Abstract
Study Objectives
This study evaluated the differences in obesity-related outcomes across multiple adolescent sleep health domains, including circadian misalignment (CM), circadian timing, and sleep duration.
Methods
53 adolescents (aged 14–18; body mass index [BMI] percentile < 95%; 53.7% female) completed a cross-sectional study that included baseline assessment of height; weight; demographics; and 10 days assessment of sleep, physical activity, and dietary outcomes. Sleep duration, sleep timing, and physical activity data were collected from all participants using wrist-worn and waist-worn actigraphs. Dietary intake was measured using the Automated Self-Administered 24 Hours dietary recalls on 3 randomized days. Circadian timing was measured using dim-light melatonin onset (DLMO), and CM was calculated as the distance of time between DLMO and the average sleep onset time. Participants were categorized into groups (early vs late circadian timing, aligned vs misaligned circadian timing, and adequate sleep vs short sleep), and differences in dietary outcomes, physical activity, and BMI were analyzed using t-tests.
Results
Adolescents with later DLMO (M = 21:30 ± 1:11) had 0.63 higher BMI and 0.47% less averaged daily percent fat consumption than adolescents with early DLMO. Adolescents with CM (M = 1:42 ± 1:06) consumed 451.77 more averaged daily kcal consumption compared with those with circadian alignment. No statistically significant differences were found in any obesity-related outcome between sleep duration groups.
Conclusions
Our cross-sectional findings indicate that focusing on sleep timing and circadian alignment, beyond sleep duration, may promote better health outcomes for healthy adolescents. The findings of this study could enhance sleep education and inform clinical models for prevention efforts for pediatric obesity.
Keywords: sleep health, circadian rhythms, adolescence, obesity, eating behaviors, exercise
Statement of Significance.
This study underscores the critical role of circadian timing and circadian misalignment (CM) on adolescent health, with implications beyond sleep duration alone. Adolescents with later circadian timing or misalignment exhibited a higher BMI and higher calorie intake compared with aligned peers. Findings suggest that focusing on circadian factors, rather than just sleep duration, may offer a targeted approach to obesity prevention and health optimization in adolescents. The use of gold-standard measures such as dim-light melatonin onset strengthens these insights, providing a robust foundation for future longitudinal and intervention-based research. These results support the inclusion of sleep timing and CM in adolescent health models, offering novel avenues for impactful obesity prevention strategies.
Introduction
Compared with other developmental periods, adolescence poses a substantial risk of obtaining short sleep duration [1–3] and decreased sleep quality [4]. It is estimated that over 40% of US adolescents sleep less than 7 hours per night [5] and less than 30% of adolescents are obtaining the 8–10 hours of sleep that is recommended for their age group [6, 7]. Many factors may contribute to these poor sleep outcomes, including pubertal changes in their circadian timing, which shift their circadian timing later [8, 9], and social factors such as earlier school start times, increasing relationship demands, and growing independence in setting bedtimes [10]. This mismatch can lead to circadian misalignment (CM), which occurs when actual sleep timing differs substantially from intrinsic circadian timing. CM is associated with a variety of negative physical and mental health outcomes [11–13], including cognitive and behavioral difficulties, poorer mental health, increased risk-taking behaviors [14], impaired executive functioning [15], lower academic performance [16], and worse self-reported physical health, including worse somatic complaints and decreased physical activity [17]. However, despite these known associations, CM remains less studied than other dimensions of sleep health in adolescents.
For example, while a myriad of research has been conducted examining the role of sleep duration on obesity risk [18, 19], less have examined how CM and circadian timing are associated with obesity risk in adolescents [20]. Within adult men and women, greater circadian alignment is related to greater overall caloric intake, carbohydrate intake, and meal frequency [21], though this has not been replicated in adolescents. Furthermore, 12%–16% reductions in energy expenditure have been observed in night shift workers, even when controlling for changes in sleep duration associated with work shift variability [22]. Recently, it has been observed that adolescent CM (often marked by irregularity in the midpoint of sleep) and/or later circadian timing in adolescents is related to obesity-related factors, including blunted heart rate variability, greater fat mass, and increased visceral adipose tissue [23, 24]. Furthermore, later bedtimes in children and adolescents (which may increase the risk of CM) have been cross-sectionally associated with increased intake of energy-dense, nutrient-poor foods, while earlier bedtimes have been associated with increased consumption of fruits and vegetables [25]. In-lab manipulation of adolescents’ sleep timing resulted in alterations in reward-associated neural activation [26], and the neural activation patterns differ from those observed in adults [27]. This variability in research findings across development, combined with the relative paucity of research regarding the specific role that circadian timing and CM have on obesity-related outcomes in adolescents, provides further evidence for the necessity to explore these relationships within this at-risk developmental period.
Because sleep issues and obesity are highly prevalent and growing public health concerns among adolescents, research that identifies the link between these two issues is important for health promotion efforts. Specifically, understanding how various domains of sleep health are related to obesity-related outcomes in adolescents can better inform obesity prevention efforts, allowing interventionists to enhance the focus of sleep-specific recommendations. As such, we investigated how multiple aspects of adolescent sleep health (i.e. circadian timing, CM, and sleep duration) relate to body mass index (BMI), physical activity, sedentary behavior, and dietary outcomes among healthy adolescents ages 14–18. We hypothesized that adolescents with later circadian timing, greater CM, and shorter sleep duration would have higher BMI, greater intake of calories, carbohydrates, sugars, and added sugars, and increased sedentary activity, compared with adolescents with earlier sleep timing, more circadian alignment, and greater sleep duration, respectively. We also explored the differences in adolescents’ circadian timing, CM, and sleep duration on the amount of fats, proteins, fruits, and vegetables consumed; total number of meals consumed across the day; timing of the last meal; and minutes of light activity and moderate-to-vigorous physical activity (MVPA).
Materials and Methods
The Institutional Review Board at the leading author’s institution approved all study procedures, which included 11 days monitoring period in which sleep, diet, weight, and physical performance data were collected. Data collection occurred between January through May of 2022 in the Western United States of America. All study appointments took place at a university campus and occurred on the weekends so as not to influence school performance. Parents consented and adolescent participants assented to all aspects of study participation at the first appointment.
Participants
Study staff recruited healthy adolescents (ages 14–18) using convenience sampling methods, including in-person booths to set up during local middle and high school lunches, flyers emailed to parents through school newsletters, flyers posted on school bulletin boards, and flyers mailed to professors at the leading author’s university. Exclusion criteria included factors that would significantly influence sleep and participants’ ability to adhere to study protocol. This included the presence of self-reported sleep disorders (e.g. insomnia and obstructive sleep apnea), the potential of a sleep disorder (e.g. persistent short sleepers, i.e. consistently sleeping less than 5 hours per night), persistent long sleepers (i.e. consistently sleeping more than 10 hours per night), and medications with known effects on sleep. Of note, no participants identified habitually short or long sleep during recruitment, and thus, none were excluded based on this criterion. Further exclusion criteria included factors that would influence eating habits, such as a self-reported history of eating disorders or bariatric surgery, medications with known effects on weight or eating behaviors (medications that have been established to inhibit/activate appetite or decrease/increase salivation), and severe food allergies. Because the aim of this study was to better inform obesity prevention efforts, individuals who met obesity criteria (BMI percentile > 95%) were also excluded. Additional exclusion criteria were used because this study took place as part of a larger study that included magnetic resonance imaging (MRI) scanning. The MRI-based exclusion criteria included left-handedness, pregnancy, claustrophobia, and any metal implants in the body (except for orthodontic retainers on the bottom teeth).
Procedures
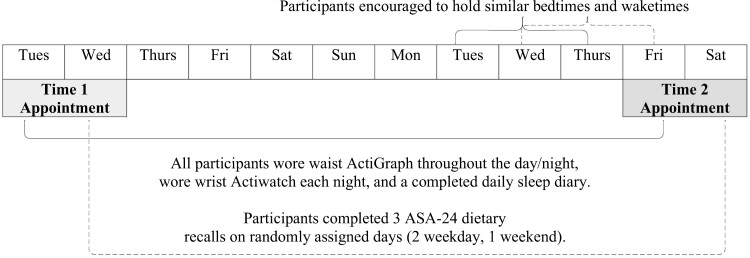
Study staff texted, emailed, or called parents of interested adolescents to give a brief overview of the study and obtain verbal consent to complete an eligibility questionnaire, which was sent to the parent and the interested adolescent via text/email. Following the determination of eligibility criteria and continued interest in participating in the study, adolescents were officially enrolled in the study. Study procedures took place over 11 days as outlined in Figure 1. First, a baseline appointment occurred on a Tuesday or a Wednesday during which study staff obtained the parent’s written consent and the adolescent’s written assent to participate in the study. Following consent, the adolescent’s height and weight were measured using a Seca scale and stadiometer, and BMI was calculated. Participants were then informed about procedures for the next 10 days, including how to wear a wrist-worn and waist-worn actigraph, complete three dietary recalls independently (to reduce desirability bias), and fill out daily sleep diaries. The wrist-worn actigraph was to be worn when getting into bed to sleep each night and to be removed when they arose for the day. The provided sleep diary was to be filled each day in partnership with actigraphy use. The waist-worn accelerometer was to be worn at all times during the study. Subjects were then given login information for the dietary recall website and assigned 3 randomized days to fill out dietary recalls (2 weekdays and 1 weekend day). Participants were informed that they could sleep; however, they preferred for the first seven nights of the study; for the final three nights, participants were encouraged to “stabilize” their preferred sleep timing (selecting a preferred, typical bedtime, and wake time that they could hold relatively constant for the three nights leading up to the final study appointment). Lastly, adolescents filled out a series of sleep and demographic questionnaires.
