Abstract
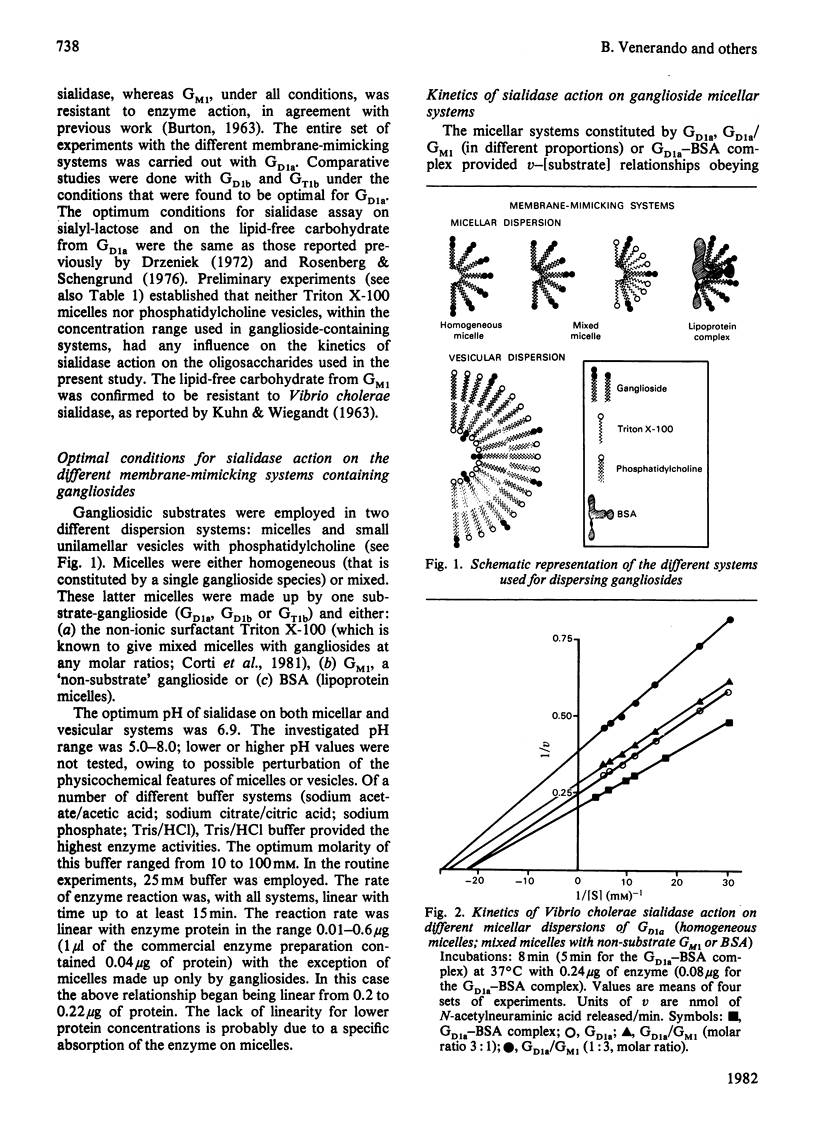
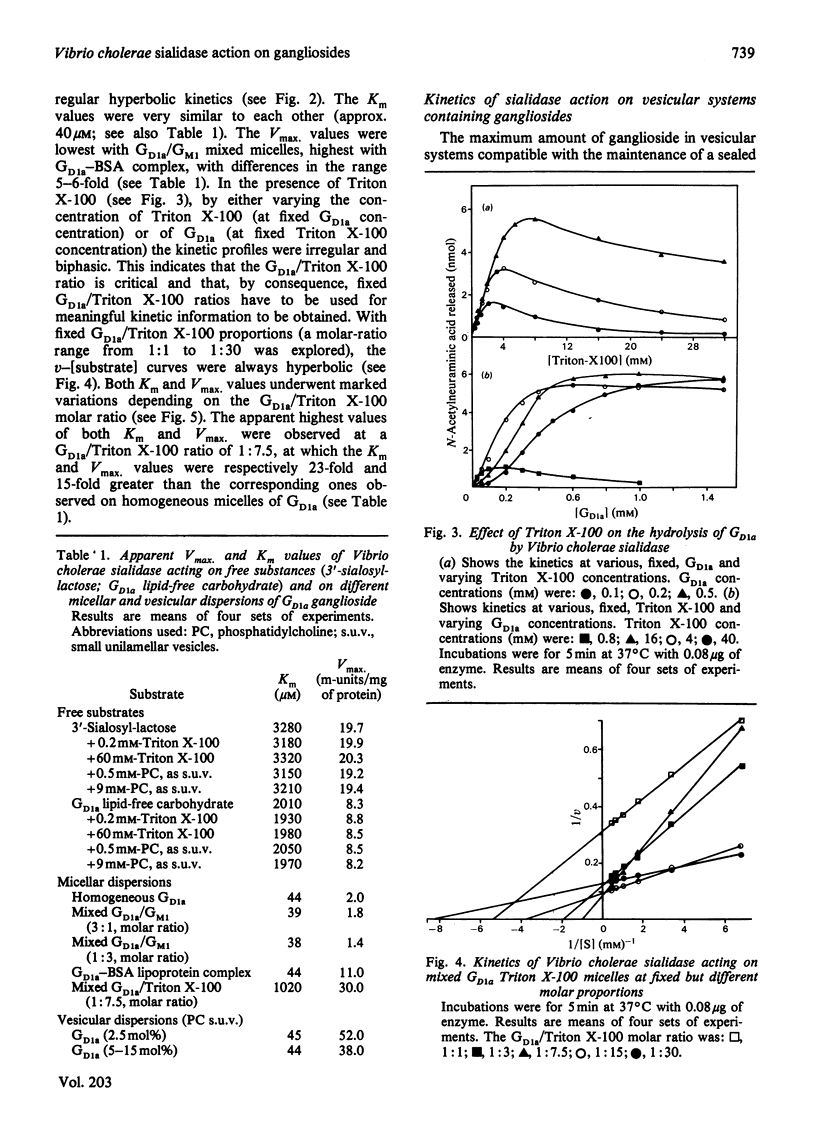
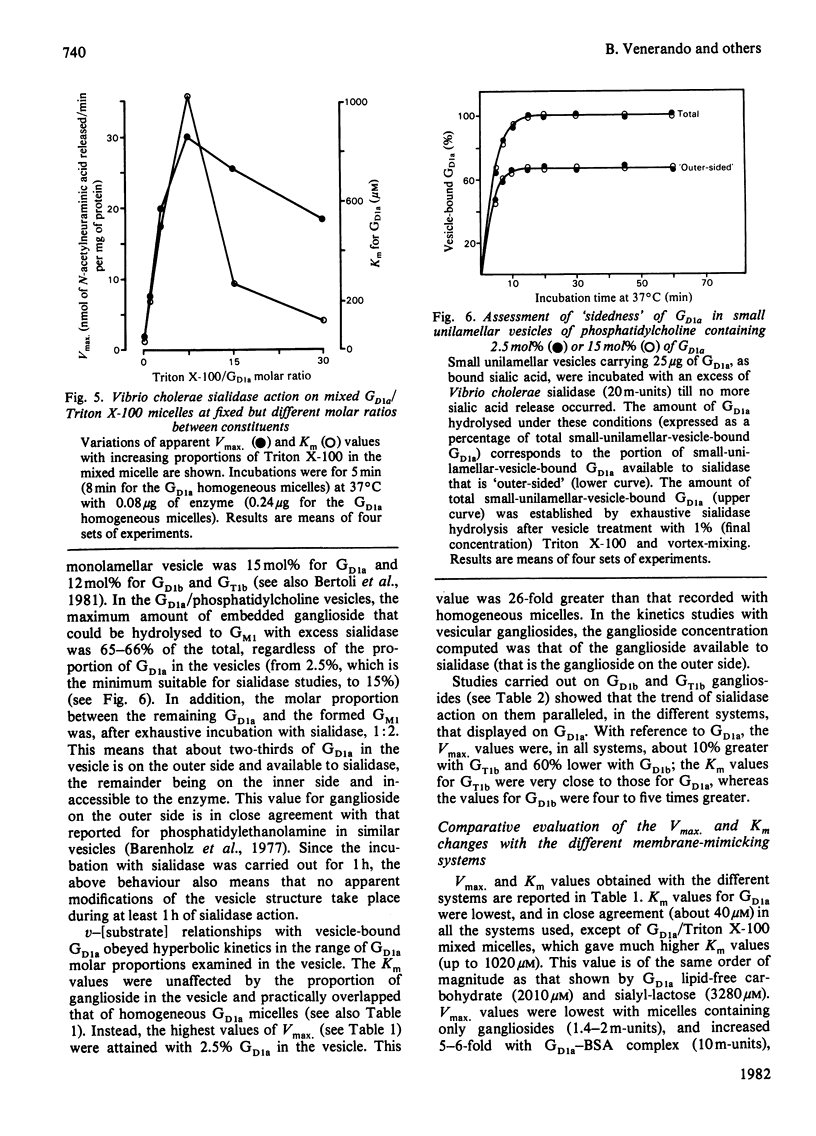
Gd1a, Gd1b and Gt1b gangliosides were dispersed in the following membrane-mimicking systems: (a) homogeneous micelles; (b) mixed micelles with Gm1 ganglioside (which is resistant to the enzyme action), Triton X-100 or bovine serum albumin; (c) small unilamellar vesicles of egg phosphatidylcholine. The effect of dispersion on sialic acid release by Vibrio cholerae sialidase was studied. As reference substrates freely interacting with the enzyme the lipid-free carbohydrates of Gd1a and 3′-sialosyl-lactose were employed. The apparent Vmax. of the enzyme was, with all the gangliosides, dependent on the type of ganglioside dispersion. It was lowest for homogeneous micelles and mixed micelles with ganglioside Gm1, and increased about 6-fold for ganglioside/bovine serum albumin lipoprotein micelles, 15-fold for mixed-ganglioside/Triton X-100 micelles (optimal molar ratio 1:7.5) and 30-fold for phosphatidylcholine vesicles containing 2.5 mol% ganglioside (this proportion was optimal for enzyme activity on the vesicles). For ganglioside Gd1a, the activity on Triton X-100 mixed micelles and on mixed vesicles was even greater (3- and 6-fold respectively) than that displayed on Gd1a lipid-free carbohydrate. With each of the used gangliosides the apparent Km values were very similar values for homogeneous micelles and vesicular dispersions, but showed marked increases for Triton X-100 mixed micelles, approaching the values exhibited by reference oligosaccharides. Triton X-100 micelles and phosphatidylcholine vesicles did not appreciably alter the kinetics of sialidase action on 3′-sialosyl-lactose and on Gd1a lipid-free carbohydrate, indicating that the above effects are dependent on the intrinsic characteristics of the membrane-like systems containing gangliosides.