Abstract
The relationship between the oxidative balance score (OBS) and the serum Klotho level has yet to be defined. We sought to investigate the potential relationship between OBS and the serum Klotho level in the U.S. population aged 40–79 years. This study included 8,145 participants from the National Health and Nutrition Examination Surveys (NHANES) database spanning from 2007 to 2016. The OBS consisted of the dietary OBS and the lifestyle OBS, based on 16 dietary components and 4 lifestyle components. Weighted multiple linear regressions were performed to explore the association between OBS and serum Klotho level. Furthermore, nonlinear relationships were analyzed through the application of restricted cubic splines (RCS). In the multivariate linear regression model with adjustment for such as demographics, economic income and dietary intake, a higher OBS was associated with a higher serum Klotho, with the beta estimate and 95%CI of 2.85 (1.03–4.68, p < 0.01). Compared with the lowest tertile group, the highest group was associated with a higher Klotho level (30.35, 3.43–57.28, p < 0.05). Furthermore, higher dietary OBS and lifestyle OBS were similarly associated with higher Klotho level (beta (95%CI): 1.27 (0.79–3.32); 14.23 (9.53–18.92), respectively). The RCS exhibited a linear dose-response association between OBS, dietary OBS and lifestyle OBS with serum Klotho concentration (Pnon−linearity>0.05). The association between OBS and serum Klotho level was consistent across age, sex, education, marital status, energy intake and poverty income ratio (PIR) (Pinteraction>0.05). The study reported significant association between OBS and klotho, indicating that adherence to antioxidant behaviors may be linked to slower aging and better health.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-80464-5.
Keywords: Oxidative balance score, Serum Klotho, NHANES
Subject terms: Geriatrics, Nutrition, Public health, Risk factors, Nutrition disorders
Introduction
Oxidative stress (OS), caused by an imbalance between pro-oxidants and antioxidants, is a significant factor affecting lifespan and is implicated in the pathogenesis of various age-related chronic diseases1–3. Research has shown that excessive oxidative stress contributes to the onset and progression of many chronic diseases, including cardiovascular disease, neurodegenerative diseases, and cancer4. Previously, the estimation of oxidative balance relied on isolated measurements of various oxidative stress and defense parameters, which only partially reflected the overall status due to the complexity and variety of pathway involved in redox homeostasis5. However, the recently proposed Oxidative Balance Score (OBS), which incorporates dietary and lifestyle factors that influence oxidative processes, provides a more comprehensive approach to assessing oxidative balance6.
The OBS provides a holistic method for evaluating oxidative stress by accounting for twenty dietary factors and four lifestyle factors, influencing the pro-oxidant/antioxidant equilibrium7. Investigations have demonstrated a substantial inverse relationship between OBS and a range of health conditions, including metabolic dysfunction-associated steatotic liver disease (MASLD)8, chronic kidney disease (CKD)9, sleep disorders6, periodontitis10, diabetes mellitus11,12, and depressive symptoms13. This substantial inverse relationship underscores the importance of OBS in reflecting antioxidant status, which is crucial for mitigating the risk of these conditions. The association between OBS and health condition was characterized by a dose-response pattern, where a higher OBS was linked to a decreased risk of adverse health outcomes. The study conducted by Zhan et al. demonstrated a linear association between OBS and stroke, with each unit increase in OBS corresponding to 2% reduction in stroke risk14. Similarly, the study from the UK Biobank involving 175,808 participants has identified a significant inverse dose-response relationship between OBS and risk of colorectal cancer15. Furthermore, A study identified a significant inverse relationship between OBS and metabolic syndrome, with a 43% and 58% reduced risk in the third and fourth quartiles compared to the first, highlighting a dose-dependent effect16. These findings collectively suggest that an increase of OBS reflects a better antioxidant status and may confer a reduced risk of these conditions, with the strength of the association intensifying as OBS increases.
The gene encoding Klotho, a protein with recognized anti-aging properties, is predominantly expressed in the kidney tissue17. Subsequent research has indicated potential associations between Klotho and oxidative stress, implying that serum Klotho could be a biomarker for assessing antioxidant capacity and vulnerability to oxidative stress-induced diseases18. Decreased Klotho expression was associated with increased reactive oxygen species (ROS) and oxidative damage, signifying its protective role against cellular injury18. An increasing body of literature has shown that an inverse associations between serum Klotho levels and cancer19, heart failure20, diabetes21, cognition impairment and dementia22. Interestingly, a U-shaped association was found between OBS with diabetes and cognitive impairment21,22. Nonetheless, the specific relationship between OBS and the concentration of Klotho in serum remains to be fully elucidated and warrants further investigation.
In this study, we aimed to explore the potential association between OBS, dietary OBS, and lifestyle OBS with serum Klotho levels in 8,145 Americans aged 40–79 years. A study on the relationship between redox equilibrium and Klotho can contribute to the scientific discourse on healthy lifestyle.
Methods
Study population
The National-Health-and-Nutrition-Examination-Survey (NHANES) is a research initiative to assess the health and nutrition of children and adults across America. This survey is conducted annually and represents a nationally diverse sample of around 5,000 individuals from 15 counties. Its distinctiveness lies in its dual approach, combining structured interviews with comprehensive physical examinations. Interviews are conducted within respondents’ homes, while physical measurements are taken at specialized and well-equipped mobile centers, which travel to locations throughout the country23.
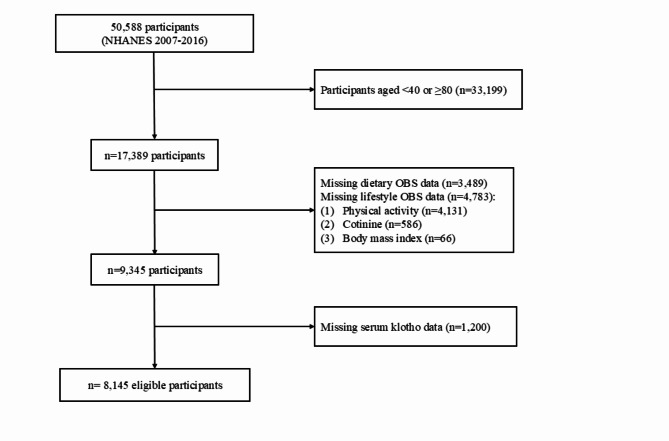
In this retrospective study, we included 50,588 participants from 5 survey cycles of the NHANES (2007–2008, 2009–2010, 2011–2012, 2013–2014, and 2015–2016). Firstly, participants with missing dietary OBS (n = 12,800) and lifestyle OBS (n = 17,858) were excluded. Next, participants with missing serum Klotho data (n = 11,785) were excluded. Finally, the analysis involved 8,145 individuals (Fig. 1). The research protocol received approval from the Ethics Review Board at the National Center for Health Statistics and all participants signed informed consent24.
Fig. 1.
The flow chart of participant selection.
