Abstract
‘Candidatus Endonucleobacter’ infects the nuclei of deep-sea mussels but it was unknown how they can prevent apoptosis of the host. A new study by Porras and colleagues, published in Nature Microbiology, suggests that the bacterium upregulates host-derived apoptosis inhibitors and genes for digesting sugars, lipids and amino acids acquired through horizontal gene transfer from the mussels.
It is very common for metazoans to live in close association with bacteria; however, only a very low number of these bacteria live inside the hosts organelles1–3. ‘Candidatus Endonucleobacter’ is a clade of the family Endozoicomonadaceae that inhabits the nuclei of deep-sea bathymodioline mussels from hydrothermal vents and cold seeps worldwide4. A single bacterium infects the mussel by entering the nucleus, where it divides until it reaches up to 80,000 cells and the nucleus is swollen up to 50 times its normal size. The infected mussel cells eventually burst, and the bacteria are released into the seawater4. Two main questions about this unique life strategy remained: 1. How do ‘Ca. Endonucleobacter’ secure energy and nutrition within the host nucleus to support their massive replication? and 2. How do ‘Ca. Endonucleobacter’ avoid an immune response from the host that would lead to cell death through apoptosis?
A recent study by Porras et al.5 provides new insight into the genetic adaptations of ‘Ca. Endonucleobacter’ to enable this unique lifestyle. The authors investigated two different species of ‘Ca. Endonucleobacter’ infecting the deep-sea mussel Bathymodiolus puteoserpentis from hydrothermal vents on the Mid-Atlantic Ridge and Gigantidas childressi from cold seeps in the Gulf of Mexico. Despite being different species and separated geographically by thousands of kilometers, this study revealed surprising similarities in the metabolic strategy of the parasite-host interactions between the mussels and ‘Ca. Endonucleobacter’.
Once inside the nucleus of a host cell (Fig. 1), ‘Ca. Endonucleobacter’ imports and consumes sugars, lipids, and amino acids from the host rather than using host DNA, RNA, or histones. At the same time, the mussels express genes for the import of sugars, amino acids and the synthesis of lipid droplets in the early and mid-infection stages. The data further suggest that the mussels initiate programmed cell death in response to the infection, but a unique feature of both ‘Ca. Endonucleobacter’ species is that they encode multiple inhibitors of apoptosis (IAPs) to prevent this process. The result is that the host and the parasite are locked in a physiological arms race over apoptosis control - but the deployment of specific IAPs allows the parasite to prevent apoptosis long enough to extract the necessary energy and nutrients for massive replication before the host cell eventually dies. While IAPs are common in animals, they have not been described in bacteria before6 and they are believed to have been acquired through horizontal gene transfer (HGT) from the host, or potentially via viruses.
Fig. 1. The ‘Ca. Endonucleobacter’ infection cycle.
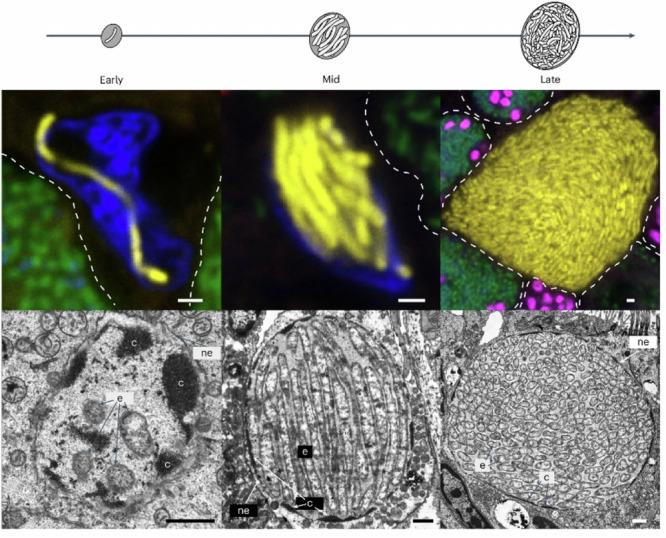
Parasite (in yellow), nuclei (DAPI-stained DNA in blue) and neighbouring cells (indicated with dotted lines) with sulfur-oxidizing symbionts (in green) and methane-oxidizing symbionts (in pink) (e, ‘Ca. Endonucleobacter’ cell; c, chromatin; ne, nuclear envelope. Scale bars, 1 μm. From ref. 5.
The use of IAPs in an intranuclear parasite raises a “chicken or egg” dilemma: Was ‘Ca. Endonucleobacter’ able to acquire IAPs because of its intranuclear lifestyle, or did the acquisition of IAPs enable the parasite to thrive within the nucleus in the first place? This study suggests that HGT from eukaryotes to bacteria may be more common than previously assumed.
Competing interests
L.H. is an Editorial Board Member at Communications Biology, but was not involved in the editorial review, nor the decision to publish this article. The other authors declare no competing interests.
References
- 1.Gruber-Vodicka, H. R. et al. Two intracellular and cell type-specific bacterial symbionts in the placozoan Trichoplax H2. Nat. Microbiol.4, 1465–1474 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salje, J. Cells within cells: Rickettsiales and the obligate intracellular bacterial lifestyle. Nat. Rev. Microbiol.19, 375–390 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Sacchi, L. et al. A symbiont of the tick Ixodes ricinus invades and consumes mitochondria in a mode similar to that of the parasitic bacterium Bdellovibrio bacteriovorus. Tissue Cell36, 43–53 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Zielinski, F. U. et al. Widespread occurrence of an intranuclear bacterial parasite in vent and seep bathymodiolin mussels. Environ. Microbiol.11, 1150–1167 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Porras, M.Á.G. et al. An intranuclear bacterial parasite of deep-sea mussels expresses apoptosis inhibitors acquired from its host. Nat. Microbiol. 10.1038/s41564-024-01808-5 (2024). [DOI] [PMC free article] [PubMed]
- 6.Clem, R. J. Viral IAPs, then and now. Semin. Cell Dev. Biol.39, 72–79 (2015). [DOI] [PubMed] [Google Scholar]


