Abstract
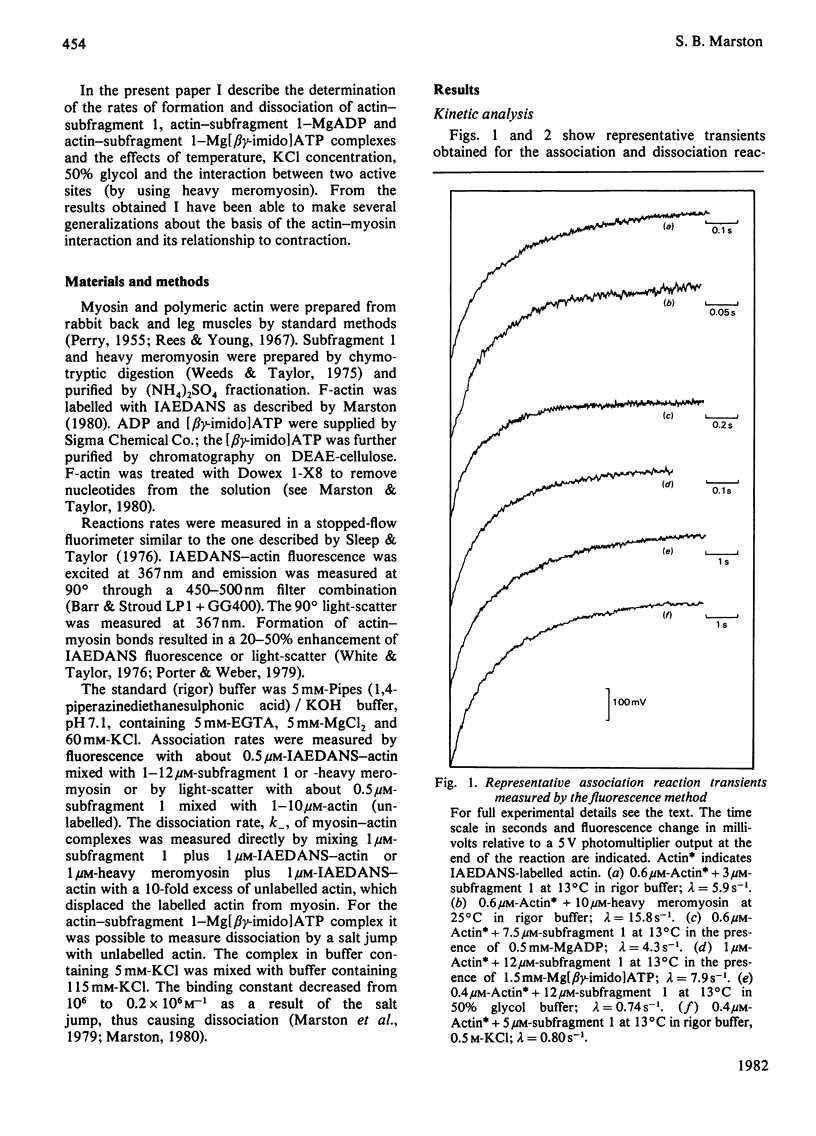
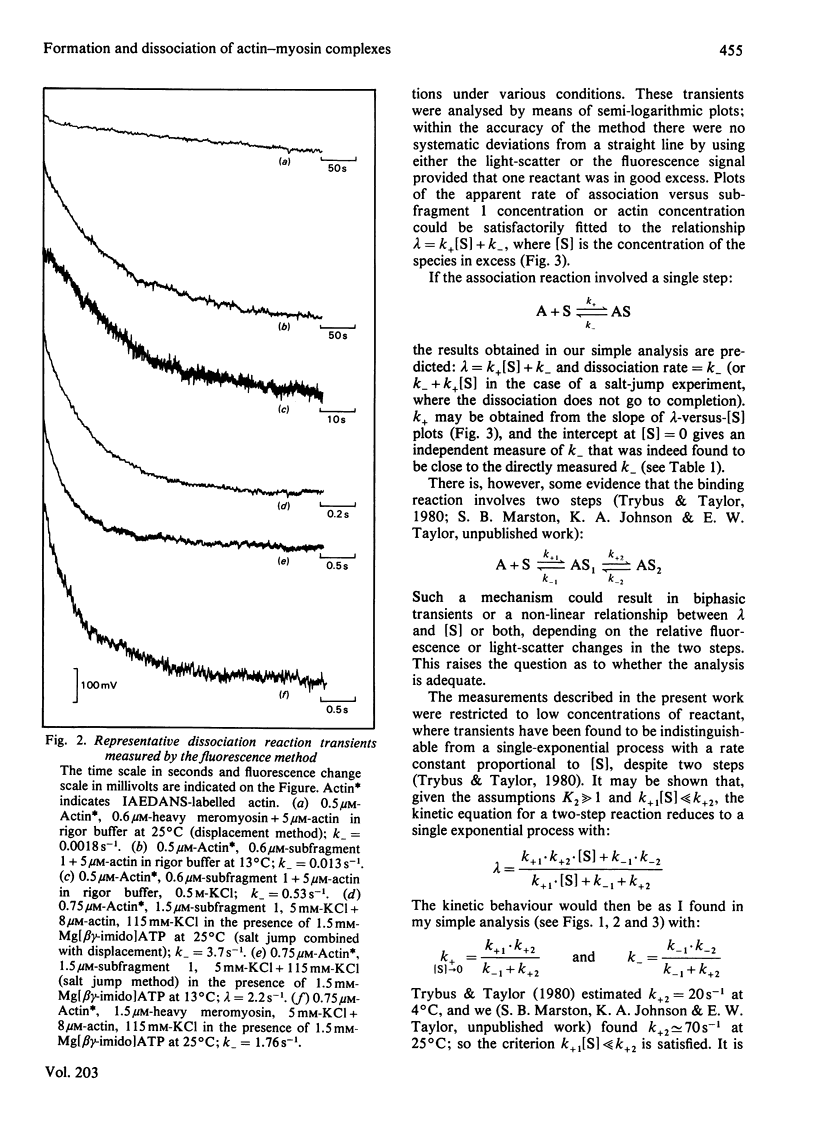
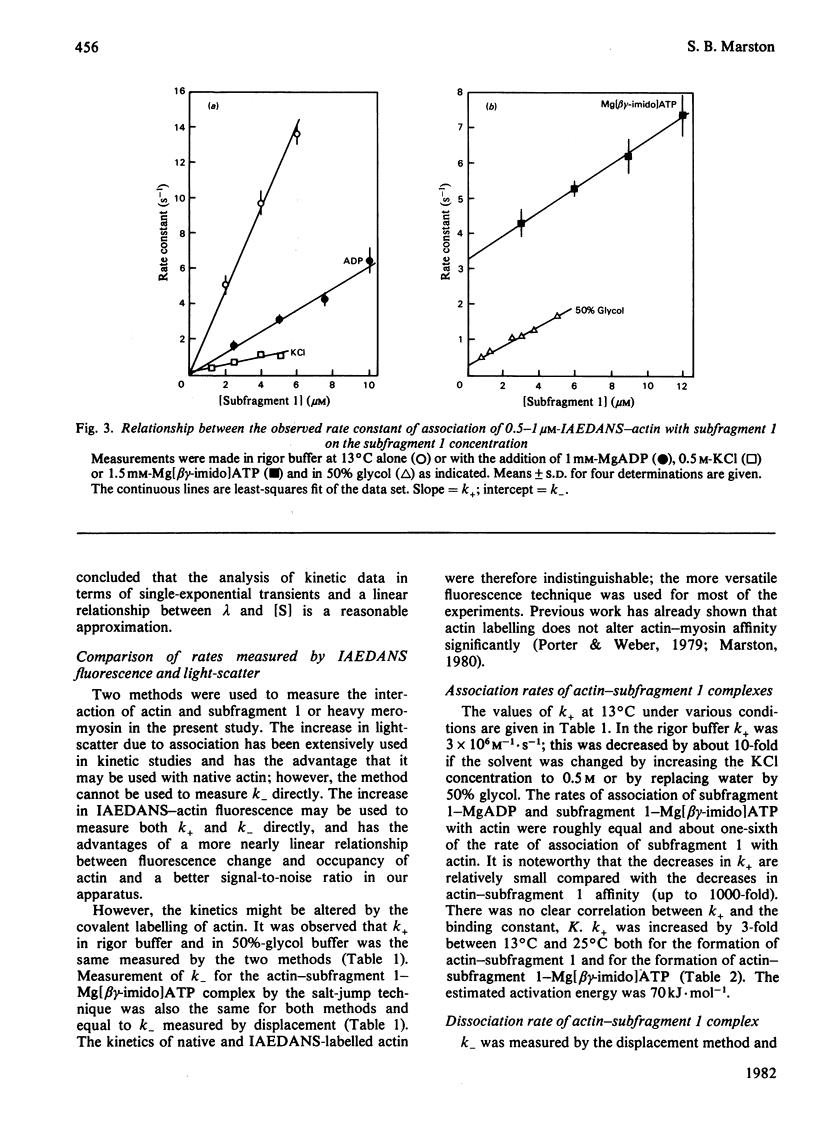
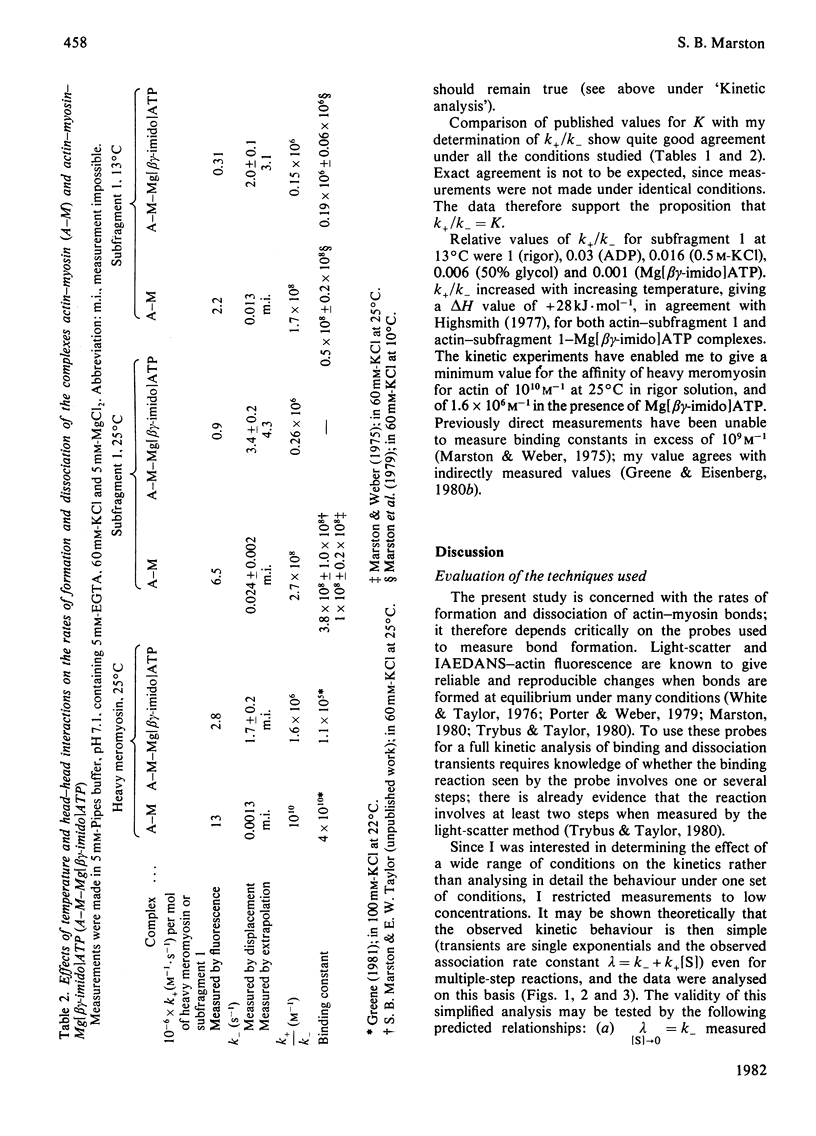
The rates of formation and dissociation of actin-subfragment 1 and actin-heavy mero-myosin complexes were measured by using light-scatter and the change in fluorescence of N-iodoacetyl-N'-(5-sulpho-1-naphthyl)ethylenediamine (IAEDANS)-labelled acting as probes. Association rate measurements were made at low protein concentration, where the transients approximated to single exponentials with rate constants proportional to the concentration of reactant in excess. Dissociation rate measurements were made by displacing IAEDANS-actin from myosin with excess native actin and by a salt jump. The second-order rate constant of association for actin-subfragment 1 was 3 x 10(6) M-1 . s-1 in 60 mM-KCl at 13 degree C. It was decreased 10-fold in 500 mM-KCl and in 50% (v/v) glycol. It was decreased 6-fold when MgADP or Mg[beta gamma-imido]ATP bound to myosin. The dissociation rate constant was 0.012 s-1 in 60 mM-KCl at 13 degree C. It was increased 4-fold by 500 mM-KCl, 25-fold by 50% glycol, 8-fold by MgADP binding and 170-fold by Mg[beta gamma-imido]ATP binding. Ea for association was 70 kJ . mol-1 and for dissociation 35 kJ . mol-1. Heavy meromyosin associated at twice the rate observed for subfragment 1 and dissociated at less than one-twentieth of the rate for subfragment 1 (60 mM-KCl, 25 degree C), but when Mg[beta gamma-imido]ATP bound actin-heavy meromyosin dissociated at one-half the rate for subfragment 1. There were significant correlations between increase in the dissociation rate constant, decrease in binding constant and increase in magnitude of conformational change. The association rate constant did not correlate with any property of the actin-myosin complex.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Greene L. E. Comparison of the binding of heavy meromyosin and myosin subfragment 1 in F-actin. Biochemistry. 1981 Apr 14;20(8):2120–2126. doi: 10.1021/bi00511a008. [DOI] [PubMed] [Google Scholar]
- Greene L. E., Eisenberg E. Dissociation of the actin.subfragment 1 complex by adenyl-5'-yl imidodiphosphate, ADP, and PPi. J Biol Chem. 1980 Jan 25;255(2):543–548. [PubMed] [Google Scholar]
- Greene L. E., Eisenberg E. The binding of heavy meromyosin to F-actin. J Biol Chem. 1980 Jan 25;255(2):549–554. [PubMed] [Google Scholar]
- Gruen D. W. A mean-field model of the alkane-saturated lipid bilayer above its phase transition. I. Development of the model. Biophys J. 1981 Feb;33(2):149–166. doi: 10.1016/S0006-3495(81)84878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highsmith S. Heavy meromyosin binds actin with negative cooperativity. Biochemistry. 1978 Jan 10;17(1):22–26. doi: 10.1021/bi00594a004. [DOI] [PubMed] [Google Scholar]
- Highsmith S. The effects of temperature and salts on myosin subfragment-1 and F-actin association. Arch Biochem Biophys. 1977 Apr 30;180(2):404–408. doi: 10.1016/0003-9861(77)90054-6. [DOI] [PubMed] [Google Scholar]
- Hill T. L., Eisenberg E. Theoretical considerations in the equilibrium binding of myosin fragments on F-actin. Biophys Chem. 1980 Apr;11(2):271–281. doi: 10.1016/0301-4622(80)80030-5. [DOI] [PubMed] [Google Scholar]
- Hofmann W., Goody R. S. The ternary complex formed between actin, myosin subfragment 1 and ATP (beta, gamma-NH). FEBS Lett. 1978 May 1;89(1):169–172. doi: 10.1016/0014-5793(78)80547-x. [DOI] [PubMed] [Google Scholar]
- Inoue A., Tonomura Y. Dissociation of acto-H-meromyosin and that of acto-subfragment-1 induced by adenyl-5'-yl-imidodiphosphate: evidence for a ternary complex of F-actin, myosin head, and substrate. J Biochem. 1980 Dec;88(6):1643–1651. doi: 10.1093/oxfordjournals.jbchem.a133140. [DOI] [PubMed] [Google Scholar]
