Abstract
Introduction:
Injuries to the liver and small bowel are common in polytrauma. While there are currently a variety of accepted damage control techniques to expeditiously manage such injuries, morbidity and mortality remains high. Pectin polymers have previously been shown to effectively seal visceral organ injuries ex-vivo through physiochemical entanglement with the glycocalyx. We sought to compare the standard of care for the management of penetrating liver and small bowel injuries with a pectin based bioadhesive patch in a live animal model.
Methods:
15 adult male swine underwent a laparotomy with standardized laceration to the liver. Animals were randomized to 1 of 3 treatment arms: packing with laparotomy pads (N=5), suture repair (N=5), or pectin patch repair (N=5). Following two hours of observation, fluid was evacuated from the abdominal cavity and weighed. Next a full thickness small bowel injury was created, and animals randomized to either a sutured repair (N=7) or pectin patch repair (N=8). The segment of bowel was then pressurized with saline, and the burst pressure recorded.
Results:
All animals survived the protocol to completion. There were not clinically significant differences between groups regarding baseline vitals or laboratory studies. On one-way ANOVA there was a statistically significant difference between groups regarding post liver repair blood loss (26ml suture vs 33ml pectin vs 142ml packing, p < 0.01). On post-hoc analysis there was no statistically significant difference between suture and pectin (p=0.9). Post repair small bowel burst pressures were similar between pectin and suture repair (234 vs 224 mmhg, p=0.7).
Conclusion:
Pectin-based bioadhesive patches performed similarly to the standard of care for the management of liver lacerations and full thickness bowel injuries. Further testing is warranted to assess the biodurability of a pectin patch repair as it may offer a simple option to effectively temporize traumatic intra-abdominal injuries.
Study type:
Therapeutic
Level of evidence:
Not applicable, basic science animal study
Keywords: Polytrauma, Damage Control Surgery, Bioadhesive Patch
Introduction
Trauma remains the lead cause of death in young patients(1), with intra-abdominal injuries occurring in 30% of severely injured trauma patients(2). The liver and spleen are the two most commonly injured intra-abdominal organs in the setting of blunt trauma(3, 4), with the small bowel being frequently injured due to penetrating trauma(5). Often these injuries coincide in the severely injured polytrauma patient, mandating operative intervention. Decompensated patients may require damage control surgery with control of hemorrhage or enteral spillage as the priority. While there are currently a variety of options to expeditiously manage such injuries, morbidity and mortality remains high(6–10). Intraoperative bleeding may occur from the raw surface of the liver in addition to post-operative complications such as delayed hemorrhage, biliary leaks, fistula development, and liver abscesses(9, 11, 12). Similarly, small bowel anastomotic failure may lead to leaks, fistula formation, infectious complications, or death(13). Numerous adjuncts have been used to prevent such complications including various biologic surgical glues, staple line reinforcements, suture reinforcements, and buttressed tissue repairs all with varying degrees of success(14–19).
Currently, there are no FDA approved or commonly used sealants for management of visceral organ injuries. Available sealants tend to fail because they are either non-adherent or weakly adherent to the mesothelial glycocalyx. Failure is also due to the forces related to natural physiologic movement of visceral organs(20–24). Additionally, many sealants are potentially allogenic and/or require special storage and employment considerations further hindering their use in the emergent or austere setting(25, 26). Pectin, a natural occurring plant-derived heteropolysaccharide responsible for plants’ tensile strength and shear resistance, has been shown to bind to the visceral glycocalyx(27, 28). Proprietary bioadhesive pectin patches have outperformed traditional sealants and films in sealing pulmonary air leaks and adhering to bowel serosa in post mortem and ex-vivo animal studies(29–31). Unlike other sealants, pectin-based bioadhesives, which are structurally similar to the underlying glycocalyx, undergo a rapid physiochemical entanglement with the mesothelial glycocalyx in a manner analogous to the hook and loop mechanism of Velcro. This results in an air tight seal which may mitigate the native physiologic movement of visceral organs.
Our lab has had success in prior investigations using pectin-based bioadhesive patches to seal pleural air leaks(31). We sought to further assess the use of pectin patches for managing mesothelial disruptions of abdominal solid and hollow viscus organs in a living porcine model. In this proof-of-concept study we compare pectin-based bioadhesive patches to the standard of care for the management of liver lacerations and penetrating small bowel injury.
Methods
Animals
Research grade male Yorkshire swine 3–5-months-old (40–50kg) were used for all experiments. Institutional Animal Care and Use Committee application approval was granted prior to all experiments. The following work was compliant with the reporting guidelines provided by the ARRIVE guidelines (see supplemental digital content, SDC1). Specifically, the NIH Principles and Guidelines for Reporting Preclinical Research and the guidelines for reporting the results of experiments on mammals were utilized. Animals were excluded from analysis if they expired prior to the completion of the experiment.
Anesthesia, Intubation, and Euthanasia
Animals were sedated with intramuscular ketamine (15–33 mg/kg, Par Pharmaceutical, Woodcliff Lake, NG) and midazolam (400–500 μg, Par Pharmaceutical, Woodcliff Lake, NJ) prior to the induction of general anesthesia. After general anesthesia, animals were intubated with a 6.5 French cuffed endotracheal tube (Medtronic, Minneapolis, MN); anesthesia was maintained with 1–3% Isoflurane (Baxter, Deerfield, IL). Following intubation, a 9 Fr cordis was placed in the external jugular vein and a 5Fr catheter was placed in the common carotid artery. Animals remained sedated, ventilated, with maintenance intravenous fluids (IVF) (0.9% normal saline) running for the duration of the procedures. Animals were euthanized underneath veterinarian supervision in accordance with established protocols following completion of all experimental protocols.
