Abstract
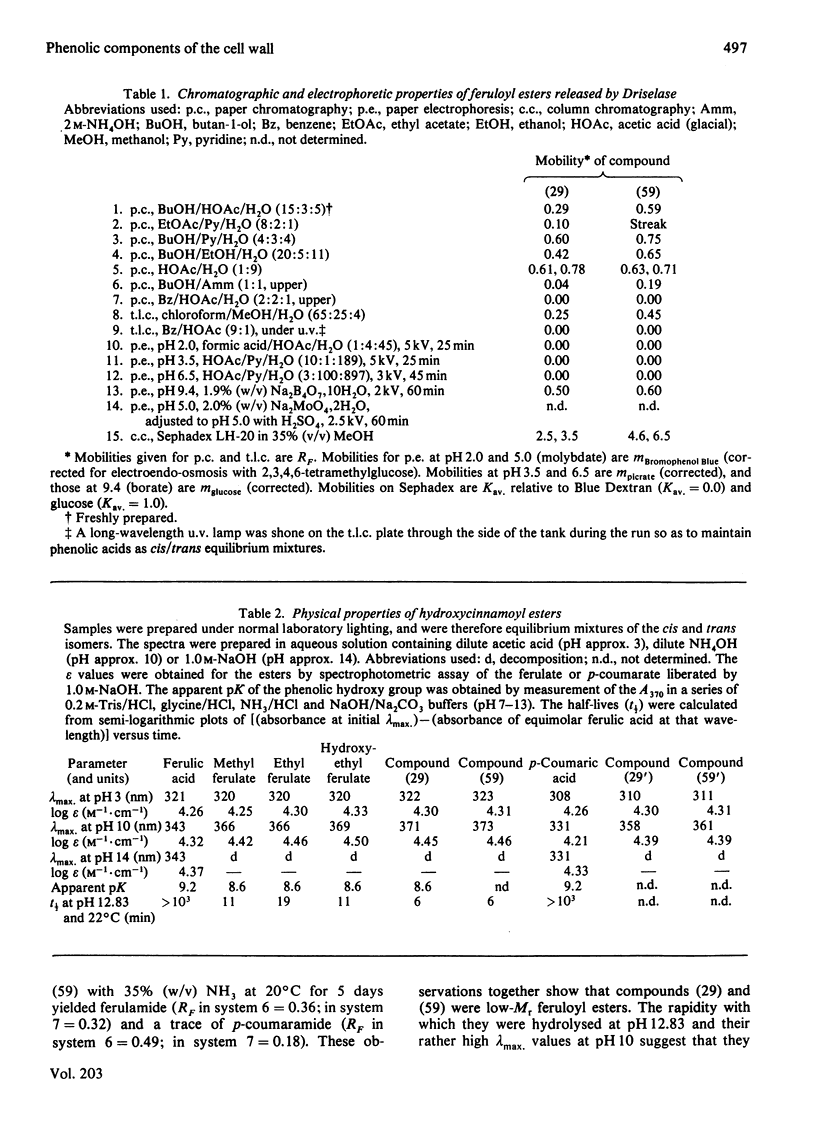
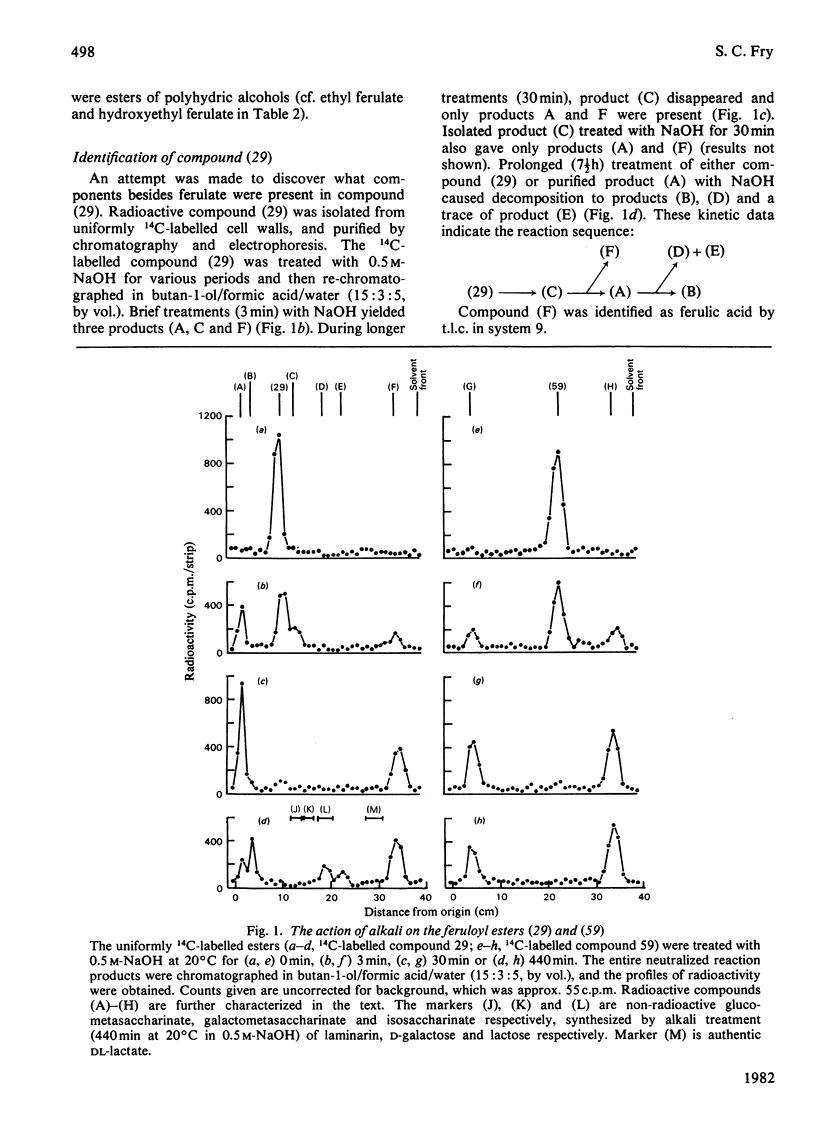
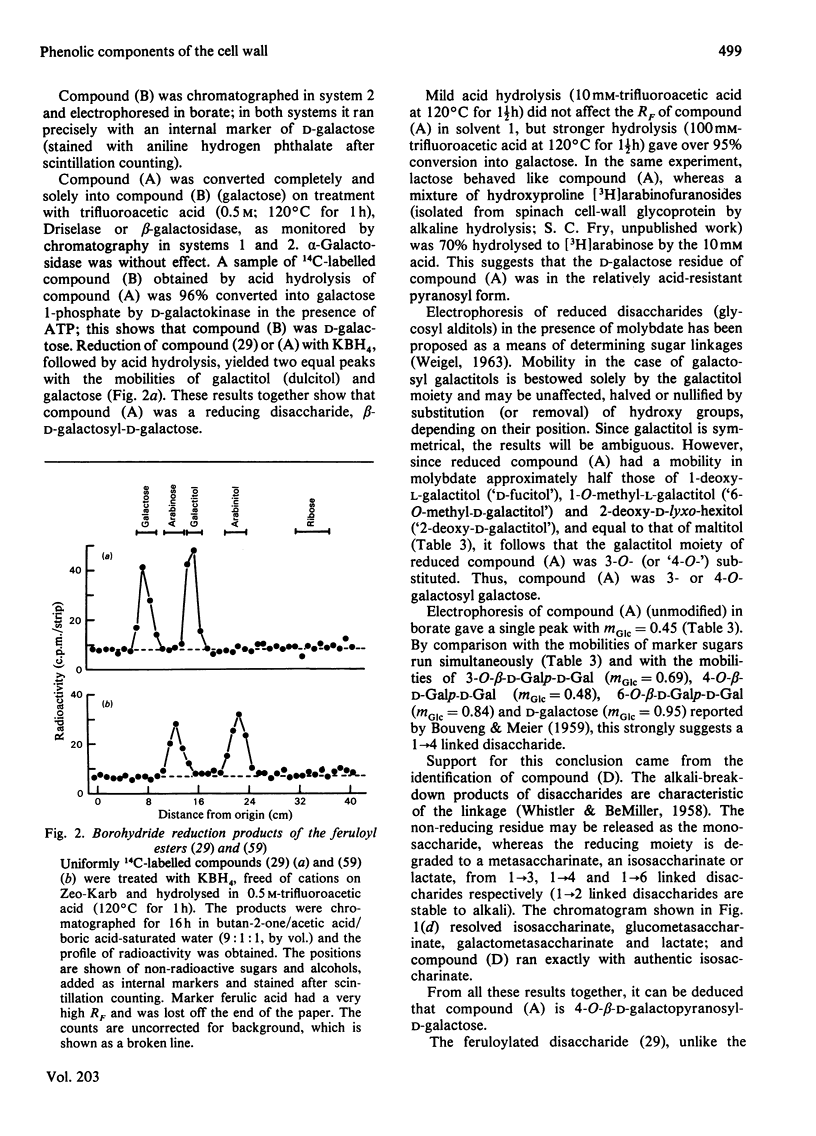
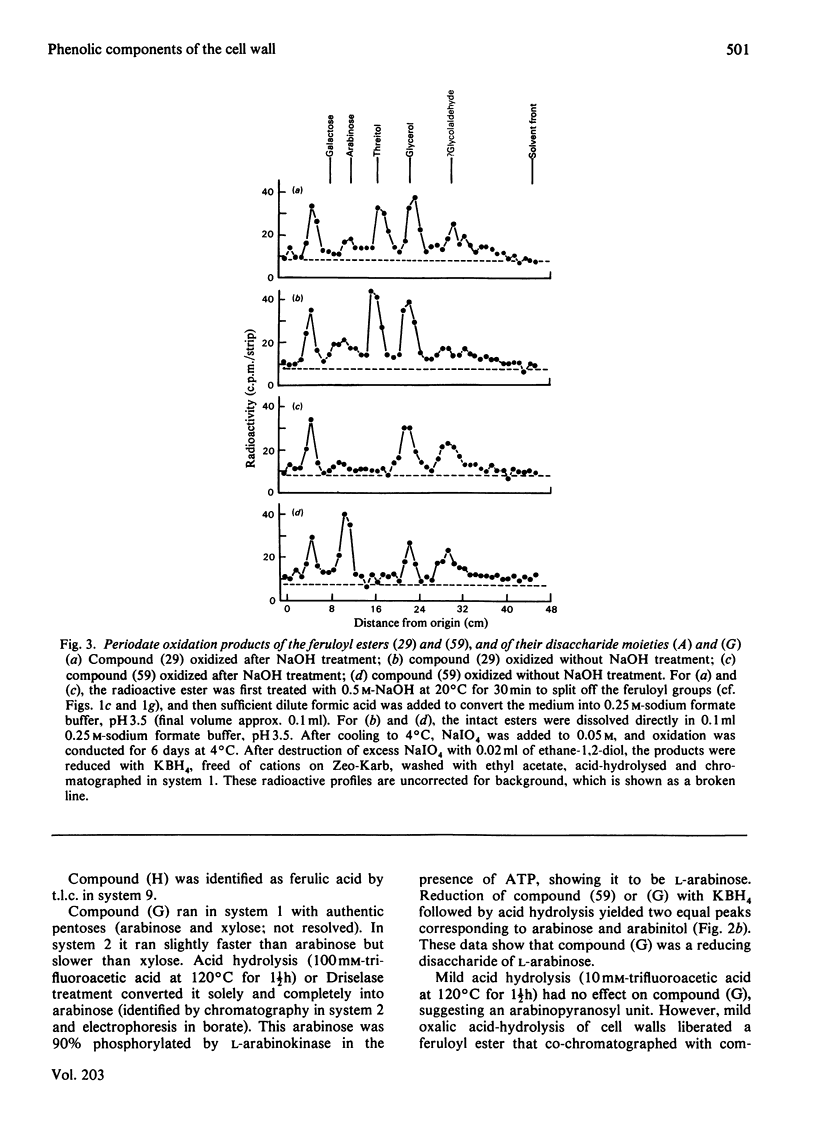
1. Cell walls from rapidly growing cell suspension cultures of Spinacia oleracea L. contained ferulic acid and p-coumaric acid esterified with a water-insoluble polymer. 2. Prolonged treatment with trypsin did not release may feruloyl esters from dearabinofuranosylated cell walls, and the polymer was also insoluble in phenol/acetic acid/water (2:1:1, w/v/v). 3. Treatment of the cell walls with the fungal hydrolase preparation "Driselase' did liberate low-Mr feruloyl esters. The major esters were 4-O-(6-O-feruloyl-beta-D-galactopyranosyl)-D-galactose and 3?-O-feruloyl-alpha-L-arabinopyranosyl)-L-arabinose. These two esters accounted for about 60% of the cell-wall ferulate. 4. It is concluded that the feruloylation of cell-wall polymers is not a random process, but occurs at very specific sites, probably on the arabinogalactan component of pectin. 