Abstract
Nanostructured materials with antibacterial activity face the same threat as conventional antibiotics - bacterial resistance, which reduces their effectiveness. However, unlike antibiotics, research into the emergence and mechanisms of bacterial resistance to antibacterial nanomaterials is still in its early stages. Here we show how Gram-positive Staphylococcus aureus and Gram-negative Escherichia coli bacteria develop resistance to silver nanoparticles, resulting in an increase in the minimum inhibitory concentration from 1.69 mg/L for S. aureus and 3.38 mg/L for E. coli to 54 mg/L with repeated exposure over 12 and 6 cultivation steps, respectively. The mechanism of resistance is the same for both types of bacteria and involves the aggregation of silver nanoparticles leading to the formation of black precipitates. However, the way in which Gram-positive and Gram-negative bacteria induce aggregation of silver nanoparticles is completely different. Chemical analysis of the surface of the silver precipitates shows that aggregation is triggered by flagellin production in E. coli and by bacterial biofilm formation in S. aureus. However, resistance in both types of bacteria can be overcome by using pomegranate rind extract, which inhibits both flagellin and biofilm production, or by stabilizing the silver nanoparticles by covalently binding them to a composite material containing graphene sheets, which protects the silver nanoparticles from aggregation induced by the bacterial biofilm produced by S. aureus. This research improves the understanding of bacterial resistance mechanisms to nanostructured materials, which differ from resistance mechanisms to conventional antibiotics, and provides potential strategies to combat bacterial resistance and develop more effective antimicrobial treatments.
Subject terms: Antimicrobial resistance, Biomedical materials
Bacteria resist AgNPs by aggregating them through the flagellin production in E. coli or biofilm formation in S. aureus. Protection from AgNPs aggregation by pomegranate rind extract or strong Ag binding to graphene sheets can overcome the resistance.
Introduction
Today, modern medicine faces a significant threat from the loss of antibiotic effectiveness against bacteria, limiting our ability to treat bacterial infections. According to a statement by the UN General Assembly in September 2016, if bacterial resistance continues to increase at its current rate, it is estimated that untreatable infections caused by multidrug-resistant bacteria will become the leading cause of death by 20501. As more and more bacterial infections fail to respond to conventional antibiotic treatment, new ways to overcome bacterial resistance must be developed. One potential approach to combat bacterial resistance is to restore the antibacterial efficacy of antibiotics by combining them with novel nanostructured antibacterial substances or by using antibacterial nanomaterials themselves. Metal or non-metal NPs such as silver, gold, copper, selenium, and metal oxide NPs such as ZnO, TiO2, CaO, and MgO nanoparticles, often referred to as nano-antibiotics, have emerged as promising antimicrobial agents for the treatment and prevention of infectious diseases, demonstrating efficacy against antibiotic-resistant bacteria2–5. Most of these nanostructured materials, regardless of their chemical composition, are able to fight bacteria through non-specific activity by various mechanisms. Common mechanisms include cell wall disruption, cytoplasmic membrane damage, and the production of reactive oxygen species (ROS) leading to oxidative stress. In addition, these materials can inhibit enzymes, alter gene expression and, to a lesser extent, inactivate proteins. Such a multi-level mode of action makes it much more difficult to develop bacterial resistance to nanoparticles, but this does not mean that it cannot be overcome6,7.
It is important to emphasize that the development and spread of bacterial resistance is an inherent and natural process that cannot be completely prevented. Many resistance mechanisms in bacteria developed long before the first modern antibacterial agents were used for treatment. Resistance mechanisms do not usually emerge randomly and suddenly but wait for conditions that allow them to succeed in the bacterial population. Therefore, due to the constant genomic changes and the ability of bacteria to adapt to adverse conditions, it is foreseeable and inevitable that bacteria will also develop countermeasures against the antibacterial effects of metal and metal oxide NPs. In the case of non-specific action of NPs, a non-specific mechanism of bacterial resistance can be expected. Indeed, recent publications have demonstrated the ease with which bacteria can develop resistance to silver NPs (AgNPs) through relatively simple genomic changes, often associated with bacterial efflux8–11, adaptation to reactive oxygen species12 or through simple phenotypic adaptation (without the need to alter the genetic information at all), such as the production of extracellular substances10,13,14, phenazine pigments15 or through the production of flagellin16, which triggers the aggregation and destabilization of AgNPs. Notably, resistance to silver nanoparticles has been observed predominantly in Gram-negative strains (mostly in Escherichia coli, Pseudomonas aeruginosa), with fewer studies focusing on Gram-positive strains17–19. In the case of Gram-positive strains, bacterial Ag+ efflux has been described as the main mechanism of action12,19, but only Valentin et al. induced bacterial resistance, and among the increase in efflux, other mechanisms mentioned were related to genetic changes involved in nucleotide acid synthesis and defense against oxidative stress and changes in cysteine metabolism17.
In this study, we intentionally induced bacterial resistance by exposing Gram-positive Staphylococcus aureus strains to sub-inhibitory concentrations of AgNPs and compared the resistance mechanisms with those observed in Gram-negative E. coli reported by Panacek et al.16. The mechanism by which Gram-positive and Gram-negative bacteria resist the potent antibacterial effect of AgNPs is identical and lies in the aggregation and colloidal destabilization of AgNPs, resulting in the loss of their potent antibacterial effect. However, the pathways by which Gram-positive and Gram-negative bacteria induce particle aggregation and destabilization of AgNPs are unique and completely different. While Gram-negative bacteria utilize flagellin to aggregate AgNPs, Gram-positive bacteria lacking flagellin induce aggregation of AgNPs through excessive production of bacterial biofilm - two different pathways in two different types of bacteria leading to identical aggregation of AgNPs and subsequent elimination of their antibacterial effect.