Figure 1.
Study procedure overview. All participants wore waist ActiGraph throughout the day/night, wore wrist Actiwatch each night, and a completed daily sleep diary. Participants completed three ASA-24 dietary recalls on randomly assigned days (two weekday, one weekend).
Following the 10 days monitoring period (7 nights of free sleep and 3 nights of encouraged stabilized sleep), participants attended a follow-up appointment on a Friday or Saturday afternoon, beginning 5 hours before the participant’s preferred bedtime (i.e. the self-selected bedtime that they were encouraged to adhere to for the prior three nights), and ending 1 hour past their preferred bedtime, for a total of 6 hours. This appointment took place in a dimly lit room (lux < 5 throughout the appointment duration, as confirmed by luxometer readings which occurred every 30 minutes). Participants were provided with several entertainment options during the appointment (e.g. watching movies or playing video games on a dimly lit television, reading large-font books, completing puzzles, coloring, or playing board games) as well as unlimited snacks and meals from a standardized menu.
Every 30 minutes, participants provided saliva samples to measure their salivary concentrations of melatonin. Ten minutes before each saliva sample collection, participants brushed and rinsed their teeth to remove any food residue and were instructed to recline in a chair. After reclining for 10 minutes, participants were given an untreated cotton swab to place in their mouth; adolescents were instructed to “soak” the cotton swab to the best of their ability and spit the cotton swab into the provided salivette at the end of 5 minutes. Study staff immediately weighed all salivettes provided to ensure that at least 2 mL of saliva was obtained for proper analysis. At the end of the appointment, participants were compensated and driven home by a legal guardian.
Measures
Potential covariates.
Demographics.
Participants completed a 12-item questionnaire that asked about age, current address, race/ethnicity, sex, annual gross income of parents/guardians, and current medications.
Daytime sleepiness.
Participants completed the Epworth Sleepiness Scale for Children and Adolescents (ESS-CHAD). The ESS-CHAD is used to understand general sleepiness levels throughout the day. The questionnaire contains eight questions. A total score is calculated by summing the value of each question’s answer, and a higher score indicates greater levels of daytime sleepiness. Using the ESS-CHAD for adolescents ages 12–18 has been shown to be reliable and valid for measuring daytime sleepiness, with a Cronbach α score of 0.73 and moderate-to-strong positive Pearson correlations for item-totals [28]. Within our study sample, we observed adequate reliability (Cronbach’s α = 0.753). We tested the ESS-CHAD total score as a potential covariate in our analyses.
Physical development.
The Pubertal Development Scale (PDS) uses five items to determine participants’ stages of physical development. The PDS has been shown to be a reliable and valid measure of physical development with an intraclass correlation coefficient of .81–.92 [29]. Consequently, the PDS is comparable to physical assessments (e.g. Tanner staging) performed by healthcare professionals [29]. Within our study sample, we observed questionable reliability for females and adequate reliability for males (Cronbach’s α = 0.507 and 0.700, respectively). We tested the PDS total score as a potential covariate in our analyses.
Primary outcomes.
bmi.
To calculate the BMI for each participant, study staff weighed and measured the height of each participant using a standardized Seca scale and stadiometer. Study staff used a sex- and age-corrected BMI calculator based on CDC norms for minors (UC San Diego Health BMI Calculator for Children and Teens). BMI was included as an outcome in our final models.
Physical activity.
During the 10 days between the baseline and DLMO appointments, physical activity was measured via waist-worn actigraphy (Actigraph GT3X+). The devices were calibrated to the participants’ height and weight, used a sample rate of 30 seconds, and recorded data using an epoch length of 15 seconds. Participants wore the devices on their dominant (right) hip continually, except in cases that put the device at risk of physical or water damage. Participants were instructed to record instances when they removed the accelerometers in their sleep diaries. Wearing accelerometers on the hip has been shown to accurately measure energy expenditure, including the type and intensity of physical activity [30, 31]. Accelerometers worn at the hip have been shown to have the highest accuracy (97.81%) compared with other single locations [32]. We used Actiware software to analyze the available data using Freedson, Pober, and Janz [33] cutoffs; specifically, we examined sedentary activity (0–25 counts/15 seconds epoch), light physical activity (26–555 counts/15 seconds epoch), and MVPA estimates (556+ counts/15 seconds epoch) for each day, and then averaged each day’s estimates across the monitoring period. Sedentary activity served as the primary outcome in this study, and light and MVPA served as exploratory outcomes.
Dietary intake.
Dietary intake was measured using the Automated Self-Administered 24-hour Dietary Assessment Tool (ASA-24) on 3 randomized days within the monitoring period, with one recall being randomized to a weekend day and 2 recalls being randomized to weekdays. ASA-24 uses the Automated Multiple Pass Method (recording all food and drink consumed across the day in a multiple-iteration approach) This has been shown to be accurate within 3% of total energy expenditure among normal-weight subjects [34] and to reduce desirability bias while reporting. Research among adolescents points to no significant variance in reporting accuracy between experimenter-administered interviews and self-completed online forms [35]. For this study, primary outcomes of interest included the averaged values (across the 3 recall days) for caloric consumption, grams of carbohydrates, sugar, and added sugar consumed; exploratory outcomes included averaged grams of total fat and protein consumed, number of fruits and vegetable servings consumed, total number of meals consumed across the day, and timing of the last meal of the day. For macronutrients (fat, protein, carbohydrates, sugars, and added sugars), we divided averaged daily grams consumed by daily kilocalorie consumption.
Predictor variables.
circadian timing (dlmo).
Participants’ circadian timing was determined by using 6 hours dim-light melatonin onset (DLMO) procedure to measure melatonin concentrations across 12 saliva samples (collection every 30 minutes). Using saliva samples to measure DLMO has been shown to be an accurate measure of circadian timing, comparable to plasma assessment of DLMO (correlation coefficient of 0.93) [36]. Immediately after sample collection, the samples were weighed to ensure they measured ≥2 mL. Samples that were <2mL were immediately discarded, and participants were instructed to provide another sample. If samples were ≥2 mL, they were centrifuged at 3000 g for 5 minutes (to remove the saliva from the cotton swab) and then immediately frozen. At the end of the study, frozen samples were shipped to SolidPhase Inc., which used Novolytix RIA kits to determine salivary melatonin concentrations. DLMO was considered reached when the melatonin levels exceeded a threshold of 4 pg/mL of saliva [37]. Similar to work conducted previously in our lab [38], adolescents were then dichotomized into two circadian phase groups: early timing (DLMO occurring before 10:00 pm) and late circadian timing (DLMO occurring at or after 10:00 pm), with 10:00 pm being selected as the cut-point as it was the closest to previously observed mean DLMO times in similarly aged adolescents [39].
Sleep duration and sleep onset.
Sleep was measured for 10 days following the baseline appointment using wrist-worn actigraphy devices (Philips Respironics Actiwatch 2) worn on the nondominant (left) wrist; data from these devices were cross-checked with the participants’ sleep diaries for bedtimes and wake times. Participants were instructed to put the devices on when they attempted to fall asleep for the night and to take the devices off when they woke up for the day. With a 0.81–0.86 epoch accuracy, wrist actigraphs have been shown to accurately measure circadian sleep/wake rhythms and are comparable to polysomnographic devices [40, 41]. However, wrist actigraphs have also been shown to underestimate total sleep time in adolescents; this can be offset by cross-checking sleep and wake times with participants’ sleep diaries [42]. The 10 days sleep diaries used in this study asked when participants got into bed, when they tried falling asleep, when they woke up, when they got out of bed, and if/when they removed the waist actigraphy devices.
For this study, ActiWatch software (Actiware 6.3) was used to download the wrist-worn accelerometer data into 30-second epoch intervals. Sleep diary data were used to inform scoring procedures to mark sleep period onset and offset. In two instances, sleep period onset and/or offset was not noted in a participant’s sleep dairy. Additionally, there were two instances of a significant mismatch between the sleep diary and the Actiwatch log. For these four instances, two reviewers jointly reviewed the Actiwatch log and marked the start of the sleep onset period as the beginning of the last downward trend of physical activity of the day and the sleep offset of the sleep period as the start of the first prolonged period of activity following sleep. All data were scored using the Sadeh algorithm [43], with sleep duration serving as a predictor variable, which has shown to be internally consistent in children [44]. Sleep duration was then dichotomized across the sample into “short sleep” (i.e. <8 hours of sleep per night) and “healthy sleep” (i.e. ≥8 hours of sleep per night) [38]. We also derived sleep onset time and averaged it across the 10 days monitoring period; we used this averaged sleep onset time to calculate CM.
