Abstract

Actinides are elements that are often feared because of their radioactive nature and potentially devastating consequences to humans and the environment if not managed properly. As such, their chemical interactions with the biosphere and geochemical environment, i.e., their “biogeochemistry,” must be studied and understood in detail. In this Review, a summary of the past discoveries and recent advances in the field of actinide biogeochemistry is provided with a particular emphasis on actinides other than thorium and uranium (i.e., actinium, neptunium, plutonium, americium, curium, berkelium, and californium) as they originate from anthropogenic activities and can be mobile in the environment. The nuclear properties of actinide isotopes found in the environment and used in research are reviewed with historical context. Then, the coordination chemistry properties of actinide ions are contrasted with those of common metal ions naturally present in the environment. The typical chelators that can impact the biogeochemistry of actinides are then reviewed. Then, the role of metalloproteins in the biogeochemistry of actinides is put into perspective since recent advances in the field may have ramifications in radiochemistry and for the long-term management of nuclear waste. Metalloproteins are ubiquitous ligands in nature but, as discussed in this Review, they have largely been overlooked for actinide chemistry, especially when compared to traditional environmental chelators. Without discounting the importance of abundant and natural actinide ions (i.e., Th4+ and UO22+), the main focus of this review is on trivalent actinides because of their prevalence in the fields of nuclear fuel cycles, radioactive waste management, heavy element research, and, more recently, nuclear medicine. Additionally, trivalent actinides share chemical similarities with the rare earth elements, and recent breakthroughs in the field of lanthanide-binding chelators may spill into the field of actinide biogeochemistry, as discussed hereafter.
Keywords: actinides, biogeochemistry, environment, radionuclides, nuclear waste, contamination, chelators, proteins
1. Nuclear Properties of Actinides and Environmental Consequences
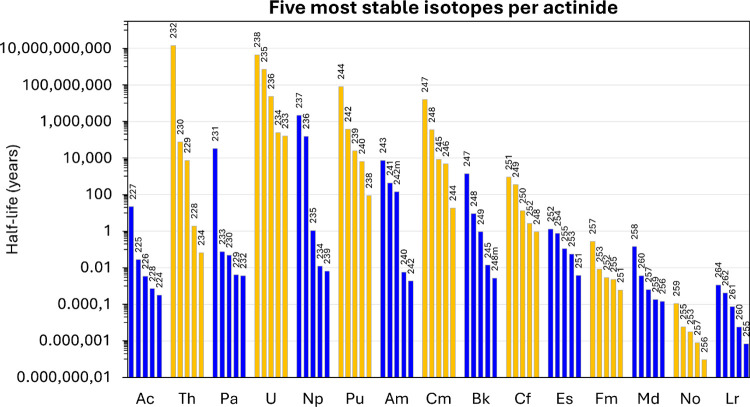
In the periodic table, the actinide series (i.e., Ac to Lr) sits below the lanthanide series, and analogies are often made between these two families, despite them having distinct physical and chemical properties in some cases. The primary difference between actinides and lanthanides is the fact that no stable actinide isotope exists, and therefore, all the actinide elements are radioactive. Among the 15 actinides, only the first four (Ac, Th, Pa, and U) are naturally present and widely distributed in the environment. Thorium and uranium are the most abundant ones because of the existence of the long-lived isotopes Th-232 (t1/2 = 14 billion years), U-238 (t1/2 = 4.5 billion years), and U-235 (t1/2 = 704 million years) (Figure 1). Actinium and protactinium do not have long-lived isotopes on the geological time scale. As a result, they are present on Earth only because some of their isotopes are intermediate products in the decay chain of natural thorium and uranium. The isotope Ac-228 (t1/2 = 6.2 h) originates from the decay chain of Th-232, while Pa-234 (t1/2 = 6.7 h) comes from U-238, and both Pa-231 (t1/2 = 32 760 years) and Ac-227 (t1/2 = 21.8 years) are decay products of the U-235 decay chain. As such, actinium and protactinium are only found near uranium and thorium sources, at trace levels (due to secular equilibrium with their parent isotopes), and do not accumulate in the environment over time.
Figure 1.
Half-lives of the five most stable isotopes that have been identified for each element of the actinide series (Ac to Lr). The mass number is indicated above each bar. Note that the y-axis is in years, is displayed as a logarithmic scale, and it spans 18 orders of magnitude.
Among the naturally occurring actinides, only actinium exhibits a +III oxidation state in solution under environmentally relevant conditions (Table 1). Consequently, actinium is the only natural actinide that has the same valence as the trivalent lanthanides and would be susceptible to undergo similar biogeochemical processes. However, because of the short half-lives of its isotopes (Figure 1), the total amount and concentrations of actinium in the environment are extremely low, and natural environmental systems (e.g., microorganisms) are unlikely to rely solely on this element. The longest-lived isotope of actinium, Ac-227, only has a 21.8 year half-life, and its average concentration in the ocean is in the low attomolar range (i.e., 10–18 mol/L), which corresponds to an estimated total inventory of actinium in the ocean of just ∼8.4 kg.1 This contrasts with the equivalent lanthanide ion, La3+, which is abundant in nature with an average content of ∼30 ppm in the continental crust2 and an average concentration in the ocean in the tens of picomoles per liter (i.e., 10–11 to 10–10 mol/L)3 —about 8 orders of magnitude higher than actinium. As a result, biological systems have had little opportunity to interact with natural trivalent actinide ions in nature while, by contrast, natural systems have had millions of years to evolve and interact with more abundant trivalent lanthanides or actinides whose dominant oxidation states are not +III (i.e., uranium and thorium; Table 1). The advent of the nuclear era in the middle of the 20th century has changed this situation dramatically and forever.
Table 1. Observed Oxidation States for the Actinide Elements in Coordination Compoundsa.
| element | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| oxidation state | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | |||||||
| +3 | +3 | +3 | +3 | +3 | +3 | +3 | +3 | +3 | +3 | +3 | +3 | +3 | +3 | ||
| +4 | +4 | +4 | +4 | +4 | +4 | +4 | +4 | +4 | |||||||
| +5 | +5 | +5 | +5 | +5 | |||||||||||
| +6 | +6 | +6 | +6 | ||||||||||||
| +7 | +7 |
The oxidation states that are the most relevant under environmental conditions are shown in bold.4 See the recent studies from MacDonald et al.,5 Windorff et al.,6 Dutkiewicz et al.,7 and Poe et al.8 for the isolation of low-valence U2+, Np2+, Pu2+, and Cf2+ complexes. Am2+ compounds have also been synthesized and characterized, mainly with small inorganic ligands.9−12 Note that elements beyond Cf are unlikely to be found in significant quantities in nature because their radioactive half-lives are too short (≤1 year); see Figure 1.
Before the discovery of transuranium elements in the 1940s,13,14 only sporadic locations on Earth contained elements heavier than uranium. For instance, this is the case of Oklo (Gabon) where, due to a unique set of geological circumstances, a natural uranium deposit underwent self-sustained nuclear reactions about 2 billion years ago and created isotopes of transuranic elements.15,16 This and other sporadic natural nuclear reactors did not produce significant quantities of new actinides that would have an impact on radioisotope inventories on Earth and associated biological processes. In contrast, the proportion of actinide elements present on Earth dramatically increased after the discovery of the first synthetic transuranic isotopes, namely Np-239 by McMillan and Abelson in 194013 (neptunium: element 93) and then Pu-238 and Pu-239 in 1941 by Seaborg, McMillan, Kennedy, and Wahl17 (plutonium: element 9417−19). As research on transuranic elements rapidly progressed within the frame of the Manhattan project and after World War II, more elements were discovered, up to americium (element 95) and curium (element 96), at which point Glenn T. Seaborg introduced the concept of the actinide series to the world.14 Most actinides after plutonium exhibit a stable +III oxidation state under environmentally and biologically relevant conditions (Table 1), and their chemistry starts to resemble, at first sight, that of the trivalent lanthanides.