Results and discussion
Antibacterial silver NPs versus adaptable and resistant bacteria
To investigate bacterial resistance to AgNPs, the same modified Tollens process20 was used to synthesize silver NPs with a diameter of 28 nm and is consistent with the methodology used in previous studies reporting resistance to silver NPs in E. coli16. The morphology and size of the silver NPs were validated by transmission electron microscopy (Supplementary Fig. 1Sa) and absorption spectroscopy, which revealed a typical narrow surface plasmon absorption peak at 407 nm (Supplementary Fig. 1Sb). A zeta potential of −35.2 mV indicates robust colloidal stability, which is essential to mitigate aggregation and maintain consistent nanoparticle performance throughout the experiments. Additional characterizations such as SEM, EDS, and XRD analysis of the AgNPs are shown and reported in the supplementary material (Supplementary Figs. 2S and 3S). The synthesized silver NPs exhibited potent antibacterial activity, exhibiting low minimum inhibitory concentrations (MICs) ranging from 1.69 to 13.5 mg/L depending on the type of bacteria (Table 1). However, repeated and prolonged exposure to subinhibitory concentrations of silver led to the development of resistance in bacteria, resulting in a gradual increase in the MICs of silver NPs over time (Table 1). Following the protocol outlined in the work of Panacek et al.16 (20 successive bacterial cultivations) allowed a direct comparison of our results for Gram-positive bacteria with those previously reported for Gram-negative bacteria.
Table 1.
Minimum inhibitory concentrations (MICs) of silver NPs against induced bacteria in each culture step compared with MICs of silver NPs against reference strains after the first, tenth, and twentieth steps
| Bacteria | 1a | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 10a | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 20a |
| S. aureus CCM 3953 | 13.5 | 13.5 | 6.75 | 13.5 | 6.75 | 13.5 | 6.75 | 6.75 | 13.5 | 13.5 | 13.5 | 27 | >54 | 27 | >54 | >54 | >54 | 27 | 27 | >54 | >54 | 13.5 |
| S. aureus 008 | 3.38 | 6.75 | 1.69 | 3.38 | 1.69 | 1.69 | 1.69 | 1.69 | 3.38 | 6.75 | 1.69 | 3.38 | 6.75 | 13.5 | 6.75 | 13.5 | 13.5 | 27 | >54 | >54 | >54 | 3.38 |
| E. coli CCM 395416 | 3.38 | 6.75 | 3.38 | 6.75 | 6.75 | 13.5 | 13.5 | >54 | >54 | >54 | 3.38 | >54 | >54 | >54 | >54 | >54 | >54 | >54 | >54 | >54 | >54 | 6.75 |
aMICs of reference (non-induced) strains—used for comparison MICs of silver NPs between induced and non-induced bacteria after the first, tenth, and twentieth step.
The similarity between Gram-negative and Gram-positive bacteria in the mechanism of bacterial resistance to silver NPs
The rate at which resistance developed in Gram-positive bacteria was slightly slower and occurred at later culture steps compared to Gram-negative bacteria. S. aureus CCM 3953 and S. aureus 008 showed resistance to silver NPs from the twelfth and thirteenth culture steps, respectively. The MIC of silver NPs against S. aureus CCM 3953 increased from 13.5 to 54 mg/L in the 12th step. Similarly, S. aureus 008, which initially had a lower MIC of 3.38 mg/L, gradually became more resistant between the 13th and 18th culture steps, while reaching a MIC of 54 mg/L in the 18th step. Conversely, bacterial resistance to AgNPs in E. coli developed more rapidly and appeared as early as the sixth culture step with a MIC value of 13.5 mg/L, as was published by Panacek et al.16. However, from the eighth culture step, the MICs against E. coli increased up to 54 mg/L and from 9th to 20th culture step even above 54 mg/L, where it remained constant and did not decrease (Table 1).
The gradual development of bacterial resistance to silver NPs, as evidenced by increasing MICs, was also accompanied by progressive precipitation of silver NPs at the bottom of microplate wells, mirroring the observations made for Gram-negative bacteria. In the presence of resistant bacterial strains, the dispersion of silver NPs changed color from yellow to gray-black due to the formation of a precipitate at the bottom of the well (Fig. 1d). Conversely, in the presence of susceptible bacterial strains, the dispersion of silver NPs diluted in Mueller–Hinton broth retained the yellow color after 8 and 24 h of cultivation, as indicated by the characteristic surface plasmon band (Fig. 1a, c, g). This band, which is typical for stable silver NPs with sizes ranging from a few to several tens of nanometers, exerts high antibacterial efficacy. However, in the presence of silver-resistant S. aureus, the characteristic surface plasmon band gradually diminishes after 8 and 24 h of cultivation, indicating the aggregation of silver NPs (Fig. 1g).
Fig. 1. AgNPs aggregation and absorption in cultures with susceptible and resistant bacteria.
Microplates before (a, b) and after (c, d) cultivation with susceptible (a, c) and resistant (b, d) S. aureus 008 with silver NPs geometrically diluted in Mueller–Hinton broth to concentrations ranging from 54 mg/L to 0.42 mg/L. TEM images of non-aggregated (e) and aggregated (f) silver NPs forming large aggregates in the wells after 24-hour culture with resistant S. aureus 008. Absorption spectra (g) of silver NPs diluted in Mueller–Hinton broth at a ratio of 1:1 before (black) and after (red, blue, green) culture of susceptible (blue) and resistant (red, green) S. aureus 008.
The aggregation stability was further confirmed by transmission electron microscopy images of silver NPs after cultivation with susceptible bacteria (Fig. 1e). The black precipitate formed after cultivation of resistant bacteria consisted of large aggregates of silver NPs with sizes on the order of hundreds of nanometers (Fig. 1f), which do not show such high antibacterial activity as the originally small silver NPs. Based on these findings, together with the results published by Panacek et al.16, it is evident that both S. aureus and E. coli have developed the ability to induce aggregation instability upon repeated exposure to AgNPs, resulting in the elimination of their antibacterial effects.
Same mechanism of resistance involving particle aggregation, but what triggers it in Gram-positive bacteria?
The results obtained clearly show that the development and mechanism of bacterial resistance to silver NPs are identical for both groups of Gram-negative and Gram-positive bacteria, centered around the aggregation of silver nanoparticles followed by the loss of their antibacterial activity at low concentrations around units of ppm. So far, there is no apparent difference between Gram-positive and Gram-negative bacteria and their resistance mechanism to silver NPs. However, Gram-negative bacteria induce the aggregation of silver NPs in a way (flagellin production) that is not possible in Gram-positive bacteria. Gram-positive bacteria do not have flagella, so they cannot produce flagellin protein as they do in the case of Gram-negative bacteria do. Therefore, flagellin-induced aggregation of silver NPs in Gram-negative bacteria is not the cause of particle aggregation and bacterial resistance in the case of Gram-positive bacteria. The course of bacterial resistance development and its mechanism is identical in Gram-positive and Gram-negative bacteria, but interestingly, this common resistance mechanism is initiated in two completely different ways that are particularly characteristic of both groups of bacteria. Gram-positive and Gram-negative bacteria have found the same weak point of the silver nanoparticles in the colloidal dispersion—their aggregation stability, but they use two different ways how to break it. From now on, a new and very interesting story is being written describing the development of bacterial resistance to AgNPs in the case of Gram-positive bacteria.