Circadian misalignment.
CM was calculated as the distance of time between DLMO and the averaged sleep onset time across the 10 days monitoring period. Similar to our previous work [38], CM estimates were then dichotomized into “aligned” (≥2 hours but <4 hours between DLMO and averaged sleep onset time, which reflects the expected delay between DLMO and sleep initiation under low-light conditions [39, 45]) and “misaligned” (<2 hours, suggesting adolescents are going to bed before they are physiologically prepared). No adolescent had a CM index that was ≥4 hours, which would have also been conceptualized as being “misaligned” (falling asleep later than the body was physiologically ready for).
Statistical analyses
We ran a series of correlations, regressions, and independent sample t-tests to determine whether our primary outcomes differed based on age, sex, race/ethnicity, income, pubertal status, or baseline daytime sleepiness (ESS). No covariate emerged as significantly related to our outcomes of interest; therefore, none were included as covariates in the final models; descriptive information regarding these variables can be found in Tables 1 and 2. We first ran a series of bivariate regressions to examine the linear relationship between all variables of interest. Next, we conducted a series of independent samples t-tests to examine whether the following variables differed across sleep outcomes across three sleep metrics (late vs early timing, aligned vs misaligned, short sleep vs adequate sleep): dietary outcomes (i.e. amount of calories consumed, % of macronutrients consumed, i.e. %fat, %sugar, %protein, %carbohydrates, and %added sugars), timing of the last meal of the day, averaged number of total daily meals, physical activity outcomes (i.e. time spent in sedentary, light, and moderate-to-vigorous physical activity), number of meals, and BMI. We confirmed that the assumptions for independent samples t-tests were met before analyses, including testing for normality using Shapiro–Wilk test. To determine statistical significance, a two-tailed p-value of .05 was selected, though we interpreted findings of any comparison with a medium or larger effect size (Cohen’s d ≥ 0.05). Post-hoc power analysis using G*Power 3.1 indicated that the study was adequately powered (achieved power of 0.80) to detect large effect sizes between groups. Due to accelerometer issues, we were unable to collect waist-worn physical activity measurements on six participants and wrist-worn accelerometry on five participants. Furthermore, we were unable to derive meaningful DLMO values from six participants. Missing data were handled with listwise deletion.
Table 1.
Sample characteristics
| Demographics | Mean ± SD or N; % |
|---|---|
| N | 53 |
| Age | 15.51 ± 1.39 |
| BMI | 21.58 ± 2.69 |
| Female (N; %) | 47; 53.7% |
| Race/Ethnicity (N; %) | |
| White, European American | 47; 88.9% |
| Native American or Alaskan Native | 1; 2.0% |
| Mexican, Mexican American, or Chicano | 2; 3.2% |
| Asian or Pacific Islander | 3; 5.9% |
| Household Income (N; %) | |
| $20 000–$49 000 | 1; 1.9% |
| $50 000–$74 000 | 10; 18.9% |
| $75 000–$99 000 | 9; 16.9% |
| $100 000–$149 000 | 17; 32.1% |
| $150 000 or more | 13; 24.5% |
| Declined to report | 3; 5.7% |
| Physical Activity | |
| Sedentary (Minutes) | 551.48 ± 52.09 |
| Light (Minutes) | 35.08 ± 11.40 |
| MVPA (Minutes) | 57.68 ± 18.05 |
| Dietary Outcomes | |
| Energy (kcal) | 2180.79 ± 669.68 |
| Protein (g) | 76.23 ± 27.58 |
| Fat (g) | 88.03 ± 33.13 |
| Carbohydrate (g) | 278.27 ± 90.57 |
| Sugar (g) | 120.55 ± 52.35 |
| Added Sugar (g) | 17.72 ± 10.42 |
| Fruit (servings) | 1.19 ± 1.79 |
| Vegetables (servings) | 1.10 ± 0.69 |
| Number of Meals (per day) | 4.67 ± 2.02 |
| Final Meal Timing (hh:mm) | 20:02:20 ± 1:27:35 |
Table 2.
Sleep characteristics
| Sleep outcome | Mean ± SD |
|---|---|
| CM* (hh:mm) | 1:42 ± 1:06 |
| DLMO (hh:mm) | 21:30 ± 1:11 |
| Actiwatch 2 Estimates | |
| Weekday Sleep Duration (hh:mm) | 7.61 ± 0.85 |
| Weekday Sleep Onset (hh:mm) | 23:11 ± 0:57 |
| Weekend Sleep Duration (hh:mm) | 8.39 ± 1.31 |
| Weekend Sleep Onset (hh:mm) | 23:52 ± 1:04 |
| ESS_CHAD Total Score | 6.75 ± 3.89 |
*CM was calculated as the distance of time between DLMO and average sleep onset time across the 10 days monitoring period.
Results
Participant characteristics
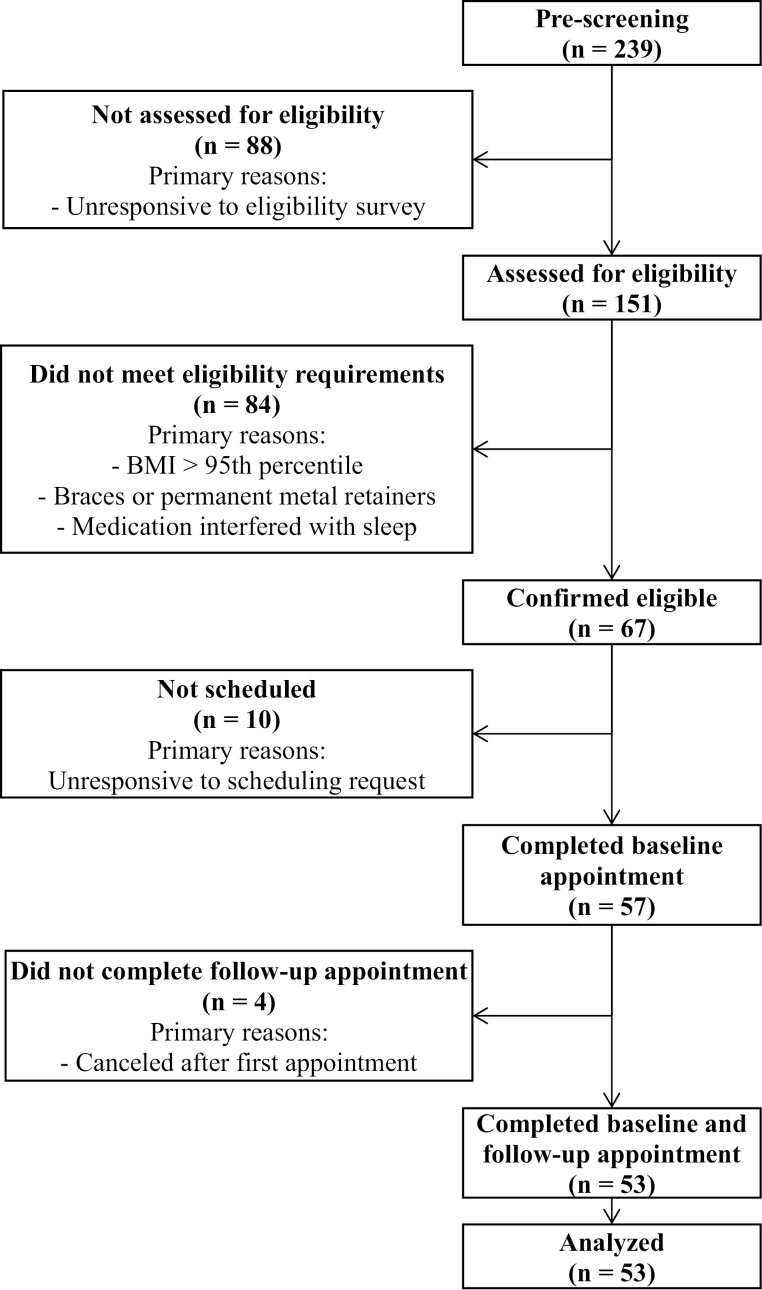
Out of 239 adolescents who expressed interest, 151 completed an eligibility survey. Of these, 67 were deemed eligible and were attempted to be recruited, and 57 of the eligible participants completed a baseline appointment; and 53 completed a baseline and follow-up appointment (see Figure 2). Our final sample included a total of 53 participants (mean age = 15.51, SD = 1.39) who completed the study protocol. The sample consisted of the following demographics: 53.7% female and 88.9% White, European American (see Table 1 for more key demographic details). Participants slept an average of 7.61 hours (SD = 0.85) on the weekdays and 8.39 hours (SD = 1.31) on the weekends. Additional averaged sleep outcomes, as well as dietary and physical activity outcomes, are also included in Tables 1 and 2. The relationship between all the predictor variables (continuous) and the dietary outcomes is presented in Table 3.
