Abstract
This study examined effects of different processing methods on phenolic compounds in flaxseed meal. The optimal SE treatment was 1.0 MPa for 3 min, and the contents of total flavonoids and phenolic acid were 2.26 times and 1.63 times of the control group, respectively. Notably, erucic acid increased 85.76 %. Optimal extrusion conditions (15 % moisture content, 140 °C, 29 hz) led to the presence of rutin and a 2.81 times increase in protocatechuic acid content over the control. Fermenting with 3 % Bacillus subtilis for 4 days yielded gallic acid in bound form and vanillic acid in free form, with protocatechuic acid increasing 40.65 % compared to the control. Among all the treatments, extrusion produced the highest levels of phenolic compounds in flaxseed meal. Each treatment significantly increased the open ring isomer ester phenol (SDG) compared to the control. Overall, various processing methods impacted the phenolic content and composition in flaxseed meal differently.
Keywords: Flaxseed meal, Phenolic compounds, Steam explosion, Extrusion, Fermentation
Highlights
-
•
Steam explosion led to increased syringic acid, quercetin, and erucic acid levels.
-
•
Extrusion enhanced the content of caffeic acid and protocatechuic acid, especially rutin.
-
•
Fermentation led to the appearance of gallic and vanillic acids, and caffeic acid was degraded.
-
•
The ring-opening isolated ester phenols of flaxseed meal rised significantly after extrusion.
1. Introduction
Flaxseed (Linum usitatissimum L.), is the seed of an annual herb of the flax family. Flaxseed was an important raw material for oil, containing 15 % to 25 % linoleic acid, with α-linolenic acid constituting over 60 %. Flaxseed meal, a byproduct from flaxseed oil extraction, typically contained 32 % to 49 % protein, 10 % to 12 % lipids, 18 % to 47 % dietary fiber, and over 7 % minerals and vitamins (Wu, Wang, Qi, & Guo, 2019). However, its use was restricted due to antinutritional components such as cyanogenic glycosides and limitations in extracting bioactive substances (Adom & Liu, 2002). Consequently, it frequently end up as animal feed, fertilizer, or waste, causing significant resource loss (Anreta et al., 2020). In truth, flaxseed meal possessed considerable nutritional benefits, containing various bioactive substances, such as amino acids, cellulose, lignan, polysaccharides, and alkaloids. Flaxseed polyphenols, in particular, have drawn attention for their potential antioxidant, anti-inflammatory, anti-atherosclerotic, and anticancer properties (Doyen et al., 2014; Mechchate et al., 2021). The milling of flaxseed meal into fine powder could be widely used in food processing. Incorporating flaxseed meal into flour enhanced dough viscosity, helping maintain the structure of baked goods (Sęczyk, Świeca, Dziki, Anders, & Gawlik-Dziki, 2017). Manthey et al. noted that adding 15 % flaxseed meal extended the shelf life of pasta and macaroni while making these items more prone to aging during storage (Manthey, Sinha, Wolf-Hall, & Hall Iii, 2008). Therefore, it was necessary to study phenolic acids, flavonoids and other functional components in flaxseed meal to improve the added value of flaxseed meal and promoted the sustainable development of flaxseed industry.
The processing methods of food raw materials are mainly divided into two categories: traditional processing methods such as fermentation, sprouting and steamcooking; and emerging processing technologies such as low-temperature plasma, ultra-micro-milling and steam explosion (SE), which are also gradually being developed (Adom & Liu, 2002; Salar, Purewal, & Bhatti, 2016). Different processing technologies will change the nutrient composition of food raw materials, causing significant differences in various bioactive compounds. Therefore, it is crucial to explore the influence of different processing methods on phenolic compounds in raw materials. SE represents an innovative and cost-effective technology that has been widely used in many areas of pretreatment such as bioactive phytochemical extraction, with time-saving, low-cost, and non-polluting advantages (Song et al., 2022). After SE pretreatment, the cell volume is enlarged and the cell wall is damaged, accelerating the release of low molecular weight substances from the cell (Chen, Shan, Cao, & Tang, 2020; Song et al., 2022). Results showed that phenolic composition and antioxidant activity of soybean bran were enhanced by SE pretreatment (Chen et al., 2020). Moreover, SE pretreatment has also been effectively employed to extract natural antioxidants and sugars from olive leaves (Romero-García et al., 2016). It also modifies the grain size of lake peel to create large cracks and micropores, thereby enhancing the extraction of flavonoids (Chen & Chen, 2011; Song et al., 2022). Furthermore, it was observed that by increasing SE pressure and extending pressure maintenance time, the content of second isoliporesin alcohol diglycoside under SE treatment was 1.73 times higher than that of untreated second isoliporesin alcohol diglycoside, significantly enhancing antioxidant activity (Noda, Asada, Sasaki, Hashimoto, & Nakamura, 2013; Wenk, 2003).
Extrusion is a high-temperature, instantaneous processing technique that occurs under conditions of elevated temperature, high pressure, and intense shear. Compared to traditional processing, extrusion processing has found wide use in the field of grain raw material processing due to its advantages of simple operation, high efficiency and low production cost (Dalbhagat, Mahato, & Mishra, 2019). Extrusion can alter the physical and chemical properties of samples, affect the dietary fiber composition, promote starch gelatinization, facilitate protein cross-linking. Deactivate endogenous enzymes, destroy anti-nutritional factors, increase the bioavailability of minerals and phenolic acids and improve the protein digestibility (Hole et al., 2013; Wang, He, & Chen, 2014). Various extrusion conditions may have varying effects on phenolic content in whole grains. On the one hand, extrusion led to decomposition of thermally unstable phenolic compounds and polymerization of some phenolic compounds, which reduced the extractable phenolic content (Wang et al., 2014). The results showed that the total phenolic content of the mixture of oat grains, oatmeal, barley flour and pomace after extrusion was reduced by 50 %, 24–46 % and 46–60 %, respectively. (Adom & Liu, 2002; Salar et al., 2016). In the extrusion of hulled buckwheat seeds, the total phenolic content was reduced by a factor of 3 due to the decomposition of phenolic compounds or the decarboxylation and polymerization of tannins during extrusion (Brennan, Brennan, Derbyshire, & Tiwari, 2011; Sharma, Gujral, & Singh, 2012). On the other hand, extrusion disrupted the cell wall matrix and high molecular weight covalent bonds in polyphenol complexes, increasing the accessibility of phenolic compounds (Wang et al., 2014). The contents of free phenolic, bound phenolic, and bound flavonoid in extruded maize were significantly higher than those in unextruded maize (Sharma et al., 2012). The solubilization of phenolic acid significantly increased in sorghum at an extrusion temperature of 180 °C, with ferulic acid being the most pronounced (> 100 %) (Sharma et al., 2012). The increased temperature causes the phenolic acid molecules to break down into smaller substances that cannot be detected as phenolic acids, resulting in a decrease in the detectable amount of phenolic acids (Adom & Liu, 2002). It was shown that extrusion increased the conjugated phenolic acid content of oats by 9 %, while reducing that of barley by 11 %. Additionally, the total free phenolic acid content of both oats and barley was significantly higher than that of unextruded grains (Wang et al., 2014).
Fermentation has been used for thousands of years as a cost-effective and lowenergy preservation process to improve the nutritional and organoleptic qualities of food products (Paucar-Menacho et al., 2022). The use of fermentation to process whole grains was not only easy to control, short cycle time and low cost, but also effectively improved the nutritional value and antioxidant properties of grains. Fermentation improved the physical and chemical properties of whole grains, and met the nutritional needs of people, which had great potential for development (Adom & Liu, 2002; Salar et al., 2016). Fermentation reduces phytic acid, which liberates minerals like calcium, magnesium, and phosphorus from their bound forms. This process boosts the absorption and utilization of these minerals in the body, enhancing their bioavailability (Yang et al., 2018). Regarding the effect of fermentation on the detoxification of flaxseed meal, the process removed cyanosides and retained flaxseed nutrients (oil, lignan and fatty acids) (Huang et al., 2023). Fermentation could increase the concentration of phenolics, enhance the bioaccessibility of proteins, reduce antinutritional factors, and improve food texture and shelf life (Hur, Lee, Kim, Choi, & Kim, 2014). Yeast and lactic acid bacteria were employed to ferment millet, resulted in a notable increase in the contents of cinnamic acid and vitexin in the fermented millet (Balli et al., 2020). Enzymatic hydrolysis occurred during the fermentation process of brown rice, which increased the total phenolic content, enhanced the antioxidant activity and improved the food quality (Adom & Liu, 2002; Salar et al., 2016).
Currently, studies on the effects of processing methods on flaxseed meal mainly focus on protein modification (Minevich, Goncharova, & Zaitseva, 2021), nutrient utilization (Yang et al., 2018), and the removal of cyanogenic glycosides, etc. (Imran, Anjum, Butt, Siddiq, & Sheikh, 2013). To the best of our knowledge, systematic studies on the effects of processing methods on phenolics in flaxseed meal are lacking. The purpose of this study was to investiage the effect of SE, extrusion and fermentation on phenolic compounds in flaxseed meal. We determined and analyzed the total phenolics, SDG, flavonoids, and phenolic acids of flaxseed meal to evaluate the effects of different processing methods. The results will provide reference for the all-round utilization of flaxseed meal in food processing industry.
2. Materials and methods
2.1. Materials and chemicals
Flaxseed meal (Zhengzhou, China), harvested in 2022, stored at −20 °C. Methanol, Na2CO3, NaCl, HCL and NaOH were purchased from Sinopharm Chemical Reagent Company (Shanghai, China). Saccharomyces cerevisiae, Lactobacillus plantarum and Bacillus subtilis were from Henan Agricultural University (Zhengzhou, Henan, China). The other reagents in liquid phase were chromatographic grade. SDG, Phenolic acid standard (purity ≥98 %) and flavonoid standard (purity ≥98 %) were primarily purchased from Sigma Aldrich (MO, USA).
