Abstract
Seaweeds, particularly the red seaweed Asparagopsis taxiformis , produce and sequester bromomethanes, which are known for mitigating methane emissions in ruminants when used as a feed supplement. Bromomethane synthesis requires hydrogen peroxide (H 2 O 2 ). We developed a staining assay utilizing 3,3′-diaminobenzidine (DAB) for identifying H 2 O 2 in three groups of seaweeds (red, brown, and green), including intensely pigmented species. Our findings indicate the previously identified "gland cell" in Asparagopsis taxiformis , responsible for bromoform synthesis and retention, is a specialized large organelle rich in H 2 O 2 . Our study introduces an effective survey tool to identify promising seaweed species abundant in bromoform from diverse marine habitats.
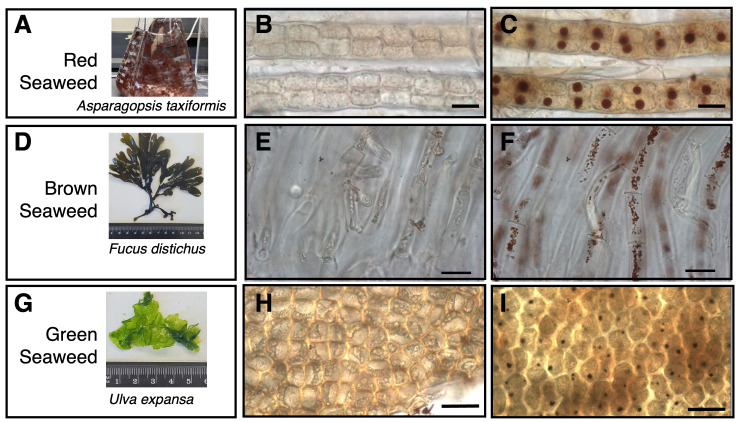
Figure 1. Detection of H 2 O 2 -containing organelles in red, brown, and green seaweeds.
A, D, and G, snapshots of seaweed organisms used in this study - red ( Asparagopsis taxiformis ), brown ( Fucus distichus ), and green ( Ulva expansa ), respectively. Fucus distichus and Ulva expansa are imaged with a ruler . B, E, H, as negative controls, 10 mM ascorbic acid was added to quench out H 2 O 2 before DAB staining in Asparagopsis taxiformis , Fucus distichus , and Ulva expansa , respectively. C, F, I, DAB staining to detect H 2 O 2 in Asparagopsis taxiformis , Fucus distichus , and Ulva expansa , respectively. Scale bar = 30 µm
Description
Methane (CH 4 ) is a significant greenhouse gas (Balcombe et al., 2018) . Considering a 100-year timescale, CH 4 holds an instantaneous global warming potential that is 120 times higher than that of carbon dioxide (CO 2 ) (Balcombe et al., 2018) . Methane now contributes to nearly 30% of the total global greenhouse gas effect (Prather and Holmes, 2017). Enteric fermentation in ruminant animals accounts for about 30.4% of global anthropogenic methane emissions, making it the primary anthropogenic source of methane. Anaerobic fermentation by ruminal microbiota is responsible for CH 4 emission (Roque et al., 2019) . Advanced mitigation technologies are sorely needed to minimize and manage CH 4 emissions in the livestock industry.
Seaweeds produce various chemical compounds that play important antimicrobial roles (Paul et al., 2006) . Bromomethanes, halogenated organic compounds, are naturally produced in most seaweeds and other algae in large quantities globally (Stemmler et al 2015). These halomethanes are shown to be effective in inhibiting fermentation in ruminant methanogens (Roque et al., 2019, Palangi et al., 2022) . Specifically, bromoform competitively binds and inhibits Coenzyme M, the second to last enzymatic step in the production of methane, resulting in a significant reduction of methane output in vitro and in agricultural ruminants (De Bhowmick et al., 2023) .
In particular, Asparagopsis taxiformis ( A. taxiformis ) , a tropical to subtropical red seaweed, synthesizes and retains high levels of bromoform, CHBr 3 . A. taxiformis has been successfully used as a feed supplement to reduce methane emissions in ruminants by up to 90% (Roque et al., 2019) . The marine bromomethane biosynthesis (mbb) locus comprises four genes ( mbb1-4 ) that encode multiple bromoperoxidases (Thapa et al., 2020) . These enzymes catalyze the reaction of bromine, an ion abundantly found in seawater, and hydrogen peroxide to produce bromomethanes. Prior studies have shown that the mbb locus is linked to CHBr 3 production in A. taxiformis and the bromoform compound is retained and concentrated in a structure previously called the “gland cell” (Thapa et al., 2020; Hargrave et al., 2024) .