Pectins
The preparation and chemical characterization of the proprietary pectin bioadhesive patch has previously been described(29). Briefly, high-methoxyl (>70% esterified) citrus pectin used in this study were obtained from a commercial source (Cargill, Minneapolis, MN, USA). The pectin powder was stored in low humidity at 25°C. The pectin patch was prepared using stepwise fluidization and dispersion with a 10,000 rpm rotor-stator mixer (L5M-A, Silverson, East Longmeadow, MA USA) for complete dissolution of powder. The 3% solution was then poured into a 10-cm diameter circular mold and cured in a 20% relative humidity environment. The films were allowed to equilibrate to ambient 40% relative humidity. To create 2-ply, conjoined films, two films were re-hydrated in a controlled humidified environment into gel form, then compressed together using custom fixtures in a materials analyzer at a force of 5N, for 30 seconds (Stable Micro Systems, Godalming, Surrey, UK). This was then placed between two weights and allowed to cure. The final 2-ply cured patch was 160um thick, translucent, and readily applied to organ surfaces with gentle pressure. Once formed the patch is simply stored at room temperature within a sealed petri dish. The 2-ply film was formed in Boston, MA, shipped in sealed petri dishes, where it was then used in the animal laboratory in Tacoma, WA.
Ex-vivo assessment of biodurability
Since the intended application would include potential exposure to bile and succus, ex-vivo experiments were conducted to determine the durability of pectin after being soaked in these media for 24 hours. Three pectin patches were cut into quarters, and a representative sample from each patch was then submerged in 10mls of freshly harvested bile or succus and stored at room temperature. Subjective evaluation was then performed to assess the biodurability of each pectin patch at 24 hours. Patches were categorized as either being dissolved, gelatinous, mixed gelatinous and solid, or solid.
Randomization
Randomization was achieved using a chit method to ensure a 1:1:1 ratio of liver treatment arms. Prior to experimentation a number was drawn regarding the planned liver treatment arm. Once removed the chit was not replaced. The same randomization processes occurred for the small bowel injury with the exception of a coin flip being used to determine which treatment arm the final animal would be assigned due to the odd number of animals for this portion of the study. The same researchers who performed randomization conducted the experiments and were not blinded to the results.
Liver injury creation and repair
Following induction, intubation, and invasive line placement, a midline laparotomy was made from the xiphoid process to the bladder. A foley catheter was placed directly into the bladder to monitor urinary output. Next a 2cm by 0.5cm deep laceration was then made sharply in the left medial lobe of the liver with #10 blade. Prior to injury creation animals were randomized to one of three liver repair arms: 1) sutured repair (n=5), 2) pectin bioadhesive patch repair (n=5, Figure 1), or 3) liver packing with laparotomy pads (n=5). Following injury creation animals underwent their respectively assigned repair.
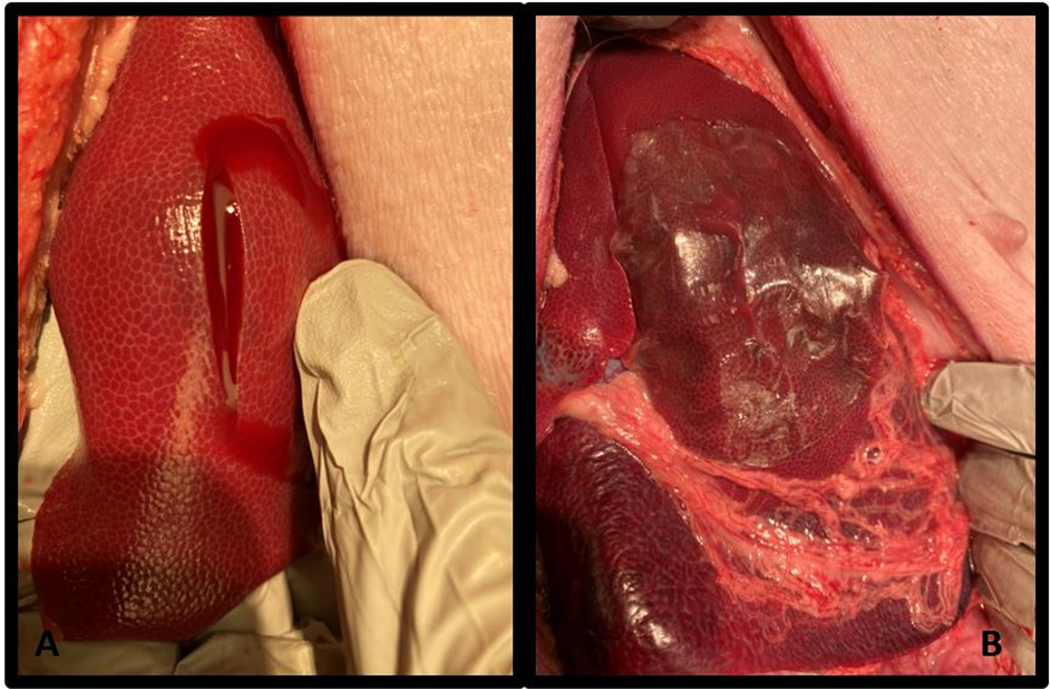
Figure 1.
Liver injury and pectin patch repair. A) Standardized liver laceration with bleeding noted from wound. B) Pectin patch repair, applied to the liver laceration is shown.
Primary suture repair was performed using a running 0 chromic suture on a CT1 needle. For pectin patch repairs, a 2-ply pectin patch was cut to size ensuring a 1–2cm circumferential overlap of the pectin to uninjured liver. Application required direct contact with dry and uninjured exposed liver surface for one minute to ensure adequate adhesion and hemorrhage control. Liver packing was performed using standard laparotomy pads placed above and below the injured lobe of liver to tamponade bleeding. Laparotomy pads were weighed prior to placement in the abdomen.
Immediately following injury repair, all remaining intra-abdominal blood and fluid was removed via suction. The midline fascia was closed with a running 0 Prolene suture and the skin edges reapproximated with towel clips. Animals remained normothermic, sedated, and ventilated for an observation period of two hours. At the end of the observation period the abdomen was reopened, and the liver examined. Any adherent blood clot over the repair was removed and weighed. Likewise, any intra-abdominal blood was evacuated via suction and weighed. For the packing arm of the study laparotomy pads were removed and weighed, with their dry weight subtracted from their post-observation weight to calculate blood loss. Vitals and labs were obtained prior to the start of the experiment and immediately prior to abdominal re-exploration. The volume of blood loss was then compared between each arm.