It should be noted that not all the actinides can be used for “bulk” chemistry or biogeochemistry experiments because the half-life of the isotopes becomes shorter and more challenging to work with as the atomic number gets higher. Figure 2 shows the actinide isotopes that are typically used for chemical or biogeochemical research purposes and their respective half-lives. In practice, the comparison between trivalent lanthanide and actinide coordination chemistries has been largely limited to the study of americium and curium and more recently extended to berkelium and californium.20−23 Under very reducing and specific conditions, plutonium(III) can also be stabilized in solution, and its interactions with the protein calmodulin have been probed,24 but in general, studies on trivalent plutonium remain rare because of its instability and low relevance to currently known biogeochemical mechanisms. In recent years, there has also been a growing interest in developing novel cancer treatments25−30 based on the synthetic and very short-lived isotope actinium-225 (225Ac3+, half-life of 9.9 days). The potential use of this alpha emitter in cancer medicine (along with short-lived lanthanide beta emitters, such as 177Lu3+) has provided strong motivation to the radiochemistry community to further probe the interactions between biological systems and trivalent f-elements.
Figure 2.
Half-life of actinide isotopes that are generally preferred in research laboratories for chemistry, biochemistry, or physics experiments. Color code: isotopes with half-lives > 100 years are displayed in green, those with half-lives of 1 to 100 years are displayed in yellow, those with half-lives < 1 year are displayed in red. Note that the y-axis is displayed as a logarithmic scale and spans 18 orders of magnitude.
2. Coordination Chemistry Properties of Actinides Compared to Environmental Metal Ions
Oftentimes, trivalent lanthanide ions are used as nonradioactive surrogates for the actinide ions. Indeed, besides exhibiting the same oxidation state in solution, trivalent lanthanides and actinides also have relatively similar size. However, the analogy is not perfect and should be used with caution as the lanthanide contraction is stronger than the actinide contraction, and for a given column, the lanthanide is smaller than its actinide counterpart (Figure 3). For example, the ionic radius of Eu3+ is slightly smaller than that of Am3+ (1.116 vs 1.157 Å31), which translates to a volume ∼10% smaller (if ions approximated to perfect spheres). Such a size difference could be significant for certain biogeochemical processes, such as incorporation into minerals or complexation to aqueous ligands. Despite this imperfect size match between lanthanides and actinides, their chemistries are still relatively similar, and biogeochemical systems amenable to interact with trivalent lanthanide ions generally also interact with trivalent actinides,32 albeit with some noticeable differences (vide infra).
Figure 3.

Comparison of the ionic radii of the trivalent ions of the lanthanide (triangles) and actinide (circles) series. Top: Ionic radius values for a coordination number of 6, as described in reference (33) for Pa to Cf and reference (34) for actinium. Bottom: Ionic radius values for a coordination number of 9, as described in reference (31).
Being large trivalent ions, f-element ions appear as outliers compared to known environmental metal ions (Fe3+, Ca2+, Zn2+, Mn2+, Cu2+, etc.; Figure 4a). However, they can still hijack environmentally relevant reactions in some cases. For example, the trivalent actinides have a higher charge density than Ca2+, but their ionic radius is in the same range (Figure 4b), so there is a potential for An3+ to substitute for Ca2+ in solids or water-soluble complexes. In contrast, in the case of ferric iron, the trivalent actinide ions are much larger, have lower charge density, and prefer higher coordination numbers (8 to 10 for An3+ vs 6 for Fe3+), which results in unfavorable conditions for substituting An3+ for Fe3+ in molecular compounds.35 As such, trivalent actinides tend to form relatively weak complexes with ligands that have naturally evolved to bind Fe3+ (also known as siderophores) due to the mismatch between their coordination chemistry properties. A characteristic example is the biological protein transferrin (Tf), which is a natural iron transporter in the blood system of mammals as it has very strong affinity for Fe3+ (log βTfFe2 = 40.1 at pH 7.436,37), but its affinity for trivalent actinides drops by several orders of magnitude (log βTfCm2 = 15.8 at pH 8.638). Nonetheless, actinides in the +III oxidation state still interact with molecules involved in the iron biological machinery, albeit with a lower affinity.
Figure 4.
Comparison of the ionic radius (a) and charge density (b) for select actinides, lanthanides, and biorelevant metals. For iron, the ionic radius shown is that of the 6-coordinated ion in order to take into account its preferred coordination mode. For the other elements, the ionic radius of the 8-coordinated ion was considered. The ionic radius values were taken from the review of Shannon,33 except for Am3+44 and Cm3+.45
A better match between f-element and iron environmental chemistries is found with tetravalent actinides (Figure 4). This is particularly important for plutonium, which is rather unstable as Pu3+ but instead forms strong complexes as Pu4+ under environmentally friendly conditions. The charge density of the Pu4+ is much higher than that of trivalent actinides or lanthanides and it starts approaching that of Fe3+. Hence, iron complexes are prone to accommodate and stabilize Pu4+. The same conclusions apply to neptunium, which is unstable as Np3+, and whose natural oxidation state is +V (NpO2+ ion), but can be reduced to Np4+ and form strong complexes with biorelevant molecules.39 In the case of plutonium, an analogy can also be made between Pu4+ and tetravalent cerium, Ce4+, as they have very similar ionic radii.33 Berkelium is an intermediate case as it can adopt both the trivalent and tetravalent oxidation states in solution with redox properties that resemble that of the Ce4+/Ce3+ couple.20,40,41 Bk4+ is the smallest tetravalent actinide that can be probed in solution, and it has been shown to form highly stable complexes with carbonate ions at near neutral pH, as well as siderophore and iodate ligands.20,23,42,43 Berkelium could, in theory, utilize both the calcium and ferric iron biogeochemical pathways, but this element is extremely rare in nature (if any), and studies of its biogeochemistry have been limited to laboratory conditions.
3. Small Natural Chelators for the Chelation of Trivalent Actinides
The large number of potential chelating molecules present in the environment, compounded by the naturally low concentration of actinides and difficulties of working with radioactive materials, has largely hampered the precise identification of the natural “actinophore” ligands involved in the complexation and transport of actinides. Small water-soluble organic chelators are ubiquitous in nature and may play a role in the transport of actinides in the environment or in vivo. Experimental studies have mainly focused on solution thermodynamics and speciation of actinides in synthetic solutions. For trivalent actinides, the vast majority of the solution-state speciation involve Cm3+ because of its convenient fluorescence properties (with detection limits below the submicromolar range46,47) and/or Am3+ because it can be probed via UV–visible spectrophotometry (in the millimolar to micromolar concentration range48−50). The other trivalent actinides either lack suitable spectroscopic properties for such studies (e.g., Ac3+ is spectroscopically silent, akin to Th4+), are unstable in solution (e.g., Np3+ and Pu3+), or are too short-lived and cost-prohibitive (e.g., Bk3+ and Cf3+; Figure 2). Structural studies involving actinide compounds usually leverage synchrotron-based X-ray absorption techniques (e.g., XANES and EXAFS51−54) as opposed to the more material-intensive techniques used for nonradioactive elements, such as crystallography, NMR, etc.
