Abstract
BACKGROUND
Recent advancements in Alzheimer's disease (AD) biomarker research and clinical trials prompt reflection on the value and consequently appropriate use of tau positron emission tomography (tau‐PET) in the future.
METHODS
We conducted an online survey among dementia and PET experts worldwide to investigate the anticipated future role of tau‐PET in clinical practice and trials.
RESULTS
Two hundred sixty‐eight dementia experts, comprising 143 clinicians and 121 researchers, covering six continents participated. The vast majority (90%) fostered a positive attitude toward the added value of tau‐PET in clinical practice, particularly for staging, diagnosing, monitoring, and prognostication in a cognitively impaired memory clinic population. Experts anticipated an important role for tau‐PET for participant selection (76%–100%) and measuring endpoints (75%–97%), in both anti‐amyloid and anti‐tau drug trials.
DISCUSSION
Our global survey study shows that dementia experts envision an important role for tau‐PET in the future, both in clinical practice and in drug trials, beyond current guidelines and practices.
Highlights
Dementia experts envision an important role for tau‐PET in the future.
Experts indicate that a tau‐PET scan could influence patient management.
Experts anticipate the utility of tau‐PET for participant selection and endpoints in drug trials.
There is a gap between the anticipated usefulness of tau‐PET and current clinical practices.
Keywords: Alzheimer's disease, positron emission tomography, tau, trials
1. BACKGROUND
Alzheimer's disease (AD) is pathologically characterized by the accumulation of amyloid‐β into plaques and of tau proteins into neurofibrillary tangles. 1 The AT(N) biomarker classification scheme identifies three biomarker categories: amyloid‐β, tau, and neurodegeneration. Tau biomarkers include quantification of insoluble neurofibrillary tangles using positron emission tomography (PET), as well as soluble phosphorylated tau (p‐tau) in cerebrospinal fluid (CSF) and plasma. 2 In the 2024 revised diagnostic criteria by the Alzheimer's Association Workgroup, tau PET (tau‐PET) is proposed as a core 2 biomarker to stage biological disease severity, provide information on prognosis, and on the likelihood that AD is contributing to symptoms. 3
Tau pathology is spatially and temporally tightly linked to neurodegeneration and the manifestation of clinical symptoms. 4 , 5 , 6 , 7 , 8 , 9 , 10 With tau‐PET, the burden, and localization of AD‐like tau pathology can be quantified and visualized in vivo. 5 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 The most widely used tau‐PET tracers (i.e., [18F]flortaucipir, [18F]MK6240, [18F]RO948, and [18F]PI2620) show specific binding to the 3R/4R isoforms of the tau protein that are characteristic of AD. 18 , 19 , 20 , 21 These common tau‐PET tracers have a lower affinity to 3R and 4R tau isoforms, observed in primary tauopathies and other neurodegenerative diseases, which enhances its diagnostic specificity. 14 , 17 , 20 , 21 , 22 , 23 Indeed, tau‐PET shows excellent diagnostic performance for distinguishing AD versus other neurodegenerative disorders (specificity ∼90%), 24 , 25 , 26 , 27 which is superior compared to other currently available biomarkers. 24 , 25 , 27 , 28 , 29 , 30 While soluble p‐tau biomarkers also reflect AD‐specific tau pathology, they appear to measure distinct neuropathological processes related to early alterations in tau metabolism, temporally much closer to amyloid abnormality. 31 , 32 , 33 , 34 Recently, the United States Food and Drug Administration (FDA) has approved the clinical use of the [18F]flortaucipir (Tauvid) PET tracer in cognitively impaired patients assessed for AD. 12 , 35 And finally, in 2024, appropriate use criteria for tau‐PET have been developed by the Alzheimer's Association and Society for Nuclear Medicine and Molecular Imaging Workgroup. 36
Nonetheless, broad clinical implementation of tau‐PET imaging is challenging due to high costs and limited availability. Moreover, the limited sensitivity of tau‐PET for the detection of early tau pathology (Braak stages I–IV) 12 , 13 , 14 may have implications regarding which individuals qualify for an examination with tau‐PET. Also, all tau‐PET tracers are characterized by various sources of off‐target binding, for example, to neuromelanin, monoamine oxidase B, and microhemorrhages. However, these off‐target binding patterns usually do not interfere with uptake patterns of AD‐specific tau pathology. 37 Currently, a common quantitative approach, cfr. the Centiloid scale for amyloid‐PET, 38 is lacking for tau‐PET. Efforts are ongoing to harmonize common tau‐PET tracers into a universal quantitative scaling method 39 , 40 and to validate various visual read methods.
RESEARCH IN CONTEXT
Systematic review: Extensive literature has demonstrated the favorable properties of tau‐PET for clinical purposes (e.g., differential diagnosis and prognostication) and drug trials. However, some questions remain for tau‐PET to be meaningfully implemented in clinical and drug development settings. This study collected expert opinions on the envisioned future role of tau‐PET.
Interpretation: Findings from our global survey study suggest that dementia experts foresee a valuable role for tau‐PET both in clinical practice and drug development trials.
Future directions: We identified a gap between the anticipated usefulness of tau‐PET and current practices. Therefore, prospective studies designed to further investigate the validity and utility of tau‐PET are needed to support the development of guidelines for the appropriate use of tau‐PET.
Recently, the advent of disease‐modifying treatments for AD has urged the importance of an accurate biomarker‐assisted diagnosis 37 and better prediction of clinical outcomes. 10 Questions remain regarding the appropriate use of tau‐PET in clinical settings, 41 also taking the emergence of high‐performing plasma p‐tau assays into consideration. Moreover, advancements in therapeutical trials have prompted critical reflection on the use of tau‐PET for participant inclusion criteria and measuring study endpoints. In this study, we investigate opinions of global dementia experts, in view of the future role of tau‐PET in clinical practice and trials. Consequently, we aim to identify gaps between experts’ perspectives and current practices and guidelines, and differences in opinions between clinicians and researchers.
2. METHODS
2.1. Population and recruitment
Individuals were eligible to participate in this survey study if they had experience in the field of dementia, with or without experience in PET imaging. We aimed to reflect the opinions of professionals globally, as these are expected to be subject to local practices and the availability of tau‐PET. To that end, recruitment for study participants occurred on social media platforms (X, LinkedIn), through international professional networks, and attendees at the Alzheimer's Association International Conference and the European Association of Nuclear Medicine Congress in 2023, were encouraged to contribute.
2.2. Survey
Study participants filled out an online survey, enquiring about expert opinions on the future role of tau‐PET (see Supplementary Materials). The survey was created in the eCastor electronic data capture system. Questions were formulated by M.R.V., R.O., and E.vdG. The survey incorporated 34–36 questions, of which 3–5 were open questions (2 questions were follow‐up questions depending on the previous answer), and all others were multiple choice. They encompassed clinical as well as trial related topics and were grouped into five categories: “Demographics”, “Tau‐PET in clinical practice”, “Tau‐PET in drug development and trials”, “Tau‐PET classification”, and “Clinical cases”. Respondents were categorized as clinician if they performed clinical work, independent of whether they also contributed to research. Respondents categorized as researchers were not involved in clinical work. Completing the survey took around 10 min.