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON R. M. THE ACTION OF NEURAMINIDASE FROM CLOSTRIDIUM PERFRINGENS ON GANGLIOSIDES. J Neurochem. 1963 Jul;10:503–512. doi: 10.1111/j.1471-4159.1963.tb09853.x. [DOI] [PubMed] [Google Scholar]
- Barenholz Y., Gibbes D., Litman B. J., Goll J., Thompson T. E., Carlson R. D. A simple method for the preparation of homogeneous phospholipid vesicles. Biochemistry. 1977 Jun 14;16(12):2806–2810. doi: 10.1021/bi00631a035. [DOI] [PubMed] [Google Scholar]
- Bertoli E., Masserini M., Sonnino S., Ghidoni R., Cestaro B., Tettamanti G. Electron paramagnetic resonance studies on the fluidity and surface dynamics of egg phosphatidylcholine vesicles containing gangliosides. Biochim Biophys Acta. 1981 Oct 2;647(2):196–202. doi: 10.1016/0005-2736(81)90246-7. [DOI] [PubMed] [Google Scholar]
- Caimi L., Lombardo A., Preti A., Wiesmann U., Tettamanti G. Optimal conditions for the assay of fibroblast neuraminidase with different natural substrates. Biochim Biophys Acta. 1979 Nov 9;571(1):137–146. doi: 10.1016/0005-2744(79)90234-1. [DOI] [PubMed] [Google Scholar]
- Cestaro B., Barenholz Y., Gatt S. Hydrolysis of di- and trisialo gangliosides in micellar and liposomal dispersion by bacterial neuraminidases. Biochemistry. 1980 Feb 19;19(4):615–619. doi: 10.1021/bi00545a002. [DOI] [PubMed] [Google Scholar]
- Corti M., Degiorgio V., Ghidoni R., Sonnino S., Tettamanti G. Laser-light scattering investigation of the micellar properties of gangliosides. Chem Phys Lipids. 1980 Apr;26(3):225–238. doi: 10.1016/0009-3084(80)90053-5. [DOI] [PubMed] [Google Scholar]