Oxidative balance scores
Calculation of OBS based on the method of the related study6, which has been used in multiple studies and has high reliability and validity in assessing oxidative stress13,25. OBS is composed of 16 dietary OBS components (including dietary iron, zinc, total fat, copper, selenium, magnesium, calcium, vitamin C, E, B6 and B12, total folate, carotene, niacin, riboflavin, and fiber) and four lifestyle OBS components (including body mass index, alcohol, smoking, and physical activity), Involving 15 antioxidants and 5 pro-oxidants.
Food and nutrient information were collected by professionals using two 24-hour dietary recall questionnaires at each survey cycle. The first interview was conducted face-to-face on a weekday, and the second was collected by phone on a weekend 3–10 days later. Dietary intakes were calculated according to the Food and Nutrient Database for Dietary Studies provided by the US Department of Agriculture26, and total intake over two days was used as the analysis. Smoking was estimated using serum cotinine. The formula, weight (kg)/height (m2), was utilized to determine the body mass index (BMI). We assessed physical activity using one-week metabolic equivalents (MET), which were calculated from specific activity data obtained based on the Global Physical Activity Questionnaire (GPAQ)27. This questionnaire includes information on the intensity and duration of different physical activity, such as work activity, recreational activity, and transportation modes. Each specific activity was assigned a MET score of 0, 4 or 8 (Supplementary Table 1). Subsequently, the weekly MET of each specific activity was obtained by multiplying the duration of each specific activity during one week by the corresponding score, and then summing the MET of each specific activity to obtain the total MET for one week28. Higher MET responded to higher level of physical activity.
We divided each component into three groups based on its tertiles, counted as 0, 1 and 2 points respectively. Higher antioxidants were represented by higher scores, and pro-oxidants by lower scores (Supplementary Table 2). Overall OBS was determined by adding the scores of these items, ranging from 0 to 40 points, with higher OBS representing more excellent antioxidant activity.
Klotho measurements
Serum Klotho level was determined by the Northwest Lipid Metabolism and Diabetes Research Laboratory of the University of Washington utilizing an ELISA kit (Immunobiology Laboratory, Japan)29. After participants were asked to fast for at least 9 h, their blood samples were collected at a mobile examination center (MEC). Blood samples were centrifuged to obtain serum and placed on dry ice, then transported to laboratories throughout the United States. All samples were stored at − 80 °C. Samples were tested in duplicate, with the final value derived from the average of the two times. In addition, quality control (QC) samples containing low and high levels of Klotho were also arranged in each ELISA plate. The sample will be re-tested if the duplicate results exceed 10% or the QC sample values are not within 2SD of the assigned value. The reference range for serum Klotho level in healthy people was 285.8-1638.6 pg/ml 20.
Covariates information
Various covariates were collected through interview questionnaires, including age, sex, race, marital status, education, poverty income ratio (PIR), energy intake and dietary intake (including whole grain, milk, red meat, cured meat and egg). Education was categorized as less than high school, high school, and more than high school. Marital status was classified as cohabiting and single. Energy and dietary intake were the sum of 2 days.
Diabetes was diagnosed based on various criteria, including (i) random glucose content ≥ 11.1 mmol/L; (ii) HbA1c concentration ≥ 6.5%; (iii) fasting glucose level ≥ 7.0 mmol/L; (iv) oral glucose tolerance test ≥ 11.1 mmol/L; or (v) the use of antidiabetic drugs30. The diagnostic criteria for hypertension included fulfilling one of the following conditions: (i) history of hypertension; (ii) taking antihypertensive medications; (iii) or with average systolic pressure ≥ 140 mmHg/average diastolic pressure ≥ 90 mmHg31. Based on criteria issued by the National Cholesterol Education Program (NCEP) Expert Panel, each of the following conditions was diagnosed as hyperlipemia: (i) total cholesterol ≥ 200 mg/dL; (ii) triglyceride ≥ 150 mg/dL; (iii) low-density lipoprotein ≥ 130 mg/dL; (iv) high-density lipoprotein ≤ 50 mg/dL for women and ≤ 40 mg/dL for man; (v) or use of cholesterol-lowering drugs32. The sample size for the covariates with missing values was 614, including PIR (n = 608), marital status (n = 4), and education (n = 2). Missing data in covariates were multiply imputed using the R package “mice”.
Statistical analyses
All analyses incorporated the dietary weight “wtdr2d”. The sample weights provided by NHANES were adjusted for different sampling rate, response rate, and coverage rate. Each respondent’s sample weight represents the estimated number of people in the target population, so that accurate national estimates can be obtained from the sample.
The Shapiro-Wilk statistical test was used to assess the normality of continuous variables. Continuous variables with normal distribution were contrasted by one-way ANOVA, and those with non-normal distribution were compared by the Kruskal-Wallis H test. Categorical variables were compared using chi-square tests.
Weighted multiple linear regression models were employed to estimate the association between OBS and serum Klotho in three different models. Age (continuous, years) and sex (female and male) were adjusted in model 1. Model 2 further adjusted for race (White, Black, Hispanic, Mexican American, and others), marital status (cohabiting, single), education (more than high school, high school, and less than high school), PIR (continuous) and daily energy intake (continuous, kcal/d). Finally, we further adjusted for whole grain (continuous, g/d), milk (continuous, cup/d), red meat (continuous, oz/d), cured meat (continuous, oz/d) and egg intake (continuous, oz/d) in model 3. In addition, we performed a trend test by designating the median of the three groups as a continuous variable. Potential nonlinear relationships were explored using restricted cubic splines (RCS) regression with three nodes at the 10th, 50th, and 90th percentiles.
Furthermore, we conducted stratified analysis by several key risk factors, including sex (< 50, ≥ 50 years), gender (female, male), education (≤ high school, > high school), PIR (< median, ≥median), marital status (cohabiting, single) and daily energy intake (< median, ≥median) by adding an interaction term in model 3. The interaction was assessed in these stratified variables using the likelihood-ratio test.
Several sensitivity analyses were performed to examine the robustness of the results. Firstly, we repeated the primary analyses by sequentially excluding each of the twenty components from OBS at a time and adjusting for the excluded component. Second, we further adjusted for chronic diseases, including diabetes and hypertension. Third, we reanalyzed the data by excluding individuals with extreme energy intake (< 1000 and > 5000 kcal/d). Fourthly, we excluded participants with comorbid chronic conditions, including diabetes and hypertension. Finally, we retrieved the participants with fewer missing items of OBS (≤ 5 items).
All statistical tests were performed with R software (v4.3.1), and P < 0.05 was deemed statistically significant.
Ethics approval and consent to participate
The National Center for Health Statistics (NCHS) Research Ethics Review Board approved all study protocols for NHANES. All participants provided written informed consent (https://www.cdc.gov/nchs/nhanes/). Protocol number: #2005–2006 and #2011–2017. We followed all relevant regulations and guidelines when performing all methods.