Biofilm formation as a cause of silver NPs aggregation
Biofilm is one of the strategies used by bacteria to resist the antibacterial effects of antimicrobial agents, including AgNPs9,14,21–23. Bacteria are known to prefer to live in colonies and form biofilms rather than live individually in their planktonic state. These bacterial communities cause 60–80% of all bacterial infections and further complicate bacterial resistance24. Biofilm is formed by irreversibly binding planktonic bacteria to any surface, maturing, dispersing around the site, and producing an exopolymer matrix. As a result, biofilm acts as a shield against the host immune system and prevents the diffusion of the antimicrobials on the bacterial surface25–27. In biofilms, bacteria produce extracellular polymeric substances (EPS) that form a protective matrix that can facilitate the aggregation of AgNPs within the biofilm28,29. Aggregation occurs because the chemical environment within the biofilm, altered by bacterial metabolic activities and EPS production, affects the stability of colloidal AgNPs, which are very sensitive to chemical changes in the environment30. As a result, the nanoparticles aggregate, reducing their effective concentration, minimizing their direct interaction with bacterial cell membranes, and decreasing their antimicrobial activity31. Staphylococci are known for their ability to form biofilm and therefore, suggesting that increased biofilm formation resulting from enhanced bacterial resistance could be a viable mechanism of bacterial resistance to silver nanoparticles13,31. Therefore, to investigate this hypothesis, the amount of biofilm formed was analyzed and quantified by several methods including SEM and Christensen method32. In our work, the Christensen method was used to quantify and compare the amount of biofilm produced and deposited on the bottom of plate wells by S. aureus 008 sensitive and resistant to silver NPs. Higher biofilm production by silver-resistant S. aureus compared to silver-sensitive strain was confirmed by measuring the optical density measurement of biofilm stained with CV (Fig. 2a) produced by S. aureus exposed to different concentrations of silver NPs. The resistant strain shows a significant increase in bacterial biofilm production at all concentrations of AgNPs up to a threshold of 13.5 mg/L, beyond which a significant decrease in biofilm production of the resistant strain is observed. It appears that the Christensen method may not provide the most accurate assessment for the higher concentrations of AgNPs. This limitation is due to the method’s primary focus on quantifying the biofilm adhering to the bottom of the wells, rather than specifically targeting biofilm formation on the surface of the AgNPs themselves. As the concentration of AgNPs increases, so does the surface area available for biofilm adhesion. However, this increased biofilm formation on the surface of AgNPs cannot be detected on the plate walls using the Christensen method because the biofilm formed is removed along with the AgNPs during the pre-staining wash steps prior to the application of crystal violet. Consequently, the method does not effectively capture all of the biofilm present at higher concentrations of AgNPs. To further support this hypothesis, a biuret and hydrocarbon assay was performed to quantify proteins and hydrocarbons, the major components of the bacterial biofilm formed on the surface of the nanoparticles. The findings demonstrate a markedly elevated accumulation of proteins and carbohydrates in the presence of the resistant strain in comparison to the sensitive strain at both AgNPs concentrations investigated (54 and 6.75 mg/L). This trend of increased biofilm production by the resistant strain is in accordance with the results of the CV staining. However, the trend of increased carbohydrate/protein (biofilm) formation with higher concentrations of AgNPs (13.5 mg/L AgNPs and higher) was not similarly reflected in the CV staining results (Fig. 2) due to preferential adsorption of bacterial biofilm to the surface of the AgNPs rather than to the bottom of the culture plate well. The higher biofilm production by silver-resistant S. aureus than the susceptible one exposed to silver NPs at subinhibitory concentrations was also clearly confirmed by scanning electron microscopy (Fig. 2b, c). Figure 2b shows a colony of susceptible bacteria without the presence of bacterial biofilm, while Fig. 2c shows the presence of biofilm surrounding Ag-resistant S. aureus. The increased biofilm production in silver-resistant S. aureus, as well as the increased flagellin production in silver-resistant E. coli which act as defense mechanisms against the effect of silver NPs, are the result of the bacteria’s efforts to adapt to an unfavorable toxic environment that reduces their viability and growth. In this case of Gram-positive bacteria, as in the case of Gram-negative bacteria, we assume that the phenotypic adaptation of bacteria to unfavorable conditions is necessary for their survival and growth (Fig. 3).
Fig. 2. Effect of AgNPs on biofilm formation in susceptible and resistant bacteria.
a Graph showing the optical density of biofilm stained by CV, formed in culture media containing different amounts of AgNPs in the presence of sensitive (black) and resistant (red) S. aureus. SEM images of susceptible (b), resistant (c) S. aureus 008 treated with sub-inhibition concentration of silver nanoparticles. Data are presented as individual points (n = 4 biologically independent samples per condition), with the median indicated by a horizontal line.
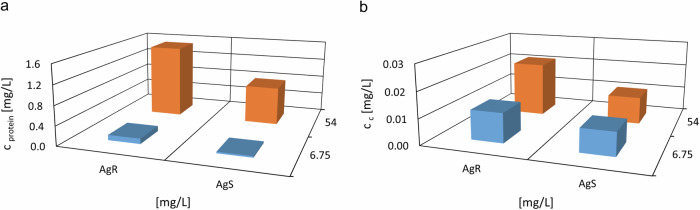
Fig. 3. Protein and carbohydrate content quantification on AgNPs surface post-cultivation with S. aureus.
Quantification of protein (a) and carbon (b) content on the surface of the AgNPs following cultivation of silver-resistant (AgR) and silver-sensitive (AgS) S. aureus 008 strains in the presence of different concentrations of AgNPs (54 (orange) and 6.75 mg/L (blue)).