Small bowel injury and repair
Following the conclusion of the liver portion of the study, animals remained intubated and sedated. If required, the liver injury was definitively repaired with a running 0 chromic suture. A 5cm portion of small bowel was isolated approximately 50cms proximal to the terminal ileum using two bowel clamps. A 20-guage rounded tip catheter was secured into the bowel for intraluminal pressure monitoring. A 5mm bunch biopsy was used to make a full thickness defect on the antimesenteric side of the isolated portion of bowel. Each animal was then randomized to undergo either a pectin patch repair (n=8) or a suture repair (n=7). Pectin patch repairs were performed in a similar manner to the liver repairs (Figure 2). Two-layer sutured repairs were performed using interrupted 3–0 Vicryl sutures. The first layer of sutures were full thickness bites which reapproximated the wound edges; with a second layer of 3–0 Vicryl seromuscular Lemberted sutures. The bowel was then infused with normal saline connected to a pressure bag until the repair failed or the bowel ruptured, and the burst pressure recorded. To serve as internal controls, the process was repeated on the same bowel, without creating an injury, approximately 10 cm proximal to the injured portion of bowel.
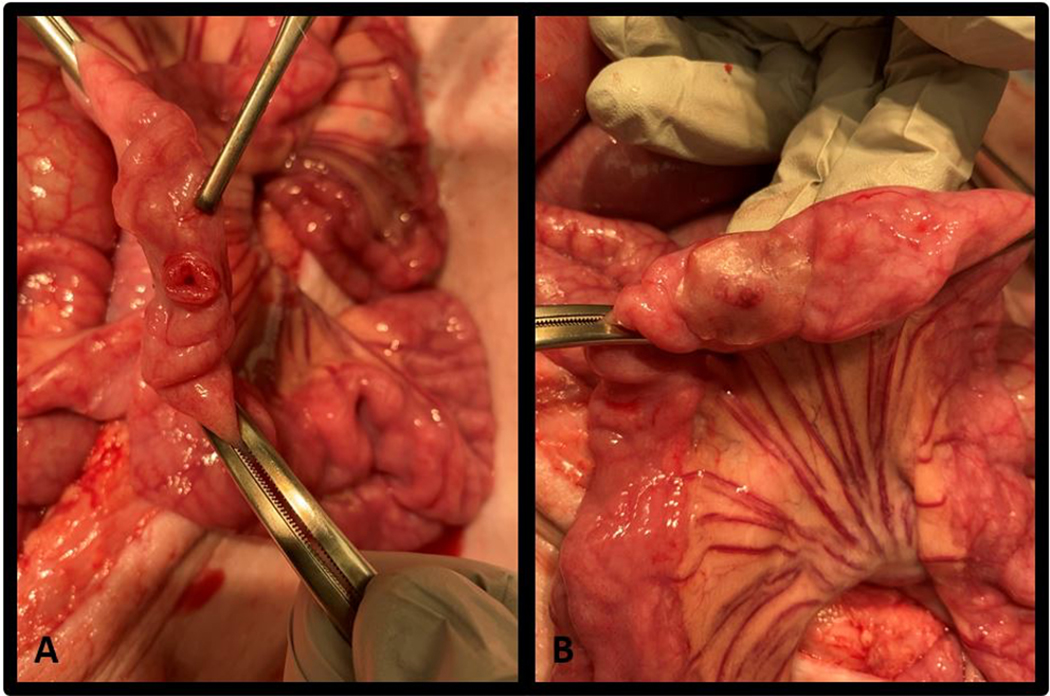
Figure 2.
Small bowel injury and pectin patch repair. A) Standardized full thickness injury to the antimesenteric border of the bowel. B) Pectin patch repair, applied to the injury prior to pressure testing is shown.
Statistics
Statistical analysis was structured as a proof-of-concept study evaluating proprietary pectin patches for use on intra-abdominal viscera. Given that there is no prior data available regarding the use of such patches on bowel injuries a power analysis could not be calculated. All data were electronically collected and stored. Linear data with normal distribution were evaluated using an analysis of variance (ANOVA) with Tukey post hoc analysis or student’s t-test. Comparisons were made between the three experimental groups for the liver data and the two experimental groups for the small bowel data. Statistical significance was set at a p value less than 0.05. Statistical analyses were performed using IBM SPSS statistics 25 (IMB Corp., Armonk, NY).
Results
Biodurability assessment
Following submersion in succus all three of the 2-ply pectin patches were noted to be in a mixed gelatinous and solid state. After soaking in bile all three of the patches were noted to be present in a gelatinous state. Representative photos of the patches following this ex-vivo experiment can be found in the supplementary materials (SDC 2).