2.3. Ethics
This survey study is in accordance with the World Medical Association Declaration of Helsinki, Ethical Principles for Medical Research Involving Human Subjects 2013, and has been reviewed by the Medical Ethics Committee from the Amsterdam UMC (Institutional Review Board [IRB] number 2023.0376). After an individual expressed their interest on a website, an online survey was sent to their e‐mail address. Informed consent was obtained from all respondents before the survey. The handling of data was in agreement with the European Union (EU) General Data Protection Regulation and the Dutch Act on Implementation of the General Data Protection Regulation. Respondent's privacy and confidentiality were respected throughout the study. Respondents had the option to either fill out the survey anonymously or leave their full name and be mentioned in the acknowledgments of this article.
2.4. Statistics
All analyses were performed in R version 4.2.1 and R Studio. Surveys with more than 50% of the questions completed were included in the analyses. For checkbox and multiple‐choice questions proportions were calculated and compared between clinicians and researchers using Fisher's exact test or Pearson's chi‐squared test. Results of questions on a 5‐point Likert scale (1 = “strongly disagree”; 2 = “disagree”; 3 = “neither agree nor disagree”, 4 = “agree”; 5 = “strongly agree”) were expressed in medians with interquartile ranges. These were compared between clinicians and researchers utilizing the Mann–Whitney U test. For the overall group and relevant subgroups, summary mean scores were calculated and compared using an independent samples t‐test. If, hypothetically, an individual responded “Neither agree nor disagree” (score = 3) to every Likert scale question (n = 8), a summary score of 24 would be reached. Consequently, a score > 24 was considered a positive attitude toward the future role of tau‐PET. For the calculation of summary scores, missing scores in Likert scale questions were recoded as a neutral score 3. Proportions with a positive attitude were compared between clinicians and researchers using Pearsons’ chi‐squared test.
3. RESULTS
3.1. Respondents
The survey was launched online on June 8th, 2023, and closed on October 6th, 2023. We included 268 respondents, comprising 143 self‐reported clinicians and 121 self‐reported researchers (Figure 1, Table 1, and Table S1). Four individuals had professional duties other than clinical or research‐related work. Experts from The United States of America were most represented with 27.6%, followed by The Netherlands (10.5%), Sweden (10.5%), Canada (6.7%), Germany (4.9%), Spain (4.9%), Brazil (4.5%), United Kingdom (4.1%), Italy (3.7%), Switzerland (3.7%), Belgium (3.4%), Argentina (3.0%), France (2.6%), Australia (1.5%), Denmark (1.5%), South Korea (1.5%) and China, Finland, Chile, Colombia, Costa Rica, Cuba, Democratic Republic of the Congo, Israel, Norway, Peru, Portugal, Singapore, Uruguay (< 1%). Overall, the sample reasonably represented demographical and professional diversity. Compared to researchers, clinicians were more often from the European or South American continent, more often specialized in neurology or neuropsychology, and had more years of professional experience.
FIGURE 1.
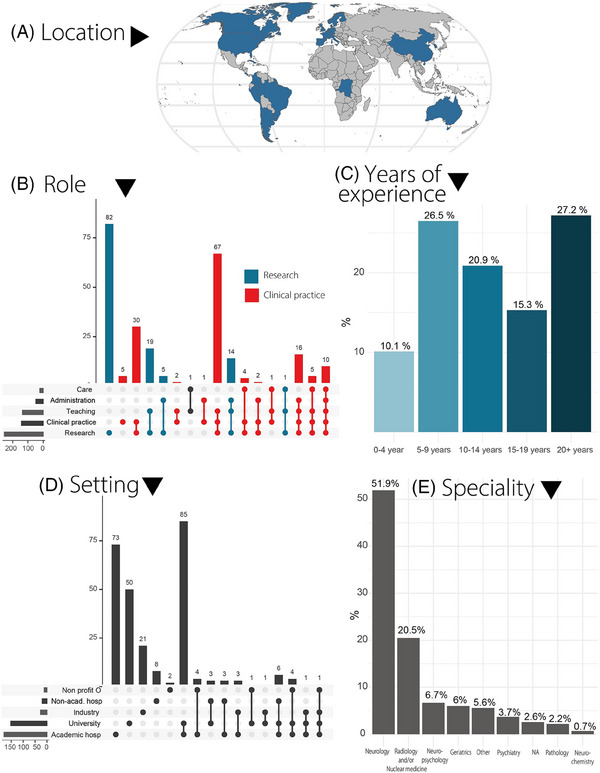
Respondent demographics. (A) Map displaying respondents' locations by country. (B) Upset plot illustrating the roles and tasks of the respondents: horizontal bars represent the frequency of each activity, while vertical bars show the frequency of task combinations. Colors differentiate between clinicians and researchers. (C) Bar graph showing the relative frequency of respondents by years of experience. (D) Upset plot summarizing the work setting of each respondent. (E) Bar plot showing the main professional specialty fields of the respondents. academic hosp, academic hospital; non‐acad. hosp, non‐academic hospital; Non profit O, non‐profit organization.
TABLE 1.
Summary of respondent demographics.
| Characteristics | Overall N = 268 * | Clinicians N = 143 * | Researchers N = 121 * | Others N = 4 * | p‐value † |
|---|---|---|---|---|---|
| Age | 0.003 | ||||
| < 35 | 68 (25.4%) | 23 (16.1%) | 43 (35.5%) | 2 (50.0%) | |
| 35 to 44 yo | 88 (32.8%) | 53 (37.1%) | 35 (28.9%) | 0 (0.0%) | |
| 45 to 54 yo | 64 (23.9%) | 37 (25.9%) | 26 (21.5%) | 1 (25.0%) | |
| 55 to 64 yo | 30 (11.2%) | 21 (14.7%) | 8 (6.6%) | 1 (25.0%) | |
| > 65 | 18 (6.7%) | 9 (6.3%) | 9 (7.4%) | 0 (0.0%) | |
| Gender | 0.10 | ||||
| Female | 102 (38.1%) | 47 (32.9%) | 53 (43.8%) | 2 (50.0%) | |
| Male | 165 (61.6%) | 95 (66.4%) | 68 (56.2%) | 2 (50.0%) | |
| Prefer not to say | 1 (0.4%) | 1 (0.7%) | 0 (0.0%) | 0 (0.0%) | |
| Continent | < 0.001 | ||||
| Africa | 1 (0.4%) | 1 (0.7%) | 0 (0.0%) | 0 (0.0%) | |
| Asia | 8 (3.0%) | 7 (4.9%) | 1 (0.8%) | 0 (0.0%) | |
| Oceania | 4 (1.5%) | 3 (2.1%) | 1 (0.8%) | 0 (0.0%) | |
| Europe | 137 (51.1%) | 81 (56.6%) | 54 (44.6%) | 2 (50.0%) | |
| North America | 92 (34.3%) | 31 (21.7%) | 61 (50.4%) | 0 (0.0%) | |
| South America | 26 (9.7%) | 20 (14.0%) | 4 (3.3%) | 2 (50.0%) | |
| Field of specialty | 0.021 | ||||
| Neurology or neuropsychology | 157 (58.6%) | 95 (66.4%) | 60 (49.6%) | 2 (50.0%) | |
| Radiology and/or nuclear medicine | 55 (20.5%) | 24 (16.8%) | 30 (24.8%) | 1 (25.0%) | |
| Other specialty | 56 (20.9%) | 24 (16.8%) | 31 (25.6%) | 1 (25.0%) | |
| Experience in dementia field | 0.021 | ||||
| 0–4 years | 27 (10.1%) | 9 (6.3%) | 18 (14.9%) | 0 (0.0%) | |
| 5–9 years | 71 (26.5%) | 35 (24.5%) | 33 (27.3%) | 3 (75.0%) | |
| 10–14 years | 56 (20.9%) | 26 (18.2%) | 30 (24.8%) | 0 (0.0%) | |
| 15–19 years | 41 (15.3%) | 25 (17.5%) | 16 (13.2%) | 0 (0.0%) | |
| 20 + years | 73 (27.2%) | 48 (33.6%) | 24 (19.8%) | 1 (25.0%) | |
Note: Respondents are grouped as “Others” if they reported not to be involved in either clinical or research related work.
n (%).