Figure 2.
Participant recruitment and eligibility.
Table 3.
Correlations between continuous sleep predictor variables and dietary outcomes
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. DLMO (hh:mm) | ||||||||||||||||
| 2. CM (hh:mm) | −.709** | |||||||||||||||
| 3. Sleep duration (hh:mm) | −.284 | −.073 | ||||||||||||||
| 4. Total Energy (kcal/day) | .068 | −.236 | .035 | |||||||||||||
| 5. Fruit (servings) | .151 | −.168 | .177 | −.017 | ||||||||||||
| 6. Vegetables (servings) | −.017 | −.164 | .170 | .305* | .388** | |||||||||||
| 7. Sedentary (min) | .179 | −.216 | −.121 | −.064 | .142 | .050 | ||||||||||
| 8. Light (min) | −.201 | .172 | .281 | .089 | −.277 | −.010 | −.089 | |||||||||
| 9. MVPA (min) | .015 | .071 | −.040 | −.307* | −.073 | −.148 | .145 | .261 | ||||||||
| 10. Protein (g/kcal) | .187 | −.054 | −.095 | −.194 | .290* | .014 | .123 | −.078 | .008 | |||||||
| 11. Fat (g/kcal) | −.307* | .133 | −.197 | .269 | −.494** | −.097 | −.024 | .175 | .098 | −.200 | ||||||
| 12. Carb (g/kcal) | .165 | −.096 | .257 | −.129 | .370** | .140 | −.023 | −.140 | −.081 | −.419** | −.797** | |||||
| 13. Sugar (g/kcal) | .261 | −.252 | .065 | .156 | .541** | .183 | .049 | −.133 | −.127 | −.153 | −.562** | .653** | ||||
| 14. Added sugar (g/kcal) | .130 | −.115 | −.074 | .395** | −.420** | −.096 | −.086 | .226 | −.033 | −.596** | .040 | .284* | .438** | |||
| 15. BMI Z-score | .294* | −.091 | −.287 | .054 | .044 | −.074 | .095 | −.218 | −.200 | .225 | −.046 | −.110 | .062 | −.020 | ||
| 16. Number of meals (per day) | −.113 | −.014 | −.039 | .336* | −.109 | .189 | .006 | .176 | −.059 | −.251 | .201 | −.028 | .004 | .206 | −.049 | |
| 17. Final Mealtime (per day) | .262 | −.100 | −.134 | .331* | .025 | −.021 | −.022 | .002 | −.310* | −.118 | −.133 | .175 | .261 | *.325 | −.078 | .251 |
CM also known as phase angle, Kcal = kilocalories, MVPA = moderate to vigorous activities; Carbs = carbohydrates; BMI = body mass index.
*Indicates a P-value < .05 and **indicates a P-value < .01.
Relationship with circadian timing
When treating DLMO timing as a continuous variable, there was a significant negative relationship between DLMO timing and %fat consumption, with later DLMO scores being associated with less %fat consumption (r(46) = −.307, p = .038; see Table 3). These findings were consistent when examining dietary differences following dichotomizing DLMO into early and late DLMO groups, with adolescents with later circadian timing having lower %fat consumption (M = 3.63%, SD = 0.09%) compared with adolescents with earlier DLMO timing (M = 4.10%, SD = 0.58%; d = 0.70). Furthermore, DLMO timing was positively correlated with BMI z-score, with later timing being associated with higher BMI z-scores (r(46) = .294, p = .047). These findings were consistent when examining BMI z-scores following dichotomizing DLMO into early and late groups; those with early DLMO had moderately lower BMI z-scores (M = 0.20, SD = 1.01) than those with later DLMO (M = 0.83, SD = 0.58; d = −0.70; see Table 4). While no other relationships between continuous DLMO and dietary and physical outcomes were noted, we did observe nonsignificant but medium effects of dichotomized DLMO in %protein and % sugar, with late DLMO consuming more %protein (M = 3.96%, SD = 1.24%) and more %sugar (M = 6.01%, SD = 2.05%) compared with early DLMO (M = 3.42%, SD = 0.89%; M = 5.22%, SD = 1.23%; d’s = −0.54 and −0.52, respectively). No other group differences emerged in dietary outcomes or physical activity outcomes across later vs earlier circadian timing.
Table 4.
Group differences in obesity-related outcomes among early circadian timing vs late circadian timing
| Early DLMO (N = 32) | Late DLMO (N = 15) | |||||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | df | t | P | Cohen’s d | |
| Total energy (kcal/day) | 2176.19 | 652.47 | 2163.79 | 676.47 | 44 | 0.06 | .953 | 0.02 (−0.61, 0.65) |
| % Protein (g/kcal per day) | 3.42 | 0.89 | 3.96 | 1.24 | 44 | -1.69 | .098 | −0.54 (−1.18, 0.10) |
| % Fat (g/kcal per day) | 4.10 | 0.58 | 3.63 | 0.85 | 44 | 2.92 | .034 | 0.70 (0.05, 1.34) |
| % Carbohydrates (g/kcal per day) | 12.68 | 1.59 | 13.22 | 2.12 | 44 | −0.97 | .338 | −0.31 (−0.94, 0.32) |
| % Sugar (g/kcal per day) | 5.22 | 1.23 | 6.01 | 2.05 | 44 | −1.62 | .113 | −0.52 (−1.15, 0.12) |
| % Added sugar (g/kcal per day) | 0.75 | 0.29 | 0.80 | 0.36 | 44 | −0.54 | .589 | −0.17 (−0.79, 0.45) |
| Fruit (serving/day) | 0.96 | 0.87 | 1.62 | 3.09 | 44 | −1.13 | .264 | −0.36 (−0.99, 0.27) |
| Vegetables (serving/day) | 1.07 | 0.72 | 1.17 | 0.72 | 44 | −0.42 | .678 | −0.13 (−0.76, 0.50) |
| Sedentary (minutes/day) | 541.78 | 64.67 | 560.36 | 16.87 | 39 | −1.05 | .300 | −0.35 (−0.99, 0.31) |
| Light (minutes/day) | 36.96 | 12.04 | 33.46 | 8.174 | 39 | 0.97 | .336 | 0.32 (−0.33, 0.97) |
| MVPA (minutes/day) | 55.53 | 19.46 | 59.70 | 15.65 | 39 | −0.69 | .492 | −0.23 (−0.88, 0.42) |
| BMI z-score | 0.20 | 1.01 | 0.83 | 0.58 | 44 | −2.18 | .034 | −0.70 (−1.34, −0.51) |
| Number of meals (per day) | 4.67 | 1.26 | 4.90 | 3.35 | 44 | −0.35 | .732 | −0.11 (−0.74, 0.52) |
| Final mealtime (per day) | 19:57 | 1:30 | 20:15 | 1:38 | 44 | −0.59 | .559 | −0.19 (−0.80, 0.43) |
*Significant (p ≤ .05). P-values were determined by a series of independent samples t-tests to examine whether variables differed for early sleep timing vs late sleep timing.
Relationship with CM
There were no relationships between continuous CM on BMI or any dietary or physical activity outcome (see Table 3). However, following dichotomizing the sample into adolescents experiencing CM versus adolescents sleeping in an aligned fashion, those experiencing CM had a moderately higher averaged daily caloric intake (2391.81 kilocalories, SD = 673.44) compared with those with circadian alignment (1940.04 kilocalories, SD = 474.88; d = −0.76; see Table 5). Interestingly, there were no differences in %macronutrient consumption, servings of fruits and vegetables consumed, numbers of meals consumed, or the timing of the last meal of the day across alignment groups (see Table 5). However, there was a nonsignificant but medium effect of CM group on minutes spent in sedentary activity, with adolescents with CM spending ~34 more minutes in sedentary activity per day compared with aligned adolescents (misaligned = 562.63 minutes, SD = 20.71; aligned = 528.00 minutes, SD = 77.17; d = −0.65), and on light physical activity, with adolescents with CM spending ~6 less minutes in light physical activity compared with aligned adolescents (misaligned = 33.27 minutes, SD = 8.29; aligned = 39.02 minutes, SD = 13.73; d = 0.52); no differences were no noted in minutes spent MVPA activity estimates across alignment groups (see Table 5).
Table 5.