- Corti M., Degriorgio V., Sonnino S., Ghidoni R., Masserini M., Tettamanti G. GM1-ganglioside-Triton X-100 mixed micelles: changes of micellar properties studied by laser-light scattering and enzymatic methods. Chem Phys Lipids. 1981 May;28(3):197–214. doi: 10.1016/0009-3084(81)90008-6. [DOI] [PubMed] [Google Scholar]
- Demel R. A., Geurts van Kessel W. S., Zwaal R. F., Roelofsen B., van Deenen L. L. Relation between various phospholipase actions on human red cell membranes and the interfacial phospholipid pressure in monolayers. Biochim Biophys Acta. 1975 Sep 16;406(1):97–107. doi: 10.1016/0005-2736(75)90045-0. [DOI] [PubMed] [Google Scholar]
- Drzeniek R. Viral and bacterial neuraminidases. Curr Top Microbiol Immunol. 1972;59:35–74. doi: 10.1007/978-3-642-65444-2_2. [DOI] [PubMed] [Google Scholar]
- Formisano S., Johnson M. L., Lee G., Aloj S. M., Edelhoch H. Critical micelle concentrations of gangliosides. Biochemistry. 1979 Mar 20;18(6):1119–1124. doi: 10.1021/bi00573a028. [DOI] [PubMed] [Google Scholar]
- Gatt S., Gazit B., Barenholz Y. Effect of bile salts on the hydrolysis of gangliosides, glycoproteins and neuraminyl-lactose by the neuraminidase of Clostridium perfringens. Biochem J. 1981 Jan 1;193(1):267–273. doi: 10.1042/bj1930267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio B., Cumar F. A., Caputto R. Configuration and interaction of the polar head group in gangliosides. Biochem J. 1980 Sep 1;189(3):435–440. doi: 10.1042/bj1890435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mraz W., Schwarzmann G., Sattler J., Momoi T., Seemann B., Wiegandt H. Aggregate formation of gangliosides at low concentrations in aqueous media. Hoppe Seylers Z Physiol Chem. 1980;361(2):177–185. doi: 10.1515/bchm2.1980.361.1.177. [DOI] [PubMed] [Google Scholar]
- Ohman R., Hygstedt O. The isolation of sialyllactosides with the aid of gel filtration. Anal Biochem. 1968 Jun;23(3):391–402. doi: 10.1016/0003-2697(68)90230-3. [DOI] [PubMed] [Google Scholar]