Results
The characteristics of participants
Table 1 displays the characteristics of the participants based on the OBS tertile. The mean age of 8,145 participants was 55.8 ± 0.2 years, 4,023 (50.3%) were females, and 75.0% identified as White. Compared to participants with lower OBS, those with higher OBS were more likely to be White, have cohabiting status, have higher educational level, income, energy intake, dietary intake (including whole grain, milk, red meat, cured meat and egg) and lower chronic disease.
Table 1.
Baseline characteristics of participants according to the oxidative balance score’s tertiles.
| OBS | P-valuea | ||||
|---|---|---|---|---|---|
| Overall | T1 | T2 | T3 | ||
| ≤ 17 | 18–24 | > 24 | |||
| OBS | 21.0(0.2) | 13.0(0.1) | 21.0(0.1) | 29.0(0.1) | |
| No. of participants | 8,145 | 2,991 | 2,504 | 2,650 | |
| Age (years) | 55.8(0.2) | 55.9(0.3) | 56.1(0.3) | 55.4(0.3) | 0.1 |
| PIR | 3.4(0.1) | 3.0(0.1) | 3.4(0.1) | 3.7(0.1) | < 0.001 |
| Energy intake(kcal/d) | 4,137(29) | 3,227(28) | 4,077(37) | 4,997(52) | < 0.001 |
| Whole grain intake(g/d) | 2.1(0.0) | 1.2(0.0) | 2.0(0.1) | 2.9(0.1) | < 0.001 |
| Milk intake(cup/d) | 1.5(0.0) | 0.8(0.0) | 1.5(0.0) | 2.2(0.1) | < 0.001 |
| Red meat intake(oz/d) | 3.2(0.1) | 2.8(0.1) | 3.3(0.1) | 3.6(0.1) | < 0.001 |
| Cured meat intake(oz/d) | 1.9(0.1) | 1.8(0.1) | 1.9(0.1) | 2.1(0.1) | 0.01 |
| Egg intake(oz/d) | 1.1(0.0) | 0.9(0.0) | 1.1(0.0) | 1.2(0.0) | < 0.001 |
| Sex, % | 0.6 | ||||
| Female | 4,023(50.3) | 1,449(50.6) | 1,228(49.2) | 1,346(51.1) | |
| Male | 4,122(49.7) | 1,542(49.4) | 1,276(50.8) | 1,304(48.9) | |
| Race, % | < 0.001 | ||||
| White | 3,874(75.0) | 1,276(71.6) | 1,201(74.8) | 1,397(78.2) | |
| Black | 1,570(8.4) | 804(13.0) | 426(7.5) | 340(5.0) | |
| Hispanic | 860(4.4) | 343(4.8) | 267(4.6) | 250(3.9) | |
| Mexican American | 1,126(6.0) | 382(6.0) | 371(6.4) | 373(5.7) | |
| Others | 715(6.2) | 186(4.6) | 239(6.7) | 290(7.3) | |
| Education, % | < 0.001 | ||||
| Less than high school | 776(4.2) | 368(5.6) | 232(4.2) | 176(2.9) | |
| High school | 2,821(29.8) | 1,232(37.8) | 859(31.6) | 730(21.2) | |
| More than high school | 4,548(66.0) | 1,391(56.5) | 1,413(64.1) | 1,744(75.9) | |
| Marital status, % | < 0.001 | ||||
| Cohabiting | 5,439(71.8) | 1,835(66.4) | 1,719(73.0) | 1,885(75.7) | |
| Single | 2,706(28.2) | 1,156(33.6) | 785(27.0) | 765(24.3) | |
| Diabetes, % | < 0.001 | ||||
| No | 6,385(84.4) | 2,219(80.7) | 1,948(83.8) | 2,218(88.2) | |
| Yes | 1,760(15.6) | 772(19.3) | 556(16.2) | 432(11.8) | |
| Hypertension, % | < 0.001 | ||||
| No | 4,053(55.3) | 1,327(51.1) | 1,235(51.3) | 1,491(62.5) | |
| Yes | 4,092(44.7) | 1,664(48.9) | 1,269(48.7) | 1,159(37.5) | |
| Hyperlipidemia, % | 0.04 | ||||
| No | 1,717(20.6) | 570(18.4) | 529(20.2) | 618(22.9) | |
| Yes | 6,428(79.4) | 2,421(81.6) | 1,975(79.8) | 2,032(77.1) | |
Data expressed as mean [SD] or n (%)
aP-value of Chi-square test, one-way ANOVA or Kruskal-Wallis H test.
OBS oxidative balance score, PIR poverty income ratio.
Association between the OBS and serum Klotho
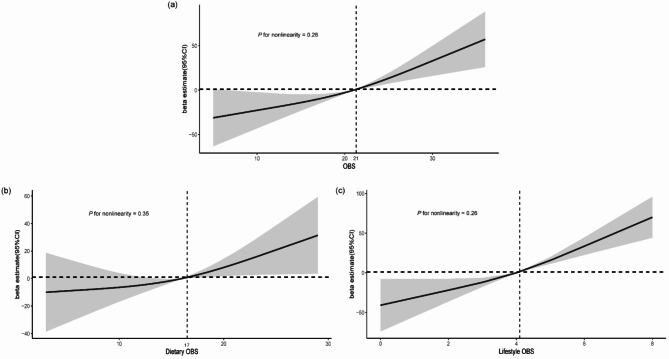
Table 2 demonstrates the relationship between OBS and serum Klotho level. OBS and serum Klotho both showed significant positive associations in the three models. After adjusting for multiple covariates such as demographics, economic status, and dietary intake (Model 3), a higher OBS was significantly associated with a higher serum Klotho (β = 2.85, CI: 1.03–4.68, p < 0.01). The significant positive association remained, with OBS as a categorical variable. Compared to the lowest OBS group, the highest group was significantly associated with higher serum Klotho levels (30.35 (3.43–57.28), p < 0.05). Furthermore, RCS showed a linear association between OBS and serum Klotho (Pnon−linearity = 0.26), with the inflection point occurring at an OBS value of approximately 21 (Fig. 2).
Table 2.
Association between oxidative balance score and serum klotho levels.
| Tertiles of OBS | P for trend | Continuous (per 1 score increase) | |||
|---|---|---|---|---|---|
| T1 | T2 | T3 | |||
| β (95%CI) | |||||
| Model 1 | Ref | 14.73(-6.15, 35.61) | 30.69(7.68, 53.70)* | 0.01 | 2.33(0.93, 3.72)** |
| Model 2 | Ref | 24.35(1.59, 47.10)* | 48.74(21.08, 76.40)*** | < 0.001 | 4.01(2.21, 5.81)*** |
| Model 3 | Ref | 15.57(-5.75, 36.90) | 30.35(3.43, 57.28)* | < 0.027 | 2.85(1.03, 4.68)** |
Model 1: age (continuous, years) and sex (female and male).
Model 2: Model 1 + race (White, Black, Hispanic, Mexican American and others), marital status (cohabiting and single), education status (less than high school, high school and more than high school), poverty income ratio (continuous) + daily energy intake (continuous, kcal/d).