Fisher's exact test; Pearson's chi‐squared test for comparison between clinicians and researchers.
3.2. General view on the importance of tau pathology
First, we enquired opinions toward tau pathology in AD in general. The majority of respondents stated that in AD neocortical tau aggregation is closely associated with neurodegenerative processes (91.0%), strongly correlated with cognitive decline (90.3%), and a central event in the pathogenesis of AD (84.0%). More than half of respondents indicated that it is secondary to the accumulation of amyloid‐β (53.0%). Most respondents indicated that tau pathology in AD is not primarily driven by amyloid‐β independent pathways (85.1%) or that it is a meta‐phenomenon in AD (93.0%). This question was answered by all participants (n = 268).
3.3. Value of tau‐PET in clinical practice
Overall, 89.9% of respondents fostered a positive attitude (mean summary score 30.2) toward the added value of tau‐PET, with no difference present between the views of clinicians (88.1%) versus researchers (91.7%, p = 0.44). In clinical practice, most experts indicated that tau‐PET is valuable for pathophysiological staging (79.7%), identifying tau aggregation patterns in suspected atypical AD (76.7%), monitoring disease progression (75.6%), predicting future cognitive decline (74.4%), and differentiating AD from non‐AD pathologies (70.7%) (Figure 2A, Table S2). A smaller proportion considered tau‐PET valuable for early detection of AD (42.5%). Compared to clinicians, researchers envisioned greater value in the role of tau‐PET for predicting cognition (67 .8% vs. 83.2% resp., p = 0.004). Nine individuals (3.4%), of which eight clinicians, responded that there is no place for tau‐PET in clinical settings.
FIGURE 2.
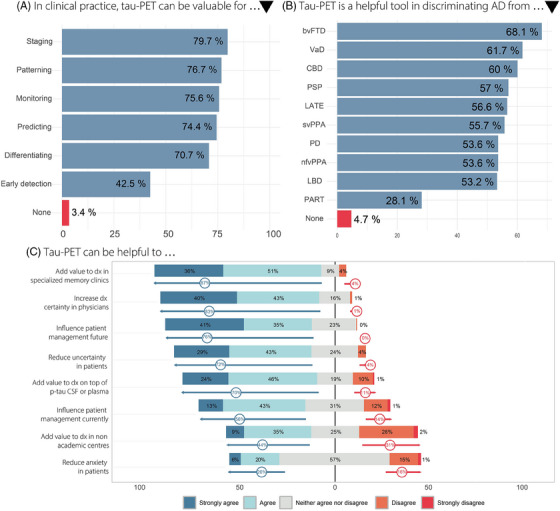
Summary findings on the envisioned future role of tau‐PET in clinical practice. (A) Envisioned value of tau‐PET per clinical purpose. (B) Envisioned utility of tau‐PET to differentiate between various neurodegenerative pathologies. (C) Proportions of responses of (dis)agreement on a Likert scale to eight statements regarding the added value of tau‐PET. Arrows indicate the combined proportion of agreement or disagreement. AD, Alzheimer's disease; CSF, cerebrospinal fluid; dx, diagnosis/diagnostic; p‐tau, phosphorylated tau; tau‐PET, tau positron emission tomography.
3.3.1. Patient population
Respondents considered tau‐PET to be most valuable for clinical use in the prodromal stage (90.7%), followed by the dementia stage (48.1%) and preclinical stage (37.1%, Table S3). Two clinicians and two researchers (1.7%) indicated that tau‐PET is not useful to them in any of these clinical stages.
3.3.2. Differential diagnosis
Clinicians and researchers largely agreed that tau‐PET is a helpful tool for discriminating symptomatic AD from behavioral variant frontotemporal dementia (68.1%), vascular dementia (61.7%), and suspected corticobasal degeneration (60.0%) (Figure 2B, Table S4). Over half of respondents indicated that tau‐PET can support in discriminating AD from limbic age‐related TDP‐43 encephalopathy (LATE, 56.6%), progressive supranuclear palsy (57.0%), semantic variant primary progressive aphasia (55.7%), non‐fluent variant primary progressive aphasia (53.6%), Parkinson's disease (53.6%), and Lewy body dementia (53.2%). The majority of clinicians and researchers agreed that tau‐PET is not helpful to discriminate AD from primary age‐related tauopathy (PART, 71.9%). Eleven individuals (4.7%) indicated that tau‐PET is of no assistance when discriminating AD from any of the aforementioned diseases.
We also presented three clinical case vignettes (Figure 3) and asked whether in current clinical practice respondents would request a tau‐PET scan. In the first case, an 80‐year‐old male patient with a typical amnestic‐predominant AD presentation and amyloid positivity in CSF, only one‐quarter (57/227) of respondents would request a tau‐PET scan (clinicians 24.5% vs. researchers 26.4%, p = 0.70). In the second case, a 55‐year‐old female patient presenting with behavioral symptoms and an abnormal amyloid‐PET scan, most experts (77.9%, 176/226) would request a tau‐PET scan (clinicians 73.4% vs. researchers 84.9%, p = 0.04). Finally in the third case, a 55‐year‐old‐male patient suspected of mixed pathology including vascular and AD dementia and an abnormal amyloid‐PET scan, a considerable proportion (65.4%, 144/220) would request a tau‐PET scan (clinicians 61.5% vs. researchers 72.6%, p = 0.09).
FIGURE 3.
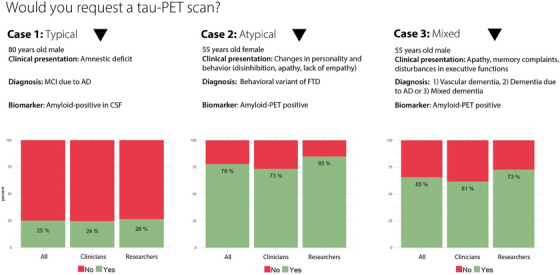
Responses to three clinical case vignettes. Proportions of respondents who would request a tau‐PET scan in a typical AD patient (case 1), a patient with an atypical presentation (case 2), and a patient with suspected mixed pathology (case 3). AD, Alzheimer's disease; amyloid‐PET, amyloid positron emission tomography; CSF, cerebrospinal fluid; MCI, mild cognitive impairment; FTD, frontotemporal dementia; tau‐PET, tau positron emission tomography.
