzik A., Allen C., Nat. Commun. 2023, 14, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Deng J., Ye Z., Zheng W., Chen J., Gao H., Wu Z., Chan G., Wang Y., Cao D., Wang Y., Lee S. M.‐Y., Ouyang D., Drug Deliv. Transl. Res. 2023, 13, 966. [DOI] [PubMed] [Google Scholar]
- 31. Damiati S. A., Damiati S., Front. Mol. Biosci. 2021, 8, 67754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang J., Heshmati Aghda N., Jiang J., Mridula Habib A., Ouyang D., Maniruzzaman M., Int. J. Pharm. 2022, 628, 122302. [DOI] [PubMed] [Google Scholar]
- 33. Wang F., Elbadawi M., Tsilova S. L., Gaisford S., Basit A. W., Parhizkar M., Mater. Des. 2022, 219, 110735. [DOI] [PubMed] [Google Scholar]
- 34. Bennett‐Lenane H., O'Shea J. P., Murray J. D., Ilie A.‐R., Holm R., Kuentz M., Griffin B. T., Pharmaceutics 2021, 13, 1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gao H., Jia H., Dong J., Yang X., Li H., Ouyang D., Acta Pharm. Sin. B 2021, 11, 3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee H., Kim J., Kim S., Yoo J., Choi G. J., Jeong Y.‐S., J. Chem. 2022, 2022, 1. [Google Scholar]
- 37. Schmitt J. M., Baumann J. M., Morgen M. M., Pharm. Res. 2022, 39, 3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gong D., Ben‐Akiva E., Singh A., Yamagata H., Est‐Witte S., Shade J. K., Trayanova N. A., Green J. J., Acta Biomater. 2022, 154, 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gao H., Kan S., Ye Z., Feng Y., Jin L., Zhang X., Deng J., Chan G., Hu Y., Wang Y., Cao D., Ji Y., Liang M., Li H., Ouyang D., Chem. Eng. J. 2022, 442, 136310. [Google Scholar]
- 40. Stiepel R. T., Pena E. S., Ehrenzeller S. A., Gallovic M. D., Lifshits L. M., Genito C. J., Bachelder E. M., Ainslie K. M., J. Controlled Release 2022, 351, 883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rebollo R., Oyoun F., Corvis Y., El‐Hammadi M. M., Saubamea B., Andrieux K., Mignet N., Alhareth K., ACS Appl. Mater. Interfaces 2022, 14, 39736. [DOI] [PubMed] [Google Scholar]
- 42. Elbadawi M., Muñiz Castro B., Gavins F. K. H., Ong J. J., Gaisford S., Pérez G., Basit A. W., Cabalar P., Goyanes A., Int. J. Pharm. 2020, 590, 119837. [DOI] [PubMed] [Google Scholar]
- 43. Muñiz Castro B., Elbadawi M., Ong J. J., Pollard T., Song Z., Gaisford S., Pérez G., Basit A. W., Cabalar P., Goyanes A., J. Controlled Release 2021, 337, 530. [DOI] [PubMed] [Google Scholar]
- 44. Ong J. J., Castro B. M., Gaisford S., Cabalar P., Basit A. W., Pérez G., Goyanes A., Int. J. Pharm. X 2022, 4, 100120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yang J., Soltan A. A. S., Clifton D. A., NPJ Digit. Med. 2022, 5, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Settles B., Synth. Lect. Artif. Intell. Mach. Learn. 2012, 6, 1. [Google Scholar]
- 47. Mockus J. presented at Proceedings of the IFIP Technical Conference, Springer‐Verlag, 400, 1974. [Google Scholar]
- 48. Rasmussen C. E., Williams C. K. I., Gaussian Processes for Machine Learning, The MIT Press, 2005.
- 49. Maffettone P. M., Friederich P., Baird S. G., Blaiszik B., Brown K. A., Campbell S. I., Cohen O. A., Davis R. L., Foster I. T., Haghmoradi N., Hereld M., Joress H., Jung N., Kwon H.‐K., Pizzuto G., Rintamaki J., Steinmann C., Torresi L., Sun S., Digit. Discov. 2023, 2, 1644. [Google Scholar]
- 50. Häse F., Roch L. M., Aspuru‐Guzik A., Trends Chem. 2019, 1, 282. [Google Scholar]
- 51. Self‐Driving Laboratories for Chemistry and Materials Science | Materials Science | ChemRxiv | Cambridge Open Engage https://chemrxiv.org/engage/chemrxiv/article‐details/65a887f29138d231612bf6df.
