Abstract
Porokeratosis is a group of chronic dermatoses characterized by the presence of cornoid lamellae. Disseminated superficial actinic porokeratosis (DSAP) is a common subtype, presenting as multiple small annular scaly lesions primarily in sun-exposed areas. While previous studies have documented DSAP in prostate cancer patients, the association with androgen deprivation therapy (ADT) has not been reported. In this report, we describe an elderly patient with advanced prostate cancer, who developed DSAP subsequent to undergoing ADT. We present the clinical, dermoscopic, and histopathological evaluations, and discuss the potential role of ADT in the pathogenesis of DSAP.
Key words: disseminated superficial actinic porokeratosis, prostate cancer, androgen deprivation therapy, goserelin
Introduction
Porokeratosis comprises a group of chronic dermatoses due to abnormal keratinization processes and is characterized histopatho-logically by the presence of cornoid lamellae. Among the subtypes of porokeratosis, disseminated superficial actinic porokeratosis (DSAP) is among the most commonly observed, manifesting as multiple to numerous small annular scaly lesions in sun-exposed areas.1,2 DSAP typically emerges in the third to fourth decade of life and is believed to be associated with genetic predisposition and exposure to ultraviolet (UV) radiation. Various factors, including immunosuppression, malignancy, radiation therapy, and medications, have also been reported to be related to this condition.1 While previous studies have reported the occurrence of DSAP in prostate cancer (PC) patients,3,4 an association between androgen deprivation therapy (ADT) and DSAP has not been documented in the literature. ADT, the standard treatment for advanced PC, has been associated with various cutaneous side effects.5 This case report aims to describe the clinical features, diagnosis, and management of DSAP in an elderly patient with PC undergoing ADT, and to explore the potential association between the two conditions.
Case Report
A 73-year-old male presented with multiple pruritic, erythematous patches on both forearms that had been present for the past 2 years. The initial lesions were the size of a corn kernel and have since expanded to the size of a coin. Itching was exacerbated during activity or sweating and improved in cool environments. No similar patches were observed on other parts of the body. The patient had a history of PC with spinal metastasis, having undergone surgery 7 years prior and received multiple sessions of chemoradiation. He had been receiving ADT in the form of goserelin injections for the past 3 years. The patient had frequent sun exposure due to his gardening activities. There was no history of similar complaints previously or among other family members. On examination, multiple annular erythematous patches with white scales were observed on the extensor surfaces of both forearms (Figure 1 A-B), while dermoscopy revealed keratin rims and branched vessels (Figure 1 C-D). The patient was provisionally diagnosed with porokeratosis, and treatment with 0.25% des oximetasone ointment was initiated. After 3 months of therapy, the patches became hyperpigmented and non-pruritic.
The patient returned after 3 months due to the recurrence of pruritus in the previously affected areas, with no new lesions reported. The patient continued to receive ADT regularly. Upon examination, multiple violaceous to hyperpigmented patches and plaques with peripheral scales were observed. Dermoscopy revealed pigmentation along the keratin rim, shiny white structures and brown dots within the lesions. Histopathological examination confirmed the presence of cornoid lamellae, in addition to interface dermatitis and dermal solar elastosis (Figure 2). Based on the clinical presentation, dermoscopic findings, and histopathology, a diagnosis of DSAP was established. Treatment with desoximetasone ointment was resumed, and the patient was educated on sun protection, including the use of protective clothing and sunscreen. Regular follow-up was scheduled to evaluate the possibility of neoplastic transformation.
Figure 1.
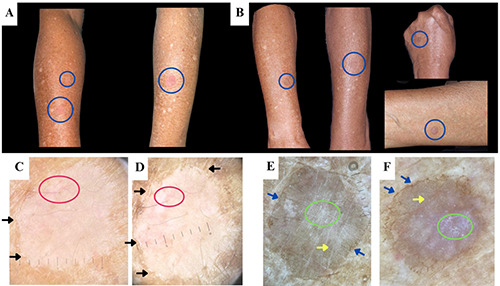
Clinical presentation of the porokeratotic lesions (blue circles) during the initial visit (A) and during pruritus recurrence after 3 months (B). Dermoscopy performed during the initial presentation (C-D) revealed the presence of keratin rims (black arrows) and branched vessels (red circles), while pigmentation along keratin rim (blue arrows), shiny white structures (green circles), and brown dots (yellow arrows) were observed during pruritus recurrence (E-F).
Figure 2.