A suitable quantity of selected and refined flaxseed meal should be measured and placed in an electric hot air drying oven. A drying temperature of 105 °C should be maintained for four hours (MM400, Retsch, Han, Germany). Once the drying process is complete, the flaxseed meal should be removed from the oven and allowed to cool to ambient temperature. Subsequently, it should be sieved through a 40-mesh filter and packaged for future use.
2.2. Extraction of phenolics
Free phenolic extraction: Initially, 20 mL of an 80 % methanol solution was introduced to 1 g of flaxseed meal, followed by stirring at ambient temperature for 30 min. The extract underwent centrifugation at 10,000 ×g and 4 °C for 10 min, resulting in the collection of the supernatant. This extraction process was repeated three times, combining all extracts, and the organic solvent was evaporated at 40 °C. The combined supernatant was dried and reconstituted in 10 mL of a 50 % (v/v) methanol/distilled water solution. To isolate soluble conjugated phenols, the residual concentrated aqueous phase was subjected to alkaline hydrolysis with 10 mL of 4 M NaOH after ethyl acetate extraction. This mixture underwent hydrolysis through oscillation for 2 h under a nitrogen atmosphere at 40 °C. Subsequently, the pH of the hydrolysate was adjusted to 2.0 using 12 M HCl and extracted three times with 15 mL of ethyl acetate. The resulting extract was evaporated to dryness and then redissolved in 10 mL of 50 % (v/v) methanol/distilled water to isolate the conjugated phenol components. Following this, the remaining residue was treated with 4 M NaOH and hydrolyzed on a shaking table (180 rpm) under nitrogen for 4 h. The solution's pH was adjusted to 2.0, and combined phenolic compounds were extracted three times with ethyl acetate. Afterward, the combined extract was evaporated to dryness and dissolved in a 50 % (v/v) methanol/distilled water solution. Finally, the three phenolic extracts were filtered through a 0.22 μm polytetrafluoroethylene (PTFE) membrane and analyzed using HPLC.
In our study, “free phenolics” refers to the soluble fraction obtained without NaOH hydrolysis; “bound phenolics” means the soluble fraction following alkaline hydrolysis and the insoluble fraction obtained from the residue following alkaline hydrolysis.
2.3. Steam explosion treatment
Steam explosion was performed using a QBS-80 batch steam explosion apparatus (Hebi Gentle Bioenergy, Hebi, China). Steam explosion was performed for pressures 0.2, 0.6, 1.0, 1.4 and 1.8 MPa and 1.0 MPa for 1, 2, 3, 4 min (Noda et al., 2013; Wenk, 2003). Flaxseed meal was placed inside the vessel and exposed to the saturated steam. After steam explosion, the product was collected and freeze-dried, and then was sieved 40 mesh for use. The flaxseed meal without SE treatment was used as a control group.
2.4. Extrusion treatment
The DS-32 twin screw extruder (Jinan Saixin expanding Machinery, Jinan, China) was used to extrude flaxseed meal according to the below experimental design conditions of water content, barrel temperature and screw speed. The feed speed was 100 g/min, the die diameter at the die head was 3.00 mm, the screw diameter in the barrel was 32 mm, and the screw length was 742 mm.
There are three independent variables in this study. These variables are water content (15, 20, 25, 30 and 35 % at 140 °C extrusion temperature and 17 hz extrusion speed), extrusion temperature (17, 20, 23, 26 and 29 hz with 15 % water content at 140 °C extrusion temperature) and screw speed (120, 130, 140, 150, and 160 °C, with 15 % water content and 17 hz extrusion speed) (Brennan et al., 2011).
2.5. Fermentation processes
The flaxseed meal were mixed 1:1 with sterile water, transferred to a 100 mL conical flask and autoclaved for 30 min at 121 °C. After cooling, Saccharomyces cerevisiae, Lactobacillus casei and Bacillus subtilis (All from Henan Agricultural University, Zhengzhou, Henan) were inoculated respectively and fermented in incubators at corresponding temperatures. The bacterial cultures were retained for further use when the optical density of each bacterial suspension reached 1.0 as measured at 600 nm. There are three independent variables in this study. These variables are fermentation strains (inoculated with 3 % Saccharomyces cerevisiae, Lactobacillus casei and Bacillus subtilis respectively, and fermented at corresponding temperature for 5 days), inoculum amount (inoculated with 1, 2, 3, 4 and 5 % Bacillus subtilis, respectively, for 5 days) and fermentation time (inoculated with 3 % Bacillus subtilis for 1, 2, 3, 4 and 5 d, respectively) (Hur et al., 2014).
2.6. Determination of total phenolic content of flaxseed meal
The total phenolic content was assessed using Folin-Ciocalteu methods, referencing the standard curve of gallic acid in line with established procedures (Zhang et al., 2015). Results are reported as milligrams of gallic acid equivalent (GAE) per gram of sample, calculated on a dry weight basis.
2.7. Determination of flavonoid content
The free and bound phenolic compounds were isolated and analyzed using High-Performance Liquid Chromatography (HPLC) both pre- and post-treatment. Initially, the phenolic sample underwent filtration through a 0.22 μm Nylon syringe filter. Subsequently, HPLC analysis was executed using a chromatographic C18 column (1.8 μm, 50.0 mm × 2.1 mm) with slight methodological adjustments. The instrumental parameters included a column temperature of 35 °C, an injection volume of 10 μL, and a flow rate of 0.3 mL/min. The elution gradient was achieved using a 0.1 % acetic acid in water (v/v, mobile phase A) and methanol (mobile phase B) with the following sequence: 0–4 min, 90 %–65 % B, 4–27 min, 65–90 % B, 27.01–30 min, 90–30 % B. The eluents' absorbance was measured at 280 nm. Finally, the identification of polyphenol compounds in the samples was performed by comparing the results with LC-spectra data and the retention times of standards.
2.8. Determination of phenolic acid content
The free and bound phenolic compounds were isolated and characterized before and after treatment using High-Performance Liquid Chromatography (HPLC). Initially, the phenolics passed through a Nylon syringe filter (0.22 μm). Subsequently, the analyses were conducted on an HPLC system equipped with a C18 chromatographic column (1.8 μm, 50.0 mm × 2.1 mm), following a slightly modified method. The instrument parameters were established as follows: column temperature at 35 °C, injection volume of 10 μL, and flow rate of 0.3 mL/min. The elution gradient employed a 0.1 % methanol acetate in methanol acetate/water solution (v/v, mobile phase A) and a 0.1 % acetic acid/water solution (mobile phase B) according to the program: 0–11 min, 9–14 % B; 11–14 min, 14–15 % B; 14–17 min, 15 % B; 17–24 min, 15–16.5 % B; 24–28 min, 16.5–19 % B; 28–30 min, 19–25 % B; 30–36 min, 25–26 % B; 36–38 min, 26–28 % B; 38–41 min, 28–35 % B; 41–46 min, 35–40 % B; 46–48 min, 40–48 % B; 48–50 min, 9 % B. The absorbance of the eluent was detected at 280 nm. Finally, the identification of polyphenolic compounds in the samples was accomplished by comparing the LC-spectra data and retention times with those of standards.
The SDG testing method was the same as above.
2.9. Statistical analysis
The experiment was set up with three biological replications and the results were expressed as mean (means) ± standard deviation (SD). Data were statistically analyzed using SPSS 19.0 (SPSS Inc., Chicago, IL, USA), with Duncan's multiple comparisons between means and significance testing at the 0.05 level (p < 0.05). Charts were completed by Origin 2021 (OriginLab Corp., Northampton, MA, USA).
3. Results
3.1. Effect of steam explosion on the phenolics of flaxseed meal
3.1.1. Effect of SE time on phenolics of flaxseed meal
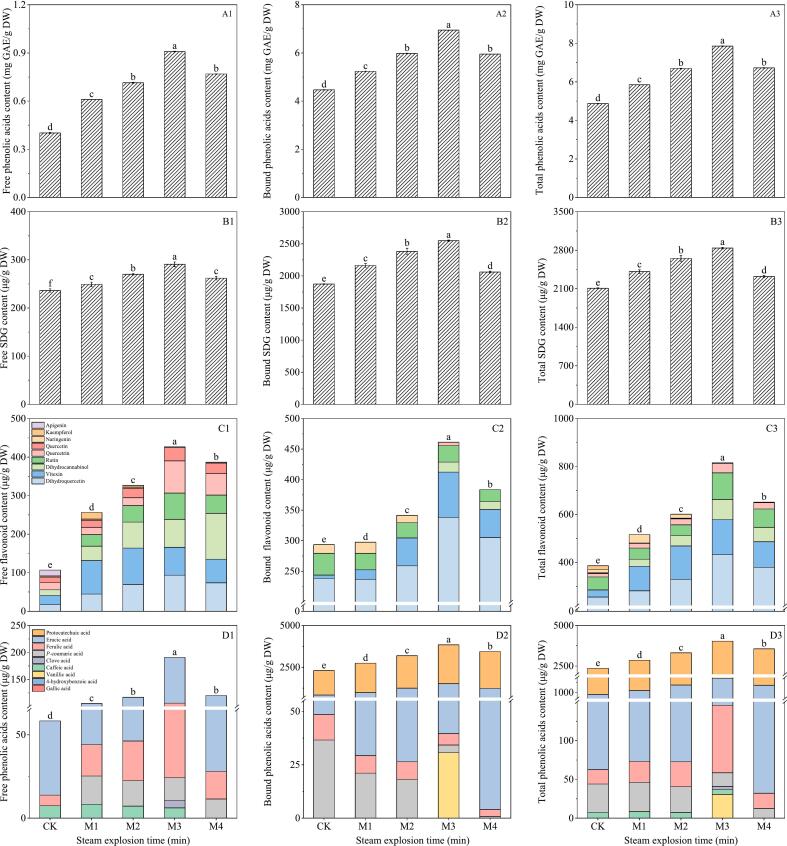
The Fig. 1A1–3 indicates that the highest concentrations of free and bound phenolic compounds in flaxseed meal were observed at 1.0 MPa for 3 min, measuring 0.91 mg GAE/g DW and 6.95 mg GAE/g DW, which represented increases of 126.24 % and 50.55 % compared to the control, respectively. Furthermore, at 1.0 MPa across durations of 1, 2, 3, and 4 min, the total phenolic concentration in flaxseed meal first rose and then declined, with values recorded at 5.85, 6.70, 7.85, and 6.62 mg GAE/g DW, respectively.