Group differences in obesity-related outcomes among adolescents with CM vs circadian alignment
| Aligned sleep (N = 18) | Misaligned sleep (N = 25) | |||||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | df | t | P | Cohen’s d | |
| Total energy (kcal/day) | 1940.04 | 474.88 | 2391.81 | 673.45 | 40 | −2.43 | .020 | −0.76 (−1.39, −0.12) |
| % Protein (g/kcal per day) | 3.48 | 1.07 | 3.72 | 1.07 | 40 | −0.73 | .467 | −0.23 (−0.84, 0.39) |
| % Fat (g/kcal per day) | 3.93 | 0.49 | 4.00 | 0.81 | 40 | −0.32 | .751 | −0.10, (−0.71, 0.51) |
| % Carbohydrates (g/kcal per day) | 13.02 | 1.59 | 12.64 | 1.93 | 40 | 0.07 | .507 | 0.21 (−0.41, 0.82) |
| % Sugar (g/kcal per day) | 5.26 | 1.15 | 5.65 | 1.86 | 40 | −0.78 | .441 | −0.24 (−0.86, 0.37) |
| % Added sugar (g/kcal per day) | 0.75 | 0.31 | 0.79 | 0.34 | 40 | −0.40 | .691 | −0.16 (−0.74, 0.49) |
| Fruit (serving/day) | 1.01 | 0.94 | 1.33 | 2.41 | 40 | −0.54 | .594 | −0.17 (−0.78, 0.45) |
| Vegetables (serving/day) | 0.91 | 0.56 | 1.17 | 0.69 | 40 | −1.26 | .213 | −0.39 (−0.99, 0.22) |
| Sedentary (minutes/day) | 528.00 | 77.16 | 562.63 | 20.71 | 36 | −1.98 | .056 | −0.65 (−1.30, −0.02) |
| Light (minutes/day) | 39.02 | 13.73 | 33.27 | 8.29 | 36 | 1.60 | .119 | 0.52 (−0.13, 1.17) |
| MVPA (minutes/day) | 61.47 | 19.59 | 53.26 | 17.81 | 36 | 1.35 | .185 | 0.44 (−0.21, 1.09) |
| BMI z-score | 0.24 | 1.06 | 0.62 | 0.65 | 40 | −1.45 | .154 | −0.45 (−1.07, 0.17) |
| Number of meals (per day) | 4.38 | 0.86 | 4.90 | 2.74 | 40 | −0.78 | .440 | −0.24 (−0.86, 0.37) |
| Final mealtime (per day) | 19:52 | 1:16 | 20:14 | 1:37 | 40 | −0.78 | .443 | −0.22 (−0.83, 0.40) |
*Significant (p ≤ .05). P-values were determined by a series of independent samples t-tests to examine whether variables differed for aligned sleep vs misaligned sleep.
Relationship with sleep duration
There were no significant relationships between continuous weekday sleep duration and BMI, dietary outcomes, and physical activity outcomes (see Table 3). There were nonsignificant but medium effects of dichotomized sleep duration (short vs adequate) on %carbohydrates consumed and servings of fruits per day. Specifically, adolescents obtaining adequate sleep consumed more %carbohydrates (M = 13.41%, SD = 1.87%) and servings of fruit (M = 1.68, SD = 2.92) compared with adolescents obtaining short sleep (M = 12.55%, SD = 1.34%; M = 0.81, SD = 0.45; d’s = −0.56 and −0.50, respectively; see Table 6). There were no significant group differences between those with short sleep versus adequate sleep duration on BMI, any other dietary outcome, or any physical activity outcome (see Table 6).
Table 6.
Group differences in obesity-related outcomes among adolescents with short sleep vs adequate sleep
| Short sleep (N = 31) | Adequate sleep (N = 16) | |||||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | df | t | P | Cohen’s d | |
| Total energy (kcal/day) | 2151.16 | 669.94 | 2285.48 | 589.42 | 44 | −0.67 | .504 | −0.21 (−0.82, 0.40) |
| % Protein (g/kcal per day) | 3.67 | 1.09 | 3.23 | 0.92 | 44 | 1.37 | .179 | 0.42 (−0.19, 1.03) |
| % Fat (g/kcal per day) | 4.04 | 0.41 | 3.88 | 0.95 | 44 | 0.76 | .448 | 0.24 (−0.37, 0.85) |
| % Carbohydrates (g/kcal per day) | 12.55 | 1.34 | 13.41 | 1.87 | 44 | −1.81 | .078 | −0.56 (−1.17, 0.06) |
| % Sugar (g/kcal per day) | 5.28 | 1.16 | 5.93 | 0.02 | 44 | −1.39 | .172 | −0.46 (−1.08), 0.18) |
| % Added sugar (g/kcal per day) | 0.79 | 0.30 | 0.81 | 0.35 | 44 | −0.23 | .816 | −0.07 (−0.68, 0.54) |
| Fruit (serving/day) | 0.81 | 0.45 | 1.68 | 2.92 | 44 | −1.62 | .113 | −0.50 (−1.11, 0.12) |
| Vegetables (serving/day) | 1.00 | 0.66 | 1.27 | 0.77 | 44 | −1.21 | .233 | −0.37 (−0.98, 0.24) |
| Sedentary (minutes) | 547.13 | 62.88 | 557.44 | 24.57 | 40 | −0.55 | .587 | −0.19 (−0.86, 0.49) |
| Light (minutes) | 34.02 | 11.35 | 37.68 | 13.23 | 40 | −0.90 | .372 | −0.31 (−0.98, 0.37) |
| MVPA (minutes) | 58.08 | 20.46 | 58.34 | 12.90 | 40 | −0.04 | .968 | −0.01 (−0.68, 0.66) |
| BMI z-score | 0.55 | 0.82 | 0.22 | 1.11 | 44 | 1.17 | .250 | 0.37 (−0.26, 0.98) |
| Number of meals (per day) | 4.78 | 2.43 | 4.46 | 1.43 | 44 | 0.49 | .626 | 0.15 (−0.46, 0.76) |
| Final mealtime (per day) | 19:53 | 1:33 | 20:09 | 1:30 | 44 | −0.56 | .573 | −0.18 (−0.78, 0.43) |
*Significant (p ≤ .05). P-values were determined by a series of independent samples t-tests to examine whether variables differed for short sleep vs adequate sleep.
Discussion
The goal of this study was to examine the unique relationship of sleep timing, CM, and sleep duration on obesity-related outcomes in healthy adolescents. More specifically, we were interested in comparing how adolescents experiencing early versus late circadian timing, aligned versus misaligned circadian timing, and adequate sleep versus short sleep duration differ in their BMI, average dietary intake, macronutrient intake, number of meals, timing of the last meal of the day, and average sedentary, light, and MVPA levels. We observed that adolescents with later circadian timing had higher BMI than adolescents with earlier circadian timing, as well as consumed less fat but more protein and sugar, compared with adolescents with earlier circadian timing. Furthermore, adolescents with CM were more likely to eat more calories, spend more time in sedentary activity, and spend less time in light physical activity, compared with adolescents with better circadian alignment. Finally, adolescents obtaining adequate sleep consumed more carbohydrates and servings of fruits compared with adolescents with short sleep. These findings offer novel information about various sleep and circadian domains uniquely related to dietary and physical activity outcomes in a critical developmental population.
Our first major finding demonstrated that adolescents with later circadian timing were more likely to have a higher BMI than those with earlier circadian timing (by an increase of 0.63 BMI z-score units). Later sleep timing has been similarly associated with higher BMI in adults [46]. A BMI z-score of 0.20 or greater is considered clinically significant by the United States Preventive Services Task Force, with such changes being associated with improved cardiometabolic health [47]. In younger children, earlier bedtimes have resulted in greater BMI z-scores following a weight-management intervention [48], and in young adults, advancing bedtime earlier resulted in decreased appetite and cravings for sweet/salty foods [49]. Our findings also suggested that timing may influence macronutrient consumption patterns in adolescents, with those with later DLMO timing eat less fat but more protein and sugar. While future studies are needed to understand how timing influences micronutrient pattern consumption, our findings paired with previous literature may suggest that promoting earlier sleep timing may potentially serve as an obesity prevention target in adolescents.
We also found that adolescents with CM were more likely to consume ~450 more calories compared with adolescents with circadian alignment. Previous manipulation studies have found similar relationships between CM and overall caloric intake and meal frequency in adults [21]. While we did not observe significant changes in the percentage of macronutrient consumption by daily caloric intake, additional research has demonstrated that increased social jetlag, which may indicate risk for CM, is related to higher sugar-sweetened beverage [50], fat and oil [51], and unhealthy food consumption [52], as well as increased BMI [52]. However, to the best of our knowledge, no other studies have used gold-standard physiological measurements to determine CM indexes to explore such relationships in adolescent groups. These mixed findings suggest that future research on this topic is warranted.
In a similar vein, adolescents with CM spent more time in sedentary behavior and less time in light physical activity compared with adolescents with circadian alignment, though no differences were noted across CM groups on physical activity. In adolescents and adults, CM has been associated with physical activity levels [21, 52], though fewer studies have explored the role of CM on sedentary activity. Recent reviews have illustrated that poor sleep may impact sedentary activity more directly than MVPA [18, 19]. Sedentary activity imparts a substantial risk for the development of obesity, independent of physical activity levels, as it significantly alters metabolic functioning [53, 54]. Given the paucity of research on CM and sedentary activity, future exploration of the intersection of CM and sedentary behavior is especially warranted.