3.3.3. Clinical setting
Most respondents agreed that tau‐PET will have added value on top of routine diagnostic procedures in specialized memory clinics (median score 4 corresponding to “agree” on a Likert scale 1–5 [IQR 4‐5], Table S5). However, for non‐academic settings, clinicians were less confident than researchers (median score 3 corresponding to “neither agree nor disagree” [2–4] vs. 3 [3–4], p = 0.006). Respondents agreed that, in the future, tau‐PET will be of additional value on top of more cost‐effective and feasible biomarkers like CSF and/or plasma p‐tau (median score 4 [3–5]). In Figure 2B proportions per Likert category are shown.
3.3.4. Clinical impact
While respondents agreed that tau‐PET can improve the diagnostic certainty of physicians (median score 4 [4–5]) and reduce uncertainty in patients (median score 4 [4–5]), reduction of anxiety in patients was deemed less certain (median score 3 [3–4], Table S5). Experts were in agreement that tau‐PET can influence patient management, currently (median score 4 [3–4]), and especially when effective disease modifying therapies are/become available (median score 5 corresponding to “strongly agree” [4–5]). In Figure 2B, proportions per Likert category are shown. While 96.8% (211/218) of respondents stated that a physician should know the tau‐PET status of their patient before initiating disease‐modifying treatment targeting tau, a smaller yet substantial proportion of 62.7% (138/220) responded that the tau‐PET status should be determined before amyloid‐β treatment.
3.3.5. TW.M.vdF.au‐PET tracer of choice in the clinic
Most experts (n = 182, 67.9%) did not express a preference for a particular tau‐PET tracer to differentiate AD dementia from other neurodegenerative disorders in the clinic. Some indicated a preference for [18F]MK6240 (n = 43, 16.0%), [18F]flortaucipir (n = 22, 8.2%), [18F]PI2620 (n = 11, 4.1%), [18F]RO948 (n = 4, 1.5%), [18F]GTP1 (n = 1, < 1%), and [18F]PM‐PBB3 (n = 1, < 1%). Four individuals valued two of the previously mentioned tracers equally.
3.3.6. Tau‐PET assessment in clinic
According to the respondents, in clinical practice tau‐PET scans should be assessed using a visual read method in combination with a quantitative measure (81.0%, 209/258). A smaller proportion (11.6%, 30/258) preferred assessment with only a quantitative measure, for example, based on a SUVR cutoff. Only 7.4% (19/258) opted for a purely visual read method.
3.4. Value of tau‐PET in therapeutic trials
3.4.1. Purpose of tau‐PET in trials
We asked whether tau‐PET would be useful for participant selection and measuring endpoints in drug trials targeting a variety of biological targets. 42 , 43 Respondents stated that tau‐PET can be used for participant selection in anti‐tau (99.6%) and anti‐amyloid trials (76.1%) and were less confident regarding other drug classes, such as inflammation and immunity (40.0%), synaptic plasticity and neuroprotection (38.4%), proteostasis and proteinopathies (32.2%), metabolism and bioenergetics (25.1%), neurotransmitter receptors (20.8%), epigenetic (18.4%), oxidative stress (18.0%), vasculature (16.5%), and neurogenesis (15.7%) (Figure 4A, Table S6). More clinicians than researchers indicated a role for tau‐PET to aid participant selection in proteostasis and proteinopathy drug trials (36.8% vs. 27.0%, p = 0.01). Similarly, experts agreed that tau‐PET can be useful to measure endpoints in anti‐tau (97.2%) and anti‐amyloid trials (75.0%) and less so for other drug classes, such as inflammation and immunity (48.0%), synaptic plasticity and neuroprotection (34.3%), proteostasis and proteinopathies (30.2%), metabolism and bioenergetics (23.8%), oxidative stress (22.6%), epigenetic (21.4%), neurotransmitter receptors (18.1%), neurogenesis (16.5%), and vasculature (15.3%) (Figure 4A, Table S7).
FIGURE 4.
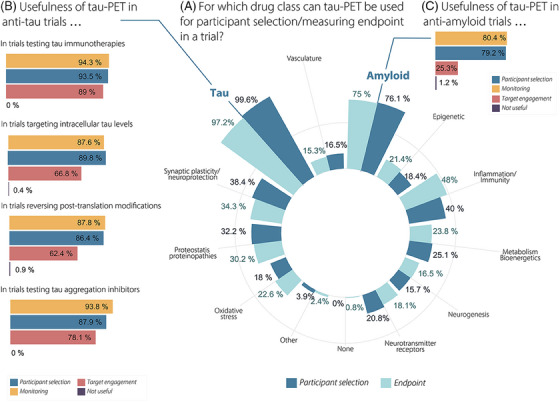
Summary findings on the envisioned future role of tau‐PET in drug trials. (A) Circular bar plot showing proportions of respondents envisioning a role for tau‐PET for participant selection and measuring endpoints across different drug classes. (B) Bar plots showing proportions of respondents envisioning a role for tau‐PET for participant selection, monitoring and target engagement within four anti‐tau trial classes specifically. (C) Bar plots showing proportions of respondents envisioning a role for tau‐PET for participant selection, monitoring and target engagement within anti‐amyloid drug trials specifically. tau‐PET, tau positron emission tomography.
When focusing on anti‐amyloid trials in particular, experts indicated a role for tau‐PET for participant selection (79.2%) and monitoring (80.4%), but less for target engagement (25.3%, Figure 4C). In anti‐tau trials testing tau immunotherapies, tau aggregation inhibitors, targeting intracellular tau levels, and reversing post‐translation modifications, 4 overall, an important role for tau‐PET was envisioned in participant selection and monitoring (all > 85%, Figure 4B). Regarding target engagement, most respondents indicated usefulness in anti‐tau trials testing tau immunotherapies (89.0%), followed by trials testing tau aggregation inhibitors (78.1%), trials targeting intracellular tau levels (66.8%), and trials reversing post‐translation modifications (62.4%).
3.4.2. Tracer of choice in trials
Most respondents (n = 190, 70.9%) did not express a preference for a particular tau‐PET tracer for use in trials. Some indicated a preference for [18F]MK6240 (n = 46, 17.2%), [18F]PI2620 (n = 12, 4.5%), [18F]flortaucipir (n = 10, 3.7%), [18F]RO948 (n = 5, 1.9%), and [18F]PM‐PBB3 (n = 1, < 1%). Four individuals valued two of the previously mentioned tracers equally.
3.4.3. Tau‐PET assessment in trials
Of all experts, 63.5% (165/260) indicated that in trials tau‐PET scans should be assessed using a visual read method in combination with a quantitative measure. A smaller proportion of 36.5% (95/260) preferred assessment with only a quantitative measure, for example, based on a SUVR cutoff. None of the respondents opted for a purely visual read method for use in trials.
4. DISCUSSION
In this survey study, 268 dementia experts from 29 different countries provided their perspectives on the future role of tau‐PET in clinic and trials. The vast majority (∼90%) fostered a positive attitude toward the added value of tau‐PET, particularly for staging, diagnosing, monitoring, and predicting in a cognitively impaired memory clinic population. From a set of clinical cases, our findings suggest that a tau‐PET scan is perceived particularly useful in patients with an atypical presentation or suspicion of mixed pathology. Furthermore, experts indicated that a tau‐PET scan could influence patient management in current practice, and stated that this would further increase when effective disease‐modifying treatments are/become available. Experts foresee great utility of tau‐PET for participant selection and measuring endpoints, in both anti‐amyloid and anti‐tau drug trials.