Fig. 1.
Influence of steam explosion time on total phenolic (A1–3), SDG (B1–3), flavonoids (C1–3) and phenolic acids (D1–3) of flaxseed meal. CK means unprocessed flaxseed meal; M1, M2, M3, and M4 mean flaxseed meal treated under steam explosion conditions of 1.0 min + 1.0 MPa, 2.0 min + 1.0 MPa, 3.0 min + 1.0 MPa and 4.0 min + 1.0 MPa respectively. The bars represent the standard deviation of the mean (n ≥ 3), and the mean values of different letters are significantly different (p < 0.05).
According to Fig. 1B1–3, SE notably elevated the levels of free, bound, and total SDG in flaxseed meal. The peak concentrations of free, bound, and total SDG in flaxseed meal occurred at 1.0 MPa for 3 min, reaching 290.76 μg/g DW, 2547.77 μg/g DW, and 2838.54 μg/g DW, respectively. These values were 1.23, 1.36, and 1.35 times greater than the control.
From Fig. 1C1–3, it can be seen that the flavonoids mainly existed in bound state.
in flaxseed meal. A total of 7 free flavonoids were detected, comprising rutin, vitexin, quercetin, naringenin, kaempferol, dihydroquercetin, apigenin, dihydrosanathenol and quercetin. Regarding bound phenolic, only vitexin, dihydroquercetin, apigenin, dihydrosanathenol and quercetin were detected. The primary flavonoids in flaxseed meal were dihydroquercetin, quercetin and vitexin. SE increased the content of dihydroquercetin in flaxseed meal. The total dihydroquercetin concentration rose from 255.35 μg/g dry weight (DW) to 378.81 μg/g DW within 1–4 min, peaking at 431.57 μg/g DW at 3 min under a steam pressure of 1.0 MPa. The quercetin content of flaxseed meal was significantly increased by the SE treatment, and reached a maximum level at 1 MPa,3 min. The free quercetin content of flaxseed meal was 2.59, 4.65, 5.01 and 8.20 times higher than that of the control at 1, 2, 3 and 4 min of 1.0 MPa SE treatment, respectively. Quercetin in flaxseed meal mainly existed in the free state. The total flavonoid content (979.51 μg/g DW) in flaxseed meal was significantly improved and was 2.44 times higher than that of the control under SE conditions of 1.0 MPa,3 min.
As shown in Fig. 1D1–3, a total of seven phenolic acids were detected in flaxseed meal: vanillic acid, caffeic acid syringic acid p-coumaric acid, erucic acid, ferulic acid and protocatechuic acid. Notably, erucic acid and protocatechuic acid were the predominant phenolic acids in flaxseed meal. Compared with the control, the total erucic acid content of flaxseed meal under 1.0 MPa and 1, 2, 3, 4 min was 1.21, 1.54, 1.85 and 1.46 times, respectively. Erucic acid in flaxseed meal mainly existed in the combined state. At the 1.0 MPa, 3 min, the total phenolic acid content amounted to 4035.83 μg/g DW, reflecting an increase to 1.71 times that of the control treatment. The SE treatment significantly enhanced the total phenolic acid content in flaxseed meal, highlighting increases in both p-coumaric acid and ferulic acid.
3.1.2. Effect of SE pressure on phenolics of flaxseed meal
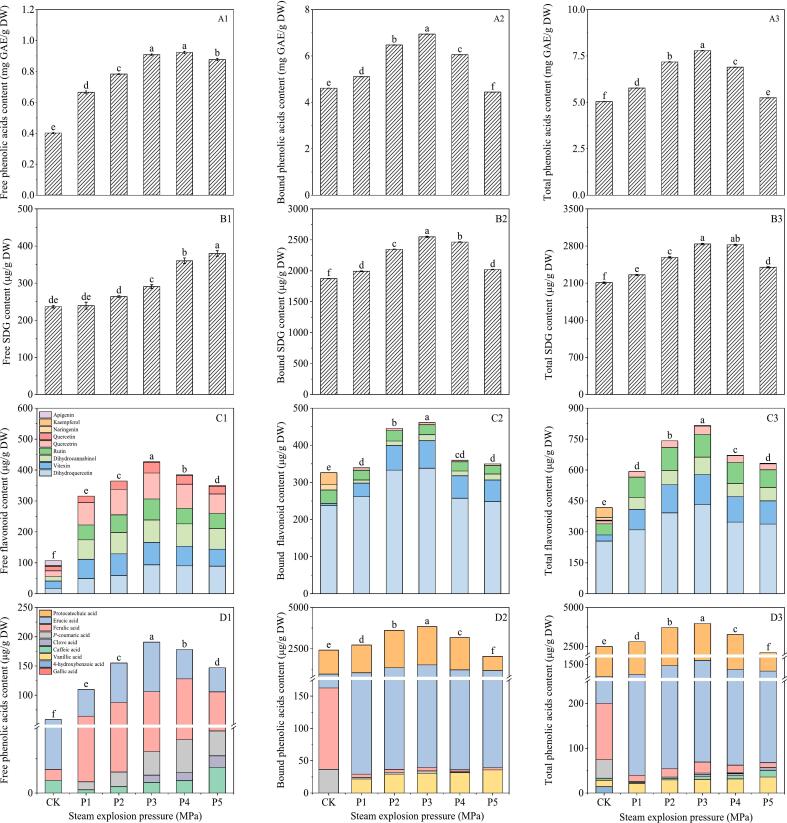
It can be seen from Fig. 2A1–3 that under SE pressure of 0.20–1.80 MPa, the total phenolic content of flaxseed meal initially increased and then decreased. At this condition, the total phenolic content was 0.69, 0.76, 0.91, 0.92 and 0.88 mg GAE/g DW, respectively. Under the treatment condition of SE pressure of 1.0 MPa, the contents of free phenolic and bound phenolic were highest, which were 0.91 mg GAE/g DW and 6.94 mg GAE/g DW, respectively.
Fig. 2.
Influence of steam explosion pressure on total phenolic (A1–3), SDG (B1–3), flavonoids (C1–3) and phenolic acids (D1–3) of flaxseed meal. CK means unprocessed flaxseed meal; M1, M2, M3, and M4 mean flaxseed meal treated under steam explosion conditions of 1 min + 0.2 MPa, 1 min + 0.6 MPa, 1 min + 1.0 MPa and 1.0 min + 1.4 MPa treatment, respectively. The bars represent the standard deviation of the mean (n ≥ 3), and the mean values of different letters are significantly different (p < 0.05).
From Fig. 2B1–3, it can be seen that SE could increase the SDG content in flaxseed meal. The free, bound and total SDG contents in flaxseed meal reached the maximum at 1.0 MPa, 3 min with 290.76 μg/g DW, 2547.77 μg/g DW and 2838.54 μg/g DW, which represent increases of 1.23 1.36 and 1.35 than that of control, respectively.
As can be seen from Fig. 2C1–3, a total of seven flavonoids were detected, namely rutin, vitexin, quercetin, naringenin, dihydroquercetin, dihydrosorcinol and quercetin. The flavonoids mainly existed in the bound state in the flaxseed meal. Under SE pressure of 0.20–1.40 MPa, SE could increase the content of dihydroquercetin in flaxseed meal, and the total dihydroquercetin content increased from 310.03 μg/g DW to 337.88 μg/g DW. SE treatment for 3 min could significantly increase the total flavonoid content in flaxseed meal. Then the total flavonoid content was 979.51 μg/g DW, which was 2.44 times that of the control group. SE effectively disrupted the barriers to extraction inherent in flaxseed meal, enhancing the solvent's contact with active components and facilitating the dissolution of phenolics (Chen et al., 2020). Additionally, the breaking of glycosidic, ester, and ether bonds during SE augmented the availability of carboxyl and phenolic hydroxyl groups, rendering bound phenolics free and subsequently increasing their content (Wan et al., 2022).
From Fig. 2D1–3, it can be seen that a total of seven phenolic acids were detected in flaxseed meal: vanillic acid, caffeic acid syringic acid p-coumaric acid, erucic acid, ferulic acid and protocatechuic acid. Notably, erucic acid and protocatechuic acid emerged as the predominant phenolic acids in the flaxseed meal. The majority of erucic acid exists in a bound state within the meal. In comparison to the control, the total erucic acid content of flaxseed meal subjected to SE treatment (1.0 MPa) for durations of 1, 2, 3, and 4 min recorded values of 1.25, 1.31, 1.14, 0.79, and 0.96 times, respectively. The SE treatment significantly enhanced the total phenolic acid content in flaxseed meal, highlighting increases in both p-coumaric acid and ferulic acid. At the 1.0 MPa, 3 min, the total phenolic acid content amounted to 4035.83 μg/g DW, reflecting an increase to 1.71 times that of the control treatment (Adom & Liu, 2002; Salar et al., 2016).