Beyond timing and alignment, we also examined whether adolescents meeting sleep recommendations [55] (i.e. obtaining an average of 8 hours of sleep during the weekday) differed from adolescents failing to meet this recommendation. We observed that adolescents obtaining adequate sleep consumed more carbohydrates and more servings of fruit compared with adolescents obtaining short sleep. While previous adolescent experimental sleep restriction research has demonstrated that adolescents consume more carbohydrates when undergoing sleep restriction than when undergoing sleep extension [56], these increases in carbohydrates were accompanied by increases in foods higher in a glycemic load. Our findings seem to suggest that adolescents getting healthy sleep may consume more carbohydrates, but rather than having these increases be driven by corresponding increases in sugar/added sugar, their carbohydrate count may be driven by increasing their fruit intake.
Over the past decade, there has been a call to move beyond simply examining sleep duration and to rather evaluate multiple dimensions of sleep and how they relate to health [57]. This study highlights the justification for such a call, demonstrating that by examining sleep from a multimodal perspective, we were able to uncover unique mechanisms that link poor sleep to obesity-related outcomes, namely CM and circadian timing. Furthermore, while we observed that the amount of sleep adolescents engaged (i.e. sleep duration) in influenced carbohydrate and fruit consumption, it did not significantly relate to BMI, the majority of dietary outcomes, or physical activity patterns. Instead, it appears that circadian timing and CM held greater predictive validity for weight and weight-related behaviors in adolescents. Previous literature has posed that CM and circadian preference could be heavily related to obesity risk due to several factors, including poorer inhibitory control (i.e. worse regulation over food choices and timing), emotional reactivity (i.e. emotional eating), metabolic disturbances, snacking, meal timing, meal skipping, and sedentary behaviors [58]. While our findings are cross-sectional and did not examine mediating factors, moderating factors, or causality, our findings affirm that circadian timing and CM may be critical factors to examine when seeking to better understand adolescent health.
Furthermore, these findings can directly inform prevention models that aim to improve adolescent health and prevent obesity. Unfortunately, most adolescent weight-related interventions have not historically included sleep as a component of the treatment [59], though there is evidence that adolescents with better baseline sleep have increased weight loss outcomes following standard weight-loss interventions [60, 61]. Furthermore, there is preliminary evidence that adolescent sleep timing may be effectively advanced earlier into the morning particularly those with circadian phase delay [62–65] and that CM can be diminished following adolescent sleep interventions [64, 66]. As such, advancing the timing of adolescent sleep and decreasing CM may serve as a salient intervention target for weight-related interventions. One recent pilot study demonstrated that by improving sleep by shifting bedtimes earlier, adolescents were able to increase their consumption of low glycemic index foods (e.g. fruits) and decrease the desire to consume higher glycemic foods compared with adolescents who were unable to shift their bedtime earlier [67]. These preliminary findings suggest that future obesity prevention efforts should consider addressing CM and sleep timing factors as part of intervention procedures to improve dietary patterns and enhance weight-loss outcomes.
Our study holds multiple strengths that set it apart in the field of adolescent sleep health research. This study is one of the first endeavors to contrast how sleep duration and circadian factors relate to physical health outcomes in adolescents, providing a unique standpoint in adolescent health research and paving the way for more nuanced studies in the future. Furthermore, the utilization of gold-standard methodologies for both predictor and outcome variables is a particular strength, including actigraphy, DLMO, and direct measurements of height and weight. Reliance on these methods reduces desirability bias in our participants.
However, this study is not without limitations. Due to the study’s cross-sectional design, causal inferences cannot be made, even as key trends are identified. Future research should continue to explore relationships between CM and obesity-related outcomes in adolescents by using similar physiological measures within experimental manipulation protocols. Additionally, while dietary recalls have been shown to accurately represent macronutrient intake in adults [34] and to be comparable to clinical interviews in adolescents [35], they are also subject to difficulties such as recall bias, underreporting, and misunderstanding of instructions. Future studies may incorporate clinically administered dietary interviews, photographic methods (taking pictures before and after consumption), or using apps/technology to continuously monitor dietary intake to minimize errors associated with self-reporting. Additionally, given this study’s explorative nature, our sample size was comparatively small and underpowered to detect small to medium effect sizes. Of note, our sample was largely homogenous, composed of predominantly high-income, white adolescents, which limits the generalizability of our study, especially given that historically minoritized groups may be especially at risk for poor sleep and obesity [68], particularly among adolescents [69]. Additionally, by excluding adolescents with obesity or severe sleep difficulty, we may have limited the variability of our data (thus limiting our power) and the generalizability of our research findings. Similarly, having adolescents maintain a consistent bedtime/waketime in the 3 days leading up to the DLMO appointment may have reduced our ecological validity, and not accounting for napping behaviors may have influenced obesity-related outcomes. Finally, our use of dichotomizing our predictor variables to examine group differences in obesity-related outcomes and having multiple comparisons limits our statistical validity. Therefore, we encourage future research to use larger sample sizes, account for multiple comparisons, seek to include more diverse populations, minimize eating/sleep/activity disruptions, and use longitudinal studies to evaluate these relationships continuously over time.
In conclusion, this study demonstrates that circadian factors such as circadian timing and the presence of CM are unique predictors of obesity-related outcomes in healthy adolescents, beyond sleep duration. Specifically, adolescents who have later circadian timing are more likely to have a higher BMI than those with earlier circadian timing and have altered macronutrient consumption. Additionally, adolescents who are experiencing CM (compared with adolescents who are relatively aligned to their circadian rhythms) are more likely to consume more calories, spend less time in light physical activity, and spend more time in sedentary activity, compared with those who are experiencing circadian alignment. Shorter sleep duration was also associated with less carbohydrate and fruit consumption, compared with obtaining adequate sleep. These findings give insight into potential mechanisms that link poor sleep with obesity outcomes in adolescents.
Acknowledgments
We extend our heartfelt gratitude to all the adolescent participants who generously contributed their time to provide insights into their sleep and health behaviors. Their invaluable participation has been crucial in advancing our understanding of the intricate relationship between sleep health and obesity risk among teenagers.
Contributor Information
Kara McRae Duraccio, Department of Psychology, Brigham Young University, Provo, UT, USA.
Lindsey Lee, Department of Psychology, Brigham Young University, Provo, UT, USA.
Isabella D Wright, Department of Psychology, Brigham Young University, Provo, UT, USA.
Sarah Kamhout, Department of Psychology, Brigham Young University, Provo, UT, USA.
Nathan Boris, Department of Psychology, Brigham Young University, Provo, UT, USA.
Victoria Zhang, Department of Psychology, Brigham Young University, Provo, UT, USA.
Isaac Wilkins, Department of Psychology, Brigham Young University, Provo, UT, USA.
Author contributions
Kara Duraccio (Conceptualization [lead], Data curation [lead], Formal analysis [lead], Funding acquisition [lead], Investigation [equal], Methodology [equal], Project administration [equal], Writing—original draft [equal], Writing—review & editing [lead]), Lindsey M. Lee (Investigation [equal], Project administration [equal], Writing—original draft [equal], Writing—review & editing [supporting]), Isabella D. Wright (Data curation [equal], Formal analysis [supporting], Investigation [equal], Methodology [equal], Project administration [equal], Writing—original draft [equal], Writing—review & editing [equal]), Sarah Kamhout (Formal analysis [supporting], Investigation [supporting], Writing—original draft [equal], Writing—review & editing [equal]), Nathan Borris (Investigation [supporting], Methodology [supporting], Writing—original draft [supporting]), Victoria Zhang (Methodology [supporting], Project administration [supporting], Writing—original draft [equal]), and Isaac Wilkins (Investigation [supporting], Methodology [supporting], Writing—original draft [supporting])
Funding
Brigham Young University funded this study through start-up funds.
Conflict of interest statement
None. The authors report there are no competing interests to declare.
Data Availability
The data that support the findings of this study are available from the corresponding author, Kara Duraccio, upon reasonable request.