While the majority of the viewpoints by the dementia experts are in line with the state‐of‐the‐art literature and/or clinical practice, there are a few areas where there is a potential disconnect between them. First, both researchers and clinicians indicated that tau‐PET is most valuable in the prodromal stage of AD (90.7%). However, the literature consistently shows that the diagnostic performance of tau‐PET is highest in the dementia stage, where the extent of tau‐PET uptake is most pronounced. In fact, between ∼33%–50% of amyloid‐positive individuals with MCI have a negative tau‐PET scan, whereas this is ∼10%–20% at the dementia stage of AD. 12 , 24 , 25 , 26 , 27 Next, while the only currently FDA‐approved method for the interpretation of tau‐PET scans in clinical practice is a visual read, 12 , 35 experts indicated that tau‐PET scans should be rated visually in combination with (non‐approved) quantitative measures like a threshold approach (81%). Such an approach could resemble how [18F]FDG PET is used in the diagnosis of neurodegenerative disorders, where a visual rating is often accompanied by an automated tool that provides additional quantitative information. 44 , 45 Likewise, amyloid‐PET scans are increasingly assessed by visual read combined with a quantitative measure such as SUVR. The question remains whether tau‐PET, given its great variability in spreading patterns, would benefit from additional quantitative information.
The outcomes of this tau‐PET survey are largely in agreement with the Updated Appropriate Use Criteria for Amyloid and Tau PET by the Alzheimer's Association and Society for Nuclear Medicine and Molecular Imaging. 36 For example, both the Workgroup and dementia experts agreed that a tau‐PET scan can be useful in the diagnosis of patients with an atypical clinical presentation and suspicion of underlying mixed pathology. Indeed, the identification of the primary etiology causing cognitive symptoms becomes increasingly important in the context of treatment decisions. Moreover, respondents and the Workgroup agreed that eligibility for anti‐amyloid drug treatment can be determined with tau‐PET. This is in line with findings from the TRAILBLAZER clinical trial, where participants with low to intermediate tau‐PET tracer uptake had a more favorable response to the treatment compared to the high tau‐PET group, suggesting a theragnostic role for tau‐PET. 46 While overall experts and the Workgroup additionally see value in tau‐PET as an aid in prognostication in clinical practice, a significantly smaller proportion of clinicians (67.8%) compared to researchers (83.2%) indicated so. Large prospective studies, in both cognitively impaired and unimpaired populations, have provided evidence that tau‐PET holds strong predictive value with clinical relevance. 47 , 48 , 49 This expert opinion may thus reflect the uncertainty of how tau‐PET can be utilized in the clinic as a prognostic tool, rather than its prognostic performance in a research setting. Finally, the survey respondents foresaw an even broader window for the clinical application of tau‐PET in the future, as they additionally anticipated tau‐PET to be used as a tool for staging disease severity and monitoring disease progression. Nonetheless, currently, wide clinical application of tau‐PET imaging is hampered by high costs and limited availability. Therefore, it will most likely be used selectively in patients benefiting most from a tau‐PET scan, in addition to more accessible tests.
Notably, experts anticipated a valuable role for tau‐PET for participant selection and measuring of endpoints in both anti‐amyloid and anti‐tau drug trials. This viewpoint is supported by the literature. For example, the aforementioned findings from the donanemab trial TRAILBLAZER suggested a putative role of tau‐PET for participant selection. 46 Also, a recent phase‐1 study with a tau‐targeting antisense oligonucleotide therapy demonstrated proof‐of‐concept for tau‐PET as a trial endpoint, as temporal tau‐PET uptake was substantially reduced following treatment. 50 On the topic of target engagement in anti‐tau trials, experts foresaw the utility of tau‐PET for this purpose in tau immunotherapies (89.0%), which gradually decreased for tau aggregation inhibitors, therapies targeting intracellular tau levels, and reversing post‐translation modifications, reflecting the differing mechanisms‐of‐action of these drug classes. In general, the experts had a positive attitude toward use of tau‐PET in anti‐tau (near‐unanimous) and anti‐amyloid (∼75%) trials, which dropped substantially for other drug classes like inflammation, synaptic plasticity, and proteostasis. This has important potential ramifications for the future role of tau‐PET in trials as the 2024 AD drug development pipeline 43 showed broad diversification of the drug portfolio, going well beyond anti‐amyloid and anti‐tau therapies.
A major strength of our survey study is that through our global outreach dementia experts from six different continents with diverse backgrounds responded. However, our recruitment strategy may constitute a participant selection bias. Moreover, experts from The Netherlands and Sweden are relatively overrepresented. In our attempt to keep the survey comprehensible and recruit a sufficient number of respondents, we were obliged to compromise on profoundness and nuances of the questions. For example, in some scenarios, it is not further specified whether alternative biomarkers, such as amyloid‐PET, FDG‐PET, or the complete CSF panel, were available.
In conclusion, our global survey study shows that dementia experts envision an important role for tau‐PET in the future, both in clinical practice and in drugs trials, beyond current guidelines and clinical practices. Prospective clinical studies investigating the impact of tau‐PET on clinical practice and identifying patients that benefit most from tau‐PET are needed to support guidelines in the appropriate use of tau‐PET. 41 Future findings from drug development will further direct meaningful implementation of tau‐PET in trials.
CONFLICT OF INTEREST STATEMENT
R.O. has received research funding/support from European Research Council, ZonMw, NWO, National Institute of Health, Alzheimer Association, Alzheimer Nederland, Stichting Dioraphte, Cure Alzheimer's fund, Health Holland, ERA PerMed, Alzheimerfonden, Hjarnfonden, Avid Radiopharmaceuticals, Janssen Research & Development, Roche, Quanterix and Optina Diagnostics, has given lectures in symposia sponsored by GE Healthcare, is an advisory board member for Asceneuron and a steering committee member for Bristol Myers Squibb. All the aforementioned have been paid to the institutions. He is an editorial board member of Alzheimer's Research & Therapy and the European Journal of Nuclear Medicine and Molecular Imaging.
E.vdG. has received research support from NWO, ZonMw, Hersenstichting, Alzheimer Nederland, Health∼Holland, and KWF. E.vdG. has performed contract research for Heuron Inc. and Roche. E.vdG. has a consultancy agreement with IXICO and Life Molecular Imaging for reading PET scans. Research programs of W.M.vdF. have been funded by ZonMW, NWO, EU‐JPND, EU‐IHI, Alzheimer Nederland, Hersenstichting CardioVascular Onderzoek Nederland, Health∼Holland, Topsector Life Sciences & Health, stichting Dioraphte, Gieskes‐Strijbis fonds, stichting Equilibrio, Edwin Bouw fonds, Pasman stichting, stichting Alzheimer & Neuropsychiatrie Foundation, Philips, Biogen MA Inc, Novartis‐NL, Life‐MI, AVID, Roche BV, Fujifilm, Eisai, Combinostics. W.M.vdF. holds the Pasman chair. W.M.vdF. is recipient of ABOARD, which is a public‐private partnership receiving funding from ZonMW (#73305095007) and Health∼Holland, Topsector Life Sciences & Health (PPP‐allowance; #LSHM20106). W.M.vdF. is recipient of TAP‐dementia (www.tap‐dementia.nl), receiving funding from ZonMw (#10510032120003). TAP‐dementia receives co‐financing from Avid Radiopharmaceuticals and Amprion. WMvdF has been an invited speaker at Biogen MA Inc, Danone, Eisai, WebMD Neurology (Medscape), NovoNordisk, Springer Healthcare, European Brain Council. W.M.vdF. is consultant to Oxford Health Policy Forum CIC, Roche, Biogen MA Inc, and Eisai. W.M.vdF. participated in advisory boards of Biogen MA Inc, Roche, and Eli Lilly. W.M.vdF. is member of the steering committee of EVOKE/EVOKE+ (NovoNordisk). All funding is paid to her institution. W.M.vdF. is member of the steering committee of PAVE, and Think Brain Health. W.M.vdF. was associate editor of Alzheimer, Research & Therapy in 2020/2021. W.M.vdF. is associate editor at Brain. M.R.V. and I.L.C. have nothing to disclose. Authors disclosures are available in the Supporting Information.