3.2. Effect of extrusion on phenolics of flaxseed meal
3.2.1. Effect of water content on phenolics of flaxseed meal
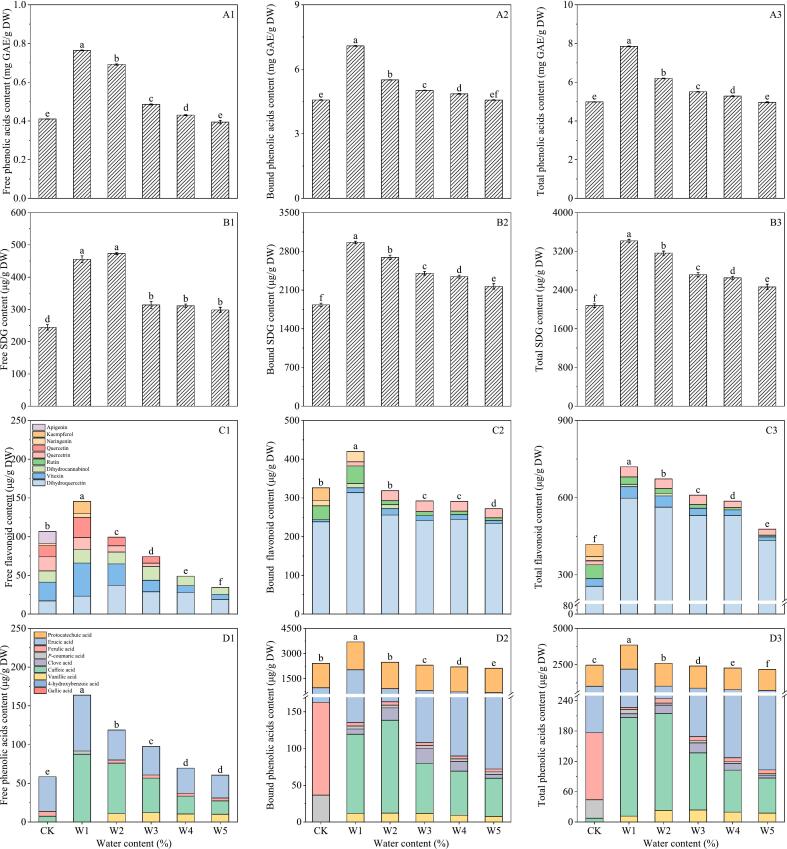
As can be seen from Fig. 3A1–3, the total phenolic content of flaxseed meal under extrusion treatment of 15 %, 20 %, 25 %, 30 % and 35 %, 140 °C and 17 hz was 7.85, 6.19, 5.51, 5.29 and 4.96 mg GAE/g DW, respectively. The variation in content initially decreased, then increased, ultimately peaking at the conditions of 15 %. Furthermore, under the treatment with 15 % water content, the contents of free phenolic and bound phenolic reached their highest, recorded at 0.75 mg GAE/g DW and 7.09 mg GAE/g DW, respectively.
Fig. 3.
Influence of water content on total phenolic (A1–3), SDG (B1–3), flavonoids (C1–3) and phenolic acids (D1–3) of flaxseed meal. CK means unprocessed flaxseed meal; W1, W2, W3, W4 and W5 mean flaxseed meal treated with 15 %, 20 %, 25 %, 30 % and 35 % water content at 140 °C extrusion temperature and 17 hz extrusion speed, respectively. The bars represent the standard deviation of the mean (n ≥ 3), and the mean values of different letters are significantly different (p < 0.05).
As it can be seen from Fig. 3B1–3, the SDG content in flaxseed meal can be increased by extrusion. SDG in flaxseed meal mainly exists in the form of bound state.
When the water content of flaxseed meal was 15 %, the total SDG content reached the highest (3416.95 μg/g DW), which was 1.65 times higher than that of the control.
The analysis presented in Fig. 3C1–3 demonstrates that flavonoids primarily existed in a bound form within flaxseed meal. A total of six flavonoids were detected, dihydroquercetin, vitexin, quercitrin, naringenin, apigenin, dihydrocannabinol and dihydrokaempferol. Extrusion treatment notably augmented the dihydroquercetin content in flaxseed meal, and the total dihydroquercetin content decreased from 313.65 μg/g DW to 233.89 μg/g DW when the water content was increased from 15 % to 35 %, and reached a maximum of 313.65 μg/g DW at the water content of 15 %. Additionally, within the 15–35 % water range, the total flavonoids content of the flaxseed meal showed a decline from 744.06 μg/g DW to 497.46 μg/g DW, indicating a negative correlation between water content and total flavonoid levels. In the SE treatment process, at a pressure of 1.0 MPa for 3 min, the cell volume expanded, the cell wall was damaged, and the release of small and medium-sized molecular substances in the cell was promoted, thus increasing the content of total flavonoids (Adom & Liu, 2002; Salar et al., 2016).
As it can be seen from Fig. 3D1–3, there were 6 phenolic acids detected in flaxseed meal, namely p-coumaric acid, caffeic acid, syringic acid, erucic acid, protocatechuic acid and ferulic acid. Among these, erucic acid and protocatechuic acid represent the predominant phenolic acids in flaxseed meal.
3.2.2. Effect of extrusion temperature on phenolics of flaxseed meal
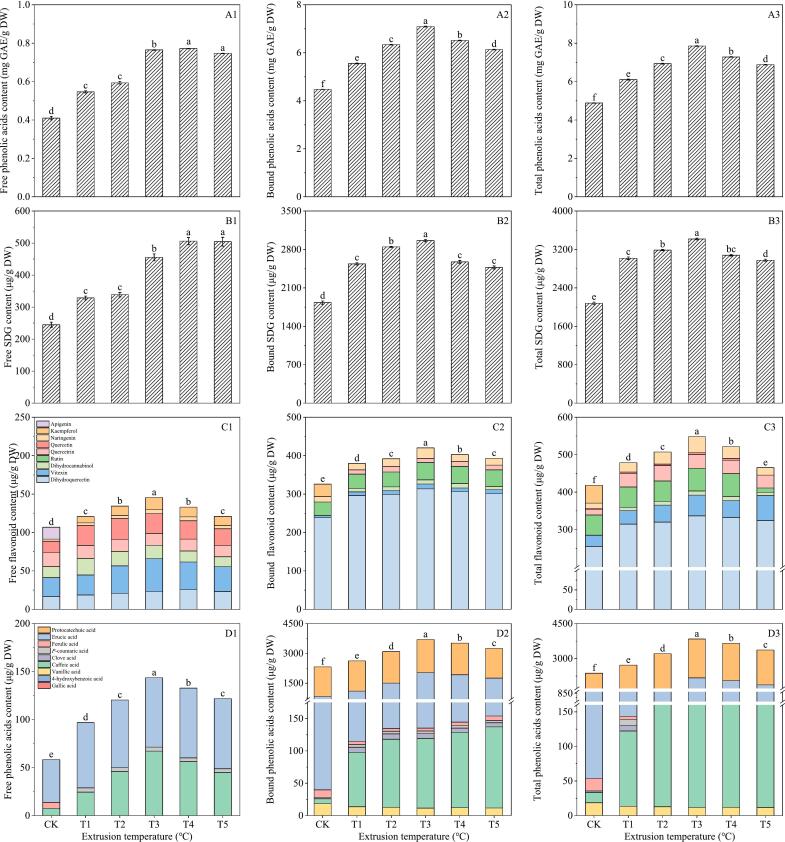
As can be seen from Fig. 4A1–3, when extrusion temperature was 120 °C, 130 °C,
Fig. 4.
Influence of extrusion temperature on total phenolic (A1–3), SDG (B1–3), flavonoids (C1–3) and phenolic acids (D1–3) of flaxseed meal. CK means unprocessed flaxseed meal; T1, T2, T3, T4 and T5 mean flaxseed meal treated with 15 % water content and 17 hz extrusion speed at 120 °C, 130 °C, 140 °C, 150 °C and 160 °C extrusion temperature, respectively. The bars represent the standard deviation of the mean (n ≥ 3), and the mean values of different letters are significantly different (p < 0.05).
140 °C, 150 °C and 160 °C, the total phenolic content of flaxseed meal measured 6.10, 6.93, 7.83, 7.28 and 6.87 mg GAE/g DW, respectively. The content displayed an initial increase followed by a decrease, peaking at 140 °C. At this temperature, the free phenolic content and bound phenolic content achieved their maximum levels at 0.74 mg GAE/g DW and 7.08 mg GAE/g DW, respectively.
From Fig. 4B1–3, it can be seen that with the rising of extrusion temperature, both the bound and total SDG content in flaxseed meal initially rise and then decline. The peak was observed at 140 °C, with values of 455.40 μg/g DW for bound SDG and 2961.55 μg/g DW for total SDG. These figures represent 1.86 and 1.62 times higher than those of of the control, respectively.
The data presented in Fig. 4C1–3 demonstrates that extrusion temperature plays a crucial role in enhancing the flavonoid content in flaxseed meal. Dihydroquercetin emerged as the predominant flavonoid, primarily existing in a bound form. A total of eight flavonoids were detected in flaxseed meal, specifically dihydroquercetin, vitexin, dihydrocannabinol, rutin, quercetin, quercetin, kaempferol and naringenin. The extrusion process, particularly at elevated temperatures, proved advantageous for increasing the flavonoid content. Notably, the concentration of dihydroquercetin in flaxseed meal significantly increased when extruded at temperatures ranging from 120 to 160 °C. At temperatures of 120, 130, 140, and 150 °C, the quercetin conjugates in the 15 %, 17 hz extruded flaxseed meal were found to be 1.25, 1.32, 1.29, and 1.26 times higher than that of the control, respectively.
As shown in Fig. 4D1–3, a total of seven phenolic acids were detected in flaxseed meal: vanillic acid, p-coumaric acid, caffeic acid, syringic acid, erucic acid, protocatechuic acid and ferulic acid. Erucic acid and protocatechuic acid were the major phenolic acids in flaxseed meal.