Model 3: Model 2 + whole grain (continuous, g/d), milk (continuous, cup/d), red meat (continuous, oz/d), cured meat (continuous, oz/d) and egg intake (continuous, oz/d).
OBS oxidative balance score, CI confidence interval.
*P < 0.05; **P < 0.01; ***P < 0.001.
Fig. 2.
Dose-response relationships between OBS, dietary OBS and lifestyle OBS and serum klotho level. (a) OBS; (b) Dietary OBS; (c) Lifestyle OBS. Median OBS is reference standard. Beta and 95%CIs are based on weighted multiple linear regression model adjusted for age and sex, race, marital status, education status, and poverty income ratio, daily energy intake, whole grain, milk, red meat, cured meat and egg intake. (b) plus lifestyle OBS; and (c) plus dietary OBS. Solid lines indicate OR and shaded areas represent 95%CI. OBS oxidative balance score, CI confidence interval.
Association between the dietary OBS/lifestyle OBS and serum Klotho
Associations between dietary OBS, lifestyle OBS and serum Klotho were also analyzed, respectively (Table 3). Similarly, we observed that both dietary OBS and lifestyle OBS showed significantly positive associations with serum Klotho. The β and 95% CI were 1.27 (0.79–3.32) for dietary OBS and 14.23 (9.53–18.92) for lifestyle OBS, respectively. Considering these two types of OBS as categorical variables, we found that dietary OBS was not significantly associated with serum Klotho (15.65 (-15.93-47.23)), but there was a significant positive relationship between lifestyle OBS and serum Klotho (46.77 (27.13–66.42)). Moreover, the RCS indicated that the dose-response associations between dietary OBS and lifestyle OBS and serum Klotho both showed linearity (Pnon−linearity>0.05). Specifically, inflection points were identified at the dietary OBS of 17 and the lifestyle OBS of 4, respectively (Fig. 2).
Table 3.
Association between dietary/lifestyle OBS with serum klotho levels.
| Tertiles of OBS | Continuous (per 1 score increase) | |||
|---|---|---|---|---|
| T1 | T2 | T3 | ||
| Dietary OBS | ||||
| Range | ≤ 12 | 13–20 | > 20 | |
| β (95%CI) | ||||
| Model 1 | Ref | 10.28(-10.62, 31.19) | 23.8(-2.16, 49.76) | 1.6(0.12, 3.08)* |
| Model 2 | Ref | 19.32(-3.90, 42.53) | 40.49( 8.95, 72.04)* | 3.01(1.00, 5.01)** |
| Model 3 | Ref | 10.58(-12.03, 33.19) | 20.27(-10.60, 51.14) | 1.61(0.40, 3.62)* |
| Model 4 | Ref | 7.79(-15.01, 30.58) | 15.65(-15.93, 47.23) | 1.27(0.79, 3.32)* |
| Lifestyle OBS | ||||
| Range | ≤ 4 | 5 | > 5 | |
| β (95%CI) | ||||
| Model 1 | Ref | 18.47(-6.30, 43.24) | 54.02(34.82, 73.21)*** | 16.01(11.63, 20.40)*** |
| Model 2 | Ref | 19.17(-6.35, 44.69) | 54.58(35.38, 73.78)*** | 16.26(11.74, 20.78)*** |
| Model 3 | Ref | 15.51(-9.97, 41.00) | 47.84(28.30, 67.38)*** | 14.48(9.83, 19.13)*** |
| Model 4 | Ref | 15.00(-10.67, 40.67) | 46.77(27.13, 66.42)*** | 14.23(9.53, 18.92)*** |
Model 1: age (continuous, years) and sex (female and male).
Model 2: Model 1 + race (White, Black, Hispanic, Mexican American and others), marital status (cohabiting and single), education status (less than high school, high school and more than high school), poverty income ratio (continuous) + daily energy intake (continuous, kcal/d).
Model 3: Model 2 + whole grain (continuous, g/d), milk (continuous, cup/d), red meat (continuous, oz/d), cured meat (continuous, oz/d) and egg intake (continuous, oz/d).
Model 4:Model 3 + lifestyle OBS or dietary OBS (continuous).
OBS oxidative balance score, CI confidence interval.
*P < 0.05; **P < 0.01; ***P < 0.001.
Stratified subgroup analysis
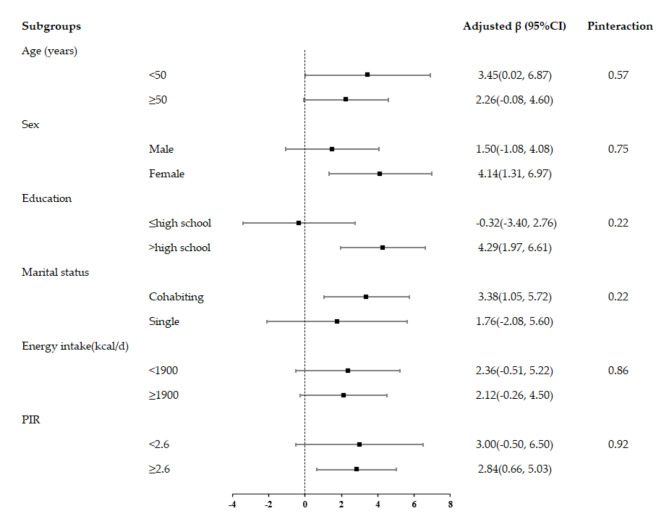
In subgroup analysis, we observed consistent associations stratified by sex (male, female), age (< 50, ≥ 50 years), education (≤ high school, > high school), PIR (< median, ≥median), marital status (cohabiting, single) and daily energy intake (< median, ≥median). No significant interactions with these risk factors were observed in the relationship between OBS and Klotho level (Pinteraction >0.05) (Fig. 3).
Fig. 3.
Adjusted β and 95%CI for OBS and serum klotho level, stratified by several key risk factors. Models were adjusted for age and sex, race, marital status, education status, and poverty income ratio, daily energy intake, whole grain, milk, red meat, cured meat and egg intake. In each stratified analysis, the stratification variable was excluded in the adjustments. Likelihood ratio tests were used for assessment of interaction, and two-sided P values (unadjusted for multiple comparisons) are reported.
Sensitivity analyses
Firstly, we systematically excluded each of the 20 components from OBS individually at a time and the results did not alter (Supplemental Table 3). Secondly, after further adjusting for chronic diseases (including diabetes, hypertension and hyperlipidemia), the results remained consistent (Supplemental Table 4). Thirdly, the exclusion of participants with extreme energy intake (< 1000 and > 5000 kcal/d) or comorbid chronic diseases (including diabetes and hypertension) strengthened the association between OBS and serum Klotho (Supplemental Table 4). Finally, our results did not change after retrieving the participants with fewer missing OBS items (≤ 5) (Supplemental Table 5).
Discussion
Our study disclosed a significantly positive relationship between the OBS and the serum Klotho level. Moreover, we observed a linear dose-response dynamic between OBS and serum Klotho level. This relationship remained consistent across subgroups analysis categorized factors by age, sex, marital status, energy intake, education, and PIR. When the OBS was further decomposed into dietary OBS and lifestyle OBS, both were found to have a robust positive link to serum Klotho level.