Liver injury
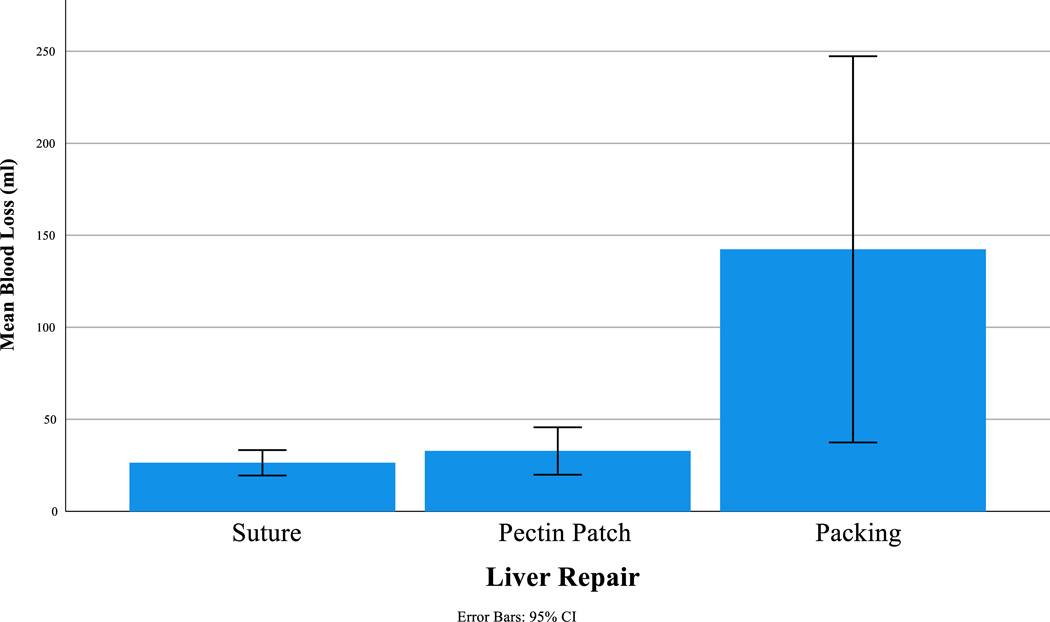
All animals survived until euthanasia. As such no animals were excluded from data analysis. No difference was seen between groups regarding weight, vitals, or labs with the exception of pH (Table 1). Similarly, following two hours of post repair observation there was no difference between groups regarding vitals or labs except for blood glucose (Table 1). On one-way ANOVA we did note that there was significantly more blood loss between liver treatment groups (p<0.01). On Tukey post-hoc analysis we found that there was significantly more blood loss from the liver packing group compared to the suture repair (142ml ± 85 vs 26ml ± 6, p<0.01) and compared to the pectin patch repair (142ml ± 85 vs 33ml ± 10, p=0.01). Pectin performed similarly to the suture repair (33ml ± 10 vs 26ml ± 6, p=0.9,) (Figure 3). At the time of re-exploration liver injuries were noted to be hemostatic for all animals in the suture and pectin arms of the study. This was also true prior to the removal of pads in the liver packing group. There was no difference between groups regarding urine output or volume of maintenance IVFs administered.
Table 1.
Liver results. Mean (standard deviation) BL= baseline.
| Suture (n=5) | Pectin Patch (n=5) | Packing ( n=5) | P-value | |
|---|---|---|---|---|
|
|
||||
| Weight (kg) | 45.7 (1.1) | 44.7 (1.2) | 44.0 (1.2) | 0.13 |
| BL Temp (F) | 99.1 (0.9) | 100.2 (1.4) | 99.8 (1.5) | 0.44 |
| BL HR (BPM) | 105 (23) | 98 (10) | 89 (16) | 0.37 |
| BL MAP (mmHg) | 70 (19) | 58 (10) | 60 (13) | 0.44 |
| BL pH | 7.41 (0.04) | 7.45 (0.06) | 7.48 (0.02) |
0.04 *s-PP 0.301 *s-P 0.04 *PP-P 0.42 |
| BL Lactate (mmol/L) | 1.1 (0.5) | 0.9 (0.2) | 1.0 (0.3) | 0.62 |
| BL Cr (mg/dL) | 0.9 (0.2) | 0.9 (0.1) | 0.8 (0.3) | 0.55 |
| BL Glucose (mg/dL) | 88 (16) | 78 (12) | 96 (40) | 0.55 |
| BL Hct (% PCV) | 22 (1) | 21 (3) | 24 (1) | 0.13 |
| BL Hgb (g/dL) | 7.6 (0.4) | 7.3 (1.0) | 8.1 (0.4) | 0.13 |
| Final Temp (F) | 98.5 (1.0) | 99.1 (2.1) | 97.9 (1.2) | 0.47 |
| Final HR (BPM) | 80 (8) | 80 (6) | 74 (5) | 0.25 |
| Final MAP (mmHg) | 52 (7) | 50 (6) | 48 (4) | 0.66 |
| Final pH | 7.45 (0.03) | 7.47 (0.03) | 7.49 (0.05) | 0.23 |
| Final Lactate (mmol/L) | 0.9 (0.2) | 0.9 (0.4) | 0.9 (0.2) | 0.96 |
| Final Cr (mg/dL) | 1.0 (0.2) | 0.9 (0.1) | 1.0 (0.1) | 0.60 |
| Final Glucose (mg/dL) | 105 (11) | 67 (27) | 39 (12) |
<0.01 *s-PP 0.012 *s-P <0.01 *PP-P 0.08 |
| Final Hct (% PCV) | 21 (1) | 20 (3) | 22 (1) | 0.27 |
| Final Hgb (g/dL) | 7.0 (0.4) | 6.8 (1.0) | 7.5 (0.4) | 0.28 |
| Total Fluids (mls) | 1382 (110) | 1316 (73) | 1308 (88) | 0.40 |
| Total UOP (mls) | 233 (45) | 224 (158) | 225 (222) | 0.99 |
| Blood loss (ml) | 26 (6) | 33 (10) | 142 (85) |
<0.01 *s-PP 0.98 *s-P <0.01 *PP-P 0.01 |
s = suture, PP= pectin patch, p= packing.
= post hoc Tuckey p-values.
Figure 3.
Post-observation blood following liver laceration repair.