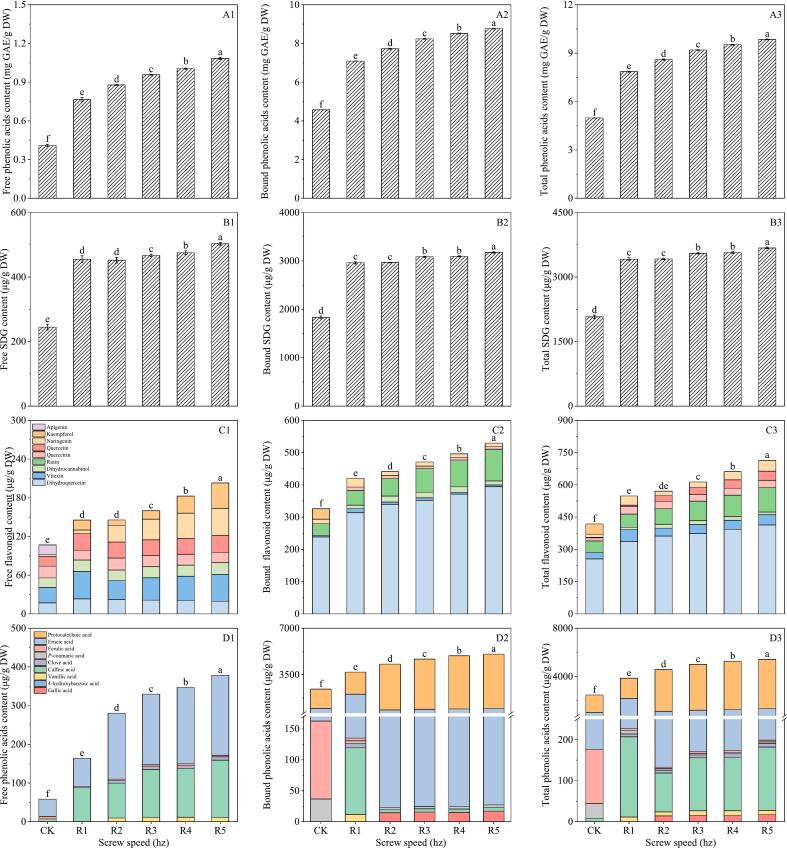
3.2.3. Effect of screw speed on phenolics of flaxseed meal
Fig. 5A1–3 illustrates that the total phenolic content of flaxseed meal measured 7.85, 8.60, 9.20, 9.53, and 9.85 mg GAE/g DW at screw speeds ranging from 17 to 29 hz, with an observable increasing trend. The peak value occurred at 29 hz during screw extrusion treatment. At this screw speed of 29 hz, the concentrations of free phenolic and bound phenolic reached their highest levels, recorded at 1.08 mg GAE/g DW and 8.77 mg GAE/g DW, respectively.
Fig. 5.
Influence of rotation speed on total phenolic (A1–3), SDG (B1–3), flavonoids (C1–3) and phenolic acids (D1–3) of flaxseed meal. CK means unprocessed flaxseed meal; R1, R2, R3, R4 and R5 mean flaxseed meal treated with 15 % water content at 140 °C extrusion temperature with 17 hz, 20 hz, 23 hz, 26 hz and 29 hz extruded speed, respectively. The bars represent the standard deviation of the mean (n ≥ 3), and the mean values of different letters are significantly different (p < 0.05).
Fig. 5B1–3 illustrates that with an increase in screw speed, the levels of free, bound, and total SDG in flaxseed meal exhibited an initial rise, peaking at 29 hz. The maximum concentrations recorded were 503.20 μg/g DW, 3175.35 μg/g DW, and 3678.55 μg/g DW, respectively. These values represent increases of 2.06, 1.73, and 1.77 times compared to the control group.
As shown in Fig. 5C1–3, the screw speed can significantly boost the flavonoid content in flaxseed meal. Dihydroquercetin acts as the main flavonoid in flaxseed meal, primarily existing in a bound form. A total of eight flavonoids were detected in flaxseed meal, including dihydroquercetin, vitexin, dihydrocannabinol, rutin, quercetrin, quercetin, kaempferol and naringenin. The extrusion process showed that higher screw speeds effectively enhanced the flavonoid levels in flaxseed meal. Specifically, the extrusion treatment increased dihydroquercetin content in flaxseed meal at screw speeds of 17, 20, 23, 26 and 29 hz. The bound quercetin content in flaxseed meal was 1.32, 1.08, 1.04, 1.05 and 1.06 times greater than the control for those respective speeds.
It can be seen from the Fig. 5D1–3, caffeic acid and erucic acid were the main phenolic acids in flaxseed meal, and mainly in the bound state. A total of seven phenolic acids were detected in flaxseed meal: gallic acid, 4-hydroxybenzoic acid, vanillic acid, caffeic acid, syringic acid, p-coumaric acid, ferulic acid, erucic acid, protocatechuic acid. The extrusion treatment demonstrated that increasing the screw speed positively impacted the phenolic acid content in flaxseed meal.
3.3. Effect of fermentation treatment on phenolics of flaxseed meal
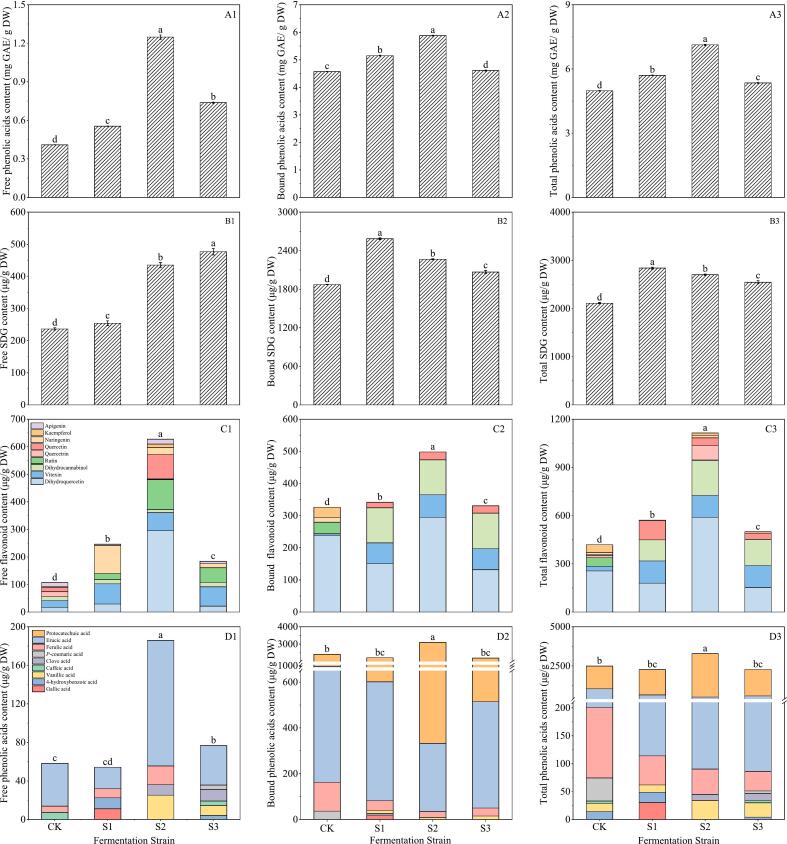
3.3.1. Effect of fermentation strains on phenolics of flaxseed meal
As can be seen in Fig. 6A1–3, among the three treatment groups, the treatment group inoculated with Bacillus subtilis had the highest content of free phenolic, bound phenolic and total phenolic, which were 1.25 mg GAE/g DW, 5.88 mg GAE/g DW and 7.13 mg GAE/g DW, respectively. In contrast, the total phenolic content for Lactobacillus plantarum and Saccharomyces cerevisiae was 5.71 mg GAE/g DW and 5.35 mg GAE/g DW, respectively, showing a trend with an initial decline followed by an increase. Notably, at five days post-inoculation under fermentation conditions with a 3 % inoculum of Bacillus subtilis, the phenolic content reached its peak. All bacterial strains contributed to an increase in the phenolic content of fermented flaxseed meal, with the treatment using Bacillus subtilis achieving a phenolic content 1.42 times greater than that of the control.
Fig. 6.
Influence of fermentation strains on total phenolic (A1–3), SDG (B1–3), flavonoids (C1–3) and phenolic acids (D1–3) of flaxseed meal. CK means unprocessed flaxseed meal; S1, S2 and S3 mean flaxseed meal inoculated with 3 % Saccharomyces cerevisiae, Lactobacillus casei and Bacillus subtilis and fermented at corresponding temperature for 5 days. The bars represent the standard deviation of the mean (n ≥ 3), and the mean values of different letters are significantly different (p < 0.05).
As can be seen from Fig. 6B1–3, after fermentation treatment, the SDG content in flaxseed meal increased significantly, which mainly existed in bound state. After Bacillus subtilis inoculation, the bound and total SDG content in flaxseed meal peaked at 2265.55 μg/g DW and 2700.74 μg/g DW, respectively. These values represent an increase of 1.21 and 1.28 times compared to the control.
As can be seen from Fig. 6C1–3, flavonoids were mainly present in the free state in flaxseed meal. A total of nine free flavonoids were detected dihydroquercetin, vitexin, dihydrocannabinol, rutin, quercetrin, quercetin, naringenin, kaempferol and apigenin. Only dihydroquercetin, vitexin, quercetin, rutin, naringenin, apigenin and dihydrocamptothecin were detected in the bound phenolic. Apigenin and vitexin were the major flavonoids in flaxseed meal. Fermentation could increase the apigenin content in flaxseed meal. Examining possible factors might indicate that the β-glucosidase generated by Bacillus subtilis aids in breaking down β-glucosidic bonds found in phenolic compounds, resulting in a heightened level of apigenin (Adom & Liu, 2002; Salar et al., 2016). After inoculation with Bacillus subtilis, Lactobacillus plantarum and Saccharomyces cerevisiae, the total apigenin content inoculated with Bacillus subtilis increased from 14.64 μg/g DW to 89.24 μg/g DW. Bacillus subtilis could increase the total flavonoids content in flaxseed meal, which elevated the content from 432.84 to 1141.20 μg/g DW.
As illustrated in Fig. 6D1–3, flaxseed meal contains five primary phenolic acids: vanillic acid, ferulic acid, erucic acid, and syringic acid. Among these, erucic acid and vanillic acid stand out as the predominant phenolic acids in flaxseed meal. Upon fermentation, the concentration of protocatechuic acid within flaxseed meal increased, where it existed in a bound form. Following the inoculation with Bacillus subtilis, the total protocatechuic acid content surged from 1453.81 μg/g DW to 2766.15 μg/g DW. In comparison to the control group, the levels of bound phenolic acid and total phenolic acid in flaxseed meal rose significantly after Bacillus subtilis inoculation, measuring 3099.14 μg/g DW and 3285.02 μg/g DW respectively, which represents increases of 1.28 and 1.31 times compared to the control.