- Marston S. B. Evidence for an altered structure of actin-S1 complexes when Mg-adenylylimidodiphosphate binds. J Muscle Res Cell Motil. 1980 Sep;1(3):305–320. doi: 10.1007/BF00711933. [DOI] [PubMed] [Google Scholar]
- Marston S. B., Rodger C. D., Tregear R. T. Changes in muscle crossbridges when beta, gamma-imido-ATP binds to myosin. J Mol Biol. 1976 Jun 14;104(1):263–276. doi: 10.1016/0022-2836(76)90012-7. [DOI] [PubMed] [Google Scholar]
- Marston S. B., Taylor E. W. Comparison of the myosin and actomyosin ATPase mechanisms of the four types of vertebrate muscles. J Mol Biol. 1980 Jun 5;139(4):573–600. doi: 10.1016/0022-2836(80)90050-9. [DOI] [PubMed] [Google Scholar]
- Marston S. B., Tregear R. T., Rodger C. D., Clarke M. L. Coupling between the enzymatic site of myosin and the mechanical output of muscle. J Mol Biol. 1979 Feb 25;128(2):111–126. doi: 10.1016/0022-2836(79)90121-9. [DOI] [PubMed] [Google Scholar]
- Marston S., Weber A. The dissociation constant of the actin-heavy meromyosin subfragment-1 complex. Biochemistry. 1975 Aug 26;14(17):3868–3873. doi: 10.1021/bi00688a021. [DOI] [PubMed] [Google Scholar]
- Pate E. F., Brokaw C. J. Cross-bridge behavior in rigor muscle. Biophys Struct Mech. 1980;7(1):51–63. doi: 10.1007/BF00538158. [DOI] [PubMed] [Google Scholar]