How to overcome bacterial resistance to silver NPs in Gram-positive bacteria
Through the bacteria—inhibition of biofilm formation
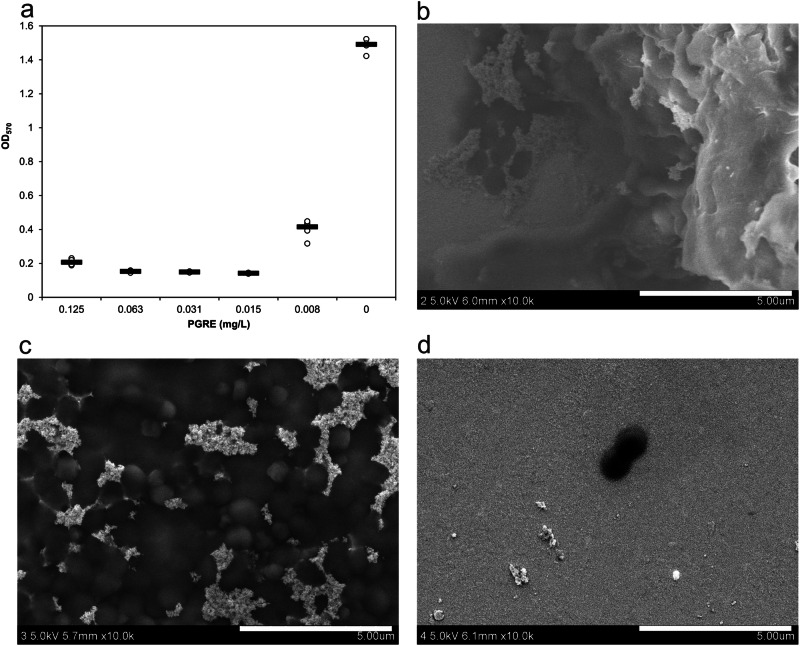
Bacterial biofilm formation has been identified as a significant contributor to silver particle aggregation, providing a resistance mechanism in S. aureus, resulting in an increase in MIC to 54 mg/L silver NPs. However, such a high concentration of silver poses a toxic threat to mammalian cells33. Therefore, there is an urgent need to develop new methods or modifications to the dispersion of silver NPs to overcome this emerging resistance mechanism and reduce the antibacterial concentration of silver NPs below the toxic threshold. One of the promising approaches to overcome this challenge is the combination of silver NPs with substances that inhibit bacterial biofilm formation. Initially, the effect of pomegranate rind extract (PGRE) has been evaluated due to its long known antimicrobial properties34–36 against planktonic bacteria and ability to inhibit biofilm formation. In addition, PGRE has shown efficacy in inhibiting flagellin production, therefore effectively overcoming resistance to AgNPs in E. coli16. Thus, PGRE as a single, simultaneous, and effective joint agent would be a very effective and clever way to overcome the resistance of E. coli and S. aureus, which induce silver aggregation in different ways (flagellin or biofilm production). As a result, this approach to overcoming bacterial resistance to AgNPs could be used to simultaneously inhibit both flagellin production and biofilm formation in E. coli and S. aureus, respectively, using only one substance to overcome both resistance mechanisms. First, the combined effect between AgNPs and PGRE and their respective MICs was investigated. Supplementary Fig. 4S illustrates the microtiter plate scheme showing different combinations of both antimicrobial agents against resistant S. aureus. While these combinations did not show a strong antibacterial effect, sub-inhibitory concentrations of PGRE did help to reduce the MIC of AgNPs from over 54 to 13.5 mg/L, which is in line with the MIC for the susceptible strain. AgNPs are known to interact with bacterial cell walls and membranes, inducing structural damage and increased permeability. This effect could be complemented by PGRE’s bioactive compounds, such as polyphenols and tannins, which are compounds known to increase reactive oxygen species production, disrupt bacterial metabolism, and release bioactive compounds, targeting multiple bacterial vulnerabilities37. Inhibition of biofilm formation in S. aureus by PGRE was also evaluated in this case using the modified Christensen method. The optical densities of the stained biofilm are shown in Fig. 4a, where the tested concentrations of PGRE solution ranged from 0.008% to 0.125%, and the MIC of PGRE was determined to be 0.031%. Figure 4a shows that the amount of biofilm formed decreases significantly when sub-inhibitory concentrations of PGRE (0.015; 0.008%) or higher are used. A decrease in biofilm formation was also observed by scanning electron microscopy. Scanning electron microscopy further supported these findings, demonstrating robust biofilm formation in silver-resistant S. aureus in the presence of silver NPs (Fig. 4b), while a reduction was observed with increasing concentrations of PGRE (Fig. 4c, d). It should be emphasized that the amount of bacterial growth does not decrease after 24 h of cultivation in a medium containing PGRE at concentrations below the MIC of PGRE (data not shown), i.e., the decrease in biofilm production is not related to the decrease in the number of bacteria, but only to the inhibitory effect of PGRE on biofilm production. PGRE may inhibit biofilm formation by several potential mechanisms. Bioactive compounds present in PGRE, such as polyphenols, flavonoids, and tannins, may disrupt the biofilm matrix by interfering with bacterial extracellular polymeric substances. These compounds may inhibit initial bacterial adhesion to surfaces and subsequent biofilm maturation38,39. By reducing the biofilm matrix, PGRE minimizes the aggregation-inducing environment of AgNPs, resulting in reduced nanoparticle aggregation. It is also important to note that PGRE contains compounds that can act as capping agents, adsorbing onto the AgNP surface and forming a protective layer that prevents particle agglomeration. This capping can result in steric hindrance and electrostatic repulsion, stabilizing the nanoparticles and preventing aggregation40,41.
Fig. 4. Biofilm formation and inhibition in resistant S. aureus with PGRE.
a Graph showing the optical density of biofilm formed in culture media containing different amounts of PGRE in the presence of resistant S. aureus 008. b SEM images of biofilm formed in the presence of 27 mg/L silver NPs without any inhibition, c inhibition by 0.039% PGRE, d inhibition by 0.624% PGRE. Data are presented as individual points (n = 4 biologically independent samples per condition), with the median indicated by a horizontal line.