There is increasing interest in developing large-scale cultivation of Asparagopsis for methane mitigation, but this seaweed can be challenging to grow at scale As such, there is a strong interest to identify other bromoform-rich seaweeds across diverse marine habitats that can potentially serve as a feed supplement. Significant production of bromomethanes occurs in other seaweeds including the giant kelp Macrocystis spp . , but the retention of these bromomethanes, including bromoform, is far lower than in Asparagopsis . Currently, gas chromatography-mass spectrometry (GC-MS) is used to quantify the concentration of bromoform. However, this method doesn't relate spatial information to determine where bromoform is produced in a cost-effective manner that can be performed in seaweeds across a variety of marine habitats. In this study, we use hydrogen peroxide as a proxy to investigate hydrogen peroxide-containing organelles as hydrogen peroxide is required for CHBr 3 production in vivo . In the presence of peroxidase, such as the bromoperoxidase, 3,3'-diaminobenzidine (DAB), a colorless compound, reacts with H 2 O 2 to form a dark purple/brown product that can be observed microscopically. DAB has been widely used to detect H 2 O 2 -containing peroxisomes in plants (Daudi et al., 2012) . Here, we probe whether other northern California seaweeds have abundant H 2 O 2 -containing organelles as potential bromoform biosynthesis sites. To do this, we utilize 3,3'-diaminobenzidine (DAB) stain, predominantly used in terrestrial Arabidopsis thaliana , as the output of a dark purple/brown substrate forms upon oxidation by hydrogen peroxide in the presence of peroxidases, such as bromoperoxidase (Daudi et al., 2012) . The biggest challenges of DAB staining in seaweeds are sample thickness and removal of natural pigments.
The hydrogen peroxide content of many seaweeds remains unknown. We are interested in identifying local species that are also high in bromoform production to provide other options for feed additives. We use hydrogen peroxide as a proxy for CHBr 3 identification in A. taxiformis as a survey tool to effectively identify CHBr 3 in various seaweed species.
Three diverse macroalgae were investigated for the presence of H 2 O 2 -containing organelles using DAB staining. Our results demonstrated the presence of diverse H 2 O 2 -containing organelles in the three seaweed species. The red seaweed, A. taxiformis , was collected in San Diego, isolated and purified in the lab at Scripps Institution of Oceanography and ultimately grown in sterile seawater in a culture flask in the lab at SFSU ( Figure 1A ). The brown seaweed, Fucus distichus ( F. distichus ), and the green seaweed, Ulva expansa ( U. expansa ), were locally collected from San Francisco Bay ( Figure 1 D and 1G). DAB is vacuum infiltrated into live seaweed tissue in a similar manner as reported in plants (Daudi et al., 2012) . As controls, all three seaweed tissues were pre-infiltrated with ascorbic acid to quench out H 2 O 2 before DAB staining. As shown in Figure 1B, the bead-like pericentral cells of A. taxiformis give a clear background after de-staining, indicating the ascorbic acid infiltration successfully removed H 2 O 2 in this filamentous seaweed. The brown seaweed, F. distichus , is thicker and more pigmented than A. taxiformis . Nonetheless, ascorbic acid infiltration effectively removed H 2 O 2 in F. distichus tissues ( Figure 1E ). As shown in Figure 1C, DAB detected a large round H 2 O 2 -containing organelle in most A. taxiforms cells. These organelles were previously described as “gland cells” that are known to be involved in bromoform biosynthesis. These H 2 O 2 -rich structures can be directly microscopically examined in whole-mounted tissues, and as seen in Figure 1C, are contained in each cell. DAB staining is exclusively detected in these round organelles. Little DAB staining is detected in pericentral cellular space ( Figure 1C ). For the brown seaweed, F. distichus , the DAB-stained tissues are thick and dark and thus incompatible with whole-tissue mount imaging. Cryo-sectioning was used to create thin sections of DAB-stained F. distichus tissue. As shown in Figure 1F , H 2 O 2 -containing organelles in F. distichus are detected as small punctates in the cytoplasmic space of the elongated cylindrical cells. The thin thallus of the green seaweed, U. expansa , comprises two layers of cells, thus can be microscopically examined without sectioning. DAB staining detected H 2 O 2 -containing organelles as individual punctate within the cytoplasm of each cell. These patterns are consistent with the reported peroxisome pattern mapped by YFP-tagged with a peroxisome targeting signal (Blomme et al. 2021) . Our studies with the three diverse macroalgae show that H 2 O 2 -containing organelles are highly diverse in their numbers and shapes across taxa. While A. taxiformis and U. expansa have one centralized H2O2-containing organelle, F. distichus displays numerous small ones around the cell.