- Porter M., Weber A. Non-cooperative response of actin-cystein 373 in cooperatively behaving regulated actin filaments. FEBS Lett. 1979 Sep 15;105(2):259–262. doi: 10.1016/0014-5793(79)80624-9. [DOI] [PubMed] [Google Scholar]
- Rees M. K., Young M. Studies on the isolation and molecular properties of homogeneous globular actin. Evidence for a single polypeptide chain structure. J Biol Chem. 1967 Oct 10;242(19):4449–4458. [PubMed] [Google Scholar]
- Sleep J. A., Taylor E. W. Intermediate states of actomyosin adenosine triphosphatase. Biochemistry. 1976 Dec 28;15(26):5813–5817. doi: 10.1021/bi00671a019. [DOI] [PubMed] [Google Scholar]
- Trybus K. M., Taylor E. W. Kinetic studies of the cooperative binding of subfragment 1 to regulated actin. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7209–7213. doi: 10.1073/pnas.77.12.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeds A. G., Taylor R. S. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975 Sep 4;257(5521):54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]
- White H. D., Taylor E. W. Energetics and mechanism of actomyosin adenosine triphosphatase. Biochemistry. 1976 Dec 28;15(26):5818–5826. doi: 10.1021/bi00671a020. [DOI] [PubMed] [Google Scholar]