Through the silver nanoparticles—stabilization against particle aggregation
Another way to overcome bacterial resistance to silver NPs could be to increase the stability of AgNPs, thus preventing their aggregation induced by the bacterial biofilm, regardless of the amount of biofilm produced by the bacteria. Besides traditional methods such as electrostatic or steric surface stabilization by polymers or surfactants, which were not successful for the AgNPs used against silver-resistant E. coli16, AgNPs can be stabilized by binding them firmly to the surface of a nanostructured substrate. One of the possibilities could be the use of cyanographene-silver (GCN/Ag) nanocomposites (Supplementary Fig. 5S), where silver NPs are strongly bound to the graphene surface, thus reducing their tendency to aggregate42,43. The strong and long-lasting antibacterial effect of the nanocomposite was attributed to the robust covalent bond between nitrile and silver in GCN/Ag, which facilitates strong interactions between the microbial membrane and the nanocomposite. These results support the hypothesis that the strong binding of silver to GCN can circumvent key bacterial resistance mechanisms, particularly the induction of aggregation observed within AgNPs colloids. In particular, the binding of nanoparticles to the cell membrane can initiate a cascade of events leading to the degradation of cell function and the production of reactive oxygen species, resulting in cell wall and membrane damage, as confirmed in our studies42. Graphene–silver nanocomposite with extremely high antibacterial activity (Age-related MIC 3.375 mg/L) should, therefore, have a good potential to withstand aggregation initiated by resistant bacteria. Therefore, the influence of biofilm production by resistant strains and its influence on the aggregation stability and antibacterial activity of the GCN/Ag composite was also investigated. Biofilm production was evaluated in the same manner as for PGRE, within the concentration range of 0.422–54 mg/L of silver amount in GCN/Ag nanocomposite. The optical densities of the stained biofilm are shown in Fig. 5a and compared with the OD of the resistant strain (i.e., 0 mg/L AgNPs).
Fig. 5. Biofilm formation and survival duration in S. aureus with GCN/Ag treatment.
Graph showing the optical density of biofilm formed in culture media containing different amounts of GCN/Ag nanocomposite in the presence of resistant S. aureus 008 (a). Data are presented as individual points (n = 4 biologically independent samples per condition), with the median indicated by a horizontal line. Graph showing the minimum duration for the killing curve of the resistant bacteria in the presence of GCN/Ag, i.e., the fact that the bacteria live for 13 hours before it dies (b). Effect of varying concentrations of AgNPs in GCN/Ag composite on bacterial killing. The data are represented as follows: blue corresponds to 0 mg/L AgNPs, orange to 3.375 mg/L AgNPs, gray to 6.75 mg/L AgNPs, yellow to 13.5 mg/L AgNPs, and green to 27 mg/L AgNPs. The experiment was performed in triplicate.
The amount of biofilm formed in the presence of GCN/Ag is very low for all tested Ag concentrations from 0.422 to 54 mg/L and is comparable to the amount of biofilm produced by the sensitive strain. The reduced biofilm formation is mainly due to the preserved high antibacterial activity of the stabilized AgNPs in the GCN/Ag composite, resulting in bacterial killing and interruption of biofilm production. It is important to note that some degree of biofilm formation was observed at all Ag-related concentrations in the GCN/Ag composite, including the highest silver concentrations from 13.5 to 54 mg/L. This biofilm production at high silver concentrations can be attributed to the presence of bacteria that survive for a period of time in the presence of GCN/Ag. This is evident from the minimum time for killing curve shown in Fig. 5b. Even at the highest silver concentration in the nanocomposite, bacteria were not killed for at least 13 h after the start of bacterial incubation, indicating that bacteria are able to form a bacterial biofilm at very low levels during this time until they are completely inhibited and killed. The same trend was observed with the addition of PGRE, as shown in Supplementary Fig. 6S, which provides further evidence by detailing the minimum duration required to kill resistant bacteria.
In summary, this work reports the rapid emergence of S. aureus resistance to AgNPs upon repeated exposure. The mechanism of S. aureus resistance is the aggregation and loss of antibacterial activity of AgNPs, which is caused by increased production of bacterial biofilm. However, this mechanism can be overcome by the application of PGRE or stabilization of AgNPs by strong binding to GCN/Ag composite material, which inhibits biofilm formation (PGRE) or preserves the high antibacterial activity of AgNPs (GCN/Ag). However, the question arises whether bacteria could develop resistance in a similar manner to AgNPs in combination with PGRE and/or to GCN/Ag. In the case of GCN/Ag, the potential emergence of bacterial resistance to the GCN/Ag nanocomposite was investigated and studied in the publication by Panacek et al.42, which showed no detectable development of resistance after 60 consecutive bacterial cultures. In the case of AgNPs combined with PGRE, bacterial resistance is currently being induced by repeated exposure, and even after 20 culture steps, no elevated MICs have been observed, i.e., no development of resistance of S. aureus to AgNPs combined with PGRE has been detected.
Conclusions
In conclusion, this study, together with the previously published work by Panacek et al.16, illustrates the rapid development of effective resistance mechanisms in both Gram-positive and Gram-negative bacteria to potent antibacterial agents against silver NPs within just twenty consecutive cultivations. In conclusion, this study, together with the previously published work by Panacek et al.16, illustrates the rapid development of effective resistance mechanisms in both Gram-positive and Gram-negative bacteria against potent antibacterial silver NPs within just twenty consecutive cultivations. The ability to develop resistance so quickly is probably because the bacteria target one of the weakest points of colloidal dispersions of silver NPs, namely their aggregation stability, which is, among other things, very sensitive to the surrounding chemical environment and physical conditions of the particles. It is most likely that the resistance mechanism of both groups of Gram-positive and Gram-negative bacteria, consisting of aggregation and destabilization of silver NPs, is identical, precisely because this is the easiest way to eliminate their high antibacterial activity. In contrast to the mechanisms of bacterial resistance to antibiotics, which often involve complex chemical, biological, and molecular interactions, often conditioned by a change in the bacterial genotype,41 this is a much simpler physical effect requiring only phenotypic adaptation. Moreover, and most interestingly in this work, each bacterial group can find its own individual way to this resistance mechanism: in the case of Gram-negative bacteria, it is flagellin production; in the case of Gram-positive bacteria, it is biofilm formation. The good news, in both cases of Gram-negative and Gram-positive bacteria resistant to silver NPs, is that we can overcome the resistance mechanism through various strategies and reignite the enhanced antibacterial potential of silver NPs. The question is, for how long? We believe that this work will stimulate a series of detailed experimental investigations involving research on the development of bacterial resistance to antibacterial nanoparticulate materials. These findings will certainly improve the understanding of bacterial resistance mechanisms to antibacterial agents, which will be useful in preventing bacterial drug resistance and fighting infectious bacteria.