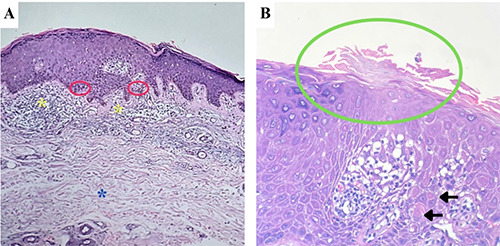
(A) Histopathological examination (H&E, 100x) revealed vacuolar alteration (red circles), lymphohistiocytic infiltrates on dermoepidermal junction (yellow asterisks), and dermal solar elastosis (blue asterisk). (B) At higher magnification (H&E, 400x), a parakeratotic column with hypogranulosis (green circle) and few dyskeratotic cells (black arrows), resembling a cornoid lamella, was observed at the edge of the biopsy specimen.
Discussion
The occurrence of DSAP in an elderly patient with PC undergoing ADT raises intriguing questions regarding the potential relationship among these three conditions. A systematic literature search on the PubMed, EMBASE, and Google Scholar databases, using search terms related to porokeratosis, DSAP, PC, and ADT, identified five reports of porokeratosis arising in PC patients (Table 1). O’Donnell et al.4 reported a case of DSAP in a series of patients undergoing suramin therapy for metastatic PC, while Hsueh et al.3 mentioned a case of DSAP associated with PC in a retrospective study of 39 patients. However, the latter did not provide detailed case information. A case of giant porokeratosis coexisting with disseminated superficial porokeratosis in an elderly patient with PC has also been described.6 Additionally, two case studies reported radiotherapy-induced localized porokeratosis.7,8 Hence, to the best of our knowledge, the specific association between DSAP and ADT has not been previously reported.
DSAP typically manifests on the extremities, although isolated facial actinic forms have rarely been reported.9 In this case, the onset of DSAP was temporally closer to the initiation of ADT compared to PC onset. The Naranjo score, indicating the likelihood of a drug side effect association, yielded a score of +2 in this case, implying the possibility of ADT-related adverse effects. While a direct causal link between ADT and DSAP in this patient cannot be definitively established, it is imperative to consider the potential association between the two conditions.
Current hypotheses suggest that genetic predisposition plays a critical role in the development of all porokeratosis subtypes, with mutations in the mevalonate kinase (MVK) pathway genes being significant contributors to DSAP.10 The pathogenesis of DSAP is proposed to involve a two-hit mechanism. In this concept, various factors, including UV radiation or immunosuppression, can act as the second “hit” or triggering event, leading to clonal keratinocyte proliferation.11 On the other hand, androgens have been demonstrated to influence keratinocyte maturation and keratinization through direct effects and modulation of growth factors.12 Consequently, androgen deprivation may potentially serve as a secondary triggering event in the development of DSAP. Further, the potential involvement of androgen deficiency in the pathogenesis of DSAP could partially account for its higher prevalence in females.
A recent study reported that the presence of keratin rim is the most commonly observed dermoscopic finding in porokeratosis, with pigmentation along keratin rim present in 38.5% of cases.13 In this case, we sequentially observed both patterns, suggesting that pigmentation along the keratin rim may represent long-standing or previously treated lesions. Brown dots and shiny white structures, which have also been described in porokeratosis,13,14 were observed. While the classic angulated parakeratotic column was not identified, the focal absence of the granular layer and the presence of dyskeratotic cells beneath a parakeratotic column supported the presence of a cornoid lamella, thus affirming the diagnosis of porokeratosis. It is worth noting that other histopathological features, such as interface dermatitis and solar elastosis, which were present in this case, can also be observed in DSAP.14,15
The treatment of DSAP is notoriously challenging. In this patient, a potent topical corticosteroid was able to reduce inflammation and pruritus associated with DSAP. However, it is important to note that treatment response varies among individuals, and some patients may require multiple modalities. Several topical medications, including vitamin D analogs, diclofenac, 5-fluorouracil, and more recently, statins, have been used with varying success.1,16 Cryotherapy and lasers have also been utilized.1 A recent study by Novice et al.17 reported the occurrence of malignant transformation in up to 29.3% of DSAP cases. UV radiation, in addition to its role in the development of DSAP, might contribute to malignant transformation of porokeratotic lesions. Therefore, educating patients about sun protection is crucial. Given the recalcitrant nature of DSAP and its potential for neo-plastic transformation, long-term follow-up is warranted.
Conclusions
This case report highlights a potential association between DSAP, PC, and ADT in an elderly patient. Clinicians should be aware of this possible association when managing elderly patients with PC. Further research is warranted to elucidate the underlying mechanisms of the potential association discussed in this paper.
Table 1.