CONSENT STATEMENT
All respondents provided informed consent.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
Leonie N. C. Visser, Ellen M. A. Smets, and Jolanda H. M. Dobbe provided valuable input on the interpretability of the survey questions from a communication point of view. We thank all respondents of the survey for their valuable contributions. The following respondents have consented to be acknowledged by name: Valentin Ourry, Raquel Sanchez‐Valle, Diego Sarasola, Colin Groot, Antoine Garnier‐Crussard, Roos Rikken, Brian Gordon, Emma Coomans, Alexandra J. Weigand, Olivier Rouaud, Alexa Pichet Binette, Juan Domingo Gispert, Mehrnaz Shekari, Paresh Malhotra, Joseph Therriault, Hanna Cho, Victor L. Villemagne, Arlette Haeger, Silvia Morbelli, Matthias Brendel, Rik Vandenberghe, Ophir Keret, Anders Martin Fjell, Pascual Sanchez Juan, Lars Lau Raket, Lyduine E. Collij, Ilse Bader, Ellen Singleton, Hanneke Rhodius‐Meester, Jacob Vogel, Floor Duits, Matteo Tonietto, David Wolk, Sara Hall, David Jones, Han‐Kyeol Kim, Sietske Sikkes, Ashvini Keshavan, Cécile Tissot, Kurt Segers, Julie Ottoy, Kristian Steen Frederiksen, Julien Lagarde, Matthijs Biesbroek, Elles Konijnenber, Alex Whittington, Konstantin Messerschmidt, Gayane Aghakhanyan, Pezzuto Salvatore, Gregory Klein, Tharick Pascoal, Hannah de Bruin, Nelleke Tolboom, Clifford Jack, Tobias Melton Axelsen, Fransje E. Reesink, Philip Scheltens, João Pedro Ferrari‐Souza, Alexis Moscoso Rial, Shannon Risacher, Michael Pontecorvo, Livia Ruffini, Gerard N. Bischof, Robert Laforce, Michelle Smulders, Oskar Hansson, Gemma Salvadó, Sylvia Villeneuve, Dhivya Srinivasan, Federica Agosta, Daniela Perani, Heidi Jacobs, Everard Vijverberg, David Cash, Marie Sarazin, Nicolai Franzmeier, José Contador, Stephanie Schultz, Nick Corriveau‐Lecavalier, Eloy Rodriguez, Etienne Aumont, Meichen Yu, Nicolas Villain, Carla Abdelnour, Pamela Lukasewicz Ferreira, Brandon Hall, EleannaVarangis, Maura Malpetti, Frédéric St‐Onge, Agathe Vrillon, Carmela Tartaglia, Maira Okada de Oliveira, Yakeel T. Quiroz, Elizabeth Head, Joseph Winer, Jesse Brown, Karine Provost, Jonathan Graff‐Radford, Marta Marquié, Antoine Verger, Leonel T. Takada, Tiago Gil Oliveira, Valentina Garibotto, Rob Durcan, Eduardo R. Zimmer, Nahuel Magrath Guimet, Claudia Kimie Suemoto, Jhony Mejia, Ian Law, Gabriel Gonzalez‐Escamilla, Javier Arbizu, Andrew W. Stephens, Nicola Spotorno, Joel Simrén, Meredith Braskie, Alexandre Bejanin, Daniele Altomare, Ruben Smith, Stijn Servaes, Michel Grothe, Ishita Batta, Amy Brodtmann, Leonardo Sacco, André Luiz Rodrigues Palmeira, Cristiano Schaffer Aguzzoli, Maartje Kester, Samuel N. Lockhart, Erik Hif, Emmanuel Epenge Djonga, Geert Jan Biessels, Dirk Saal, Luiza Machado, Leonard Pieperhoff, Micaela Hernández, Nilton Custodio, Paulo Caramelli, Tom den Heijer, Liana Lisboa Fernandez, Elisa de Paula França Resende, Sonia Brucki, Ricardo Allegri, Michael Scholl, Sebastian Palmqvist, Leonardo Iaccarino, Niklas Mattsson‐Carlgren, Dirk Beher, Ryan Schubert, Gil Rabinovici, Renaud La Joie, David N. Soleimani‐Meigooni, William Jagust, Susan Landau, Robert Perneczky, Isadora Lopes Alves, Vincent Dore, Sang Won Seo, Chul Hyoung Lyoo, Michael Ewers, Bernard Han, Tobey J. Betthauser, Hwamee Oh, Lea T. Grinberg, Henryk Barthel, Agneta Nordberg, Ann Cohen, Georges El Fakhri, Serge Gauthier, Koen van Laere, Marianne Chapleau, Jennifer Whitwell, Irene Sintini, Neha Singh, Mark Battle, Konstantinos Chiotis, Alexander Drzezga, Pallavi Sachdev, Philipp Meyer, Duygu Tosun, Juha Rinne, Deepti Putcha, Jonathan M. Schott, Keir Yong, Carolyn Fredericks, Edilio Borroni, Rosaleena Mohanty, Eric Westman, Alberto Lleó, Juan Fortea, Jose Luis Molineuvo, Marc Suárez‐Calvet, Arthur C. Macedo, Yi‐Ting Wang, Jean‐Paul Soucy, Paolo Vitali, Pedro Rosa Neto, Douglas Teixeira Leffa, Bruna Bellaver, Wyllians Borelli, Natasha Krishnadas, Catalina Bensi, Priscila Elliott, Sophie Mastenbroek, Juan J. Llibre Rodriguez, Antoine Leuzy, Nikolaos Karvelas, Ricardo Nitrini, Chetelat, Erik Stomrud, David Aguillon, Sudhir Sivakumaran, Norm Mazer, David Knopman, Edmond Teng, Maria Carillo, Liana Apostolova, Justin Sanchez, Adam Brickman, Debora Elisa Peretti, Christopher Chen, Feng‐Tao Liu, Giovanni Frisoni, Tao Sun, Michael C. Irizarry, Francesca Mangialasche, Bengt Winblad, Daniel Ferreira, Fernando Coto‐Yglesias, Johannes Kornhuber, John Obrien, Ron Petersen, Linus Jönsson, Hilkka Soininen, Elisabet Londos, Anders Wimo, Anne‐Marie De Cock, Peter De Deyn, and Mirko Petrovic. Research of the Alzheimer Center Amsterdam is part of the neurodegeneration research program of Amsterdam Neuroscience. The Alzheimer Center Amsterdam is supported by Stichting Alzheimer Nederland and Stichting VUmc fonds. W.M.vdF. holds the Pasman chair. M.R.V. is appointed at TAP‐dementia (www.tap‐dementia.nl), receiving funding from ZonMw (#10510032120003) in the context of Onderzoeksprogramma Dementie, part of the Dutch National Dementia Strategy. TAP‐dementia receives co‐financing from Avid Radiopharmaceuticals, Roche Diagnostics and Amprion. Timely, Accurate, and Personalized Diagnosis of Dementia (TAP‐dementia) receives funding from ZonMw (#10510032120003) in the context of Onderzoeksprogramma Dementie, which is part of the Dutch National Dementia Strategy. Amsterdam UMC locations VUmc and AMC, Erasmus MC, UMCU, University Maastricht, UMCG, VU University, Elisabeth‐TweeSteden Ziekenhuis Tilburg and Vilans participate in TAP‐dementia (www.tap‐dementia.nl) TAP‐dementia receives co‐financing from Avid Radiopharmaceuticals, Roche Diagnostics and Amprion. Gieskes‐Strijbis fonds also contributes to TAP‐dementia.