5. The possible role of such phenolic substituents in cell-wall architecture and growth is discussed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dalton C. C., Street H. E. The role of the gas phase in the greening and growth of illuminated cell suspension cultures of spinach (Spinacia oleracea, L.). In Vitro. 1976 Jul;12(7):485–494. doi: 10.1007/BF02796491. [DOI] [PubMed] [Google Scholar]
- Darvill A. G., McNeil M., Albersheim P. Structure of Plant Cell Walls: VIII. A New Pectic Polysaccharide. Plant Physiol. 1978 Sep;62(3):418–422. doi: 10.1104/pp.62.3.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry S. C., Street H. E. Gibberellin-sensitive Suspension Cultures. Plant Physiol. 1980 Mar;65(3):472–477. doi: 10.1104/pp.65.3.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD B. H. Hydrolysis of the soluble pentosans of wheat flour and Rhodymenia pa'mata by ruminal micro-organisms. Biochem J. 1957 Dec;67(4):643–651. doi: 10.1042/bj0670643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune D. C., Galston A. W. Inverse Effects of Gibberellin on Peroxidase Activity and Growth in Dwarf Strains of Peas and Corn. Plant Physiol. 1959 Jul;34(4):416–418. doi: 10.1104/pp.34.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort A. J., Lamport D. T. Anhydrous hydrogen fluoride deglycosylates glycoproteins. Anal Biochem. 1977 Oct;82(2):289–309. doi: 10.1016/0003-2697(77)90165-8. [DOI] [PubMed] [Google Scholar]
- NEUFELD E. F., FEINGOLD D. S., HASSID W. Z. Phosphorylation of D-galactose and L-arabinose by extracts from Phaseolus aureus seedlings. J Biol Chem. 1960 Apr;235:906–909. [PubMed] [Google Scholar]
- O'Neill M. A., Selvendran R. R. Glycoproteins from the cell wall of Phaseolus coccineus. Biochem J. 1980 Apr 1;187(1):53–63. doi: 10.1042/bj1870053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter T. J., Neukom H. The mechanism of oxidative gelation of a glycoprotein from wheat flour. Evidence from a model system based upon caffeic acid. Biochim Biophys Acta. 1968 Jun 24;158(3):363–381. doi: 10.1016/0304-4165(68)90291-2. [DOI] [PubMed] [Google Scholar]
- Panayotatos N., Villemez C. L. The formation of a -(1 leads to 4)-D-galactan chain catalysed by a Phaseolus aureus enzyme. Biochem J. 1973 Jun;133(2):263–271. doi: 10.1042/bj1330263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overbeek J. The Growth Hormone and the Dwarf Type of Growth in Corn. Proc Natl Acad Sci U S A. 1935 May;21(5):292–299. doi: 10.1073/pnas.21.5.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIGEL H. PAPER ELECTROPHORESIS OF CARBOHYDRATES. Adv Carbohydr Chem. 1963;18:61–97. doi: 10.1016/s0096-5332(08)60240-4. [DOI] [PubMed] [Google Scholar]
- WHISTLER R. L., BEMILLER J. N. Alkaline degradation of polysaccharides. Adv Carbohydr Chem. 1958;13:289–329. doi: 10.1016/s0096-5332(08)60359-8. [DOI] [PubMed] [Google Scholar]
- Whitmore F. W. Phenolic acids in wheat coleoptile cell walls. Plant Physiol. 1974 May;53(5):728–731. doi: 10.1104/pp.53.5.728. [DOI] [PMC free article] [PubMed] [Google Scholar]