References
- 1. Maslowsky J, Ozer EJ.. Developmental trends in sleep duration in adolescence and young adulthood: evidence from a national United States sample. J Adolesc Health. 2014;54(6):691–697. doi: https://doi.org/ 10.1016/j.jadohealth.2013.10.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Keyes KM, Maslowsky J, Hamilton A, Schulenberg J.. The great sleep recession: changes in sleep duration among US adolescents, 1991-2012. Pediatrics. 2015;135(3):460–468. doi: https://doi.org/ 10.1542/peds.2014-2707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Matricciani L, Olds T, Petkov J.. In search of lost sleep: secular trends in the sleep time of school-aged children and adolescents. Sleep Med Rev. 2012;16(3):203–211. doi: https://doi.org/ 10.1016/j.smrv.2011.03.005 [DOI] [PubMed] [Google Scholar]
- 4. Fatima Y, Doi SAR, Al Mamun A.. Sleep problems in adolescence and overweight/obesity in young adults: is there a causal link? Sleep Health. 2018;4(2):154–159. doi: https://doi.org/ 10.1016/j.sleh.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 5. Twenge JM, Krizan Z, Hisler G.. Decreases in self-reported sleep duration among U.S. adolescents 2009-2015 and association with new media screen time. Sleep Med. 2017;39:47–53. doi: https://doi.org/ 10.1016/j.sleep.2017.08.013 [DOI] [PubMed] [Google Scholar]
- 6. Paruthi S, Brooks LJ, D’Ambrosio C, et al. Recommended amount of sleep for pediatric populations: a consensus statement of the american academy of sleep medicine. J Clin Sleep Med. 2016;12(6):785–786. doi: https://doi.org/ 10.5664/jcsm.5866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wheaton AG, Jones SE, Cooper AC, Croft JB.. Short sleep duration among middle school and high school students - United States, 2015. MMWR Morb Mortal Wkly Rep. 2018;67(3):85–90. doi: https://doi.org/ 10.15585/mmwr.mm6703a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Crowley SJ, Fournier CL, Eastman CI.. Late bedtimes prevent circadian phase advances to morning bright light in adolescents. Chronobiol Int. 2018;35(12):1748–1752. doi: https://doi.org/ 10.1080/07420528.2018.1504784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lucien JN, Ortega MT, Shaw ND.. Sleep and puberty. Curr Opin Endocr Metab Res. 2021;17:1–7. doi: https://doi.org/ 10.1016/j.coemr.2020.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Crowley SJ, Wolfson AR, Tarokh L, Carskadon MA.. An update on adolescent sleep: new evidence informing the perfect storm model. J Adolesc. 2018;67:55–65. doi: https://doi.org/ 10.1016/j.adolescence.2018.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kuula L, Halonen R, Lipsanen J, Pesonen AK.. Adolescent circadian patterns link with psychiatric problems: a multimodal approach. J Psychiatr Res. 2022;150:219–226. doi: https://doi.org/ 10.1016/j.jpsychires.2022.03.056 [DOI] [PubMed] [Google Scholar]
- 12. Dolsen EA, Harvey AG.. Dim light melatonin onset and affect in adolescents with an evening circadian preference. J Adolesc Health. 2018;62(1):94–99. doi: https://doi.org/ 10.1016/j.jadohealth.2017.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hasler BP, Clark DB.. Circadian misalignment, reward-related brain function, and adolescent alcohol involvement. Alcohol Clin Exp Res. 2013;37(4):558–565. doi: https://doi.org/ 10.1111/acer.12003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hisler GC, Dickinson DL, Bruce SA, Hasler BP.. Preliminary evidence that misalignment between sleep and circadian timing alters risk‐taking preferences. J Sleep Res. 2023;32(2):e13728. doi: https://doi.org/ 10.1111/jsr.13728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hahn C, Cowell JM, Wiprzycka UJ, et al. Circadian rhythms in executive function during the transition to adolescence: The effect of synchrony between chronotype and time of day. Dev Sci. 2012;15(3):408–416. doi: https://doi.org/ 10.1111/j.1467-7687.2012.01137.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Russo PM, Biasi V, Cipolli C, Mallia L, Caponera E.. Sleep habits, circadian preference, and school performance in early adolescents. Sleep Med. 2017;29:20–22. doi: https://doi.org/ 10.1016/j.sleep.2016.09.019 [DOI] [PubMed] [Google Scholar]
- 17. Dolsen EA, Wyatt JK, Harvey AG.. Sleep, circadian rhythms, and risk across health domains in adolescents with an evening circadian preference. J Clin Child Adolesc Psychol. 2019;48(3):480–490. doi: https://doi.org/ 10.1080/15374416.2017.1416620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duraccio KM, Simmons DM, Beebe DW, Byars KC.. Relationship of overweight and obesity to insomnia severity, sleep quality, and insomnia improvement in a clinically referred pediatric sample. J Clin Sleep Med. 2022;18(4):1083–1091. doi: https://doi.org/ 10.5664/jcsm.9806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krietsch KN, Chardon ML, Beebe DW, Janicke DM.. Sleep and weight-related factors in youth: a systematic review of recent studies. Sleep Med Rev. 2019;46:87–96. doi: https://doi.org/ 10.1016/j.smrv.2019.04.010 [DOI] [PubMed] [Google Scholar]
- 20. Miller AL, Lumeng JC, LeBourgeois MK.. Sleep patterns and obesity in childhood. Curr Opin Endocrinol Diabetes Obes. 2015;22(1):41–47. doi: https://doi.org/ 10.1097/MED.0000000000000125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baron KG, Reid KJ, Kim T, et al. Circadian timing and alignment in healthy adults: associations with BMI, body fat, caloric intake and physical activity. Int J Obes (Lond). 2017;41(2):203–209. doi: https://doi.org/ 10.1038/ijo.2016.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Broussard JL, Van Cauter E.. Disturbances of sleep and circadian rhythms: novel risk factors for obesity. Curr Opin Endocrinol Diabetes Obes. 2016;23(5):353–359. doi: https://doi.org/ 10.1097/MED.0000000000000276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morales-Ghinaglia N, Fernandez-Mendoza J.. Sleep variability and regularity as contributors to obesity and cardiometabolic health in adolescence. Obesity (Silver Spring). 2023;31(3):597–614. doi: https://doi.org/ 10.1002/oby.23667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jankovic N, Schmitting S, Stutz B, Kruger B, Buyken A, Alexy U.. Alignment between timing of “highest caloric intake” and chronotype in relation to body composition during adolescence: the DONALD Study. Eur J Nutr. 2024;63(1):253–265. doi: https://doi.org/ 10.1007/s00394-023-03259-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Golley RK, Maher CA, Matricciani L, Olds TS.. Sleep duration or bedtime? Exploring the association between sleep timing behaviour, diet and BMI in children and adolescents. Int J Obes (Lond). 2013;37(4):546–551. doi: https://doi.org/ 10.1038/ijo.2012.212 [DOI] [PubMed] [Google Scholar]
- 26. Hasler BP, Soehner AM, Wallace ML, et al. Experimentally imposed circadian misalignment alters the neural response to monetary rewards and response inhibition in healthy adolescents. Psychol Med. 2021;52:1–9. doi: https://doi.org/ 10.1017/S0033291721000787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hasler BP, Soehner AM, Wallace ML, et al. Experimentally imposed circadian misalignment alters the neural response to monetary rewards and response inhibition in healthy adolescents. Psychol Med. 2022;52(16):3939–3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Janssen KC, Phillipson S, O’Connor J, Johns MW.. Validation of the Epworth Sleepiness Scale for children and adolescents using Rasch analysis. Sleep Med. 2017;33:30–35. doi: https://doi.org/ 10.1016/j.sleep.2017.01.014 [DOI] [PubMed] [Google Scholar]
- 29. Koopman-Verhoeff ME, Gredvig-Ardito C, Barker DH, Saletin JM, Carskadon MA.. Classifying pubertal development using child and parent report: comparing the pubertal development scales to tanner staging. J Adolesc Health. 2020;66(5):597–602. doi: https://doi.org/ 10.1016/j.jadohealth.2019.11.308 [DOI] [PubMed] [Google Scholar]
- 30. Berlin JE, Storti KL, Brach JS.. Using activity monitors to measure physical activity in free-living conditions. Phys Ther. 2006;86(8):1137–1145. [PubMed] [Google Scholar]
- 31. Migueles JH, Cadenas-Sanchez C, Ekelund U, et al. Accelerometer data collection and processing criteria to assess physical activity and other outcomes: a systematic review and practical considerations. Sports Med. 2017;47(9):1821–1845. doi: https://doi.org/ 10.1007/s40279-017-0716-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cleland I, Kikhia B, Nugent C, et al. Optimal placement of accelerometers for the detection of everyday activities. Sensors (Basel). 2013;13(7):9183–9200. doi: https://doi.org/ 10.3390/s130709183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Freedson P, Pober D, Janz KF.. Calibration of accelerometer output for children. Med Sci Sports Exerc. 2005;37(11 Suppl):S523–S530. doi: https://doi.org/ 10.1249/01.mss.0000185658.28284.ba [DOI] [PubMed] [Google Scholar]
- 34. Moshfegh AJ, Rhodes DG, Baer DJ, et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr. 2008;88(2):324–332. doi: https://doi.org/ 10.1093/ajcn/88.2.324 [DOI] [PubMed] [Google Scholar]