Vermeiren MR, Calandri IL, van der Flier WM, van de Giessen E, Ossenkoppele R. Survey among experts on the future role of tau‐PET in clinical practice and trials. Alzheimer's Dement. 2024;16:e70033. 10.1002/dad2.70033
Contributor Information
Marie R. Vermeiren, Email: m.r.vermeiren@amsterdamumc.nl.
Rik Ossenkoppele, Email: r.ossenkoppele@amsterdamumc.nl.
REFERENCES
- 1. Scheltens P, Blennow K, Breteler MMB, et al. Alzheimer's disease. Lancet. 2016;388(10043):505–517. doi: 10.1016/S0140-6736(15)01124-1 [DOI] [PubMed] [Google Scholar]
- 2. Jack CR, Bennett DA, Blennow K, et al. NIA‐AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimer's Dement. 2018;14(4):535–562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jack CR, Andrews JS, Beach TG, et al. Revised criteria for diagnosis and staging of Alzheimer's disease: Alzheimer's Association Workgroup. Alzheimer's Dement. 2024;20(8):5143–5169. doi: 10.1002/alz.13859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ossenkoppele R, van der Kant R, Hansson O. Tau biomarkers in Alzheimer's disease: towards implementation in clinical practice and trials. Lancet Neurol. 2022;21(8):726–734. doi: 10.1016/S1474-4422(22)00168-5 [DOI] [PubMed] [Google Scholar]
- 5. Macedo AC, Therriault J, Tissot C, et al. Predicting functional decline in aging and Alzheimer's disease with PET‐based Braak staging. Brain Commun. 2024;6(2). doi: 10.1093/braincomms/fcae043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ossenkoppele R, Schonhaut DR, Schöll M, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease. Brain. 2016;139(pt 5):1551–1567. doi: 10.1093/brain/aww027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johnson KA, Schultz A, Betensky RA, et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol. 2016;79(1):110–119. doi: 10.1002/ana.24546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jack CR, Wiste HJ, Schwarz CG, et al. Longitudinal tau PET in ageing and Alzheimer's disease. Brain. 2018;141(5):1517–1528. doi: 10.1093/brain/awy059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ossenkoppele R, Smith R, Ohlsson T, et al. Associations between tau, Aβ, and cortical thickness with cognition in Alzheimer disease. Neurology. 2019;92(6):e601–e612. doi: 10.1212/WNL.0000000000006875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Therriault J, Schindler SE, Salvadó G, et al. Biomarker‐based staging of Alzheimer disease: rationale and clinical applications. Nat Rev Neurol. 2024;20(4):232–244. doi: 10.1038/s41582-024-00942-2 [DOI] [PubMed] [Google Scholar]
- 11. Leuzy A, Chiotis K, Lemoine L, et al. Tau PET imaging in neurodegenerative tauopathies‐still a challenge. Mol Psychiatry. 2019;24(8):1112–1134. doi: 10.1038/s41380-018-0342-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fleisher AS, Pontecorvo MJ, Devous MD, et al. Positron emission tomography imaging with [ 18 F]flortaucipir and postmortem assessment of Alzheimer disease neuropathologic changes. JAMA Neurol. 2020;77(7):829. doi: 10.1001/jamaneurol.2020.0528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lowe VJ, Lundt ES, Albertson SM, et al. Tau‐positron emission tomography correlates with neuropathology findings. Alzheimers Dement. 2020;16(3):561–571. doi: 10.1016/j.jalz.2019.09.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Soleimani‐Meigooni DN, Iaccarino L, La Joie R, et al. 18F‐flortaucipir PET to autopsy comparisons in Alzheimer's disease and other neurodegenerative diseases. Brain. 2020;143(11):3477–3494. doi: 10.1093/brain/awaa276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pascoal TA, Shin M, Kang MS, et al. In vivo quantification of neurofibrillary tangles with [ 18 F]MK‐6240. Alzheimers Res Ther. 2018;10(1):74. doi: 10.1186/S13195-018-0402-Y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Therriault J, Pascoal TA, Lussier FZ, et al. Biomarker modeling of Alzheimer's disease using PET‐based Braak staging. Nat Aging. 2022;2(6):1–10. doi: 10.1038/s43587-022-00204-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Groot C, Villeneuve S, Smith R, Hansson O, Ossenkoppele R. Tau PET imaging in neurodegenerative disorders. J Nucl Med. 2022;63(suppl 1):20S–26S. doi: 10.2967/jnumed.121.263196 [DOI] [PubMed] [Google Scholar]
- 18. Lowe VJ, Curran G, Fang P, et al. An autoradiographic evaluation of AV‐1451 Tau PET in dementia. Acta Neuropathol Commun. 2016;4(1):58. doi: 10.1186/s40478-016-0315-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aguero C, Dhaynaut M, Normandin MD, et al. Autoradiography validation of novel tau PET tracer [F‐18]‐MK‐6240 on human postmortem brain tissue. Acta Neuropathol Commun. 2019;7(1):37. doi: 10.1186/s40478-019-0686-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yap SY, Frias B, Wren MC, et al. Discriminatory ability of next‐generation tau PET tracers for Alzheimer's disease. Brain. 2021;144(8):2284–2290. doi: 10.1093/brain/awab120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aguero C, Dhaynaut M, Amaral AC, et al. Head‐to‐head comparison of [18F]‐Flortaucipir, [18F]‐MK‐6240 and [18F]‐PI‐2620 postmortem binding across the spectrum of neurodegenerative diseases. Acta Neuropathol. 2024;147(1):25. doi: 10.1007/s00401-023-02672-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu FT, Lu JY, Li XY, et al. 18 F‐florzolotau positron emission tomography imaging of tau pathology in the living brains of patients with corticobasal syndrome. Mov Disord. 2023;38(4):579–588. doi: 10.1002/mds.29338 [DOI] [PubMed] [Google Scholar]
- 23. Santillo AF, Leuzy A, Honer M, et al. 18F]RO948 tau positron emission tomography in genetic and sporadic frontotemporal dementia syndromes. Eur J Nucl Med Mol Imaging. 2023;50(5):1371–1383. doi: 10.1007/s00259-022-06065-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ossenkoppele R, Rabinovici GD, Smith R, et al. Discriminative accuracy of [18F]flortaucipir positron emission tomography for Alzheimer disease vs other neurodegenerative disorders. JAMA. 2018;320(11):1151‐1162. doi: 10.1001/jama.2018.12917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jack CR, Wiste HJ, Botha H, et al. The bivariate distribution of amyloid‐β and tau: relationship with established neurocognitive clinical syndromes. Brain. 2019;142(10):3230–3242. doi: 10.1093/brain/awz268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pascoal TA, Therriault J, Benedet AL, et al. 18F‐MK‐6240 PET for early and late detection of neurofibrillary tangles. Brain. 2020;143(9):2818–2830. doi: 10.1093/BRAIN/AWAA180 [DOI] [PubMed] [Google Scholar]