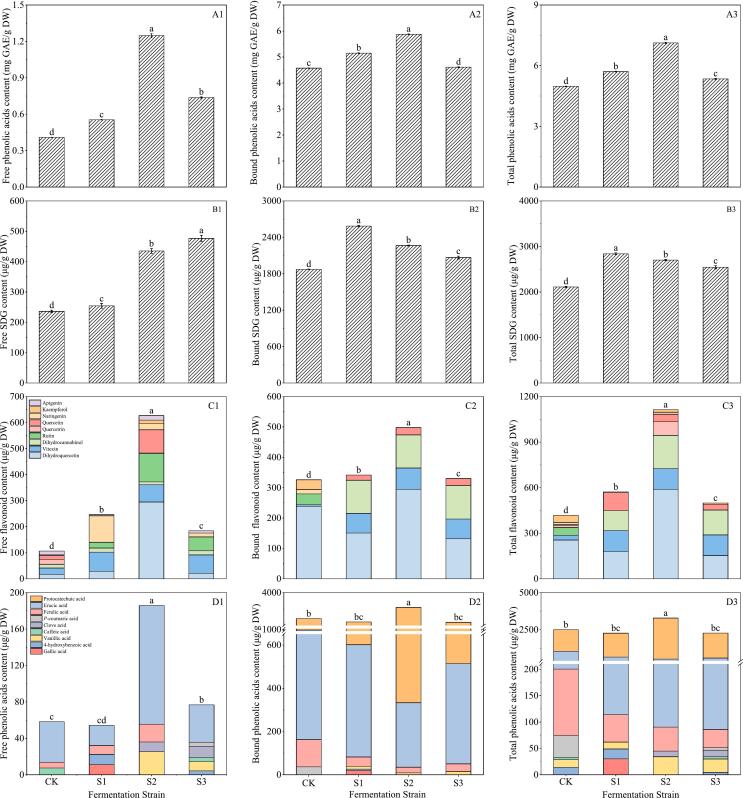
3.3.2. Effect of inoculum level on phenolics of flaxseed meal
From Fig. 7A1–3, it can be seen that inoculation with Bacillus subtilis, after 5 days of fermentation, the highest free phenolic, bound phenolic and total phenolic contents were 1.24 mg GAE/g DW, 6.34 mg GAE/g DW, and 7.59 mg GAE/g DW, respectively, for inoculum levels of up to 3 % under the 1–5 % fermentation treatments. After inoculation with 1 %, 2 %, 3 %, 4 % and 5 % Bacillus subtilis, the total phenolic content of flaxseed meal was 4.92, 5.98, 7.59, 5.86 and 5.08 mg GAE/g DW, respectively.
Fig. 7.
Influence of inoculum amount on total phenolic (A1–3), SDG (B1–3), flavonoids (C1–3) and phenolic acids (D1–3) of flaxseed meal. CK means unprocessed flaxseed meal; I1, I2, I3, I4 and I5 mean flaxseed meal inoculated with 1 %, 2 %, 3 %, 4 % and 5 % Bacillus subtilis, respectively, for 5 days. The bars represent the standard deviation of the mean (n ≥ 3), and the mean values of different letters are significantly different (p < 0.05).
Fig. 7B1–3 illustrates that after fermentation treatment, the SDG content in flaxseed meal initially increased and then decreased as the inoculum amount rose. The SDG primarily existed in a bound state within the flaxseed meal. When the inoculum reached 3 %, the free, bound, and total SDG content peaked at 435.19, 2265.55 μg/g DM, and 2700.74 μg/g DM, respectively. These values represented increases of 1.84, 1.46, and 2.59 times compared to the corresponding control.
The data depicted in Fig. 7C1–3 illustrates that flavonoids predominantly existed in their free form within flaxseed meal. A total of 9 free flavonoids were detected, which included rutin, vitexin, quercetin, naringenin, kaempferol, dihydroquercetin, apigenin, dihydrokaurinol and quercetin. In the bound phenolic, only dihydroquercetin, vitexin, quercetin, rutin and dihydrokaurinol were detected. Dihydroquercetin and vitexin emerged as the principal flavonoids in flaxseed meal, with apigenin following closely. Fermentation could increase the dihydroquercetin content in flaxseed meal, showing a rise from 208.63 μg/g DW to 589.95 μg/g DW when the inoculum concentration increased from 1 % to 5 %. In comparison to the control group, varying inoculum amounts significantly influenced the free apigenin content. The total flavonoid concentration in flaxseed meal, subjected to 1, 2, 3, 4 and 5 % Bacillus subtilis fermentation treatments was 5.29, 5.82, 6.10, 5.08 and 4.88 times higher than that of the control, respectively. The total flavonoid content in flaxseed meal was markedly escalated with the magnify of inoculation rate, peaking at 1141.20 μg/g DW when the inoculation amount was 3 %.
As shown in Fig. 7D1–3, a total of four free phenolic acids were detected in flaxseed meal: caffeic acid, p-coumaric acid, erucic acid and protocatechuic acid. A total of four bound phenolic acids were detected: vanillic acid, ferulic acid, erucic acid and protocatechuic acid. Erucic acid and protocatechuic acid were the main phenolic acids in flaxseed meal. The inoculum amount can increase the content of protocatechuic acid in flaxseed meal. The total protocatechuic acid content increased from 1984.33 μg/g DW to 2766.15 μg/g DW. The total phenolic acid content of flaxseed meal inoculated with 1 %, 2 %, 3 %, 4 % and 5 % Bacillus subtilis fermentation treatments was 0.96, 1.17, 1.32, 1.14 and 0.95 times higher than that of the control, respectively.
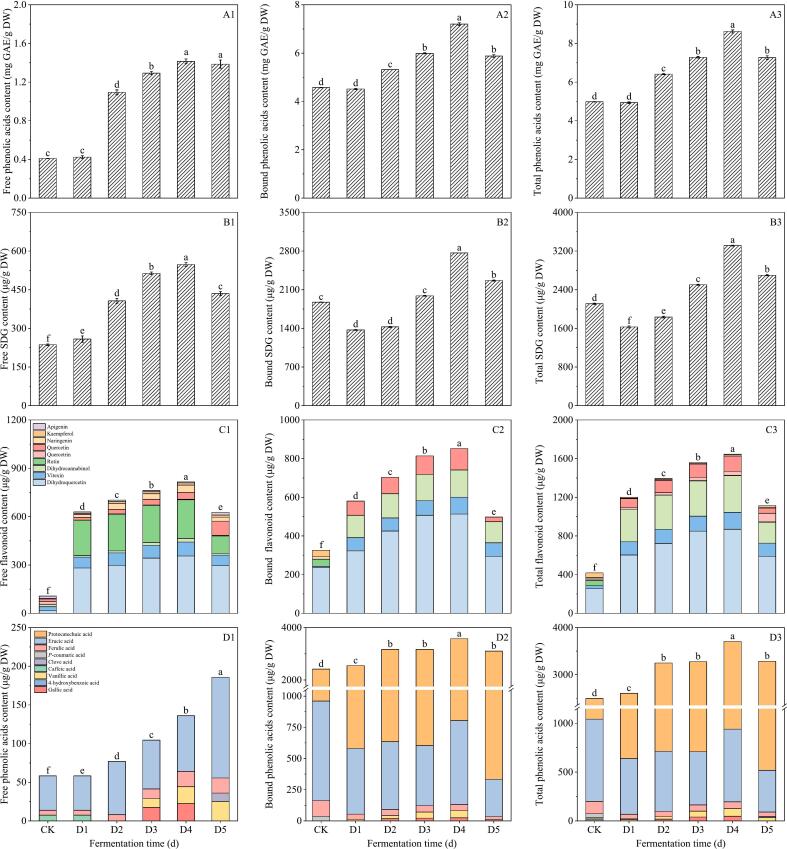
3.3.3. Effect of fermentation time on phenolics of flaxseed meal
It can be seen in Fig. 8A1–3, after inoculation with 3 % of Bacillus subtilis, the content of free phenolic, bound phenolic and total phenolic was the highest when the fermentation time reached 4 days. They were 1.41 mg GAE/g DW, 7.20 mg GAE/g DW and 8.62 mg GAE/g DW respectively. Following 3 % Bacillus subtilis inoculation, the total phenolic content of flaxseed meal varied by fermentation duration, yielding 4.93, 6.41, 7.28, 8.61, and 7.26 mg GAE/g DW after 1, 2, 3, 4, and 5 days, respectively.
Fig. 8.
Influence of fermentation time on total phenolic (A1–3), SDG (B1–3), flavonoids (C1–3) and phenolic acids (D1–3) of flaxseed meal. CK means unprocessed flaxseed meal; D1, D2, D3, D4 and D5 mean flaxseed meal inoculated with 3 % Bacillus subtilis for 1 d, 2 d, 3 d, 4 d and 5 d, respectively. The bars represent the standard deviation of the mean (n ≥ 3), and the mean values of different letters are significantly different (p < 0.05).
From Fig. 8B1–3, it can be seen the SDG content in flaxseed meal initially increased and subsequently decreased with prolonged fermentation time. On 4 d, the free, bound and total SDG content in flaxseed meal attained their peak levels of 547.56 μg/g DW, 2765.55 μg/g DW and 3313.11 μg/g DW, respectively. These values were 2.32, 1.48 and 1.57 times higher than those observed in the control.
From Fig. 8C1–3, it can be seen that flavonoids mainly existed in the free state in flaxseed meal. A total of 9 free flavonoids were detected namely rutin, vitexin, quercetin, naringenin, kaempferol, dihydroquercetin, apigenin, dihydrokaempferol, quercetin. Only dihydroquercetin, vitexin, quercetin, rutin, dihydrosannabinol were detected in the bound phenolic. Dihydroquercetin, argemone and dihydrosorbinol stood out as the predominant flavonoids in flaxseed meal, with quercetin following. Fermentation significantly enhanced the dihydroquercetin content in flaxseed meal, increasing from 603.41 μg/g DW to 868.92 μg/g DW over 1 to 5 days of fermentation. Additionally, fermentation greatly elevated vitexin levels, peaking at 174.66 μg/g DW after 5 days. Compared to the control, varying fermentation durations substantially impacted the concentration of bound dihydrosorbinol. Under a 3 % Bacillus subtilis fermentation treatment, the total flavonoid content of flaxseed meal after 1, 2, 3, 4, and 5 days increased by 2.83, 3.29, 3.68, 3.89, and 2.64 times compared to control, respectively. Extending the fermentation period markedly increased total flavonoid content. Notably, after 4 days of Bacillus subtilis fermentation, the total flavonoid content reached its peak at 1683.08 μg/g DW.