Methods
Synthesis of silver NPs
Silver NPs were synthesized by a modified Tollens process, which involved the reduction of the [Ag(NH3)2]+ cation by d-maltose (p.a., Sigma Aldrich). The concentrations of all the reaction components were as follows: silver nitrate 1 × 10−3 mol dm−3 (p.a., Fagron); ammonia 5 × 10−3 mol dm−3 (28–30% w/w, p.a., Sigma-Aldrich); sodium hydroxide 9.6 × 10−3 mol dm−3 (p.a., Lach-Ner) and d-maltose as a reducing agent 1 × 10−2 mol dm−3. All the reaction components were, at the laboratory temperature (23 °C), stirred continuously with a magnetic stirrer, while the reduction was initiated by injection of reducing agent and finished 5 min after the change of the dispersion color to typical dark yellow.
Repeated bacterial culture in the presence of silver NPs
Bacterial strains used for the study were as follows: S. aureus CCM 3953 and E. coli CCM 3954 obtained from the Czech Collection of Microorganisms, Masaryk University Brno, S. aureus 008 obtained from the culture collection of the Department of Microbiology, Faculty of Medicine and Dentistry, Palacký University Olomouc. All the tested microorganisms were stored in cryotubes (ITEST plus, Czech Republic) at −80 °C. Bacteria in microplates were, in twenty successive culture steps, repeatedly exposed to subinhibitory concentrations of silver. Silver NPs dispersion with a silver concentration of 1 mmol/L (108 mg/L of silver) were in geometric progression diluted by Mueller–Hinton Broth (Becton, Dickinson, and Company) and inoculated with a bacterial strain at a concentration of 106 CFU/mL. Through geometric dilution, we gained the following silver concentrations: 54, 27, 13.5, 6.75, 3.38, 1.68, and 0.84 mg/L. Bacteria were incubated at 37 °C for 24 h. After the incubation, the MICs of silver were read as the lowest silver concentrations inhibiting the visible growth of microorganisms. Immediately after the 24-h cultivation, 10 µL of Mueller–Hinton broth containing the surviving bacteria were taken from wells with subinhibitory concentrations of silver (concentrations below MIC) and consequently sub-cultured on blood agar (TRIOS) at 37 °C for 24 h. Bacteria grown on blood agar were used for inoculum preparation at a density of 106 CFU/mL and for the next culture step. The described procedure, by now, was considered to be one culture step in an experiment studying the development of bacterial resistance. In order to confirm the development of bacterial resistance to silver NPs, the MICs of silver NPs against the reference strains were determined and compared with those of induced strains ten and twenty times exposed to subinhibitory concentrations of silver.
Modified Christensen method
S. aureus 008 biofilm was grown in 96-well plates in Muller–Hilton broth, with and without the presence of silver nanoparticles, or PGRE used for the biofilm inhibition. After 24 h incubation (37 °C), wells were rinsed to remove broth with planktonic cells. The remaining biofilm was fixed with methanol (p.a., 99%, Merck) and stained with CV (p.a., 1% w/w, Sigma Aldrich)44. The optical density OD570 of each well was determined by a spectrometer (BioTek ELx808). Blank for each concentration was contracted, and values for silver-sensitive and resistant S. aureus were compared.
Protein and hydrocarbon quantification
For protein and hydrocarbon quantification, samples were prepared in larger volumes. Bacterial strains were grown overnight at 37 °C in larger flasks, where 1 L of AgNPs water dispersion at the appropriate concentration was mixed with 1 L of MH broth. After cultivation, aggregated AgNPs formed a black precipitate at the bottom of the flask. A reference sample of aggregated and precipitated AgNPs was prepared by the addition of 2 M CaNO3 (p.a., Penta) to the water dispersion. All the precipitated samples were then centrifuged for 3 min at 4000 rpm to separate the solid precipitated and liquid fractions. The liquid portion was discarded, and aggregated AgNPs in the solid precipitate were resuspended in 1 mL of distilled water and left overnight on a rotary shaker. The following day, protein and hydrocarbon content released and extracted from the surface of AgNPs to distilled water were quantified. Proteins were quantified using the Biuret method. In this method, 250 μL of the liquid fraction containing extracted proteins and hydrocarbons was mixed with Biuret reagent (0.075 g CuSO4 (p.a., Penta), 0.248 g sodium potassium tartrate (p.a., Sigma Aldrich), 0.375 g NaOH (p.a., Lachner), diluted in 50 mL of distilled water) and the absorbance was measured at 540 nm. Hydrocarbons were quantified using a colorimetric method involving oxidation by potassium dichromate (p.a., Sigma Aldrich) in the presence of sulfuric acid (p.a., Sigma Aldrich). Specifically, 125 μL of the sample containing extracted proteins and hydrocarbons was mixed with 250 μL of 0.4 M potassium dichromate, followed by the slow addition of 250 μL concentrated sulfuric acid. The mixture was left to stand for 20 min, then it was diluted with 1.875 mL of water, and the absorbance was measured at 600 nm. The concentration of proteins and hydrocarbons (expressed as carbon content) was determined by the calibration curve method, where bovine serum albumin (p.a., Sigma Aldrich) and maltose (p.a., Sigma Aldrich) were used as standards, respectively.
MDR minimal duration for killing
The time required for the lethal effect of GCN/Ag nanocomposite and PGRE (PGRE in combination with AgNPs) has been studied against resistant S. aureus 008 with a concentration of 106 CFU/ml. The microdilution method for the determination of MIC on microtitration plates has been done as usual. Every hour for 16 h, 10 µl of the tested sample was taken from different rows of microtitration plates (containing different concentrations of antimicrobials) and inoculated/spread by defined way onto a surface of blood agar plates (Trios s.r.o., Czech Republic). Bacteria was then incubated at 37 °C for 24 h and grown colonies were counted and recalculated to the number of CFU/ml.
SEM characterization of bacteria
Sensitive and resistant S. aureus 008, respectively, were cultivated in 5 ml of MH broth with silver NPs (27 mg/L) in the presence of gold-coated glass, which served as the main surface for biofilm formation. After 24-h long of incubation, the glass was washed in PBS and water, air-dried and characterized by scanning electron microscopy. For biofilm inhibition evaluation, the same approach has been established, but besides silver NPs also PGRE has been added at different concentrations. All the samples prepared by this approach were observed by Scanning electron microscope (Hitachi SU6600) with acceleration voltage 2 kV.