Our findings have important clinical implications, suggesting that enhancing antioxidant status may help mitigate the aging process and reduce the risk of chronic diseases. In elderly individuals, an elevated intake of antioxidant carotenoids, particularly α-carotene, β-carotene, lutein and zeaxanthin, was linked to higher plasma soluble Klotho levels, potentially alleviating oxidative stress33. A study found increased Klotho concentrations in the moderate intensity training group, suggesting that a positive association between regular aerobic exercise and higher levels of the anti-aging protein Klotho, along with enhanced anti-oxidative capacity34. Klotho protein, as a potential biomarker in lifestyle medicine, can quantify and monitor an individual’s health status, which can assist physicians and healthcare providers in evaluating the impact of lifestyle modifications on health, thereby facilitating more targeted clinical decision-making35.
This study was the first to explore the relationship between the OBS and serum Klotho level, a novel approach grounded in our ongoing quest for knowledge. A well-established metric to evaluate the effects of diet and lifestyle on oxidative stress is the OBS, with higher OBS indicating higher antioxidant level. Currently, there are no relevant studies evaluating the relationship between OBS and Klotho. However, numerous studies have explored the impact of enhanced antioxidant capacity on Klotho and aging. For instance, a research, also from NHANES, demonstrated a notably inverse relationship between the Composite Dietary Antioxidant Index (CDAI) and serum Klotho level in US adult population, underscoring the potential capacity of antioxidant-enriched dietary measures to decelerate the aging process36. In addition, another study showed that healthy behavior, such as aerobic exercise, was positively associated with both higher antioxidant capacity and Klotho level37. These studies are consistent with our results, responding to the health benefits of antioxidant measures. However, the OBS in our study integrated dietary and lifestyle behaviors to provide a more comprehensive and precise assessment of oxidative stress.
Compared to other oxidative stress markers, such as ROS, which focus on individual biomarkers, Malondialdehyde (MDA), which concentrates on lipid peroxidation38, and Superoxide Dismutase (SOD) and Catalase (CAT), which are related to cellular antioxidant defense mechanisms39, the OBS provides a more comprehensive assessment of oxidative stress, not confined to a single biomarker. The OBS delivers a holistic evaluation of the body’s antioxidant and oxidative status, reflecting the strength of the antioxidant capacity. Traditional biomarkers often necessitate the collection of serum, urine, or tissue samples and demand stringent conditions for sample handling and storage, which restricts their use in routine clinical practice40. In contrast, the OBS score transcends these limitations, offering a broader perspective on oxidative stress that is not bound by the constraints of single biomarker analysis.
OBS has emerged as a sensitive and applicable tool for assessing oxidative stress levels across diverse populations. Among Japanese adults, a negative association between OBS and urinary 8-hydroxydeoxyguanosine (8-OHdG) levels indicated the OBS’s effectiveness in reflecting changes in oxidative stress41. Furthermore, studies in Korean populations have confirmed that a higher OBS was associated with a lower risk of new-onset hypertension, underscoring the potential of healthy lifestyles and antioxidant-rich diets for prevention42. In Chinese adults, OBS was positively correlated with vascular endothelial function, further validating OBS as a sensitive indicator of oxidative stress43. Data from the UK Biobank indicated that higher OBS was linked to reduced incidence of colorectal cancer and its subtypes, highlighting the potential of dietary and lifestyle adjustments to mitigate cancer risk15. A study in the Spanish population revealed that higher OBS was significantly associated with lower risks of mortality from all-causes, cardiovascular disease, and cancer, emphasizing the role of oxidative balance in preventing premature death44. Based on these findings, OBS appeared to be a promising tool for assessing oxidative stress with broad application prospects in different populations, providing a scientific basis for prevention strategies.
The occurrence of oxidative stress is characterized by an elevated generation of ROS coupled with a decline in the body’s antioxidant defense mechanism. Evidence has revealed an association between the α-Klotho protein and oxidative stress onset, suggesting that inadequate Klotho level could exacerbate intrinsic ROS production, intensifying oxidative stress conditions. Mechanistically oxidative stress primarily originates from an imbalance between pro-oxidant species such as superoxide anions, hydroxyl radicals, and hydrogen peroxide, with defensive antioxidants comprising superoxide dismutase, glutathione peroxidase, and a suite of vitamins including vitamins A, C, and E45. The possibility that Vitamin E may upregulate Klotho expression could contribute to the inhibition of dendritic cell maturation mediated by NF-κB, potentially enhancing the immunotherapeutic effect of Vitamin E against Klotho-related disease46. Future mechanistic studies will be crucial to illuminate these complex underlying processes.
In general, antioxidant factors exert a positive influence, whereas pro-oxidant factors are detrimental. A higher OBS indicates a more significant exposure to antioxidants as opposed to pro-oxidant substances. The original OBS was derived from the combination intake of antioxidants vitamin C and β-carotene, alongside the inclusion of, iron, a known dietary pro-oxidant47. A critical appraisal of the definition and creation process of OBS could offer insight into the capacity and utility of these scores in demonstrating how various factors modulate the incidence of chronic disease associated with oxidative stress. It is generally accepted across numerous studies that an excess of oxidative stress, indicated by a decrease in OBS, leads to adverse health consequences. Most OBS were established by considering over ten components, including dietary intake and lifestyle, although a few considered are based solely on dietary factors48.
The dietary OBS serves as an index calculated based on the pro-oxidant and antioxidant effects of dietary nutrients. Generally, a diet with higher dietary OBS is characterized by an ample supply of fruits, vegetables, nuts, fish, and fiber, which aids in the reduction of oxidative stress. Conversely, a diet characterized by a lower dietary OBS often includes an excess intake of foods high in sugar, salt, fat, cholesterol, trans fatty acids, and processed foods, contributing to an increased in oxidative stress. Prior studies have confirmed that a lower dietary OBS was associated with a higher risk of mortality from all-cause49. In the United States, dietary fiber consumption and serum Klotho level were significantly associated among individuals aged 40–79 years50. The dietary vitamin C intake was found to correlate with serum Klotho concentrations across the general U.S. population51. Moreover, a positive relationship was observed between total carotenoid consumption and the plasma soluble Klotho (S-Klotho) level in elderly, with particularly strong associations for α-carotene, β-carotene, and lutein with zeaxanthin complex33. Higher intakes of carbohydrates, total sugars, and dietary fibers were found to correspond with increased Klotho levels, alongside trends for positive associations with intakes of vitamin D, total folates, and copper52. Among U.S. adults with optimal, an increase in serum Klotho concentration was significantly linked to an increased level of vitamin B1252. To date, there is a lack of research validating the association between the nutrient intake of riboflavin, vitamin B6, zinc, and selenium with serum Klotho level.