- 35. Hughes AR, Summer SS, Ollberding NJ, Benken LA, Kalkwarf HJ.. Comparison of an interviewer-administered with an automated self-administered 24 h (ASA24) dietary recall in adolescents. Public Health Nutr. 2017;20(17):3060–3067. doi: https://doi.org/ 10.1017/S1368980017002269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pandi-Perumal SR, Smits M, Spence W, et al. Dim light melatonin onset (DLMO): a tool for the analysis of circadian phase in human sleep and chronobiological disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(1):1–11. doi: https://doi.org/ 10.1016/j.pnpbp.2006.06.020 [DOI] [PubMed] [Google Scholar]
- 37. Carskadon MA, Acebo C, Richardson GS, Tate BA, Seifer R.. An approach to studying circadian rhythms of adolescent humans. J Biol Rhythms. 1997;12(3):278–289. doi: https://doi.org/ 10.1177/074873049701200309 [DOI] [PubMed] [Google Scholar]
- 38. Duraccio KM, Kamhout S, Wright ID, Rugh KE, Miskin J, Amdal M.. Multimodal assessment of circadian sleep health in predicting mental health outcomes in adolescents. Front Sleep. 2023;2:1177878. [Google Scholar]
- 39. Crowley SJ, Van Reen E, LeBourgeois MK, et al. A longitudinal assessment of sleep timing, circadian phase, and phase angle of entrainment across human adolescence. PLoS One. 2014;9(11):e112199. doi: https://doi.org/ 10.1371/journal.pone.0112199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Quante M, Kaplan ER, Cailler M, et al. Actigraphy-based sleep estimation in adolescents and adults: a comparison with polysomnography using two scoring algorithms. Nat Sci Sleep. 2018;10:13–20. doi: https://doi.org/ 10.2147/NSS.S151085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP.. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. doi: https://doi.org/ 10.1093/sleep/26.3.342 [DOI] [PubMed] [Google Scholar]
- 42. Short MA, Gradisar M, Lack LC, Wright H, Carskadon MA.. The discrepancy between actigraphic and sleep diary measures of sleep in adolescents. Sleep Med. 2012;13(4):378–384. doi: https://doi.org/ 10.1016/j.sleep.2011.11.005 [DOI] [PubMed] [Google Scholar]
- 43. Sadeh A, Sharkey M, Carskadon MA.. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994;17(3):201–207. doi: https://doi.org/ 10.1093/sleep/17.3.201 [DOI] [PubMed] [Google Scholar]
- 44. van Kooten J, Jacobse STW, Heymans MW, de Vries R, Kaspers GJL, van Litsenburg RRL.. A meta-analysis of accelerometer sleep outcomes in healthy children based on the Sadeh algorithm: the influence of child and device characteristics. Sleep. 2021;44(4):zsaa231. [DOI] [PubMed] [Google Scholar]
- 45. Pandi-Perumal SR, Smits M, Spence W, et al. Dim light melatonin onset (DLMO): a tool for the analysis of circadian phase in human sleep and chronobiological disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(1):1–11. doi: https://doi.org/ 10.1016/j.pnpbp.2006.06.020 [DOI] [PubMed] [Google Scholar]
- 46. Baron KG, Reid KJ, Kern AS, Zee PC.. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring). 2011;19(7):1374–1381. doi: https://doi.org/ 10.1038/oby.2011.100 [DOI] [PubMed] [Google Scholar]
- 47. Grossman DC, Bibbins-Domingo K, US Preventive Services Task Force, et al. Screening for obesity in children and adolescents: US Preventive Services Task Force recommendation statement. JAMA. 2017;317(23):2417–2426. doi: https://doi.org/ 10.1001/jama.2017.6803 [DOI] [PubMed] [Google Scholar]
- 48. Simon SL, Goetz AR, Meier M, Brinton J, Zion C, Stark LJ.. Sleep duration and bedtime in preschool‐age children with obesity: relation to BMI and diet following a weight management intervention. Pediatr Obes. 2019;14(11):e12555. doi: https://doi.org/ 10.1111/ijpo.12555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tasali E, Chapotot F, Wroblewski K, Schoeller D.. The effects of extended bedtimes on sleep duration and food desire in overweight young adults: a home-based intervention. Appetite. 2014;80:220–224. doi: https://doi.org/ 10.1016/j.appet.2014.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang KGM, Conklin A.. Association between social jetlag and sugar-sweetened beverages (SSBs) in adolescents in Western Canada. Eur J Public Health. 2022;33(2):287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Borisenkov MF, Polugrudov AS, Paderin NM, Bakutova LA.. Young inhabitants of the North with late chronotype and social jetlag consume more high-calorie foods and alcohol. Biol Rhythm Res. 2019;50(3):418–428. doi: https://doi.org/ 10.1080/09291016.2018.1455867 [DOI] [Google Scholar]
- 52. Liang F, Fu J, Xu Y, et al. Associations of social jetlag with dietary behavior, physical activity and obesity among Chinese adolescents. Nutrients. 2022;14(3):510. doi: https://doi.org/ 10.3390/nu14030510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Julian V, Bergsten P, Forslund A, et al. Sedentary time has a stronger impact on metabolic health than moderate to vigorous physical activity in adolescents with obesity: a cross-sectional analysis of the Beta-JUDO study. Pediatr Obes. 2022;17(7):e12897. doi: https://doi.org/ 10.1111/ijpo.12897 [DOI] [PubMed] [Google Scholar]
- 54. Miller MA, Kruisbrink M, Wallace J, Ji C, Cappuccio FP.. Sleep duration and incidence of obesity in infants, children, and adolescents: a systematic review and meta-analysis of prospective studies. Sleep. 2018;41(4). [DOI] [PubMed] [Google Scholar]
- 55. Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep Health. 2015;1(4):233–243. doi: https://doi.org/ 10.1016/j.sleh.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 56. Duraccio KM, Whitacre C, Krietsch KN, et al. Losing sleep by staying up late leads adolescents to consume more carbohydrates and a higher glycemic load. Sleep. 2022;45(3):zsab269. doi: https://doi.org/ 10.1093/sleep/zsab269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Meltzer LJ, Williamson AA, Mindell JA.. Pediatric sleep health: it matters, and so does how we define it. Sleep Med Rev. 2021;57:101425. doi: https://doi.org/ 10.1016/j.smrv.2021.101425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Duraccio KM, Krietsch KN, Chardon ML, Van Dyk TR, Beebe DW.. Poor sleep and adolescent obesity risk: a narrative review of potential mechanisms. Adolesc Health Med Ther. 2019;10:117–130. doi: https://doi.org/ 10.2147/AHMT.S219594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Thomason DL, Lukkahatai N, Kawi J, Connelly K, Inouye J.. A systematic review of adolescent self-management and weight loss. J Pediatr Health Care. 2016;30(6):569–582. doi: https://doi.org/ 10.1016/j.pedhc.2015.11.016 [DOI] [PubMed] [Google Scholar]
- 60. Valrie CR, Bond K, Lutes LD, Carraway M, Collier DN.. Relationship of sleep quality, baseline weight status, and weight-loss responsiveness in obese adolescents in an immersion treatment program. Sleep Med. 2015;16(3):432–434. doi: https://doi.org/ 10.1016/j.sleep.2014.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sallinen BJ, Hassan F, Olszewski A, et al. Longer weekly sleep duration predicts greater 3-month BMI reduction among obese adolescents attending a clinical multidisciplinary weight management program. Obes Facts. 2013;6(3):239–246. doi: https://doi.org/ 10.1159/000351819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lau T, Lovato N, Lack L.. Evaluation of a portable light device for phase advancing the circadian rhythm in the home environment. Sleep Biol Rhythms. 2018;16:405–411. doi: https://doi.org/ 10.1007/s41105-018-0167-5 [DOI] [Google Scholar]
- 63. Misiunaite I, Eastman CI, Crowley SJ.. Circadian phase advances in response to weekend morning light in adolescents with short sleep and late bedtimes on school nights. Front Neurosci. 2020;14:99. doi: https://doi.org/ 10.3389/fnins.2020.00099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Harvey AHK, Dolsen E, Dong L, et al. Modifying the impact of eveningness chronotype (“night-owls”) in youth: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2018;57(10):742–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Crowley SJ, Velez SL, Killen LG, Cvengros JA, Fogg LF, Eastman CI.. Extending weeknight sleep of delayed adolescents using weekend morning bright light and evening time management. Sleep. 2023;46(1):zsac202. doi: https://doi.org/ 10.1093/sleep/zsac202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gasperetti CE, Dong L, Harvey AG.. The effect of the transdiagnostic sleep and circadian intervention (TranS-C) on actigraphic estimates of sleep and rest-activity rhythms in adolescents with an evening circadian preference. Sleep Health. 2022;8(2):191–194. doi: https://doi.org/ 10.1016/j.sleh.2021.10.007 [DOI] [PubMed] [Google Scholar]
- 67. Asarnow LD, Greer SM, Walker MP, Harvey AG.. The impact of sleep improvement on food choices in adolescents with late bedtimes. J Adolesc Health. 2017;60(5):570–576. doi: https://doi.org/ 10.1016/j.jadohealth.2016.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Guglielmo DGJ, Chung J, Rogers AE, Hale L.. Racial/ethnic sleep disparities in US school-aged children and adolescents: a review of the literature. Sleep Health. 2017;4(1):68–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yip T, Cheon YM, Wang Y, Cham H, Tryon W, El-Sheikh M.. Racial disparities in sleep: associations with discrimination among ethnic/racial minority adolescents. Child Dev. 2020;91(3):914–931. doi: https://doi.org/ 10.1111/cdev.13234 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Kara Duraccio, upon reasonable request.