The simplest environmentally relevant ligands that can be studied are carbonate and bicarbonate ions. However, even for these simple and ubiquitous chelators, the species formed in solution under relevant conditions with trivalent actinides are still relatively unknown. Early studies55,56 that developed speciation models for the Am–CO3–H2O system proposed the formation of [AmCO3]+, [Am(CO3)2]−, [AmOHCO3], [Am(OH)(CO3)2]2–, [Am(OH)2(CO3)]−, [AmHCO3]2+, and [Am(HCO3)2]+. However, more recent experiments using Cm3+ luminescence57,58 concluded that [CmCO3]+, [Cm(CO3)2]−, [Cm(CO3)3]3–, [Cm(CO3)4]5–, and [Cm(CO3)4(H2O)]5– are present in solution. A recent theoretical study59 also indicated that the 1:2 complex is more stable if the coordination sphere of the metal is completed with two water molecules, i.e., [Am(CO3)2(H2O)2]−. Beyond curium, the only data available is a single electrochemical study of berkelium40 that showed the potential oxidation of Bk3+ to Bk4+ in concentrated carbonate media (i.e., 2 to 5 M), but the stoichiometry of the complexes could not be determined. These conditions are not completely relevant to the environmental chemistry of berkelium but hint at a speciation similar to that of Ce4+/Ce3+ in carbonate media. A recent and thorough computational study60 corroborated the oxidation of Bk3+ to Bk4+ due to stabilization via carbonate complexation and proposed the formation of [BkIV(CO3)4]5– and [BkIV(CO3)3(OH)2]4–, i.e., a stoichiometry that mirrors the trivalent curium–carbonate species. The trivalent actinide–carbonate complexes exhibit relatively moderate stability constants (Table 2) and are likely not strong enough to compete with other natural chelators at ambient pressure and circumneutral pH.
Table 2. Stability Constants Experimentally Determined for Complexes of Trivalent Actinium, Americium, and Curium with Small Biorelevant Chelators (DFOB = Desferrioxamine B. PYOV = Pyoverdine. SHA = Salicylhydroxamate. BHA = benzohydroxamate).
| chelator | reaction | formation constant | conditions | ref |
|---|---|---|---|---|
| carbonate | Am3+ + CO32– = [AmCO3]+ | log β11 = 5.97 | I = 0.1–0.3 M (NaClO4), T = 25 °C | (55) |
| Am3+ + 2 CO32– = [Am(CO3)2]− | log β12 = 9.58 | |||
| [Am2(CO3)3](s) = 2 Am3+ + 3 CO32– | log Ksp = −29.70 | |||
| [Cm(CO3)2]− + CO32– = [Cm(CO3)3]2– | log K3 = 2.01 | I = 3 M (NaClO4), T = 25 °C | (57) | |
| phosphate | Ac3+ + H2PO4– = [AcH2PO4]2+ | log K = 1.85 | I = 0, T = 30 °C | (78) |
| Am3+ + H2PO4– = [AmH2PO4]2+ | log K = 2.13 | I = 0, T = 30 °C | (79) | |
| Am3+ + HPO42– = [AmHPO4]+ | log K = 4.14 | |||
| Cm3+ + 2 H+ + PO43– = [CmH2PO4]2+ | log β121 = 22.02 | I = 0.1 M (NaClO4), T = 24 °C | (80) | |
| Cm3+ + H+ + PO43– = [CmHPO4]+ | log β111 = 18.56 | |||
| Cm3+ + H3PO4 = [CmH2PO4]2+ + H+ | log K = −0.14 | I = 1.1 M (NaClO4), T = 25 °C | (81) | |
| oxalate | Ac3+ + C2O42– = [AcC2O4]+ | log β11 = 3.56 | I = 1 M (NaClO4), T = 25 °C | (82) |
| Ac3+ + 2 C2O42– = [Ac(C2O4)2]− | log β12 = 6.16 | |||
| Am3+ + C2O42– = [AmC2O4]+ | log β11 = 4.63 | I = 1 M (NaClO4), T = 25 °C | (82) | |
| Am3+ + 2 C2O42– = [Am(C2O4)2]− | log β12 = 8.35 | |||
| Am3+ + 3 C2O42– = [Am(C2O4)3]3– | log β13 = 11.15 | |||
| Am3+ + C2O42– = [AmC2O4]+ | log β11 = 5.34 | I = 0.1 M (NaClO4), T = 23–26 °C | (62) | |
| Am3+ + 2 C2O42– = [Am(C2O4)2]− | log β12 = 9.14 | |||
| Am3+ + 3 C2O42– = [Am(C2O4)3]3– | log β13 = 11.49 | |||
| acetate | Cm3+ + Acetate– = [CmAcetate]2+ | log β11 = 3.18 | I = 0, T = 20 °C | (83) |
| Cm3+ + 2 Acetate– = [Cm(Acetate)2]+ | log β12 = 4.80 | |||
| Cm3+ + 3 Acetate– = [Cm(Acetate)3] | log β13 = 5.19 | |||
| malonate | Cm3+ + Malonate2– = [CmMalonate]+ | log β11 = 5.26 | I = 0, T = 20 °C | (84) |
| Cm3+ + 2 Malonate2– = [Cm(Malonate)2]− | log β12 = 8.38 | |||
| propionate | Cm3+ + Propionate2– = [CmPropionate]2+ | log β11 = 3.24 | I = 0, T = 25 °C | (85) |
| Cm3+ + 2 Propionate2– = [Cm(Propionate)2]+ | log β12 = 4.63 | |||
| citrate | Cm3+ + HCitrate3– = [CmHCitrate] | log K1 = 7.4 | I = 0.1 M (NaClO4), T = 23–25 °C | (46) |
| Cm3+ + 2 HCitrate3– = [Cm(HCitrate)2]3– | log K2 = 11.3 | |||
| Cm3+ + H2Citrate2– + HCitrate3–= [Cm(H2Citrate)HCitrate]2– | log K = 11.0 | |||
| desferrioxamine B (DFOB) | Cm3+ + DFOB3– = [CmDFOB] | log β101 = 16.80 | I = 0.1 M (NaClO4), T = 25 °C | (73) |
| Cm3+ + DFOB3– + H+ = [CmHDFOB]+ | log β111 = 25.73 | |||
| Cm3+ + DFOB3– + 2 H+ = [CmH2DFOB]2+ | log β121 = 31.62 | |||
| Am3+ + DFOB3– + H+ = [AmHDFOB)]+ | log β111 = 25.5 | I = 1 M (KNO3), T = 25 °C | (86) | |
| Am3+ + DFOB3– + 2 H+ = [AmH2DFOB]2+ | log β121 = 32.2 | |||
| Am3+ + DFOB3– + 3 H+ = [AmH3DFOB]3+ | log β131 = 37.9 | |||
| Am3+ + 2 DFOB3– + 4 H+ = [AmH4(DFOB)2]4+ | log β142 = 60.5 | |||
| pyoverdin (PYOV) | Cm3+ + PYOV4– = [CmPYOV]− | log β 101 = 19.30 | I = 0.1 M (NaClO4), T = 25 °C | (74) |
| Cm3+ + HPYOV3– + H+ = [CmHPYOV] | log β 111 = 27.40 | |||
| Cm3+ + PYOV2– + 2 H+ = [CmH2PYOV]+ | log β 121 = 32.50 | |||
| salicylhydroxamate (SHA) | Cm3+ + SHA2+ + H+= [CmSHA]2+ | log β 111 = 16.52 | I = 0.1 M (NaClO4), T = 25 °C | (75) |
| Cm3+ + 2 SHA– + H+ = [CmH(SHA)2] | log β 112 = 24.09 | |||
| benzohydroxamate (BHA) | Cm3+ + BHA2+ = [CmBHA]2+ | log β 111 = 6.52 | I = 0.1 M (NaClO4), T = 25 °C | (75) |
| Cm3+ + 2 BHA– = [CmH(BHA)2] | log β 112 = 11.60 | |||
| humic acid (HA) | Am3+ + HAx– = [AmHA]x−3 | log β 11 = 6.27 | I = 0.1 M (NaClO4), pH 6. Note: Two different sources of humic acids (Aldrich and Bradford – see original reference) | (70) |
| log β 11 = 6.36 |
Other water-soluble small chelators have been experimentally investigated for the complexation of trivalent actinides (i.e., Am3+ and Cm3+) at near-neutral pH. In solution, the proposed complexes are often based on prior speciation models determined for Eu3+ (using fluorescence spectroscopy), and the associated stability constants and complexes stoichiometry for Am3+ and Cm3+ are typically very consistent with those of Eu3+. Several small ligands bearing carboxylate binding groups, such as acetate, oxalate, and citrate, have been studied with trivalent americium.61−63 Water-soluble molecules derived from the decomposition of natural organic matter have also been studied with trivalent actinides. The vast majority of the literature focuses on aqueous ligands that belong to the humic acid substance family.64−72 These molecules are ubiquitous in nature and play a critical role in soil and plant ecosystems. However, the range of formulas and structures of molecules within the humic acid family is broad and often varies based on their origin, and these compounds are often ill-defined. As there have been several studies published on humic acid substances, their affinity for trivalent actinides has been known since the 1980s, and it appears that it is relatively weak (e.g., Kim et al. measured log β11 values of ∼6.2 for Am3+ at pH 6 for two different humic acids70), even weaker that other natural small molecules like citrates. Unless for specific conditions where a high concentration of humic acid is present, it appears unlikely that humic acids could outcompete some of the other natural chelators that have been identified. As shown in Table 2, the stability constants of the Am3+ and Cm3+ complexes with the ubiquitous biorelevant chelators are relatively modest. Hence, while abundant in nature, these particular ligands may not be able to compete with more elaborate molecules that may be present at a lower concentration but with stronger binding affinities. It therefore becomes difficult to provide general speciation models for actinides in the environment as their typically low concentrations make them susceptible to be bound by strong chelators that are site-specific and not necessarily present at high concentrations.