In the context of lifestyle factors, both antioxidant and pro-oxidant elements were considered. Independent of the exercise intensity or type, engaging in physical activity consistently increases antioxidant markers while decreasing those of pro-oxidants54. Notably, alcohol consumption and smoking behavior are two primary lifestyle factors acting as pro-oxidants. A consistent inverse relationship was observed between daily alcohol intake and level of serum α-Klotho among U.S. adults aged above 40 years55. In a cross-sectional study, cigarette smoking demonstrated an inverse relationship with serumα-Klotho level among US adults56. Additionally, a high body mass index has been identified as a pro-oxidant factor. Research has found that the expression level of plasma Klotho is reduced in overweight patients (BMI > 25 kg/m2)57.
The complex mechanisms of oxidative stress impacting Klotho levels have not been fully elucidated. In Chronic Obstructive Pulmonary Disease (COPD), oxidative stress damaged the Klotho protein, potentially affecting its stability and function. It activated inflammatory pathways through the production of cytokines and chemokines, modulated various signaling pathways such as MAPK, NF-κB, and PI3K/Akt, and contributed to reduced Klotho expression based on ROS-induced DNA damage, genetic mutations or epigenetic alterations58. Meanwhile, oxidative stress, as part of the upstream regulatory processes, could enhance the expression of microRNAs (miRNAs) and promote the methylation of Specific Protein 1 (Sp1), thereby reducing Klotho expression levels in chronic kidney disease59. In addition, antioxidant dietary intake can reduce Klotho expression and oxidative stress damage mediated through SIRT160. Future mechanistic studies are essential to illuminate these complex underlying processes.
Klotho, as a biomarker of aging, demonstrates high robustness, with its levels decreasing as age advances and being associated with various disease states such as renal diseases, cardiovascular diseases, pulmonary diseases, metabolic/endocrine disorders, cancer, neurodegenerative diseases, and more. It can be reliably measured in multiple sample types, including blood, cerebrospinal fluid, urine, and tissue samples. These characteristics render Klotho a potentially valuable biomarker for aging and disease management61. Studies from Spain have indicated that serum Klotho levels was significantly reduced in patients with cardiovascular diseases, coincident with a marked increase in inflammatory markers such as Tumor Necrosis Factor-alpha (TNF-α)/Interleukin-10 (IL-10) ratio and C-reactive protein (CRP) levels62. This suggested that inflammation may play a substantial role in the association between Klotho and cardiovascular diseases, and this role might be related to genetic backgrounds and inflammatory responses that vary among different ethnic groups. Furthermore, in patients with type 2 diabetes, Klotho levels were positively correlated with the incidence and severity of coronary artery disease, an association not observed in non-diabetic individuals, indicating a possible link with ethnic differences in diabetes prevalence and inflammatory levels63. Another study within the Chinese population revealed that patients with multiple system atrophy (MSA) had significantly lower serum Klotho and vitamin D levels compared to a healthy control group while exhibiting significantly higher homocysteine levels, indicating a potential association between Klotho levels and the severity of MSA64. These results provide valuable understanding into the role and impact of Klotho across diverse populations and lay the groundwork for further investigation into ethnic differences. Future studies should consider factors such as genetic background, dietary habits, and lifestyle, which may influence OBS and Klotho levels and contribute to ethnic disparities.
Our study exhibits several important vital strengths. Principally, our analysis was predicated on a study that was nationally representative and population-based, conferring upon our findings both generalizability and representationalism. Secondly, subgroup and sensitivity analyses increased the reliability of the results. While our study offers valuable preliminary findings, it is important to acknowledge its several limitations. Firstly, the dietary data obtained through recall questionnaires are inevitably prone to recall bias, potentially leading to the misclassification of OBS. However, we used 2 dietary recalls to assess dietary intake. Theoretically, multiple dietary surveys could more accurately respond to OBS. Secondly, the cross-sectional design of the study does not allow for the determination of causality. Thirdly, residual confounding cannot be completely ruled out, even though the model has adjusted for relevant factors as much as possible. Finally, considering the study’s focus on the U.S. population, our results cannot extend to other racial and national contexts, necessitating further investigation. To address these constraints, future studies should consider a longitudinal design and include diverse populations, which would not only reduce the impact of these constraints but also provide a more robust validation of our findings.
Conclusion
We observed an association between higher OBS and higher serum Klotho level in a nationally representative cohort of US population, indicating that adherence to antioxidant behaviours may be linked to slower aging and better health. Further studies are needed to determine the causal relationship between OBS and serum Klotho.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Kang Wang carried out the data analysis. Zhongbiao Jiang wrote the main manuscript. Qin Zhou prepared Figs. 1, 2 and 3; Tables 1, 2 and 3, while Hanfen Tang conceived the study and reviewed the manuscript. All authors reviewed the manuscript. Both Qin Zhou and Hanfen Tang served as corresponding authors, contributing equally to the research. All listed authors contributed to the development of the paper and have approved the final version for submission.
Funding
This study was supported by the Natural Science Foundation of Hunan Province of China (Grant Nos. 2022JJ30838).
Data availability
The datasets used and analyzed during the current study available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Qin Zhou, Email: zhouqin7089@csu.edu.cn.
Hanfen Tang, Email: tanghanfen0826@csu.edu.cn.