Small bowel injury
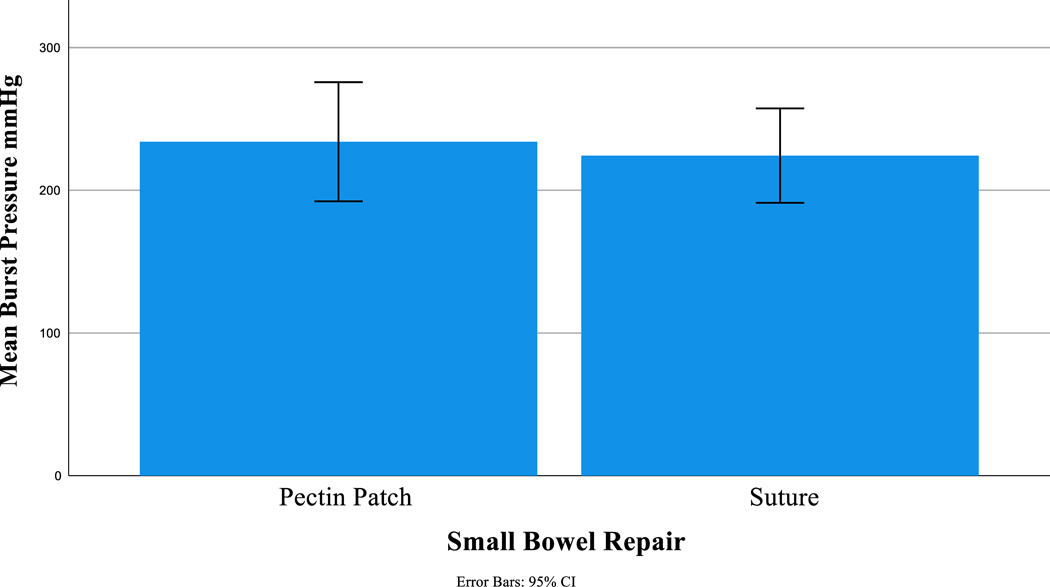
A new baseline was recorded at the beginning of the small bowel portion of the experiment and there remained no difference between groups regarding weight, vitals, or labs (Table 2). Small bowel burst pressures were supra-physiologic and similar between pectin and suture repairs (234 mmHg ± 50 vs 224 mmHg ± 36, p=0.7, Figure 4). Additionally, small bowel burst pressures were similar between the pectin group and non-injured controls (234mmHg ± 50 vs 222 mmHg ± 31, p=1.0, Table 2).
Table 2.
Small bowel results. BL=base line.
| Pectin (n=8) | Suture (n=7) | P-value | |
|---|---|---|---|
|
|
|||
| Weight (kg) | 44.3 (1.2) | 45.4 (1.3) | 0.09 |
| BL Temp (F) | 100.2 (1.1) | 99.1 (1.3) | 0.11 |
| BL HR (BPM) | 96 (20) | 99 (15) | 0.70 |
| BL MAP (mmHg) | 61 (10) | 65 (19) | 0.65 |
| BL pH | 7.45 (0.05) | 7.45 (0.06) | 0.99 |
| BL Lactate (mmol/L) | 0.9 (0.3) | 1.2 (0.4) | 0.10 |
| BL Cr (mg/dL) | 0.8 (0.3) | 0.9 (0.1) | 0.56 |
| BL Glucose (mg/dL) | 80 (11) | 96 (34) | 0.21 |
| BL Hct (% PCV) | 23 (2) | 22 (1) | 0.65 |
| BL Hgb (g/dL) | 7.7 (0.8) | 7.6 (0.5) | 0.67 |
| Final Temp (F) | 98.4 (1.5) | 98.6 (1.5) | 0.86 |
| Final HR (BPM) | 76 (7) | 80 (7) | 0.36 |
| Final MAP (mmHg) | 49 (5) | 51 (6) | 0.59 |
| End pH | 7.47 (0.04) | 7.47 (0.04) | 0.87 |
| Final Lactate (mmol/L) | 1.0 (0.3) | 0.9 (0.2) | 0.76 |
| Final Cr (mg/dL) | 1.0 (0.2) | 1.0 (0.1) | 0.79 |
| Final Glucose (mg/dL) | 61 (29) | 81 (36) | 0.26 |
| Final Hct (%PCV) | 21 (2) | 20 (2) | 0.44 |
| Final Hgb (g/dL) | 7.2 (0.8) | 6.9 (0.5) | 0.22 |
| Total Fluids (mls) | 1321 (92) | 1352 (95) | 0.53 |
| Total UOP (mls) | 232 (203) | 222 (53) | 0.90 |
| Repair Burst Pressure (mmHg) | 234 (50) | 224 (36) | 0.68 |
| Control Burst Pressure (mmHg) | 222 (31) | 222 (37) | 0.99 |
Figure 4.
Post repair burst pressure.
Discussion
In this proof-of-concept study we evaluated a proprietary 2-ply pectin-based bio-adhesive patch against standard of care for the management of liver lacerations and small bowel injuries in an in-vivo porcine model with a short observation period. We found that the pectin patches effectively adhere to the glycocalyx of both liver lacerations and full thickness bowel perforations following appropriate placement and application pressure. Importantly the pectin patches appeared to have similar results to standard interventions in the containment of blood and intraluminal contents for our liver laceration and bowel perforation. These studies findings were encouraging that pectin-based bio-adhesive patches have significant potential to be an effective product bolstering the repairs or even possibly act as a standalone treatment for solid organ and hollow viscus injuries, providing an innovative means for managing traumatic intra-abdominal injuries.
Achieving hemostasis can be particularly challenging in hepatic injuries due to its extensive vascularity and relatively large sinusoidal structures. The liver may sustain injuries that result in multiple or large raw surface areas that may not be amendable to suturing or ligation(32, 33). Hepatic hemostasis may be further hindered by trauma induced coagulopathy or underlying liver dysfunction. Disruption of the hepatic biliary tree may also lead to biliary leaks resulting in increased morbidity and mortality(34). Studies evaluating the use of fibrin-based adjuncts have reported >80% success rates at achieving hemostasis on raw liver surfaces and have been found to be effective in patients with underlying coagulopathies(19, 35, 36). Unfortunately, fibrin-based sealants typically require specialized equipment for deployment and require special storage considerations(37). They are also expensive to product and some carry a tangible risk for viral transmission or allergic reactions ranging from skin reactions to anaphylaxis(37, 38). The 2-ply pectin patches used in this study are stored at room temperature and are easy to apply without the need for specialized equipment. This and the fact that pectin is a naturally occurring and abundant material makes this product a potentially far cheaper alternative to currently available sealants. Pectin may have a theoretical advantage to packing as it may be able to serve as a definitive solution without the need for re-exploration or reintervention as is often needed with abdominal packing. We have shown in this study that pectin is able to uniquely form a sustained binding to the glycocalyx around an intra-abdominal solid organ injury to seal and control hemorrhage during a two-hour observation period.