- 52. Stach E., DeCost B., Kusne A. G, Hattrick‐Simpers J., Brown K. A., Reyes K. G., Schrier J., Billinge S., Buonassisi T., Foster I., Gomes C. P., Gregoire J. M., Mehta A., Montoya J., Olivetti E., Park C., Rotenberg E., Saikin S. K., Smullin S., Stanev V., Maruyama B., Matter 2021, 4, 2702. [Google Scholar]
- 53. Coley C. W., Eyke N. S., Jensen K. F., Angew. Chem., Int. Ed. 2020, 59, 23414. [DOI] [PubMed] [Google Scholar]
- 54. Coley C. W., Eyke N. S., Jensen K. F., Angew. Chem., Int. Ed. 2020, 59, 22858. [DOI] [PubMed] [Google Scholar]
- 55. The Fourth Paradigm: Data‐Intensive Scientific Discovery – Microsoft Research, https://www.microsoft.com/en‐us/research/publication/fourth‐paradigm‐data‐intensive‐scientific‐discovery/.
- 56. Hickman R. J., Bannigan P., Bao Z., Aspuru‐Guzik A., Allen C., Matter 2023, 6, 1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tamasi M. J., Gormley A. J., Cell Rep. Phys. Sci. 2022, 3, 101041. [Google Scholar]
- 58. Tamasi M. J., Patel R. A., Borca C. H., Kosuri S., Mugnier H., Upadhya R., Murthy N. S., Webb M. A., Gormley A. J., Adv. Mater. 2022, 34, 2201809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dalal R. J., Oviedo F., Leyden M. C., Reineke T. M., Chem. Sci., 15, 7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Leading edge Solutions for Lab Automation & Digitalization Chemspeed, https://www.chemspeed.com/.
- 61. Automated Liquid Handling Equipment | Hamilton Robotics, https://www.hamiltoncompany.com/automated‐liquid‐handling.
- 62. AG T. T., Tecan Liquid Handling & Automation, https://lifesciences.tecan.com/products/liquid_handling_and_automation.
- 63. Opentrons | Lab Automation | Lab Robots for Life Scientists Opentrons.com, https://opentrons.com/.
- 64. Bao Z., Yung F., Hickman R. J., Aspuru‐Guzik A., Bannigan P., Allen C., Drug Deliv. Transl. Res. 2024, 14, 1872. [DOI] [PubMed] [Google Scholar]
- 65. Rutherford M. L., Stinger T., Curr. Opin. Drug Discov. Devel. 2001, 4, 343. [PubMed] [Google Scholar]
- 66. Jämsä‐Jounela S.‐L., Annu. Rev. Control 2007, 31, 211. [Google Scholar]
- 67. Singh R. 2018. in Computer Aided Chemical Engineering Process Systems Engineering for Pharmaceutical Manufacturing, (Eds: Singh R., Yuan Z.), Elsevier, Amsterdam, The Netherlands: 431. [Google Scholar]
- 68. Ghosh R., Kempf D., Pufko A., Barrios Martinez L. F., Davis C. M., Sethi S., Pharm. Med. 2020, 34, 7. [DOI] [PubMed] [Google Scholar]
- 69. Han Y., Makarova E., Ringel M., Telpis V., McKinsey Comp. Rep. 2019. [Google Scholar]
- 70. Singh A. V., Varma M., Rai M., Singh S. P., Bansod G., Laux P., Luch A., Adv. Intell. Syst. 2024, 6, 2300366. [Google Scholar]
- 71. Raccuglia P., Elbert K. C., Adler P. D. F., Falk C., Wenny M. B., Mollo A., Zeller M., Friedler S. A., Schrier J., Norquist A. J., Nature 2016, 533, 73. [DOI] [PubMed] [Google Scholar]
- 72. Barwich A.‐S., Front. Neurosci. 2019, 13, 1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Christensen M., Yunker L. P. E., Shiri P., Zepel T., Prieto P. L., Grunert S., Bork F., Hein J. E., Chem. Sci. 2021, 12, 15473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Shiri P., Lai V., Zepel T., Griffin D., Reifman J., Clark S., Grunert S., Yunker L. P. E., Steiner S., Situ H., Yang F., Prieto P. L., Hein J. E., iScience 2021, 24, 102176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ribeiro M. T., Singh S., Guestrin C., presented at Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining (ACM) 2016, 1135.