References
- 1.Hajam, Y. A. et al. Oxidative stress in human pathology and aging: Molecular mechanisms and perspectives. Cells (2022). 10.3390/cells11030552 [DOI] [PMC free article] [PubMed]
- 2.Vatner, S. F. et al. Healthful aging mediated by inhibition of oxidative stress. Ageing Res. Rev.10.1016/j.arr.2020.101194 (2020). [DOI] [PMC free article] [PubMed]
- 3.Jomova, K. et al. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol.97, 2499–2574. 10.1007/s00204-023-03562-9 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mozzini, C. & Pagani, M. Oxidative stress in chronic and age-related diseases. Antioxidants (2022). 10.3390/antiox11030521 [DOI] [PMC free article] [PubMed]
- 5.Vassalle, C., Maltinti, M. & Sabatino, L. Targeting oxidative stress for Disease Prevention and Therapy: Where do we stand, and where do we go from Here. Molecules10.3390/molecules25112653 (2020). [DOI] [PMC free article] [PubMed]
- 6.Lei, X., Xu, Z. & Chen, W. Association of oxidative balance score with sleep quality: NHANES 2007–2014. J. Affect. Disord.339, 435–442. 10.1016/j.jad.2023.07.040 (2023). [DOI] [PubMed] [Google Scholar]
- 7.Goodman, M. et al. Oxidative stress score as a combined measure of pro-oxidant and antioxidant exposures. Ann. Epidemiol.17, 394–399. 10.1016/j.annepidem.2007.01.034 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Tan, Z. et al. Trends in oxidative balance score and prevalence of Metabolic Dysfunction-Associated Steatotic Liver Disease in the United States: National Health and Nutrition Examination Survey 2001 to 2018. Nutrients10.3390/nu15234931 (2023). [DOI] [PMC free article] [PubMed]
- 9.Son, D. H., Lee, H. S., Seol, S. Y., Lee, Y. J. & Lee, J. H. Association between the oxidative balance score and incident chronic kidney disease in adults. Antioxidants (2023). 10.3390/antiox12020335 [DOI] [PMC free article] [PubMed]
- 10.Qu, H. The association between oxidative balance score and periodontitis in adults: A population-based study. Front. Nutr.10.3389/fnut.2023.1138488 (2023). [DOI] [PMC free article] [PubMed]
- 11.Wu, C. et al. Gender-specific effects of oxidative balance score on the prevalence of diabetes in the US population from NHANES. Front. Endocrinol.10.3389/fendo.2023.1148417 (2023). [DOI] [PMC free article] [PubMed]
- 12.Xu, Z. et al. Association between the oxidative balance score and all-cause and cardiovascular mortality in patients with diabetes and prediabetes. Redox Biol.10.1016/j.redox.2024.103327 (2024). [DOI] [PMC free article] [PubMed]
- 13.Li, H. et al. Oxidative balance scores and depressive symptoms: Mediating effects of oxidative stress and inflammatory factors. J. Affect. Disord.334, 205–212. 10.1016/j.jad.2023.04.134 (2023). [DOI] [PubMed] [Google Scholar]
- 14.Zhan, F. et al. Higher oxidative balance score decreases risk of stroke in US adults: Evidence from a cross-sectional study. Front. Cardiovasc. Med.10.3389/fcvm.2023.1264923 (2023). [DOI] [PMC free article] [PubMed]
- 15.Chang, Y. et al. Oxidative balance score: A potential tool for reducing the risk of colorectal cancer and its subsites incidences. Front. Endocrinol.10.3389/fendo.2024.1397512 (2024). [DOI] [PMC free article] [PubMed]
- 16.Lu, Y., Wang, M., Bao, J., Chen, D. & Jiang, H. Association between oxidative balance score and metabolic syndrome and its components in US adults: A cross-sectional study from NHANES 2011–2018. Front. Nutr.10.3389/fnut.2024.1375060 (2024). [DOI] [PMC free article] [PubMed]
- 17.Kurosu, H. et al. Suppression of aging in mice by the hormone Klotho. Science309, 1829–1833. 10.1126/science.1112766 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donate-Correa, J. et al. Oxidative Stress, and mitochondrial damage in kidney disease. Antioxidants (2023). 10.3390/antiox12020239 [DOI] [PMC free article] [PubMed]
- 19.Qiao, Y. et al. Association of serum klotho levels with cancer and cancer mortality: Evidence from National Health and Nutrition Examination Survey. Cancer Med.12, 1922–1934. 10.1002/cam4.5027 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai, J. et al. Association between serum Klotho concentration and heart failure in adults, a cross-sectional study from NHANES 2007–2016. Int. J. Cardiol.370, 236–243. 10.1016/j.ijcard.2022.11.010 (2023). [DOI] [PubMed] [Google Scholar]
- 21.Wang, K. et al. Association between serum klotho levels and the prevalence of diabetes among adults in the United States. Front. Endocrinol.10.3389/fendo.2022.1005553 (2022). [DOI] [PMC free article] [PubMed]
- 22.Wu, Y. et al. Relationship of Klotho with cognition and dementia: Results from the NHANES 2011–2014 and mendelian randomization study. Translational Psychiatry. 10.1038/s41398-023-02632-x (2023). [DOI] [PMC free article] [PubMed]
- 23.Centers for Disease Control and Prevention [CDC]. About the National Health and Nutrition Examination Survey (2024). https://www.cdc.gov/nchs/nhanes/about_nhanes.htm
- 24.Centers for Disease Control and Prevention [CDC]. NCHS Research Ethics Review Board Approval (2024). https://www.cdc.gov/nchs/nhanes/irba98.htm
- 25.Seon, J. H., Kim, N. Y., Kim, J. E., Choi, C. H. & Chung, K. H. Oxidative balance scores in Korean adults are associated with periodontitis. J. Periodontol.10.1002/jper.24-0164 (2024). [DOI] [PubMed] [Google Scholar]
- 26.Ahuja, J. K. C., Moshfegh, A. J., Holden, J. M. & Harris, E. U. S. D. A. Food and nutrient databases provide the infrastructure for food and nutrition research, policy, and practice. J. Nutr.143, 241S–249S. 10.3945/jn.112.170043 (2013). [DOI] [PubMed] [Google Scholar]
- 27.Bull, F. C., Maslin, T. S. & Armstrong, T. Global physical activity questionnaire (GPAQ): Nine Country reliability and validity study. J. Phys. Act. Health. 6, 790–804. 10.1123/jpah.6.6.790 (2009). [DOI] [PubMed] [Google Scholar]
- 28.Feng, X. et al. Association between physical activity and kidney stones based on dose-response analyses using restricted cubic splines. Eur. J. Pub. Health. 30, 1206–1211. 10.1093/eurpub/ckaa162 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Alkalbani, M., Prabhu, G., Lagbo, J. & Qayyum, R. Serum Klotho and pulse pressure; insight from NHANES. Int. J. Cardiol.355, 54–58. 10.1016/j.ijcard.2022.02.021 (2022). [DOI] [PubMed] [Google Scholar]
- 30.Zhou, Y., Gu, K. & Zhou, F. Dietary flavonoid intake and Cancer mortality: A population-based cohort study. Nutrients10.3390/nu15040976 (2023). [DOI] [PMC free article] [PubMed]
- 31.Zhou, N. et al. The dietary inflammatory index and its association with the prevalence of hypertension: A cross-sectional study. Front. Immunol.10.3389/fimmu.2022.1097228 (2023). [DOI] [PMC free article] [PubMed]
- 32.Grundy, S. M. et al. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation106, 3143–3421. 10.1161/circ.106.25.3143 (2002). [PubMed] [Google Scholar]
- 33.He, X., Yin, X., Chen, X. & Chen, X. Aging and antioxidants: The impact of dietary carotenoid intakes on soluble klotho levels in aged adults. Front. Endocrinol.10.3389/fendo.2023.1283722 (2023). [DOI] [PMC free article] [PubMed]