In this regard, there has been a particular effort to study molecules of the hydroxamate families, such as desferrioxamine (e.g., DFO-B) and pyoverdine, as they are multidentate natural chelators that form relatively strong complexes with the trivalent actinide ions. Extensive studies performed by Moll and co-workers73−75 using synthetic solutions showed that Cm3+ forms water-soluble complexes with desferrioxamine and pyoverdine with very high stability constants, i.e., log β11 ≈ 17–19, which is many orders of magnitude higher than in the case of carboxylate ligands (Table 2). However, because of the high pKa of the hydroxamate function (e.g., the three hydroxamate groups of DFO-B have pKa values of 8.4, 9.0, and 9.776), such ligands only form their most stable complexes with the actinides at pH above 8–9, which offsets their potential near-neutral pH. The predominance window of actinide–hydroxamate complexes is, therefore, relatively narrow and limited by the acidity of the hydroxamate groups on one side of the pH scale and by the formation of actinide hydroxides on the other side. Hence, despite their strong potential for trivalent actinide binding, it remains unclear whether or not such ligands play an important role in the environmental chemistry of actinides, especially under neutral or slightly acidic conditions.
The role of small chelators on the speciation of trivalent actinides in the environment still remains an open question.77 Many decades after the discovery of heavy actinides, there is still no consensus on the existence of natural “actinophore” ligands that could drive the speciation of trivalent actinides even in the presence of many other potential ligands, akin to siderophores for ferric iron. Multidentate hydroxamates, such as desferrioxamine and pyoverdine, are natural starting points to study natural chelators for actinides. However, despite their affinity for Am3+ and Cm3+, these molecules are, by design, meant to bind small ions like Fe3+ and not actinides. It is therefore possible that other natural molecules with much higher affinity for trivalent actinides exist or have existed in nature. Given the low abundance of trivalent actinides in the environment, the identification of actinophore molecules will likely be derived from the study of biogeochemical processes that involve more abundant trivalent lanthanide ions.
4. Existence and Relevance of Actinide–Protein Complexes
Metalloproteins have been a particular subject of attention for lanthanide and actinide binding. In line with the general trend observed in actinide sciences, the vast majority of the studies dedicated to actinide–protein interactions concern tetravalent actinides (Th4+, Np4+, Pu4+) and uranium (UO22+).39,53,87,88 Studies of metalloproteins able to complex trivalent lanthanides, and by extension trivalent actinides, have been mainly focused on mammalian proteins present in the bloodstream, organs, or skeleton87 because of the importance of understanding these reactions in the human body. These studies are generally framed in the context of potential internal contamination with nuclear materials with the rationale being that the transport of metal ions in vivo implies their solubilization and circulation in the bloodstream via strong water-soluble ligands, including metalloproteins. In should be noted that the likelyhood of accidental contamination scenarios is very low, even in the event of a major nuclear power plant accident (which mainly involve insoluble actinide compounds, such as UO2 and PuO2). Mammalian proteins are also unlikely to play a significant role in the management of nuclear waste.
The main proteins studied for actinide binding include the mammalian proteins transferrin,89,90,39,91,92,38,93−96,35,53,39 ferritin,53,88 fetuin,97−99 albumin,100 calmodulin,24,101 siderocalin,23,102 and ostepontin.103,104 A handful of very recent studies105−107 have also investigated α-amylase, one of the most important digestive proteins that is also present in human saliva and catalyzes the hydrolysis of polysaccharides. The most studied class of metalloproteins for actinide binding is transferrins (Tf). Transferrin is a glycoprotein of about 80 kDa and it is one of main iron transporters in vivo in its trivalent oxidation state. Given the similar charge/radius ratio between ferric iron and plutonium (vide supra), iron transporter proteins have been the main target for actinide–protein studies. Human transferrin consists of 679 amino acids108 folded into two lobes (C and N), each able to carry one Fe3+ ion. In human serum, its total concentration is ∼35 μM, and about 40% of it is in its apo form (i.e., not bound to metal ions109,110); hence, it is susceptible to binding other metals entering the bloodstream, including trivalent actinides, even if they have lower affinity than the native ferric iron.36
Early investigations111,112 showed association of plutonium with glycoproteins under laboratory conditions, which prompted several studies into the role of transferrin for the in vivo behavior of tetravalent actinides.39,90−92,96 It was established that the majority of soluble plutonium in the blood plasma is associated with transferrin (Figure 5) with the assumption that the +IV oxidation state of plutonium is predominant under these conditions. This is consistent with the high liver uptake for tetravalent actinides observed in small rodents following internal contamination.113 As a reminder, transferrin is the mammalian proteins that transport Fe3+ in the bloodstream. It is composed of 679 amino acids and folds into two homologous lobes called the N-lobe and C-lobe, which are connected with a short bridge. Each lobe has one binding pocket naturally containing up to one Fe3+ ion each. The two lobes are not identical, with only 40% sequence match, and can react differently.108In vitro solution thermodynamic experiments have showed that the affinity of apo-transferrin for Pu4+ can be a few orders of magnitude higher than that of Fe3+ under certain conditions (Table 3). Importantly, the metal binding mode of transferrin is not direct but instead requires a synergistic ion that coordinates the metal ion in the protein’s binding pocket. In vitro studies have used bicarbonate, nitrilotriacetate, or citrate39,95,38 as coligands to bind actinides to transferrin. Going one step further in the actinide uptake mechanism, the recognition of actinide-loaded transferrin with its receptors has been also studied in vitro.91,92 Critical results obtained by Jensen et al.91 showed that among the three species that can be formed with plutonium (Pu2Tf, PuCFeNTf, FeCPuNTf), only the one with Pu4+ in the C-lobe and Fe3+ in the N-lobe (i.e., PuCFeNTf) adopts a conformation similar to the natural Fe2Tf, highlighting how the biomechanism can be highly selective, at multiple levels, beyond the short-range coordination sphere of the metal ion. The distinct behavior among the three Tf–Fe–Pu species was also confirmed in cells.91
Figure 5.