Prevention of anastomotic leak and reinforcement of bowel repairs has long been an area of interest with multiple interventions and techniques attempted. The objective of any such adjunct is to reinforce serosal apposition(39) as breakdown of an anastomosis or repair can be catastrophic resulting in intra-abdominal sepsis, fistula development, and death. Most anastomotic failures occur 5–10 days following surgery, during tissue remodeling (40, 41). The natural dynamic nature of bowel can be disruptive to the anastomotic healing processes(30). Standard sutured or stapled repairs may also result in local ischemia at the anastomotic site further pre-disposing the repair to leak(42). Again, the 2-ply pectin patch in our study demonstrated an ability to maintain a seal despite supra-physiologic distension and contact with the intraluminal contents.
In a prior study evaluating pectin to multiple commercially available sealants for sealing pressurized bowel injuries, Zhang et al found that only SepraFilm (Baxter, Deerfield, IL) and Pectin were able to withstand 100cm H2O for >10 minutes without failing(30). Other commercially available sealants including DuraSeal (Integra Life Sciences, Plainsboro, NJ) Evicel (Ethicon, Somerville, NJ), Surgicel (Ethicon), and Coseal (Baxter) all failed in <20 seconds at such pressures. Although it is difficult to quantify in our results, we also found the pectin patch to be robust, easy to handle and apply. Unlike other commercially available films it does not crumple or undesirably adhere immediately to the operator or surrounding surfaces, making exacting application quite possible. We were able to demonstrate the ability of a pectin patch repair to seal a full thickness bowel injury at pressures, nearly three times greater than the pressure used by Zheng et al. This study adds to the body of literature that demonstrates pectin-based patches perform equal or superiorly to commercially available sealants.(30, 31) Importantly, it is the first study to report on pectin’s use for managing intra-abdominal injuries in-vivo using a living large animal model.
Pectin-based bioadhesive patches have several potential benefits for managing intra-abdominal visceral injuries. In a damage control setting they may offer an effective means to rapidly temporize both solid organ and hollow viscus disruptions, as application requires little more than applying it directly to the injured site with pressure. Additionally, pectin which is biocompatible and bioabsorbable also serves as a scaffold fore mesothelial regeneration(28, 29) while also avoiding the use of permanent foreign bodies. Due to the mechanism by which pectin interacts with the underlying glycocalyx, pectin can distribute tissue strain across a broad reinforcement area thus bolstering the repair site and maximizing perfusion(24). Also unlike fibrin glues, allergic reactions to pectin are virtually nonexistent(43). Contrary to traditional sealants, pectin does not require special instruments for employment nor do the patches require special storage or shipping considerations, which make it particularly useful in austere settings such as those experienced by the military. In a fully equipped and robust surgical setting pectin patches may have a future as an adjunct to other definitive interventions akin to staple line reinforcements or hemostatic adjuncts. However, in a resource limited setting, pectin patches may offer a light weight, inexpensive, rapidly effective, and expeditious option to temporize solid organ hemorrhage and bowel spillage.
Limitations
There are several important limitations of our study. In this study we tested a single injury pattern to both the liver and the small bowel. While our liver injury model is very reproducible, the mechanism by which we made our injury is not representative of many traumatic hepatic injuries. The same critique may be made of our small bowel injuries as we tested a single full thickness injury to the antimesenteric wall of the bowel. Further, we only tested burst pressure which may have limited our ability to identify microleaks or air leaks. Blood loss in the packing arm of the liver study was also likely skewed due to absorption of peritoneal fluid in the laparotomy pads. Nonetheless blood loss was statistically similar between the suture and pectin patch arms of the study. With our animals remaining ventilated and sedated during the observational period, we are limited in our ability to assess the effect of external forces, such as shifting weight with walking, on pectin adherence. Additionally, with the observational period limited to two hours post-repair numerous questions remain regarding the bio-durability of intra-abdominal pectin patch repairs, as well as, any sequalae, such as adhesions, may result following the intra-abdominal use of pectin. Ultimately, future large animal survival studies are needed to better understand the performance of pectin-based bioadhesive patches over a more clinically relevant time course of at least on to two weeks.
Conclusion
Pectin-based bioadhesive patches appear to adhere to the mesothelial glycocalyx of the liver and small bowel with containment of hemorrhage and intraluminal contents in the short term for managing intra-abdominal hemorrhage and enteric spillage from solid organ and hollow viscus injuries, performing similarly to the standard of care. These pectin patches may offer a novel means for managing such injuries without requiring the placement of permanent foreign bodies. Further testing is warranted to better assess the biodurability of a pectin patch repair as it may offer a rapid and simple option to effectively temporize intra-abdominal traumatic injuries.
Supplementary Material
1) The ARRIVE guidelines 2.0: author check list. Check list of the essential 10 minimum items required for reporting animal studies.
2) Biodurability testing of pectin patch. A) Pectin patch sample prior to submersion. B) Pectin patch after 24 hours in bile. C) Pectin patch after 24 hours in succus. D) Experimental layout with three pieces of pectin each submerged in 10mls of normal saline (NS), bile, and succus. E) Gelatinous pectin patch poured onto chucks pad following 24-hour submersion in bile. F) Remainder of pectin patch removed from succus following 24 hours.
Acknowledgements
We thank the Madigan Army Medical Center Department of Clinical Investigations staff including LTC Branden Maxwell, Mr. Branden Hubbard, Mrs. Joanna Dandeneau, Mrs. Christa Barta, Mr. Juan Tercero-Leiva, and Ms. Angela Fornell for their contributions to this work.
Funding
This work was supported by the Advanced Medical Technology Initiative (AMTI) at the Telemedicine and Advanced Technology Research Center (TATRC), US Army Medical Research and Development Command (USAMRDC). Opinions, interpretations, conclusions, and recommendations are those of the author(s) and are not necessarily endorsed by the Defense Health Agency (DHA).