- 76. Lipton Z. C., Queue 2018, 16, 31. [Google Scholar]
- 77. Carvalho D. V., Pereira E. M., Cardoso J. S., Mac. Learn. Interpret. 2019, 8, 832. [Google Scholar]
- 78. Turner B. E., Steinberg J. R., Weeks B. T., Rodriguez F., Cullen M. R., Lancet Reg. Health – Am. 2022, 11, 100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hamel L. M., Penner L. A., Albrecht T. L., Heath E., Gwede C. K., Eggly S., Cancer Control J. Moffitt Cancer Cent. 2016, 23, 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Baltrušaitis T., Ahuja C., Morency L.‐P., IEEE Trans. Pattern Anal. Mach. Intell. 2019, 41, 423. [DOI] [PubMed] [Google Scholar]
- 81. Zhang C., Yang Z., He X., Deng L., IEEE J. Sel. Top Signal Proc. 2020, 14, 478. [Google Scholar]
- 82. Schweinar A., Wagner F., Klingner C., Festag S., Spreckelsen C., Brodoehl S., Appl. Clin. Inform. 2024, 15, 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Acosta J. N., Falcone G. J., Rajpurkar P., Topol E. J., Nat. Med. 2022, 28, 1773. [DOI] [PubMed] [Google Scholar]
- 84. Vargas S., Zamirpour S., Menon S., Rothman A., Häse F., Tamayo‐Mendoza T., Romero J., Sim S., Menke T., Aspuru‐Guzik A., J. Chem. Educ. 2020, 97, 689. [Google Scholar]
- 85. Zhang K., Aslan A. B., Comput. Educ. Artif. Intell. 2021, 2, 100025. [Google Scholar]
- 86. Predicting the future of healthcare and life sciences in 2025 Deloitte Switz, https://www2.deloitte.com/ch/en/pages/life‐sciences‐and‐healthcare/articles/predicting‐the‐future‐of‐healthcare‐and‐life‐sciences‐in‐2025.html.
- 87. Pollice R., dos Passos Gomes G., Aldeghi M., Hickman R. J., Krenn M., Lavigne C., Lindner‐D'Addario M., Nigam A., Ser C. T., Yao Z., Aspuru‐Guzik A., Acc. Chem. Res. 2021, 54, 849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Singh A. V., Bansod G., Mahajan M., Dietrich P., Singh S. P., Rav K., Thissen A., Bharde A. M., Rothenstein D., Kulkarni S., Bill J., ACS Omega 2023, 8, 21377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Singh A. V., Bhardwaj P., Upadhyay A. K., Pagani A., Upadhyay J., Bhadra J., Tisato V., Thakur M., Gemmati D., Mishra R., Zamboni P., Explor. BioMat‐X 2024, 1, 124. [Google Scholar]
- 90. C. Health for D. and R. 2022. Computer Software Assurance for Production and Quality System Software, https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/computer‐software‐assurance‐production‐and‐quality‐system‐software 2022.
- 91. C. Health for D. and R. 2024. Artificial Intelligence and Machine Learning in Software as a Medical Device. FDA. . 2024
- 92. Bernstein A., Raman A., Harv. Bus Rev. 2015; [Google Scholar]
- 93. Frey C. B., Osborne M. A., Technol. Forecast Soc. Change 2017, 114, 254. [Google Scholar]
- 94. Hole G., Hole A. S., McFalone‐Shaw I., Int. J. Pharm. X 2021, 3, 100095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Regulation –2016/679 – EN – gdpr – EUR‐Lex, https://eur‐lex.europa.eu/eli/reg/2016/679/oj.
- 96. Rights (OCR), O. for C. 2021. Health Information Privacy, https://www.hhs.gov/hipaa/index.html, 2021.
- 97. Bonaccorsi A., Rossi C., Res. Policy 2003, 32, 1243. [Google Scholar]
- 98. Fortunato L., Galassi M., Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2021, 379, 20200079. [DOI] [PubMed] [Google Scholar]
- 99. Keesey R., LeSuer R., Schrier J., HardwareX 2022, 12, e00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Baird S. G., Sparks T. D., STAR Protoc. 2023, 4, 102329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Baird S. G., Sparks T. D., Matter 2022, 5, 4170. [Google Scholar]