Altogether, our findings show that our modified DAB staining protocol effectively detects H 2 O 2 -containing organelles in macroalgae. Additionally, our findings indicate that the previously described “gland cell” of A. taxiformis is a large, specialized H 2 O 2 -containing organelle. Our DAB protocol provides a feasible survey tool that can be used to identify H2O2-rich species across all three macroalgae efficiently. This approach is cost-effective to screen for seaweeds that potentially have high bromoform production. Our DAB imaging data suggest that the staining intensity and stained surface area of the H 2 O 2 -containing organelles in A. taxiformis are much more than those in U. expansa and F. distichus . Future studies will compare the actual bromoform concentrations in various macroalgal species to determine if there is a strong relationship between the bromoform concentrations and the sizes/numbers of these H 2 O 2 -containing organelles. While H 2 O 2 -rich organelles are likely peroxisomes, further experiments are needed to isolate DAB-stained organelles to determine their cellular characteristics.
Methods
Seaweed collection and culture:
Ulva expansa and Fucus distichus were collected in Tiburon, CA, at the San Francisco State University Estuary and Ocean Science Center during low tide. They were transported and cultured at SFSU. Cold-water seaweed culture was maintained under 14°C, a 16h light / 8h dark photoperiod, and was supplemented with Guillards F2 media (Guillard 1975) in ~35ppt Seawater (Instant Ocean mixed SW) with aeration. Asparagopsis taxiformis was collected from San Diego, CA and isolated and purified over the course of several months in Dr. Jennifer Smith's Lab (Scripps Institution of Oceanography of UC San Diego) and sub-cultured at SFSU. Prior to experiments, Asparagopsis was cultured at 23°C, 16h light / 8h dark photoperiod, in ~35 ppt Seawater with Guillards F4 media (Instant Ocean mixed SW, supplemented with Germanium Dioxide, filter sterilized and autoclaved) and aeration.
3,3'-diaminobenzidine (DAB) Stain Procedure:
DAB Stain was prepared by adding 1 mg of 3,3'-diaminobenzidine (Sigma) per each mL of pH 5 seawater (pH adjusted with 1M HCL). DAB stain solution was mixed overnight and made fresh for each experiment. A sample of Asparagopsis / Ulva / Fucus was removed from the culture, rinsed with deionized water, and dried with a kimwipe. The blade was cut into five-millimeter pieces with a razor blade to allow for better DAB infiltration. These pieces were submerged in 1mg/mL DAB, and placed under a vacuum for ten minutes, the vacuum was released and repeated three times. The DAB was aspirated off with a pipette and deionized water was added to stop the DAB reaction, by diluting and washing away residual reagents (H 2 O 2 and DAB) and removing unreacted DAB, thereby reducing the availability of ions that drive the peroxidase-catalyzed reaction. The sample was submerged in pure ethanol and boiled for ten minutes as a fixative and to remove pigment from the tissue. Negative controls were pre-treated with 10 mM ascorbic acid prior to DAB staining. DAB stained & Negative controls were stored in 100% EtOH until mounting.
Sample embedding and Cryosectioning:
Samples were mounted in OCT (Optimal Cutting Temperature Compound, Fisher) and submerged in an ethanol dry ice bath until fully frozen. OCT-embedded samples were cryo-sectioned into thirty-micron sections to mount on microscope slides.
Imaging:
All samples were brightfield imaged at 20X on a Nikon 80i Microscope in the SFSU Cell & Molecular Imaging Center. Images were collected in QCapture Pro as TIFFs.
Acknowledgments
Acknowledgments
We would like to thank Anna Calamonaci for her help in editing the manuscript. We thank Dr. Annette Chan and the Cell Molecular Imaging Center at SF State for helping with the images. We would also like to thank Joanne Brown, Jennifer Thompson, and Debra Singer for this research's administrative support, project management, and development.
Funding Statement
This work was sponsored by the Center for Cellular Construction, National Science Foundation DBI-1548297. Funding for the Smith laboratory focusing on Asparagopsis cultivation was provided by a grant from the Builder’s Initiative.