Footnotes
Authorship
All authors conducted and contributed to the literature search. All authors contributed to study design. J.W., B.P, A.F., M.W., M.P., B.L collected the data. J.W., B.P., A.F., M.L., E.R., J.B., J.K., interpreted the data. J.W., B.P., A.F., M.L., B.L., J.K., wrote the article. All authors critically revised the final article.
Membership
M.L. is a member of EAST.
Media Summary
Pectin-based bioadhesive patches performed similar to suture repair for the management of liver lacerations and full thickness bowel injuries in a living porcine model. Further testing is warranted as a pectin patch repair as it may offer a rapid and simple option to effectively temporize traumatic intra-abdominal injuries.
Social Media Handles
#MadiganSurgery
@BrighamThoracic
@johnpkuckelman
Disclosure
The authors declare no conflicts of interest.
The views expressed are those of the author(s) and do not reflect the official policy of the Department of the Army, the Defense Health Agency, the Department of Defense, or the U.S. Government.
References
- 1.Søreide K. Epidemiology of major trauma. Journal of British Surgery. 2009;96(7):697–8. [DOI] [PubMed] [Google Scholar]
- 2.Gaarder C, Skaga NO, Eken T, Pillgram-Larsen J, Buanes T, Naess PA. The impact of patient volume on surgical trauma training in a Scandinavian trauma centre. Injury. 2005;36(11):1288–92. [DOI] [PubMed] [Google Scholar]
- 3.Malhotra AK, Latifi R, Fabian TC, Ivatury RR, Dhage S, Bee TK, et al. Multiplicity of solid organ injury: influence on management and outcomes after blunt abdominal trauma. Journal of Trauma and Acute Care Surgery. 2003;54(5):925–9. [DOI] [PubMed] [Google Scholar]
- 4.Wiik Larsen J, Søreide K, Søreide JA, Tjosevik K, Kvaløy JT, Thorsen K. Epidemiology of abdominal trauma: An age- and sex-adjusted incidence analysis with mortality patterns. Injury. 2022;53(10):3130–8. [DOI] [PubMed] [Google Scholar]
- 5.Ivatury RR, Simon RJ, Stahl WM. A CRITICAL EVALUATION OF LAPAROSCOPY IN PENETRATING ABDOMINAL TRAUMA. Journal of Trauma and Acute Care Surgery. 1993;34(6). [DOI] [PubMed] [Google Scholar]
- 6.Heuer M, Hussmann B, Kaiser G, Nast-Kolb D, Ruchholtz S, Lefering R, et al. Hollow organ injury and multiple trauma: treatment, course and outcome-an organ-specific evaluation of 1127 patients from the trauma registry of the DGU. Zentralblatt fur Chirurgie. 2012;139(4):445–51. [DOI] [PubMed] [Google Scholar]
- 7.Ochiai T, Igari K, Yagi M, Ito H, Kumagai Y, Iida M, et al. Treatment strategy for blunt hepatic trauma: analysis of 183 consecutive cases. Hepatogastroenterology. 2011;58(109):1312–5. [DOI] [PubMed] [Google Scholar]
- 8.Richardson JD, Franklin GA, Lukan JK, Carrillo EH, Spain DA, Miller FB, et al. Evolution in the management of hepatic trauma: a 25-year perspective. Annals of surgery. 2000;232(3):324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carmona RH, Lim RC Jr, Clark GC. Morbidity and mortality in hepatic trauma: a 5 year study. The American Journal of Surgery. 1982;144(1):88–94. [DOI] [PubMed] [Google Scholar]
- 10.Watts DD, Fakhry SM, for the EM-IHVIRG. Incidence of Hollow Viscus Injury in Blunt Trauma: An Analysis from 275,557 Trauma Admissions from the EAST Multi-Institutional Trial. Journal of Trauma and Acute Care Surgery. 2003;54(2). [DOI] [PubMed] [Google Scholar]
- 11.Pretre R, Mentha G, Huber O, Meyer P, Vogel J, Rohner A. Hepatic trauma: risk factors influencing outcome. Journal of British Surgery. 1988;75(6):520–4. [DOI] [PubMed] [Google Scholar]
- 12.Jin S, Fu Q, Wuyun G, Wuyun T. Management of post-hepatectomy complications. World journal of gastroenterology: WJG. 2013;19(44):7983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salim A, Teixeira PG, Inaba K, Brown C, Browder T, Demetriades D. Analysis of 178 penetrating stomach and small bowel injuries. World journal of surgery. 2008;32(3):471–5. [DOI] [PubMed] [Google Scholar]
- 14.De Boer MT, Boonstra EA, Lisman T, Porte RJ. Role of fibrin sealants in liver surgery. Digestive surgery. 2012;29(1):54–61. [DOI] [PubMed] [Google Scholar]
- 15.Nordentoft T, Pommergaard HC, Rosenberg J, Achiam MP. Fibrin glue does not improve healing of gastrointestinal anastomoses: a systematic review. Eur Surg Res. 2015;54(1–2):1–13. [DOI] [PubMed] [Google Scholar]
- 16.Mohan HM, Winter DC. Autobuttressing of colorectal anastomoses using a mesenteric flap. Updates Surg. 2013;65(4):333–5. [DOI] [PubMed] [Google Scholar]
- 17.Gagner M, Kemmeter P. Comparison of laparoscopic sleeve gastrectomy leak rates in five staple-line reinforcement options: a systematic review. Surg Endosc. 2020;34(1):396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zilling T, Holmin T. Surglcel® Reinforced Resection Lines in Left-sided Hepatectomy with Linear Stapling Device. An Experimental Study on Pigs. HPB Surgery. 1996;9(2):97–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kohno H, Nagasue N, Chang Y-C, Taniura H, Yamanoi A, Nakamura T. Comparison of topical hemostatic agents in elective hepatic resection: a clinical prospective randomized trial. World journal of surgery. 1992;16(5):966–9. [DOI] [PubMed] [Google Scholar]
- 20.Pedersen TB, Honge JL, Pilegaard HK, Hasenkam JM. Comparative study of lung sealants in a porcine ex vivo model. The Annals of thoracic surgery. 2012;94(1):234–40. [DOI] [PubMed] [Google Scholar]