- Op den Kamp J. A., de Gier J., van Deenen L. L. Hydrolysis of phosphatidylcholine liposomes by pancreatic phospholipase A2 at the transition temperature. Biochim Biophys Acta. 1974 Apr 29;345(2):253–256. doi: 10.1016/0005-2736(74)90263-6. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957 Jun;24(3):604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. THE GANGLIOSIDES. J Lipid Res. 1964 Apr;5:145–155. [PubMed] [Google Scholar]
- Sandhoff K., Pallmann B. Membrane-bound neuraminidase from calf brain: regulation of oligosialoganglioside degradation by membrane fluidity and membrane components. Proc Natl Acad Sci U S A. 1978 Jan;75(1):122–126. doi: 10.1073/pnas.75.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnino S., Ghidoni R., Galli G., Tettamanti G. On the structure of a new, fucose containing ganglioside from pig cerebellum. J Neurochem. 1978 Oct;31(4):947–956. doi: 10.1111/j.1471-4159.1978.tb00132.x. [DOI] [PubMed] [Google Scholar]
- Tettamanti G., Bonali F., Marchesini S., Zambotti V. A new procedure for the extraction, purification and fractionation of brain gangliosides. Biochim Biophys Acta. 1973 Jan 19;296(1):160–170. doi: 10.1016/0005-2760(73)90055-6. [DOI] [PubMed] [Google Scholar]
- Tomasi M., Roda L. G., Ausiello C., D'Agnolo G., Venerando B., Ghidoni R., Sonnino S., Tettamanti G. Interaction of GMI ganglioside with bovine serum albumin: formation and isolation of multiple complexes. Eur J Biochem. 1980 Oct;111(2):315–324. doi: 10.1111/j.1432-1033.1980.tb04944.x. [DOI] [PubMed] [Google Scholar]
- WIEGANDT H., BASCHANG G. DIE GEWINNUNG DES ZUCKERANTEILES DER GLYKOSPHINGOLIPIDE DURCH OZONOLYSE UND FRAGMENTIERUNG. Z Naturforsch B. 1965 Feb;20:164–166. [PubMed] [Google Scholar]