Pomegranate rind extract (PGRE)
Pomegranate rind extract has been prepared as published by Asadishad et al.45. Pomegranate rind was cut into small pieces and dried at 50 °C for 24 h. In total, 15 g of dried rind was added to 200 ml of distilled water and placed in a shaker (at 80 rpm) at laboratory temperature for 24 h. The crude extract was then filter-sterilized using Whatman No. 1 filter paper. A sample of the PGRE was freeze-dried to determine its dry weight content.
GCN/Ag
The silver-graphene nanocomposite was synthesized under constant stirring at room temperature by reduction of silver ions on the cyanographene (GCN) surface. The synthesis of cyanographene was based on the procedure published by Bakandritsos et al.46, where 4 g of fluorographene (extent of labeling: >61 wt% F) were stirred in 240 ml of dimethylformamide (p.a., Sigma Aldrich). After three days of steering, the mixture was sonicated for 4 h (Bandelin Sonorex, type DT 255H, frequency 35 kHz, power 640 W, effective power 160 W) in a nitrogen atmosphere. Finally, 5.1 g NaCN (p.a., Sigma Aldrich) was added to the dispersion, and the mixture was stirred and heated at 130 °C for 48 h and then purified by several washing steps, centrifuged, and dialyzed. After that, 1.5 ml of the suspension containing 5 mg GCN was vigorously stirred with 10 ml of AgNO3 solution (2.2 × 10−3 mol/L, p.a., Fagron) at room temperature for 24 h. Silver ions, which were not firmly anchored to the cyanographene plates, were removed by centrifugation at 15,000 rpm and washed with distilled water 3 times. After purification, the GCN modified with silver ions was dispersed in 10 ml of distilled water, 10 ml of 3.2 × 10−3 mol/L ammonium hydroxide (28–30%, p.a., Sigma Aldrich), and 10 ml of 6.9 × 10−3 mol/L sodium citrate (p.a., Sigma Aldrich), which resulted in the formation of diammonium salt and stabilization, respectively. Formed silver salt was subsequently reduced by the addition of 2 ml of 2.2 × 10−3 mol/L sodium borohydride solution (p.a., Sigma Aldrich) and left in the dark for an hour. The final GCN/Ag product with bound silver nanoparticles was washed three times with distilled water, centrifuged at 10,000 rpm, and dried. At the end, the dried GCN/Ag was resuspended in the appropriate volume of water to give a final concentration of 2 g/L.
Statistics and reproducibility
For biofilm quantification (using the Christensen method) and biofilm staining assays, each condition was tested in quadruplicate (n = 4), and all results are shown as individual dots. The median is calculated and shown as a horizontal line. The data have been summarized descriptively to provide a clear representation of variability across replicates. No formal statistical analyses were performed. Experiments were independently repeated three times to confirm reproducibility. Consistent findings were observed across repeated experiments, underscoring the reproducibility of the results under the conditions described.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
The authors gratefully acknowledge financial support from the internal Student Grant Agency of the Palacký University in Olomouc, Czech Republic (IGA_ PrF_2024_020 and IGA_LF_2024_034), and the project National Institute of Virology and Bacteriology (Program EXCELES, ID Project No. LX22NPO5103)—Funded by the European Union—Next Generation EU.
Author contributions
A.P., L.H., L.K., and M.K. conceptualized and designed the study and wrote the paper. A.P. and R.P. synthesized and characterized AgNPs. D.P. synthesized and characterized GCN/Ag particles. L.V. was responsible for SEM measurements. M.K., R.V., and L.H. are responsible for the microbial part of the study. All authors reviewed and approved the final version of the paper.
Peer review
Peer review information
Communications Biology thanks Cindy Gunawan and the other, anonymous, reviewer for their contribution to the peer review of this work. Primary Handling Editor: Tobias Goris. A peer review file is available.
Data availability
All data supporting the findings of this study are available within the paper, its Supplementary Information and Supplementary Data File. Numerical source data for graphs were also deposited to Zenodo repository as Supplementary Data File47.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-024-07266-3.
References
- 1.O´Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations, (2014).
- 2.Baptista, P. V. et al. Nano-strategies to fight multidrug resistant bacteria-“A Battle of the Titans. Front. Microbiol.9, 1–26 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muzammil, S. et al. Nanoantibiotics: Future nanotechnologies to combat antibiotic resistance. Front. Biosci.10, 352–374 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Makabenta, J. M. V. et al. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol.19, 23–36 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frei, A., Verderosa, A. D., Elliott, A. G., Zuegg, J. & Blaskovich, M. A. T. Metals to combat antimicrobial resistance. Nat. Rev. Chem.7, 202–224 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slavin, Y. N., Asnis, J., Häfeli, U. O. & Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol.15, 1–20 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith, W. P. J., Wucher, B. R., Nadell, C. D. & Foster, K. R. Bacterial defences: mechanisms, evolution and antimicrobial resistance. Nat. Rev. Microbiol.21, 519–534 (2023). [DOI] [PubMed] [Google Scholar]
- 8.Graves, J. L. Jr et al. Rapid evolution of silver nanoparticle resistance in Escherichia coli. Front. Genet.6, 42 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salas-Orozco, M. et al. Mechanisms of resistance to silver nanoparticles in endodontic bacteria: a literature review. J. Nanomater2019, 7630316 (2019).