Summary of the general partition of actinide ions between the proteins and other components of blood plasma. Percentages were taken from the review by Taylor.114
Table 3. Overview of Experimentally Determined Thermodynamic Constants Describing the Affinity of Metalloproteins for Select Trivalent Actinides (Ac3+, Am3+, Cm3+, and Cf3+), as well as for Tetravalent Actinides (Th4+ and Pu4+) and Uranyl (UO22+)a.
| protein | reaction | thermodynamic constant | conditions | ref |
|---|---|---|---|---|
| transferrin (Tf) | Pu4+ + Tf = PuCTf | log K11 = 24.8 | pH 6.0, synergistic ion = NTA. I = 0.1 M NaCl, MES buffer | (96) |
| Pu4+ + Tf = PuCTf | log K11 = 26.44 | pH 7.0, synergistic ion = NTA. I = 0.1 M | (99) | |
| Fe3+ + Tf = Fe2Tf | log K11 = 21.4 | pH 7.4, Ambient pCO2. | (37) | |
| Fe3+ + FeTf = Fe2Tf | log K21 = 20.3 | |||
| 2 Fe3+ + Tf = Fe2Tf | log β21 = 41.7 | |||
| Cm3+ + Tf = CmCTf | log K11 = 8.8 (C-lobe) | pH 8.6, 50 mM Tris, 150 mM NaCl, 5 mM NaHCO3 | (38) | |
| Cm3+ + CmCTf = Cm2Tf | log K21 = 7.0 (N-lobe) | |||
| 2 Cm3+ + Tf = Cm2Tf | log β21 = 15.8 | |||
| 2 Ac3+ + Tf = Ac2Tf | log β21 = 10.9 | Estimate based on literature data and a linear free-energy relationship. | (119) | |
| fetuin (Fet) | Pu4+ + Fet = PuFet | log K11 = 26.2 | pH 7.0. I = 0.1 M | (99) |
| UO22+ + Fet = UO2Fet | log K11 = 7.5 (Kd = 30 nM) | pH 7.4, 140 mM NaCl, pCO2 | (97) | |
| human serum albumin (HSA) | Th4+ + HSA = ThHSA | log K11 = 4.12 | pH ∼7.4, 5 mM HEPES, 150 mM NaCl | (100) |
| UO22+ + HSA = UO2HSA | log K11 = 4.27 | pH ∼7.4, 5 mM HEPES, 150 mM NaCl | (100) | |
| lanmodulin (LanM) | wild-type lanmodulin: 3 Ac3+ + LanM = Ac3LanM | log β31 = 36.2 | pH 7.0, 90 mM NaCl, 10 mM HEPES | (119) |
| wild-type lanmodulin: 3 Am3+ + LanM = Am3LanM | log β31 = 35.6 | pH 5.0, 75 mM NaCl, 25 mM acetate | (50) | |
| 3 Cm3+ + LanM = Cm3LanM | log β31 = 35.8 | pH 5.0, 75 mM NaCl, 25 mM acetate | (50) | |
| lanmodulin variants: | log β31 = 34.4 | pH 5.0, 75 mM NaCl, 25 mM acetate | (120) | |
| 3 Am3+ + 3D9N = Am33D9N | ||||
| 3 Am3+ + 3D9A = Am33D9A | log β31 = 33.1 | |||
| 3 Am3+ + 3D9M = Am33D9M | log β31 = 32.3 | |||
| 3 Am3+ + 3D9SeMet = Am33D9SeMet | log β31 = 32.4 | |||
| 3 Am3+ + 3D9H = Am33D9H | log β31 = 32.6 | |||
| alpha amylase (Amy) | Cm3+ + Amy = CmAmy | log K11 = 4.76 | pH 5.5, I = 0.1 M | (105) |
| Cm3+ + 3 Amy = Cm(Amy)3 | log β13 = 12.14 | |||
| UO22+ + Amy = UO2Amy | log K11 = 5.67 | I = 0.1 M (NaCl) | (107) | |
| UO22+ + 2 Amy = UO2(Amy)2 | log β12 = 10.39 | |||
| UO22+ + Amy + HO– = UO2(HO)Amy | log K11–1 = 0.64 | |||
| UO22+ + Amy +2 HO– = UO2(HO)2Amy | log β11–2 = −6.28 | |||
| osteopontin (OPN) | x UO22+ + OPN = (UO2)xOPN | (Kd = 26 nM)* | *Number of binding sites undetermined. | (103) |
| siderocalin (Scn) | [PuIVEnterobactin]2– + Scn = [PuIVEnterobactin]/Scn | log K11 = 10.0 (Kd = 0.09 nM) | pH 7.4, TBS buffer, 10 μg/mL ubiquitin, 5% DMSO. Actinide precomplexed to enterobactin. | (102) |
| [FeIIIEnterobactin]2– + Scn = [FeIiiEnterobactin]/Scn | log K11 = 9.4 (Kd = 0.41 nM) | pH 7.4, TRIS buffer, 32 μg/mL ubiquitin. | (121) | |
| [AmEnterobactin]3– + Scn = [AmEnterobactin]/Scn | log K11 = 9.6 (Kd = 0.24 nM) | pH 7.4, TBS buffer, 10 μg/mL ubiquitin, 5% DMSO. Actinide precomplexed to enterobactin. | (102) | |
| [CmEnterobactin]3– + Scn = [CmEnterobactin]/Scn | log K11 = 9.7 (Kd = 0.20 nM) | pH 7.4, TBS buffer, 10 μg/mL ubiquitin, 5% DMSO. Actinide precomplexed to enterobactin. | (102) | |
| [Am343-LI(1,2-HOPO)]− + Scn = [Am343-LI(1,2-HOPO)]/Scn | log K11 = 7.5 (Kd = 29 nM) | pH 7.4, TBS buffer, 10 μg/mL ubiquitin, 5% DMSO. Actinide precomplexed to ligand. | (102) | |
| [Cm343-LI(1,2-HOPO)]− + Scn = [Cm343-LI(1,2-HOPO)]/Scn | log K11 = 7.5 (Kd = 32 nM) | pH 7.4, TBS buffer, 10 μg/mL ubiquitin, 5% DMSO. Actinide precomplexed to ligand. | (102) | |
| [Cf343-LI(1,2-HOPO)]− + Scn = [Cf343-LI(1,2-HOPO)]/Scn | log K11 = 7.3 (Kd = 50 nM) | pH 7.4, TBS buffer, 10 μg/mL ubiquitin, 5% DMSO. Actinide precomplexed to ligand. | (23) | |
| synthetic peptides (LBT) | Am3+ + LBT = AmLBT | log K11 = 7.35 (Kd = 45 nM) | pH 7, 10 mM HEPES/100 mM NaCl buffer, | (122) |
Corresponding constants for ferric iron (Fe3+) are also given for comparison. Although not proteins, synthetic lanthanide peptides (LBTs) are also shown for the sake of comparison. The Kd values reported in this table correspond to protein binding constants, not solubility products (also abbreviated Kd in the literature).
However, while there is ample evidence that transferrin can play an important role in the in vivo transport of tetravalent actinides, the picture is far less clear for the trivalent ones. Early studies noticed that Am3+ and Pu4+ were not associated to the same species in the blood.115,116 As shown in Figure 5, transferrin only accounts for ∼30% of the speciation of americium and curium in the blood, and in fact, the main fraction of trivalent actinide species is still unknown. Nonetheless, multiple studies92,38,93−95,35 with transferrin and Cm3+ have been conducted because of the convenient fluorescence properties of this ion. In accordance with the early observations,115,116 solution thermodynamic data showed weak affinity of transferrin for Cm3+ relative to its native ferric ion (Table 3). Competitive binding of Cm3+ to bicarbonate has also been shown94,95 to decrease the overall fraction of transferrin bound to Cm3+ at pH ≥ 7.4 suggesting that transferrin may not be a very strong chelator for trivalent actinides in vivo.