Previous reports of porokeratosis in patients with prostate cancer.
| Author (Year) | Ref. | Porokeratosis subtype | Age | Cancer duration | Cancer treatment | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| O’Donnell et al. (1992) | 4 | DSAP | NR | NR | Suramin | NR | NR |
| Batchelor et al. (2010) | 7 | LP | NR | 6 years | Radiotherapy, bicalutamide, leupror | elin Liquid nitrogen cryotherapy | Complete resolution |
| Shah et al. (2018) | 8 | LP | 68 | NR | Radiotherapy | Topical imiquimod and cryotherapy | Complete resolution |
| Itoi-Ichi et al. (2020) | 6 | GP + DSAP | 66 | 4 years | NR | Topical steroids, oral etretinate & antiallergic agents | Partial response |
| Hsueh YT, et al. (2020) | 3 | DSAP | NR | NR | NR | NR | NR |
NR, not reported; DSAP, diffuse superficial actinic porokeratosis; GP, giant porokeratosis; LP, localized porokeratosis.
Availability of data and materials
All data underlying the findings are fully available.
References
- 1.Vargas-Mora P, Morgado-Carrasco D, Fustà-Novell X. Porokeratosis: A review of its pathophysiology, clinical manifestations, diagnosis, and treatment. Actas Dermosifiliogr. 2020;111:545–60. [DOI] [PubMed] [Google Scholar]
- 2.Tan LS, Chong WS. Porokeratosis in Singapore: an Asian perspective. Australas J Dermatol. 2012;53:40–4. [DOI] [PubMed] [Google Scholar]
- 3.Hsueh YT, Hsu TC, Hsu CK, et al. Disseminated superficial porokeratosis and disseminated superficial actinic porokeratosis: A case series of 39 patients. Dermatologica Sin. 2020;38:221–4. [Google Scholar]
- 4.O’Donnell BP, Dawson NA, Weiss RB. Suramin-induced skin reactions. Arch Dermatol. 1992;128:75. [PubMed] [Google Scholar]
- 5.Teo MY, Rathkopf DE, Kantoff P. Treatment of advanced prostate cancer. Annu Rev Med. 2019;70:479–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Itoi-Ochi S, Tanemura A, Arase N, et al. A case of giant porokeratosis coexisting disseminated superficial porokeratosis. J Cutan Immunol Allergy. 2020;3:139–41. [Google Scholar]
- 7.Batchelor JM, Fife K, Burrows NP. Localized porokeratosis secondary to ionizing radiotherapy for prostate carcinoma. Arch Dermatol. 2010;146. [DOI] [PubMed] [Google Scholar]
- 8.Shah PR, Richardson CT, Scott G, et al. Radiation-induced genital porokeratosis and basal cell carcinoma. J Clin Urol. 2018;11:440–1. [Google Scholar]
- 9.Chua IY, Lee J, Chiam L. Pruritic papules on the nose in a 25-year-old female. Indian J Dermatol. 2014;59:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang S-Q, Jiang T, Li M, et al. Exome sequencing identifies MVK mutations in disseminated superficial actinic porokeratosis. Nat Genet. 2012;44:1156–60. [DOI] [PubMed] [Google Scholar]
- 11.Atzmony L, Choate KA. Second-hit somatic mutations in mevalonate pathway genes underlie porokeratosis. J Invest Dermatol. 2019;139:2409–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumtornrut C, Yamauchi T, Koike S, et al. Androgens modulate keratinocyte differentiation indirectly through enhancing growth factor production from dermal fibroblasts. J Dermatol Sci. 2019;93:150–8. [DOI] [PubMed] [Google Scholar]
- 13.Zaar O, Polesie S, Navarrete-Dechent C, et al. Dermoscopy of porokeratosis: results from a multicentre study of the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2021;35:2091–6. [DOI] [PubMed] [Google Scholar]
- 14.Waqar MU, Cohen PR, Fratila S. Disseminated superficial actinic porokeratosis (DSAP): A case report highlighting the clinical, dermatoscopic, and pathology features of the condition. Cureus. 2022;14:e26923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joshi R, Mesquita L. Interface dermatitis without cornoid lamellae is a pitfall in the diagnosis of porokeratosis: A report of three cases. Indian J Dermatol Venereol Leprol. 2016;82:70. [DOI] [PubMed] [Google Scholar]
- 16.Santa Lucia G, Snyder A, Lateef A, et al. Safety and efficacy of topical lovastatin plus cholesterol cream vs topical lovastatin cream alone for the treatment of disseminated superficial actinic porokeratosis. JAMA Dermatol. 2023;159:488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novice T, Nakamura M, Helfrich Y. The malignancy potential of porokeratosis: single-center retrospective study. Cureus. 2021;13:e13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data underlying the findings are fully available.