- 34.Middelbeek, R. J. W. et al. Exercise intensity regulates cytokine and klotho responses in men. Nutr. Diabetes. 10.1038/s41387-020-00144-x (2021). [DOI] [PMC free article] [PubMed]
- 35.Paquette, J. S. et al. The longevity protein klotho: A Promising Tool to monitor lifestyle improvements. Metabolites10.3390/metabo13111157 (2023). [DOI] [PMC free article] [PubMed]
- 36.Longevity, O. M. Retracted: Composite Dietary Antioxidant Index and Plasma Levels of Soluble Klotho: Insights from NHANES. Oxidative medicine and cellular longevity (2024). 10.1155/2024/9835324 (2024). [DOI] [PMC free article] [PubMed]
- 37.Nava, R. et al. Relationship between aerobic fitness, antioxidant capacity and the anti-aging hormone, Klotho. Gazz. Med. Italiana Archivio per Le Scienze Mediche. 178, 886–893. 10.23736/s0393-3660.18.03972-4 (2019). [Google Scholar]
- 38.Mas-Bargues, C., Escrivá, C., Dromant, M., Borrás, C. & Viña, J. Lipid peroxidation as measured by chromatographic determination of malondialdehyde. Human plasma reference values in health and disease. Arch. Biochem. Biophys.10.1016/j.abb.2021.108941 (2021). [DOI] [PubMed]
- 39.Jomova, K. et al. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol.98, 1323–1367. 10.1007/s00204-024-03696-4 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Munteanu, I. G. & Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci.10.3390/ijms22073380 (2021). [DOI] [PMC free article] [PubMed]
- 41.Nanri, H. et al. The Association between oxidative balance score and urinary levels of 8-Hydroxydeoxyguanosine among Japanese adults. Nutrients10.3390/nu15214533 (2023). [DOI] [PMC free article] [PubMed]
- 42.Lee, J. H., Son, D. H. & Kwon, Y. J. Association between oxidative balance score and new-onset hypertension in adults: A community-based prospective cohort study. Front. Nutr.10.3389/fnut.2022.1066159 (2022). [DOI] [PMC free article] [PubMed]
- 43.Liu, J. et al. Oxidative balance score reflects vascular endothelial function of Chinese community dwellers. Front. Physiol.10.3389/fphys.2023.1076327 (2023). [DOI] [PMC free article] [PubMed]
- 44.Talavera-Rodriguez, I. et al. Association between an oxidative balance score and mortality: A prospective analysis in the SUN cohort. Eur. J. Nutr.62, 1667–1680. 10.1007/s00394-023-03099-8 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arroyave-Ospina, J. C., Wu, Z., Geng, Y. & Moshage, H. Role of oxidative stress in the pathogenesis of non-alcoholic fatty liver disease: Implications for Prevention and Therapy. Antioxidants10.3390/antiox10020174 (2021). [DOI] [PMC free article] [PubMed]
- 46.Nguyen Thi, X. et al. Klotho sensitive regulation of dendritic cell functions by vitamin E. Biol. Res.10.1186/s40659-016-0105-4 (2016). [DOI] [PMC free article] [PubMed]
- 47.Van Hoydonck, P. G. A., Temme, E. H. M. & Schouten, E. G. A dietary oxidative balance score of vitamin C, β-carotene and iron intakes and mortality risk in male smoking belgians. J. Nutr.132, 756–761. 10.1093/jn/132.4.756 (2002). [DOI] [PubMed] [Google Scholar]
- 48.Hernandez-Ruiz, A. et al. A review of a Priori defined oxidative balance scores relative to their components and impact on Health outcomes. Nutrients10.3390/nu11040774 (2019). [DOI] [PMC free article] [PubMed]
- 49.Wang, X. et al. Association of Dietary Inflammatory Index and Dietary oxidative balance score with all-cause and Disease-Specific Mortality: Findings of 2003–2014 National Health and Nutrition Examination Survey. Nutrients10.3390/nu15143148 (2023). [DOI] [PMC free article] [PubMed]
- 50.Liu, S. et al. The Association between Dietary Fiber Intake and Serum Klotho Levels in Americans: A cross-sectional study from the National Health and Nutrition Examination Survey. Nutrients (2023). 10.3390/nu15143147 [DOI] [PMC free article] [PubMed]
- 51.Wang, Y. et al. Association of Dietary Vitamin C Consumption with serum klotho concentrations. Foods10.3390/foods12234230 (2023). [DOI] [PMC free article] [PubMed]
- 52.Ostojic, S. M., Hillesund, E. R., Overby, N. C., Vik, F. N. & Medin, A. C. Individual nutrients and serum klotho levels in adults aged 40–79 years. Food Sci. Nutr.11, 3279–3286. 10.1002/fsn3.3310 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi, J. Y., Min, J. Y. & Min, K. B. Anti-aging protein klotho was associated with vitamin B12 concentration in adults. Medicine10.1097/md.0000000000030710 (2022). [DOI] [PMC free article] [PubMed]
- 54.de Sousa, C. V. et al. The antioxidant effect of exercise: A systematic review and meta-analysis. Sports Med.47, 277–293. 10.1007/s40279-016-0566-1 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Jiang, M., Tang, X., Wang, P., Yang, L. & Du, R. Association between daily alcohol consumption and serum alpha klotho levels among US adults over 40 years old: A cross-sectional study. Bmc Public. Health. 10.1186/s12889-023-16830-1 (2023). [DOI] [PMC free article] [PubMed]
- 56.Du, R. et al. Association between cigarette smoking and serum alpha klotho levels among US adults over 40-years-old: A cross-sectional study. Sci. Rep.10.1038/s41598-023-46698-5 (2023). [DOI] [PMC free article] [PubMed]
- 57.Amitani, M. et al. Plasma klotho levels decrease in both anorexia nervosa and obesity. Nutrition29, 1106–1109. 10.1016/j.nut.2013.02.005 (2013). [DOI] [PubMed] [Google Scholar]
- 58.Miklos, Z. & Horvath, I. The role of oxidative stress and antioxidants in Cardiovascular comorbidities in COPD. Antioxidants10.3390/antiox12061196 (2023). [DOI] [PMC free article] [PubMed]
- 59.Li, S. S. et al. Upstream and downstream regulators of Klotho expression in chronic kidney disease. Metabolism-Clinical Experimental. 10.1016/j.metabol.2023.155530 (2023). [DOI] [PubMed]
- 60.Chen, C. C., Chang, Z. Y., Tsai, F. J. & Chen, S. Y. Resveratrol pretreatment ameliorates Concanavalin A-Induced Advanced Renal glomerulosclerosis in aged mice through Upregulation of Sirtuin 1-Mediated Klotho expression. Int. J. Mol. Sci.10.3390/ijms21186766 (2020). [DOI] [PMC free article] [PubMed]
- 61.Abraham, C. R. & Li, A. Aging-suppressor Klotho: Prospects in diagnostics and therapeutics. Ageing Res. Rev.10.1016/j.arr.2022.101766 (2022). [DOI] [PubMed]
- 62.Martin-Nunez, E. et al. Association between serum levels of Klotho and inflammatory cytokines in cardiovascular disease: A case-control study. Aging-Us12, 1952–1964. 10.18632/aging.102734 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Donate-Correa, J. et al. Association of Klotho with coronary artery disease in subjects with type 2 diabetes Mellitus and preserved kidney function: A case-control study. Int. J. Mol. Sci.2410.3390/ijms241713456 (2023). [DOI] [PMC free article] [PubMed]
- 64.Guo, Y. et al. Serum Klotho, vitamin D, and homocysteine in combination predict the outcomes of Chinese patients with multiple system atrophy. CNS Neurosci. Ther.23, 657–666. 10.1111/cns.12711 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study available from the corresponding author on reasonable request.