Perhaps one aspect of transferrin’s biochemistry that remains understudied is its potential role, or lack thereof, regarding actinium binding. This is especially important in regard to numerous anticancer drugs that are being developed and that rely on the in vivo chelation of 225Ac3+.26,27,29,30,117,118 In a recent study,119 our team estimated, based on literature data for other metals combined with solution thermodynamic considerations for Ac3+, that the formation constant (log β21) of the Ac2Tf complex is 10.9. This corresponds to ∼5 orders of magnitude lower than that in the case of curium (Table 3). This low stability constant suggests that, similarly to Am3+ and Cm3+, transferrin is certainly only a minor contributor, if any, to the speciation of Ac3+in vivo. Despite the strong commercial potential for the use of actinium in medicine, the biogeochemical speciation of this particular actinide (the largest +III cation of the periodic table; Figure 3) is still largely unknown, even less so than Am3+ and Cm3+.
Other human protein studies involving trivalent actinides, such as albumin, fetuin, and alpha amylase, showed that all have fairly low binding constants (Table 3), in the same range as the citrate or humic acid complexes (Table 2). Recently, the bacterial protein siderocalin, which naturally binds Fe3+, has also been explored as a potential actinide transporter.123,102 This protein does not coordinate the metal ion directly but requires precomplexation of the metal to specific chelators, typically a siderophore ligand bearing catechol functions, such as enterobactin. This makes the uptake mechanism highly conditional, and the overall stability of the actinide–siderophore–siderocalin complexes mainly depends on the small siderophore ligand rather than the actinide, itself. For example, the stability of the Am3+ and Cm3+ complexes with the 3,4,3-LI(1,2-HOPO)-siderocalin adduct is 3 orders of magnitude lower than with the corresponding enterobactin–siderocalin adduct (Table 3). Moreover, the observed stability constants of the actinide–enterobactin–siderocalin complexes are in the same range as that of the Fe3+–enterobactin–siderocalin complex (log K11 ≈ 9.5; Table 3). Therefore, it remains unclear how important siderocalin is for actinide complexation in vivo. Based on the current published literature, no study has been performed to directly compare actinide binding to transferrin and siderocalin.
5. Case of the Metalloprotein Lanmodulin and Its Potential Impact on Actinide Chemistry
Despite the academic and historical interest for metalloproteins that are able to interact with actinides (Table 3), they remain unlikely to participate in the chemistry of metal ions in the environment as these are derived from in vivo systems rather than biogeochemical ones. In this context, the discovery of natural proteins that are stable ex vivo and able to bind f-elements under geochemical conditions appears particularly crucial. Such macrochelators would also represent a higher level of complexity relative to the traditional small chelators studied with actinides (Table 2) and may present a better picture of the biogeochemical diversity of natural systems.
The first such protein was discovered in 2018 by Cotruvo Jr. et al.124 in the context of lanthanide biochemistry studies. The natural variant of the protein was named “lanmodulin” (short for “lanthanide modulated protein” and abbreviated “LanM”). Our team then led the effort to extend the study of wild-type LanM and its variants to radioisotopes.50,119,120,125 LanM is, by far, the strongest actinide-binding protein characterized to date (Table 3), including all of the mammalian proteins previously studied at length for f-elements. Figure 6 gives a summary of the dissociation constants (Averaged Kd per binding site) that have been determined for protein complexes of actinides (Note: on the biochemistry Kd scale, the smaller the value, the stronger the complex. This Kd must not be mistaken with the geochemical Kd scale used for sorption reactions). Although they are not proteins, the synthetic peptides of “lanthanide binding tag” (LBT) have been included in Figure 6 for comparison as peptides may also exist in nature. Özçubukçu et al.122 determined the Kd values of 10 variants of the original LBT126 with Am3+. Only three variants of LBT are represented in Figure 6 as they encompass the entire stability range observed for the different Am-LBT complexes.122 In detail, LanM has three relatively similar binding sites with Kd in the picomolar range (i.e., log β31 of 34–36 at pH 7). For comparison, LanM’s affinity for the trivalent f-elements is about 2–4 orders of magnitude higher than that of the siderophore/siderocalin complexes, 4–6 orders of magnitude higher than that of transferrin, and ∼8 orders of magnitude higher than α-amylase (Figure 6). LanM even has very high affinity for the usually hard-to-complex actinium with a Kd at pH 7 of 865 fM (i.e., Kd = 8.65 × 10–13 M or log βAc3LanM of 36.2),119 representing arguably one of the strongest actinium complexes characterized to date.
Figure 6.
Comparison of the general affinity of the proteins studied for actinide(III) complexation. Note that in the biochemistry field, the thermodynamic affinity is usually reported in terms of the dissociation constant, Kd. On the Kd scale, the lower the value, the stronger the complex. Also note that the x-axis is a logarithmic scale and spans 10 orders of magnitude. In this figure, the different protein systems are organized from the strongest (LanM) to the weakest (α-amylase) from top to bottom. Although the synthetic peptides “lanthanide binding tags” (LBTs) are not proteins, they have been included in this figure for comparison.122 See Table 3 for details about chemical conditions under which the Kd values have been determined. LanM = lanmodulin. Scn = siderocalin. Tf = transferrin. LBT = lanthanide binding tag.
More importantly, LanM can sustain acidic conditions and remains bound to the trivalent actinides from pH = ∼2.5 to at least 10,119,120,125,127 which makes it a strong contender for complexing actinides in the environment over a broad acidity range. The other proteins studied with actinides are unable to bind under acidic conditions. The complexes of LanM with Am3+ or Cm3+ also remain stable even in the presence of thousands of equivalent of the siderophore DFOB,50 which was considered up to now as one of the strongest natural chelators for actinides and lanthanides.
Our team also showed that LanM can efficiently bind Ac3+ even when its concentration is in the low femtomolar range (tested down to 0.3 × 10–15 M) and remains bound to Ac3+ even in the presence of 10+12 equivalents of competing environmental cations (Ca2+, Mg2+, Zn2+, Mn2+, Cu2+) or large excess of organic ligands (carbonates, sulfates, phosphates, etc.). Such a unique affinity for actinium also allows for the efficient separation of actinium (Ac3+) and radium (Ra2+) over a broad pH range, including under environmental conditions.119 The peerless resilience of LanM at low pH, combined with its f-element selectivity, has also led to its use as a scavenger for next-generation hydrometallurgical processes in rare earth extraction and separation.128,129 Similar to the Ac3+/Ra2+ separation, our team also showed that LanM can be used to efficiently separate americium (Am3+) from neptunyl (NpO2+) via a simple macromolecular screen (e.g., size-exclusion columns) as the protein binds only to the trivalent f-elements. This kind of protein-based separation of trivalent actinides/lanthanide from other metals could challenge our understanding of the mobility of radioisotopes in the environment since natural proteins are currently not taken into account in speciation models.130,131
As shown in Figure 7a, under laboratory conditions, it was also observed that the LanM complexes with trivalent actinides are stable in the presence of a large excess of carbonate/bicarbonate ions and at high pH, hinting that such complexes could persist in the environment. Furthermore, it was demonstrated that LanM can compete with environmentally relevant minerals (i.e., quartz, calcite, kaolinite, montmorillonite clay) and significantly reduces the sorption of americium.125 Under the tested conditions with calcite, LanM, and 243Am3+ (Figure 7b), the soluble fraction of americium jumps from ∼3% to ∼80% in the presence of just 200 nM LanM and keeps increasing at higher LanM concentration. However, the presence of LanM had no impact on the sorption of neptunyl (NpO2+), which highlights the specificities of this protein for trivalent f-elements. While, these recent results50,119,120,125 represent a first chapter in the discovery of metalloproteins that are relevant to the biogeochemistry of actinides, thus far, all the evidence points to the formation of very stable and resilient actinide–LanM complexes that could exist under environmental conditions. Further research is needed in this area, but it is clear that if such proteins (or related compounds) are present in the vicinity of nuclear waste, their impact on the speciation of actinides cannot be ignored. As the inventory of nuclear waste keeps growing, if new locations are being considered for above-ground or subsurface storage, beyond the traditional risk assessment studies performed for such sites, it could be interesting to screen for the presence of LanM-like biomolecules.
Figure 7.