References
- Balcombe Paul, Speirs Jamie F., Brandon Nigel P., Hawkes Adam D. Methane emissions: choosing the right climate metric and time horizon. Environmental Science: Processes & Impacts. 2018;20(10):1323–1339. doi: 10.1039/c8em00414e. [DOI] [PubMed] [Google Scholar]
- Blomme Jonas, Liu Xiaojie, Jacobs Thomas B, De Clerck Olivier. A molecular toolkit for the green seaweed Ulva mutabilis . Plant Physiology. 2021 Apr 27;186(3):1442–1454. doi: 10.1093/plphys/kiab185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daudi Arsalan, Cheng Zhenyu, O’Brien Jose A., Mammarella Nicole, Khan Safina, Ausubel Frederick M., Bolwell G. Paul. The Apoplastic Oxidative Burst Peroxidase in Arabidopsis Is a Major Component of Pattern-Triggered Immunity . The Plant Cell. 2012 Jan 1;24(1):275–287. doi: 10.1105/tpc.111.093039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bhowmick Goldy, Hayes Maria. Potential of Seaweeds to Mitigate Production of Greenhouse Gases during Production of Ruminant Proteins. Global Challenges. 2023 Apr 8;7(5) doi: 10.1002/gch2.202200145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghassemi Nejad Jalil, Ju Mun-Su, Jo Jang-Hoon, Oh Kyung-Hwan, Lee Yoon-Seok, Lee Sung-Dae, Kim Eun-Joong, Roh Sanggun, Lee Hong-Gu. Advances in Methane Emission Estimation in Livestock: A Review of Data Collection Methods, Model Development and the Role of AI Technologies. Animals. 2024 Jan 29;14(3):435–435. doi: 10.3390/ani14030435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillard Robert R. L. Culture of Phytoplankton for Feeding Marine Invertebrates. Culture of Marine Invertebrate Animals. 1975:29–60. doi: 10.1007/978-1-4615-8714-9_3. [DOI]
- Hargrave Matthew S., Islam Shahima, Agarwal Vinayak, Smith Jennifer E. The influence of light stress on bromoform synthesis and concentration in the red seaweed Asparagopsis taxiformis. Journal of Applied Phycology. 2024 Jan 4;36(1):321–329. doi: 10.1007/s10811-023-03129-2. [DOI] [Google Scholar]
- Anthropogenic and Natural Radiative Forcing. Climate Change 2013 – The Physical Science Basis. 2014 Mar 24;:659–740. doi: 10.1017/cbo9781107415324.018. [DOI]
- Palangi Valiollah, Lackner Maximilian. Management of Enteric Methane Emissions in Ruminants Using Feed Additives: A Review. Animals. 2022 Dec 7;12(24):3452–3452. doi: 10.3390/ani12243452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul Nicholas A., Cole Louise, De Nys Rocky, Steinberg Peter D. ULTRASTRUCTURE OF THE GLAND CELLS OF THE RED ALGA ASPARAGOPSIS ARMATA (BONNEMAISONIACEAE) 1 . Journal of Phycology. 2006 May 25;42(3):637–645. doi: 10.1111/j.1529-8817.2006.00226.x. [DOI] [Google Scholar]
- Ponte José M. S., Seca Ana M. L., Barreto Maria Carmo. Asparagopsis Genus: What We Really Know About Its Biological Activities and Chemical Composition. Molecules. 2022 Mar 9;27(6):1787–1787. doi: 10.3390/molecules27061787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roque Breanna Michell, Brooke Charles Garrett, Ladau Joshua, Polley Tamsen, Marsh Lyndsey Jean, Najafi Negeen, Pandey Pramod, Singh Latika, Kinley Robert, Salwen Joan King, Eloe-Fadrosh Emiley, Kebreab Ermias, Hess Matthias. Effect of the macroalgae Asparagopsis taxiformis on methane production and rumen microbiome assemblage. Animal Microbiome. 2019 Feb 12;1(1) doi: 10.1186/s42523-019-0004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roque Breanna M., Salwen Joan K., Kinley Rob, Kebreab Ermias. Inclusion of Asparagopsis armata in lactating dairy cows’ diet reduces enteric methane emission by over 50 percent. Journal of Cleaner Production. 2019 Oct 1;234:132–138. doi: 10.1016/j.jclepro.2019.06.193. [DOI] [Google Scholar]
- Thapa Hem R., Lin Zhenjian, Yi Dongqi, Smith Jennifer E., Schmidt Eric W., Agarwal Vinayak. Genetic and Biochemical Reconstitution of Bromoform Biosynthesis in Asparagopsis Lends Insights into Seaweed Reactive Oxygen Species Enzymology . ACS Chemical Biology. 2020 May 26;15(6):1662–1670. doi: 10.1021/acschembio.0c00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Peng, Li Dapeng, Yang Qi, Su Peng, Wang Hui, Heimann Kirsten, Zhang Wei. Commercial cultivation, industrial application, and potential halocarbon biosynthesis pathway of Asparagopsis sp. Algal Research. 2021 Jun 1;56:102319–102319. doi: 10.1016/j.algal.2021.102319. [DOI] [Google Scholar]