- 10.Faghihzadeh, F., Anaya, N. M., Astudillo-Castro, C. & Oyanedel-Craver, V. Kinetic, metabolic and macromolecular response of bacteria to chronic nanoparticle exposure in continuous culture. Environ. Sci. Nano5, 1386–1396 (2018). [Google Scholar]
- 11.Stabryla, L. M. et al. Role of bacterial motility in differential resistance mechanisms of silver nanoparticles and silver ions. Nat. Nanotechnol.16, 996–1003 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Gunawan, C., Teoh, W. Y., Marquis, C. P. & Amal, R. Induced adaptation of Bacillus sp. to antimicrobial nanosilver. Small9, 3554–3560 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Khan, S., Mukherjee, A. & Chandrasekaran, N. Silver nanoparticles tolerant bacteria from sewage environment. J. Environ. Sci.23, 346–352 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Yang, Y. & Alvarez, P. J. J. Sublethal concentrations of silver nanoparticles stimulate biofilm development. Environ. Sci. Technol. Lett.2, 221–226 (2015). [Google Scholar]
- 15.Ellis, D. H., Maurer-Gardner, E. I., Sulentic, C. E. W. & Hussain, S. M. Silver nanoparticle antibacterial efficacy and resistance development in key bacterial species. Biomed. Phys. Eng. Express5, 1–13 (2019). [Google Scholar]
- 16.Panáček, A. et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol.13, 65–71 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Valentin, E. et al. Heritable nanosilver resistance in priority pathogen: a unique genetic adaptation and comparison with ionic silver and antibiotics. Nanoscale12, 2384–2392 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Elbehiry, A., Al-Dubaib, M., Marzouk, E. & Moussa, I. Antibacterial effects and resistance induction of silver and gold nanoparticles against Staphylococcus aureus-induced mastitis and the potential toxicity in rats. Microbiologyopen8, e698 (2019). [DOI] [PMC free article] [PubMed]
- 19.Kędziora, A. et al. Consequences of long-term bacteria’s exposure to silver nanoformulations with different physicochemical properties. Int. J. Nanomed.15, 199–213 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panáček, A. et al. Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J. Phys. Chem. B110, 16248–16253 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Jacqueline, C. & Caillon, J. Impact of bacterial biofilm on the treatment of prosthetic joint infections. J. Antimicrob. Chemother.69, 37–40 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Usui, M., Yoshii, Y., Thiriet-Rupert, S., Ghigo, J. M. & Beloin, C. Intermittent antibiotic treatment of bacterial biofilms favors the rapid evolution of resistance. Commun. Biol.6, 275 (2023). [DOI] [PMC free article] [PubMed]
- 23.Harrison, J. J., Ceri, H. & Turner, R. J. Multimetal resistance and tolerance in microbial biofilms. Nat. Rev. Microbiol.5, 928–938 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Black, C. E. & Costerton, J. W. Current concepts regarding the effect of wound microbial ecology and biofilms on wound healing. Surg. Clin. North Am.90, 1147–1160 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Hall, C. W. & Mah, T. F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev.41, 276–301 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Venkatesan, N., Perumal, G. & Doble, M. Bacterial resistance in biofilm-associated bacteria. Fut. Microbiol.10, 1743–1750 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Craft, K. M., Nguyen, J. M., Berg, L. J. & Townsend, S. D. Methicillin-resistant: Staphylococcus aureus (MRSA): antibiotic-resistance and the biofilm phenotype. Medchemcomm10, 1231–1241 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dsouza, F. P., Dinesh, S. & Sharma, S. Understanding the intricacies of microbial biofilm formation and its endurance in chronic infections: a key to advancing biofilm-targeted therapeutic strategies. Arch. Microbiol.206, 1–34 (2024). [DOI] [PubMed] [Google Scholar]
- 29.El et al. Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions. Environ. Sci. Technol.44, 1260–1266 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Joshi, A. S., Singh, P. & Mijakovic, I. Interactions of gold and silver nanoparticles with bacterial biofilms: molecular interactions behind inhibition and resistance. Int. J. Mol. Sci.21, 1–24 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mann, R. et al. Evolution of biofilm-forming pathogenic bacteria in the presence of nanoparticles and antibiotic: adaptation phenomena and cross-resistance. J Nanobiotechnol.19, 291 (2021). [DOI] [PMC free article] [PubMed]
- 32.Christensen, G. D. et al. Adherence of coagulase-negative Staphylococci to plastic tissue culture plates: a quantitative model for the adherence of Staphylococci to medical devices. J. Clin. Microbiol.22, 996–1006 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panáček, A. et al. Silver nanoparticles strongly enhance and restore bactericidal activity of inactive antibiotics against multiresistant Enterobacteriaceae. Colloids Surf. B Biointerfaces142, 392–399 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Meléndez, P. A. & Capriles, V. A. Antibacterial properties of tropical plants from Puerto Rico. Phytomedicine13, 272–276 (2006). [DOI] [PubMed] [Google Scholar]
- 35.Braga, L. C. et al. Pomegranate extract inhibits Staphylococcus aureus growth and subsequent enterotoxin production. J. Ethnopharmacol.96, 335–339 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Braga, L. C. et al. Synergic interaction between pomegranate extract and antibiotics against Staphylococcus aureus. Can. J. Microbiol.51, 541–547 (2005). [DOI] [PubMed] [Google Scholar]
- 37.Sorrenti, V. et al. Beneficial effects of pomegranate peel extract and probiotics on pre-adipocyte differentiation. Front. Microbiol.10, 660 (2019). [DOI] [PMC free article] [PubMed]
- 38.Er-rahmani, S. et al. Plant-derived bioactive compounds for the inhibition of biofilm formation: a comprehensive review. Environ. Sci. Pollut. Res. Int.31, 34859–34880 (2024). [DOI] [PubMed]
- 39.Salim, A. et al. Antimicrobial and antibiofilm activities of pomegranate peel phenolic compounds: varietal screening through a multivariate approach. J. Bioresour. Bioprod.8, 146–161 (2023). [Google Scholar]
- 40.Hassan, M. G. et al. Pomegranate extract-mediated synthesis of silver nanoparticles: a potential dual-anticancer and antimicrobial agent. Inorg. Chem. Commun.10.1016/j.inoche.2024.112853 (2024).
- 41.Nasiriboroumand, M., Montazer, M. & Barani, H. Preparation and characterization of biocompatible silver nanoparticles using pomegranate peel extract. J. Photochem. Photobiol. B179, 98–104 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Panáček, D. et al. Silver covalently bound to cyanographene overcomes bacterial resistance to silver nanoparticles and antibiotics. Adv. Sci.2003090, 3–10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hochvaldová, L. et al. Restoration of antibacterial activity of inactive antibiotics via combined treatment with a cyanographene/Ag nanohybrid. Sci. Rep.12, 1–9 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stepanovic, S. et al. Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. J. Compil.115, 891–900 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Asadishad, B., Hidalgo, G. & Tufenkji, N. Pomegranate materials inhibit flagellin gene expression and flagellar-propelled motility of uropathogenic Escherichia coli strain CFT073. FEMS Microbiol Lett.334, 87–94 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Bakandritsos, A. et al. Cyanographene and graphene acid: emerging derivatives enabling high-yield and selective functionalization of graphene. ACS Nano11, 2982–2991 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hochvaldova, L. et al. Numerical source data for graphs [Data set]. Zenodo. 10.5281/zenodo.14054828 (2024).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All data supporting the findings of this study are available within the paper, its Supplementary Information and Supplementary Data File. Numerical source data for graphs were also deposited to Zenodo repository as Supplementary Data File47.