Example of the potential impact of lanmodulin (LanM) on the speciation and sorption of trivalent actinides. (a) Fraction of the curium–lanmodulin complex (248Cm3LanM) formed as a function of the ratio carbonate/LanM at different pH values. The speciation of curium was determined via fluorescence spectroscopy as previously in ref (125). [LanM] = 1.0 μM. [Cm] = 2.0 μM. [CO3]total = ambient concentration up to 300 mM. The x-axis is shown with a logarithmic scale and gives the ratio between the total concentration of HCO3–/CO32– and the concentration of LanM. Dotted lines are for eye guidance only. (b) Soluble fraction of americium (243Am3+) and neptunyl (239NpO2+) in the presence of calcite (CaCO3) and with or without LanM. Note the strong increase in the soluble fraction of Am3+ (from 3 to 79–93%) upon addition of LanM. pH = 8.5. See ref (125) for experimental details.
6. Conclusion and Outlook
Actinide chemistry has been a fascinating topic since its inception and has forever changed the world, especially after the discovery of transuranic elements. However, now ∼80 years following the introduction of the actinide series concept by Glenn T. Seaborg,14 the biogeochemistry of actinides is still far from being completely understood. Scientists embarking on this field of research face several barriers, including the dearth of basic data (when compared to natural elements), the limited availability of certain radioisotopes, the logistical hurdles for experiments involving radioactive samples, and the financial burden associated with research isotopes. Compounding these effects, actinides exhibit distinct physicochemical properties, and even if we consider them as a series, almost every one of them represents a unique case in the periodic table with a distinct combination of redox, nuclear, spectroscopic, and chelation properties. For the trivalent ones, while lanthanides are often regarded as nonradioactive surrogates, the analogy is far from perfect, and more research using actinide isotopes directly instead of surrogates is needed. In this regard, the recent push, both by academics and private companies, to develop actinium chemistry for cancer medicine (i.e., 225Ac-targeted alpha therapies132,133) is a positive step forward that will bring more attention to the biogeochemistry of actinides. Lessons learned from this field will likely be informative about the chemistry of trivalent actinides found in the environment and vice versa. One of the biggest questions in the field of actinide biogeochemistry concerns their speciation in nature and whether or not specific “actinophore” chelators exist. Given the typically low concentration of actinides in the environment, the wide variety of sites where they can be found (solid matrices, oceans, dry areas, oxidized or anoxic conditions, etc.), and the near limitless range of natural compounds that exist (small aqueous chelators, proteins, minerals, etc.), this question will likely remain open for some time.
Acknowledgments
This work was performed under the auspices of the U.S. Department of Energy (DOE) by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344. Part of this work was supported by the Office of Defense Nuclear Nonproliferation Research and Development within the U.S. Department of Energy’s National Nuclear Security Administration. The author is thankful to LLNL’s Glenn T. Seaborg Institute (GTSI) for continued support and for providing a safe and efficient environment to perform actinide research. Release number: LLNL-JRNL-865950.
Author Contributions
CRediT: Gauthier J.-P. Deblonde conceptualization, writing - review & editing.
The author declares no competing financial interest.
Dedication
This article is dedicated to Annie Kersting, Dan M. Park, Joseph Cotruvo Jr., Mavrik Zavarin, and Yongqin Jiao for their exceptional mentoring and scientific leadership.
Special Issue
Published as part of ACS Environmental Auspecial issue “2024 Rising Stars in Environmental Research”.
References
- Geibert W.; Charette M.; Kim G.; Moore W. S.; Street J.; Young M.; Paytan A. The release of dissolved actinium to the ocean: A global comparison of different end-members. Marine Chemistry 2008, 109, 409–420. 10.1016/j.marchem.2007.07.005. [DOI] [Google Scholar]
- Taylor S. R. Abundance of chemical elements in the continental crust: a new table. Geochim. Cosmochim. Acta 1964, 28, 1273–1285. 10.1016/0016-7037(64)90129-2. [DOI] [Google Scholar]
- Alibo D. S.; Nozaki Y. Rare earth elements in seawater: particle association, shale-normalization, and Ce oxidation. Geochim. Cosmochim. Acta 1999, 63, 363–372. 10.1016/S0016-7037(98)00279-8. [DOI] [Google Scholar]
- The Chemistry of the Actinide and Transactinide Elements, 3rd ed.; Springer Nature, 2006. https://link.springer.com/book/10.1007/1-4020-3598-5 (accessed June 16, 2021).
- MacDonald M. R.; Fieser M. E.; Bates J. E.; Ziller J. W.; Furche F.; Evans W. J. Identification of the + 2 Oxidation State for Uranium in a Crystalline Molecular Complex, [K(2.2.2-Cryptand)][(C5H4SiMe3)3U]. J. Am. Chem. Soc. 2013, 135, 13310–13313. 10.1021/ja406791t. [DOI] [PubMed] [Google Scholar]
- Windorff C. J.; Chen G. P.; Cross J. N.; Evans W. J.; Furche F.; Gaunt A. J.; Janicke M. T.; Kozimor S. A.; Scott B. L. Identification of the Formal + 2 Oxidation State of Plutonium: Synthesis and Characterization of {PuII[C5H3(SiMe3)2]3}–. J. Am. Chem. Soc. 2017, 139, 3970–3973. 10.1021/jacs.7b00706. [DOI] [PubMed] [Google Scholar]
- Dutkiewicz M. S.; Apostolidis C.; Walter O.; Arnold P. L. Reduction chemistry of neptunium cyclopentadienide complexes: from structure to understanding. Chem. Sci. 2017, 8, 2553–2561. 10.1039/C7SC00034K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poe T. N.; Ramanantoanina H.; Sperling J. M.; Wineinger H. B.; Rotermund B. M.; Brannon J.; Bai Z.; Scheibe B.; Beck N.; Long B. N.; Justiniano S.; Albrecht-Schönzart T. E.; Celis-Barros C. Isolation of a californium(II) crown–ether complex. Nat. Chem. 2023, 15, 722–728. 10.1038/s41557-023-01170-9. [DOI] [PubMed] [Google Scholar]
- Abraham M. M.; Boatner L. A.; Finch C. B.; Reynolds R. W.; Zeldes H. Electron Paramagnetic Resonance Investigations of Divalent Americium and Trivalent Curium in Strontium Chloride. Phys. Rev. B 1970, 1, 3555–3560. 10.1103/PhysRevB.1.3555. [DOI] [Google Scholar]
- Baybarz R. D.; Asprey L. B.; Strouse C. E.; Fukushima E. Divalent americium: The crystal structure and magnetic susceptibility of AmI2. Journal of Inorganic and Nuclear Chemistry 1972, 34, 3427–3431. 10.1016/0022-1902(72)80237-9. [DOI] [Google Scholar]
- Baybarz R. D. The preparation and crystal structures of americium dichloride and dibromide. Journal of Inorganic and Nuclear Chemistry 1973, 35, 483–487. 10.1016/0022-1902(73)80560-3. [DOI] [Google Scholar]