As shown in Fig. 8D1–3, a total of five phenolic acids were detected in flaxseed meal: gallic acid, caffeic acid, p-coumaric acid, erucic acid and protocatechuic acid. Among these, erucic acid and protocatechuic acid were the main phenolic acids in flaxseed meal. The total content of protocatechuic acid increased from 1966.13 μg/g DW to 2765.55 μg/g DW, reaching a maximum value of 2765.55 μg/g DW at 4 d.
4. Discussion
The study assessed the impact of SE, extrusion, and fermentation on the phenolic compounds in cold-pressed flaxseed meal. Results indicated that as SE pressure increased and extraction time extended, phenolic compounds typically rose initially, followed by a decline. The SE showcased a physicochemical synergy, where the formation and degradation of phenolic substances coexisted, necessitating an equilibrium state. Excessively high or prolonged SE pressure could lead to the thermal decomposition of these compounds (Chen et al., 2020; Wan et al., 2022).
Optimal treatment conditions for extracting phenolics from flaxseed meal were detected as 1.0 MPa for 3 min, resulting in the detection of nine free flavonoids and five bound flavonoids (Fig. 1C1–3). Six phenolic acids were detected, with erucic acid and protocatechuic acid being predominant. In the control sample, only four free phenolic acids and six flavonoids were detected (Fig. 1D1–3). Under the treatment conditions, the SE process yielded a phenolic content 1.36 times that of the control, with free phenolic compounds showing a 92.23 % increase compared to the control, aligning with findings by Chen et al. (2020). SE effectively disrupted the barriers to extraction inherent in flaxseed meal, enhancing the solvent's contact with active components and facilitating the dissolution of phenolics (Chen et al., 2020). Additionally, the breaking of glycosidic, ester, and ether bonds during SE augmented the availability of carboxyl and phenolic hydroxyl groups, rendering bound phenolics free and subsequently increasing their content (Wan et al., 2022). At optimal conditions of 1.0 MPa for 3 min, the free, bound, and total SDG contents in flaxseed meal surmounted control levels by factors of 1.26, 1.93, and 1.85, respectively. Similar observations were made by Waszkowiak, Mikołajczak, Gliszczyńska-Świgło, and Niedźwiedzińska (2020). The induction of SE augmented the electron cloud density of the methoxy hydroxyl group in flaxseed lignan, facilitating reactions between phenolic hydroxyl groups and free radicals, thus forming extensive conjugated systems that enhanced the dispersion of free radical electrons. Consequently, flaxseed lignan exhibited notable heat resistance (Noda et al., 2013; Wenk, 2003).
The optimal processing conditions for phenolic compounds in flaxseed meal were detected as follows: water content at 15 %, extrusion temperature at 140 °C, and screw speed at 29 hz. Reducing the water content in flaxseed meal led to the release of bound caffeic acid and syringic acid, which increased by 137.55 and 12.36 μg/g DW, respectively, compared to the control. Additionally, Chen et al. (2019) reported a notable increase in bound phenolic acids during the extrusion process. An adequate water content allows for proper wetting and swelling of the material during extrusion, aiding in the disruption and reorganization of entangled macromolecular structures under applied forces (Chen et al., 2019). At the extrusion temperature of 140 °C, the presence of caffeic acid and syringic acid in flaxseed meal increased to 100.50 μg/g DW and 7.16 μg/g DW, respectively, when compared to the control. Wang et al. (2014) also noted a significant rise in syringic acid in extruded whole grain. The variations in individual phenolic content post-extrusion are attributed to the breakdown of free phenolics and the release of phenolics from soluble conjugated or bound forms, alongside alterations in extractability due to textural changes (Nayak, Liu, Berrios, Tang, & Derito, 2011). When the screw speed reached 29 hz, increased in gallic acid, naringenin, and caffeic acid were observed in flaxseed meal, which were 1.27 and 1.43 times higher than the control, respectively. Sharma et al. (2012) similarly detected a significant increase in gallic acid in extruded wheat bran. Following extrusion, both bound and free phenolic levels in flaxseed meal increased significantly. The mechanical and thermal effects of extrusion facilitate the release of soluble conjugated forms of phenolics, enhancing the free phenolic content (Hu et al., 2018; Zhang, Jin, & Ryu, 2023).
Under the fermentation treatment, the optimal conditions were detected as an inoculation of 3 % Bacillus subtilis and a fermentation period of 4 days. The inoculation with Bacillus subtilis resulted in a decrease in total quercetin content in flaxseed meal by 41.27 μg/g DW (Fig. 6C1–3). Conversely, the content of oysterin and SDG significantly increased by 141.66 μg/g DW (Fig. 6D1–3) and 438.28 μg/g DW (Fig. 6B1–3), respectively. Balli et al. (2020) also observed a substantial rise in oysterin content in fermented millet. Analyzing the underlying causes may reveal a connection to the β-glucosidase produced by Bacillus subtilis. This enzyme facilitates the hydrolysis of β-glucoside bonds within phenolic compounds, leading to an increase in polyphenol concentration (Adom & Liu, 2002; Salar et al., 2016). Following the 3 % inoculation, ferulic acid and syringic acid concentrations rose by 35.52 and 10.80 μg/g DW, respectively, when compared to the control (Fig. 7D1–3). Furthermore, rutin and SDG contents increased by 360.63 μg/g DW (Fig. 7C1–3) and 2752.89 μg/g DW (Fig. 7B1–3), respectively, relative to the control. Katina et al. (2007) noted a significant increase in ferulic acid in fermented rye bran. Variations in inoculum amounts influenced Bacillus subtilis's spore-producing ability. Excessively high inoculum levels heightened strain competition and resulted in greater metabolism of waste products (Balli et al., 2019). Fermentation liberated the bound phenolic compounds from the structure of polymerized flaxseed meal and enhanced the concentration of free phenolics (Paesani, Bravo-Núñez, & Gómez, 2021). After 4 days of fermentation, apigenin and gallic acid emerged in the flaxseed meal compared to the control, increasing by 26.58 (Fig. 8C1–3) and 25.15 μg/g DW (Fig. 8D1–3) respectively and erucic acid rose by 36.42 and 378.87 μg/g DW respectively (Fig. 8D1–3). At this stage, the total SDG content compared to the control rose 1.68 times (Fig. 8B1–3). The analysis indicated that Bacillus subtilis hydrolyzed several flavonoids, leading to an increase in gallic acid and naringin as hydrolysis progressed. This was consistent with the study by Yang et al. (2018). Research has demonstrated that during the course of fermentation, hydroxycinnamate undergoes hydrolysis by esterase to yield hydroxycinnamic acids, including caffeic acid, p-coumaric acid, and ferulic acid (Paesani et al., 2021). Caffeic acid subsequently decarboxylates under the influence of phenolic acid decarboxylase, while ferulic acid generates novel compounds. Additionally, both caffeic acid and protocatechuic acid are metabolized into dihydrocaffeic acid and catechuic acid (Adom & Liu, 2002; Salar et al., 2016).
5. Conclusion
The treatment of SE, extrusion, and fermentation improved the content and composition of phenolics in flaxseed meal to some extent. Results indicated that the optimal condition for SE was 1.0 MPa for 3 min, yielding seven varieties of phenolic acids and seven variants of flavonoids. Under this condition, the total phenolic content was 1.36 times higher than the control, while the total SDG content was 1.85 times greater than the control. The total flavonoid content and phenolic acid content stood at 1.71 times and 2.44 times that of control, respectively. The optimal extrusion parameters consist of a water level of 15 %, an extrusion temperature of 140 °C, and a screw speed of 29 hz, which enabled the detection of eight flavonoids and seven phenolic acids. Under this condition, the content of total phenolic, SDG, flavonoid and phenolic acid were 1.97, 1.77, 1.71, and 2.19 times higher than that of the control, respectively. The best fermentation conditions involved inoculating with 3 % Bacillus subtilis and fermenting for 4 days, during which nine types of flavonoids and five types of phenolic acids were detected. In this scenario, the total phenolic, the total SDG, the total flavonoid, and total phenolic acid contents are 1.97, 1.57, 3.89, and 1.49 times greater than the control, respectively. Each treatment method has its advantages and limitations, so it needs to be considered comprehensively in practical applications. These findings provide a scientific basis for the development of functional food from flaxseed meal.
CRediT authorship contribution statement
Lin Cheng: Writing – original draft, Validation, Data curation. Xiaoyong Liu: Supervision, Conceptualization. Yan Ma: Writing – review & editing, Funding acquisition. Xianqing Huang: Supervision, Funding acquisition. Xinru Zhang: Writing – review & editing, Formal analysis. Jinrui Liu: Writing – review & editing, Formal analysis. Lianjun Song: Project administration. Mingwu Qiao: Project administration. Tiange Li: Methodology, Data curation. Tianlin Wang: Methodology, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Financial support was provided by the Henan Province key research and development program (No. 241111110600), 2024 Henan Agricultural University College Students Innovation and Entrepreneurship Training Program Project (No. 105), the Special Support Fund for High-level Talents of Henan Agricultural University (No. 30501350), the Postgraduate Joint Training Base of Henan Province (No. YJS2022JD16), and the Innovative Research Team (in Science and Technology) in University of Henan Province (No. 23IRTSTHN023).
Data availability
Data will be made available on request.