- 27. Leuzy A, Smith R, Ossenkoppele R, et al. Diagnostic performance of RO948 F 18 tau positron emission tomography in the differentiation of Alzheimer disease from other neurodegenerative disorders. JAMA Neurol. 2020;77(8):955–965. doi: 10.1001/jamaneurol.2020.0989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leuzy A, Pascoal TA, Strandberg O, et al. A multicenter comparison of [18F]flortaucipir, [18F]RO948, and [18F]MK6240 tau PET tracers to detect a common target ROI for differential diagnosis. Eur J Nucl Med Mol Imaging. 2021;48(7):2295–2305. doi: 10.1007/s00259-021-05401-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ossenkoppele R, Jansen WJ, Rabinovici GD, et al. Prevalence of amyloid PET positivity in dementia syndromes: a meta‐analysis. JAMA. 2015;313(19):1939–1949. doi: 10.1001/jama.2015.4669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jansen WJ, Ossenkoppele R, Knol DL, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta‐analysis. JAMA. 2015;313(19):1924–1938. doi: 10.1001/jama.2015.4668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Therriault J, Vermeiren M, Servaes S, et al. Association of phosphorylated tau biomarkers with amyloid positron emission tomography vs tau positron emission tomography. JAMA Neurol. 2023;80(2):188–199. doi: 10.1001/jamaneurol.2022.4485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pichet Binette A, Franzmeier N, Spotorno N, et al. Amyloid‐associated increases in soluble tau relate to tau aggregation rates and cognitive decline in early Alzheimer's disease. Nat Commun. 2022;13(1):6635. doi: 10.1038/s41467-022-34129-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Groot C, Smith R, Stomrud E, et al. Phospho‐tau with subthreshold tau‐PET predicts increased tau accumulation rates in amyloid‐positive individuals. Brain. 2023;146(4):1580–1591. doi: 10.1093/brain/awac329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ossenkoppele R, Reimand J, Smith R, et al. Tau PET correlates with different Alzheimer's disease‐related features compared to CSF and plasma p‐tau biomarkers. EMBO Mol Med. 2021;13(8):e14398. doi: 10.15252/emmm.202114398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. TauvidTM (flortaucipir F 18 injection) . Avid Radiopharmaceuticals, a wholly‐owned subsidiary of Eli Lilly and Co. Accessed June 17, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212123s000lbl.pdf
- 36. Rabinovici GD, Knopman D, Arbizu J, et al. 2024 AUC for Amyloid and Tau PET: Updated Appropriate Use Criteria for Amyloid and Tau PET in Alzheimer's Disease. Vol 17. Butler Hospital Memory and Aging Program; 2024. [Google Scholar]
- 37. Ossenkoppele R, Hansson O. Towards clinical application of tau PET tracers for diagnosing dementia due to Alzheimer's disease. Alzheimer's Dement. 2021;17(12):1998–2008. doi: 10.1002/alz.12356 [DOI] [PubMed] [Google Scholar]
- 38. Klunk WE, Koeppe RA, Price JC, et al. The Centiloid Project: standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement. 2015;11(1):1–15.e1‐4. doi: 10.1016/j.jalz.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Villemagne VL, Leuzy A, Bohorquez SS, et al. CenTauR: toward a universal scale and masks for standardizing tau imaging studies. Alzheimer's Dement: DADM. 2023;15(3):e12454. doi: 10.1002/dad2.12454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Head‐to‐Head Harmonization of Tau Tracers in Alzheimer's Disease (HEAD). NIH. Accessed June 17, 2024. https://clinicaltrials.gov/study/NCT05361382 [Google Scholar]
- 41. Wolters EE, Dodich A, Boccardi M, et al. Clinical validity of increased cortical uptake of [ 18 F]flortaucipir on PET as a biomarker for Alzheimer's disease in the context of a structured 5‐phase biomarker development framework. Eur J Nucl Med Mol Imaging. 2021;48:2097–2109. doi: 10.1007/s00259-020-05118-w/Published [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cummings J, Lee G, Nahed P, et al. Alzheimer's disease drug development pipeline: 2022. Alzheimer's Dement: Transl Res Clin Intervent. 2022;8(1):e12295. doi: 10.1002/trc2.12295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cummings J, Zhou Y, Lee G, Zhong K, Fonseca J, Cheng F. Alzheimer's disease drug development pipeline: 2024. Alzheimers Dement. 2024;10(2):e12465. doi: 10.1002/trc2.12465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen K, Ayutyanont N, Langbaum JBS, et al. Characterizing Alzheimer's disease using a hypometabolic convergence index. Neuroimage. 2011;56(1):52–60. doi: 10.1016/j.neuroimage.2011.01.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Herholz K, Salmon E, Perani D, et al. Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. Neuroimage. 2002;17(1):302‐316. doi: 10.1006/nimg.2002.1208 [DOI] [PubMed] [Google Scholar]
- 46. Sims JR, Zimmer JA, Evans CD, et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER‐ALZ 2 randomized clinical trial. JAMA. 2023;330(6):512–527. doi: 10.1001/jama.2023.13239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ossenkoppele R, Pichet Binette A, Groot C, et al. Amyloid and tau PET‐positive cognitively unimpaired individuals are at high risk for future cognitive decline. Nat Med. 2022;28(11):2381–2387. doi: 10.1038/s41591-022-02049-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lu M, Pontecorvo MJ, Devous MD, et al. Aggregated tau measured by visual interpretation of flortaucipir positron emission tomography and the associated risk of clinical progression of mild cognitive impairment and Alzheimer disease: results from 2 phase III clinical trials. JAMA Neurol. 2021;78(4):445–453. doi: 10.1001/jamaneurol.2020.5505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Groot C, Smith R, Collij LE, et al. Tau positron emission tomography for predicting dementia in individuals with mild cognitive impairment. JAMA Neurol. 2024;81(8):845–856. doi: 10.1001/jamaneurol.2024.1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Edwards AL, Collins JA, Junge C, et al. Exploratory tau biomarker results from a Multiple Ascending‐Dose Study of BIIB080 in Alzheimer disease: a randomized clinical trial. JAMA Neurol. 2023;80(12):1344‐1352. doi: 10.1001/jamaneurol.2023.3861 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