- 21.Fuller C. Reduction of intraoperative air leaks with Progel in pulmonary resection: a comprehensive review. J Cardiothorac Surg. 2013;8:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hewitt CW, Marra SW, Kann BR, Tran HS, Puc MM, Chrzanowski FA Jr., et al. BioGlue surgical adhesive for thoracic aortic repair during coagulopathy: efficacy and histopathology. Ann Thorac Surg. 2001;71(5):1609–12. [DOI] [PubMed] [Google Scholar]
- 23.Petrella F, Borri A, Brambilla D, Calanca G, Vezzani N, Colantoni A, et al. Efficacy and safety of Innoseal for air leak after pulmonary resection: a case-control study. J Surg Res. 2016;206(1):22–6. [DOI] [PubMed] [Google Scholar]
- 24.Pierce A, Zheng Y, Wagner WL, Scheller HV, Mohnen D, Tsuda A, et al. Pectin biopolymer mechanics and microstructure associated with polysaccharide phase transitions. J Biomed Mater Res A. 2020;108(2):246–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oswald A-M, Joly L-M, Gury C, Disdet M, Leduc V, Kanny G. Fatal intraoperative anaphylaxis related to aprotinin after local application of fibrin glue. The Journal of the American Society of Anesthesiologists. 2003;99(3):762–3. [DOI] [PubMed] [Google Scholar]
- 26.Spotnitz WD, Burks S. Hemostats, sealants, and adhesives III: a new update as well as cost and regulatory considerations for components of the surgical toolbox. Transfusion. 2012;52(10):2243–55. [DOI] [PubMed] [Google Scholar]
- 27.Scheller HV, Jensen JK, Sørensen SO, Harholt J, Geshi N. Biosynthesis of pectin. Physiologia plantarum. 2007;129(2):283–95. [Google Scholar]
- 28.Servais AB, Kienzle A, Valenzuela CD, Ysasi AB, Wagner WL, Tsuda A, et al. Structural heteropolysaccharide adhesion to the glycocalyx of visceral mesothelium. Tissue Engineering Part A. 2018;24(3–4):199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng Y, Pierce AF, Wagner WL, Khalil HA, Chen Z, Servais AB, et al. Functional Adhesion of Pectin Biopolymers to the Lung Visceral Pleura. Polymers (Basel). 2021;13(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng Y, Pierce AF, Wagner WL, Khalil HA, Chen Z, Funaya C, et al. Biomaterial-Assisted Anastomotic Healing: Serosal Adhesion of Pectin Films. Polymers (Basel). 2021;13(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuckelman J, Conner J, Zheng Y, Pierce A, Jones I, Lammers D, et al. Improved outcomes utilizing a novel pectin-based pleural sealant following acute lung injury. J Trauma Acute Care Surg. 2020;89(5):915–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beal SL. Fatal hepatic hemorrhage: an unresolved problem in the management of complex liver injuries. The Journal of trauma. 1990;30(2):163–9. [PubMed] [Google Scholar]
- 33.Clark WR Jr, Leather RP. Hemostasis during liver resections. Surgery. 1970;67(3):556–7. [PubMed] [Google Scholar]
- 34.Tsao JI, Loftus JP, Nagorney DM, Adson MA, Ilstrup DM. Trends in morbidity and mortality of hepatic resection for malignancy. A matched comparative analysis. Annals of surgery. 1994;220(2):199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ochsner MG, Maniscalco-Theberge ME, Champion HR. Fibrin glue as a hemostatic agent in hepatic and splenic trauma. The Journal of trauma. 1990;30(7):884–7. [DOI] [PubMed] [Google Scholar]
- 36.Davidson B, Burnett S, Javed M, Seifalian A, Moore D, Doctor N. Experimental study of a novel fibrin sealant for achieving haemostasis following partial hepatectomy. Journal of British Surgery. 2000;87(6):790–5. [DOI] [PubMed] [Google Scholar]
- 37.Goczyńska P, Lasocka J, Lachert E. Fibrin glues—the current state of knowledge. Journal of Transfusion Medicine. 2021;14(4):215–25. [Google Scholar]
- 38.Busuttil RW. A comparison of antifibrinolytic agents used in hemostatic fibrin sealants. Journal of the American College of Surgeons. 2003;197(6):1021–8. [DOI] [PubMed] [Google Scholar]
- 39.Boschung U. Milestones in the history of intestinal anastomosis. Swiss Surgery= Schweizer Chirurgie= Chirurgie Suisse= Chirurgia Svizzera. 2003;9(3):99–104. [DOI] [PubMed] [Google Scholar]
- 40.Hyman N, Manchester TL, Osler T, Burns B, Cataldo PA. Anastomotic leaks after intestinal anastomosis: it’s later than you think. Ann Surg. 2007;245(2):254–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Philllips B. Reducing gastrointestinal anastomotic leak rates: review of challenges and solutions. 2016. [Google Scholar]
- 42.Chung RS. Blood flow in colonic anastomoses. Effect of stapling and suturing. Annals of surgery. 1987;206(3):335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Capucilli P, Kennedy K, Kazatsky AM, Cianferoni A, Spergel JM. Fruit for thought: anaphylaxis to fruit pectin in foods. The Journal of Allergy and Clinical Immunology: In Practice. 2019;7(2):719–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1) The ARRIVE guidelines 2.0: author check list. Check list of the essential 10 minimum items required for reporting animal studies.
2) Biodurability testing of pectin patch. A) Pectin patch sample prior to submersion. B) Pectin patch after 24 hours in bile. C) Pectin patch after 24 hours in succus. D) Experimental layout with three pieces of pectin each submerged in 10mls of normal saline (NS), bile, and succus. E) Gelatinous pectin patch poured onto chucks pad following 24-hour submersion in bile. F) Remainder of pectin patch removed from succus following 24 hours.