References
- Adom K.K., Liu R.H. Antioxidant activity of grains. Journal of Agricultural and Food Chemistry. 2002;50:6182–6187. doi: 10.1021/jf0205099. [DOI] [PubMed] [Google Scholar]
- Balli D., Bellumori M., Paoli P., Pieraccini G., Di Paola M., De Filippo C., Innocenti, M. Study on a fermented whole wheat: Phenolic content, activity on PTP1B enzyme and in vitro prebiotic properties. Molecules. 2019;24:1120. doi: 10.3390/molecules24061120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balli D., Bellumori M., Pucci L., Gabriele M., Longo V., Paoli P., Innocenti, M. Does fermentation really increase the phenolic content in cereals? A study on millet. Foods. 2020;9:303. doi: 10.3390/foods9030303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan C., Brennan M., Derbyshire E., Tiwari B.K. Effects of extrusion on the polyphenols, vitamins and antioxidant activity of foods. Trends in Food Science &Technology. 2011;22:570–575. doi: 10.1016/j.tifs.2011.05.007. [DOI] [Google Scholar]
- Chen G., Chen H. Extraction and deglycosylation of flavonoids from sumac fruits using steam explosion. Food Chemistry. 2011;126:1934–1938. doi: 10.1016/j.foodchem.2010.12.025. [DOI] [PubMed] [Google Scholar]
- Chen Y., Ma Y., Dong L., Jia X., Liu L., Huang F.M.…Zhang R. Extrusionand fungal fermentation change the profile and antioxidant activity of free and bound phenolics in rice bran together with the phenolic bioaccessibility. LWT-Food Science and Technology. 2019;115 doi: 10.1016/j.lwt.2019.108461. [DOI] [Google Scholar]
- Chen Y., Shan S., Cao D., Tang D. Steam flash explosion pretreatment enhances soybean seed coat phenolic profiles and antioxidant activity. Food Chemistry. 2020;319 doi: 10.1016/j.foodchem.2020.126552. [DOI] [PubMed] [Google Scholar]
- Dalbhagat C.G., Mahato D.K., Mishra H.N. Effect of extrusion processing on physicochemical, functional and nutritional characteristics of rice and rice-based products: A review. Trends in Food Science & Technology. 2019;85:226–240. doi: 10.1016/j.tifs.2019.01.001. [DOI] [Google Scholar]
- Doyen A., Udenigwe C.C., Mitchell P.L., Marette A., Aluko R.E., Bazinet L. Anti-diabetic and antihypertensive activities of two flaxseed protein hydrolysatefractions revealedfollowing their simultaneous separation by electrodialysis with ultrafiltration membranes. Food Chemistry. 2014;145:66–76. doi: 10.1016/j.foodchem.2013.07.108. [DOI] [PubMed] [Google Scholar]
- Hole A.S., Kjos N.P., Grimmer S., Kohler A., Lea P., Rasmussen B., Sahlstrøm S. Extrusion ofbarley and oat improves the bioaccessibility of dietary phenolic acids in growing pigs. Journal of Agricultural and Food Chemistry. 2013;61:2739–2747. doi: 10.1021/jf3045236. [DOI] [PubMed] [Google Scholar]
- Hu Z., Tang X., Zhang M., Hu X., Yu C., Zhu Z., Shao Y. Effects of different extrusion temperatures on extrusion behavior, phenolic acids, antioxidant activity, anthocyanins and phytosterols of black rice. RSC Advances. 2018;8:7123–7132. doi: 10.1039/C7RA13329D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Tse T.J., Purdy S.K., Chicilo F., Shen J., Meda V., Reaney M. Depletionof cyanogenic glycosides in whole flaxseed via. Lactobacillaceae fermentation.FoodChemistry. 2023;403 doi: 10.1016/j.foodchem.2022.134441. [DOI] [PubMed] [Google Scholar]
- Hur S.J., Lee S.Y., Kim Y.-C., Choi I., Kim G.-B. Effect of fermentation onthe antioxidant activity in plant-based foods. Food Chemistry. 2014;160:346–356. doi: 10.1016/j.foodchem.2014.03.112. [DOI] [PubMed] [Google Scholar]
- Imran M., Anjum F.M., Butt M.S., Siddiq M., Sheikh M.A. Reduction of cyanogenic compounds in flaxseed (Linum usitatissimum L.) meal using thermal treatment. International Journal of Food Properties. 2013;16:1809–1818. doi: 10.1080/10942912.2011.608914. [DOI] [Google Scholar]
- Katina K., Laitila A., Juvonen R., Liukkonen K.-H., Kariluoto S., Piironen V., Poutanen K. Bran fermentation as a means to enhance technological propertiesandbioactivity of rye. Food Microbiology. 2007;24:175–186. doi: 10.1016/j.fm.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Manthey F.A., Sinha S., Wolf-Hall C.E., Hall Iii C.A. Effect of flaxseed flour and packaging on shelf life of refrigerated pasta. Journal of Food Processingand Preservation. 2008;32:75–87. doi: 10.1111/j.1745-4549.2007.00166.x. [DOI] [Google Scholar]
- Mechchate H., Es-safi I., Conte R., Hano C., Amaghnouje A., Jawhari F.Z., Bousta D. In vivo and in vitro antidiabetic and anti-inflammatory properties of flax (Linum usitatissimum L.) seed polyphenols. Nutrients. 2021;13:2759. doi: 10.3390/nu13082759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minevich I., Goncharova A., Zaitseva L. Influence of extrusion on the feed value of flax seeds. Agrarian science. 2021;57-61 doi: 10.32634/0869-8155-2021-352-9-57-61. [DOI] [Google Scholar]
- Nayak B., Liu R.H., Berrios J.D.J., Tang J., Derito C. Bioactivity of antioxidants in extruded products prepared from purple potato and dry pea flours. Journal of Agricultural and Food Chemistry. 2011;59:8233–8243. doi: 10.1021/jf200732p. [DOI] [PubMed] [Google Scholar]
- Noda Y., Asada C., Sasaki C., Hashimoto S., Nakamura Y. Extraction method for increasing antioxidant activity of raw garlic using steam explosion. Biochemical Engineering Journal. 2013;73:1–4. doi: 10.1016/j.bej.2013.01.013. [DOI] [Google Scholar]
- Paesani C., Bravo-Núñez Á., Gómez M. Effect of stabilized wholegrain maizeflours on the quality characteristics of gluten-free layer cakes. LWT-Food Scienceand Technology. 2021;135 doi: 10.1016/j.lwt.2020.109959. [DOI] [Google Scholar]
- Paucar-Menacho L.M., Castillo-Martínez W.E., Simpalo-Lopez W.D., Verona-Ruiz A., Lavado-Cruz A., Martínez-Villaluenga C. Performance of thermoplastic extrusion, germination, fermentation, and hydrolysis techniques on phenolic compoundsin cereals and pseudocereals. Foods. 2022;11:1957. doi: 10.3390/foods11131957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-García J.M., Lama-Muñoz A., Rodríguez-Gutiérrez G., Moya M., Ruiz E., Fernández-Bolaños J., Castro E. Obtaining sugars and natural antioxidants from olive leaves by steam-explosion. Food Chemistry. 2016;210:457–465. doi: 10.1016/j.foodchem.2016.05.003. [DOI] [PubMed] [Google Scholar]
- Salar R.K., Purewal S.S., Bhatti M.S. Optimization of extraction conditions and enhancement of phenolic content and antioxidant activity of pearl millet fermented with aspergillus awamori MTCC-548. Resource-Efficient Technologies. 2016;2:148–157. doi: 10.1016/j.reffit.2016.08.002. [DOI] [Google Scholar]
- Sęczyk Ł., Świeca M., Dziki D., Anders A., Gawlik-Dziki U. Antioxidant, nutritional and functional characteristics of wheat bread enriched with ground flaxseedhulls. Food Chemistry. 2017;214:32–38. doi: 10.1016/j.foodchem.2016.07.068. [DOI] [PubMed] [Google Scholar]
- Sharma P., Gujral H.S., Singh B. Antioxidant activity of barley as affected by extrusion cooking. Food Chemistry. 2012;131:1406–1413. doi: 10.1016/j.foodchem.2011.10.009. [DOI] [Google Scholar]
- Song G., Liu J., Shui R., Sun J., Weng Q., Qiu S., Zhang F. Effect of steam explosion pretreatment on the composition and bioactive characteristic of phenolic compounds in chrysanthemum morifolium Ramat cv. Hangbaiju powder with various sievefractions. Food Science & Nutrition. 2022;10:1888–1898. doi: 10.1002/fsn3.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan F., Feng C., Luo K., Cui W., Xia Z., Cheng A. Effect of steam explosion on phenolics and antioxidant activity in plants: A review. Trends in Food Science & Technology. 2022;124:13–24. doi: 10.1016/j.tifs.2022.04.003. [DOI] [Google Scholar]
- Wang T., He F., Chen G. Improving bioaccessibility and bioavailability of phenolic compounds in cereal grains through processing technologies: A concise review. Journal of Functional Foods. 2014;7:101–111. doi: 10.1016/j.jff.2014.01.033. [DOI] [Google Scholar]
- Waszkowiak K., Mikołajczak B., Gliszczyńska-Świgło A., Niedźwiedzińska K. Effect of thermal pre-treatment on the phenolic and protein profiles and oil oxidation dynamics of golden flaxseeds. International Journal of Food Science & Technology. 2020;55:1272–1280. doi: 10.1111/ijfs.14393. [DOI] [Google Scholar]
- Wenk C. Herbs and botanicals as feed additives in monogastric animals. Animal Bioscience. 2003;16(2):282–289. doi: 10.5713/ajas.2003.282. [DOI] [Google Scholar]
- Wu S., Wang X., Qi W., Guo Q. Bioactive protein/peptides of flaxseed: A review. Trends in Food Science & Technology. 2019;92:184–193. doi: 10.1016/j.tifs.2019.08.017. [DOI] [Google Scholar]
- Yang L., Wang W., Zhai S., Ye H., Zhu Y., Li M. PSIII-34 comparison of fermentedand unfermented flax seed cake on the nutrient values and the utilization inducks. Journal of Animal Science. 2018;96:313. doi: 10.1093/jas/sky404.688. [DOI] [Google Scholar]
- Zhang Y., Jin T., Ryu G.-H. Physicochemical and antioxidant properties of extruded rhodiola as affected by twin-screw extrusion. International Journal of Food Properties. 2023;26:614–627. doi: 10.1080/10942912.2023.2174699. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.