- Maria L.; Marçalo J.; Gibson J. K.. Divalent Actinides and Transactinides: Inorganic and Organometallic Complexes. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons, Ltd, 2018: pp 1–13. [Google Scholar]
- McMillan E.; Abelson P. H. Radioactive Element 93. Phys. Rev. 1940, 57, 1185–1186. 10.1103/PhysRev.57.1185.2. [DOI] [Google Scholar]
- Seaborg G. T. The Transuranium Elements. Science 1946, 104, 379–386. 10.1126/science.104.2704.379. [DOI] [PubMed] [Google Scholar]
- Bodu R.; Bouzigues H.; Morin N.; Pfiffelmann J.-P. Isotopic anomalies in U from Gabon. Compt. Rend., Ser. D 1972, 275, 1731–1732. [Google Scholar]
- Ruffenach J. C.; Menes J.; Devillers C.; Lucas M.; Hagemann R. Etudes chimiques et isotopiques de l’uranium, du plomb et de plusieurs produits de fission dans un echantillon de minerai du reacteur naturel d’Oklo. Earth and Planetary Science Letters 1976, 30, 94–108. 10.1016/0012-821X(76)90011-X. [DOI] [Google Scholar]
- Seaborg G. T.; Mcmillan E. M.; Kennedy J. W.; Wahl A. C. Radioactive Element 94 from Deuterons on Uranium. Phys. Rev. 1946, 69, 366–367. 10.1103/PhysRev.69.366.2. [DOI] [Google Scholar]
- Seaborg G. T.; Wahl A. C.; Kennedy J. W. Radioactive Element 94 from Deuterons on Uranium. Phys. Rev. 1946, 69, 367–367. 10.1103/PhysRev.69.367. [DOI] [Google Scholar]
- Kennedy J. W.; Seaborg G. T.; Segrè E.; Wahl A. C. Properties of 94(239). Phys. Rev. 1946, 70, 555–556. 10.1103/PhysRev.70.555. [DOI] [Google Scholar]
- Antonio M. R.; Williams C. W.; Soderholm L. Berkelium redox speciation. Radiochim. Acta 2002, 90, 851–856. 10.1524/ract.2002.90.12_2002.851. [DOI] [Google Scholar]
- Cary S. K.; Vasiliu M.; Baumbach R. E.; Stritzinger J. T.; Green T. D.; Diefenbach K.; Cross J. N.; Knappenberger K. L.; Liu G.; Silver M. A.; DePrince A. E.; Polinski M. J.; Cleve S. M. V.; House J. H.; Kikugawa N.; Gallagher A.; Arico A. A.; Dixon D. A.; Albrecht-Schmitt T. E. Emergence of californium as the second transitional element in the actinide series. Nat. Commun. 2015, 6, 6827. 10.1038/ncomms7827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver M.A.; Cary S.K.; Johnson J.A.; Baumbach R.E.; Arico A.A.; Luckey M.; Urban M.; Wang J.C.; Polinski M.J.; Chemey A.; Liu G.; Chen K.-W.; Cleve S. M. V.; Marsh M.L.; Eaton T.M.; van de Burgt L. J.; Gray A.L.; Hobart D.E.; Hanson K.; Maron L.; Gendron F.; Autschbach J.; Speldrich M.; Kögerler P.; Yang P.; Braley J.; Albrecht-Schmitt T. E. Characterization of berkelium(III) dipicolinate and borate compounds in solution and the solid state. Science 2016, 353, aaf3762. 10.1126/science.aaf3762. [DOI] [PubMed] [Google Scholar]
- Deblonde G.J.-P.; Sturzbecher-Hoehne M.; Rupert P. B.; An D. D.; Illy M.-C.; Ralston C. Y.; Brabec J.; de Jong W. A.; Strong R. K.; Abergel R. J. Chelation and stabilization of berkelium in oxidation state + IV. Nat. Chem. 2017, 9, 843–849. 10.1038/nchem.2759. [DOI] [PubMed] [Google Scholar]
- Seeger P. A.; Rokop S. E.; Palmer P. D.; Henderson S. J.; Hobart D. E.; Trewhella J. Neutron Resonance Scattering Shows Specific Binding of Plutonium to the Calcium-Binding Sites of the Protein Calmodulin and Yields Precise Distance Information. J. Am. Chem. Soc. 1997, 119, 5118–5125. 10.1021/ja9633124. [DOI] [Google Scholar]
- Miederer M.; Scheinberg D. A.; McDevitt M. R. Realizing the potential of the Actinium-225 radionuclide generator in targeted alpha particle therapy applications. Adv. Drug Delivery Rev. 2008, 60, 1371–1382. 10.1016/j.addr.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratochwil C.; Bruchertseifer F.; Giesel F. L.; Weis M.; Verburg F. A.; Mottaghy F.; Kopka K.; Apostolidis C.; Haberkorn U.; Morgenstern A. 225Ac-PSMA-617 for PSMA-Targeted α-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2016, 57, 1941–1944. 10.2967/jnumed.116.178673. [DOI] [PubMed] [Google Scholar]
- Thiele N. A.; Brown V.; Kelly J. M.; Amor-Coarasa A.; Jermilova U.; MacMillan S. N.; Nikolopoulou A.; Ponnala S.; Ramogida C. F.; Robertson A. K. H.; Rodríguez-Rodríguez C.; Schaffer P.; Williams C. Jr.; Babich J. W.; Radchenko V.; Wilson J. J. An Eighteen-Membered Macrocyclic Ligand for Actinium-225 Targeted Alpha Therapy. Angew. Chem., Int. Ed. 2017, 56, 14712–14717. 10.1002/anie.201709532. [DOI] [PubMed] [Google Scholar]
- McDevitt M. R.; Thorek D. L. J.; Hashimoto T.; Gondo T.; Veach D. R.; Sharma S. K.; Kalidindi T. M.; Abou D. S.; Watson P. A.; Beattie B. J.; Timmermand O. V.; Strand S.-E.; Lewis J. S.; Scardino P. T.; Scher H. I.; Lilja H.; Larson S. M.; Ulmert D. Feed-forward alpha particle radiotherapy ablates androgen receptor-addicted prostate cancer. Nat. Commun. 2018, 9, 1–11. 10.1038/s41467-018-04107-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele N. A.; Wilson J. J. Actinium-225 for Targeted α Therapy: Coordination Chemistry and Current Chelation Approaches. Cancer Biotherapy and Radiopharmaceuticals 2018, 33, 336–348. 10.1089/cbr.2018.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu A.; Wilson J.J. Advancing Chelation Strategies for Large Metal Ions for Nuclear Medicine Applications. Acc. Chem. Res. 2022, 55, 904. 10.1021/acs.accounts.2c00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg D.; Persson I. The size of actinoid(III) ions – structural analysis vs. common misinterpretations. Coord. Chem. Rev. 2016, 318, 131–134. 10.1016/j.ccr.2016.04.003. [DOI] [Google Scholar]
- Singer H.; Steudtner R.; Klein A. S.; Rulofs C.; Zeymer C.; Drobot B.; Pol A.; Cecilia Martinez-Gomez N.; Op den Camp H. J. M.; Daumann L. J. Minor Actinides Can Replace Essential Lanthanides in Bacterial Life**. Angew. Chem., Int. Ed. 2023, 62, e202303669 10.1002/anie.202303669. [DOI] [PubMed] [Google Scholar]
- Shannon R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr., Sect. A 1976, 32, 751–767. 10.1107/S0567739476001551. [DOI] [Google Scholar]
- Deblonde G.J.-P.; Zavarin M.; Kersting A. B. The coordination properties and ionic radius of actinium: A 120-year-old enigma. Coord. Chem. Rev. 2021, 446, 214130 10.1016/j.ccr.2021.214130. [DOI] [Google Scholar]
- Adam N.; Trumm M.; Smith V. C.; MacGillivray R. T. A.; Panak P. J. Incorporation of transuranium elements: coordination of Cm(III) to human serum transferrin. Dalton Trans. 2018, 47, 14612–14620. 10.1039/C8DT02915F. [DOI] [PubMed] [Google Scholar]
- Sun H.; Li H.; Sadler P. J. Transferrin as a Metal Ion Mediator. Chem. Rev. 1999, 99, 2817–2842. 10.1021/cr980430w. [DOI] [PubMed] [Google Scholar]
- Aisen P.; Leibman A.; Zweier J. Stoichiometric and site characteristics of the binding of iron to human transferrin. J. Biol. Chem. 1978, 253, 1930–1937. 10.1016/S0021-9258(19)62337-9. [DOI] [PubMed] [Google Scholar]
- Sturzbecher-Hoehne M.; Goujon C.; Deblonde G.J.-P.; Mason A. B.; Abergel R. J. Sensitizing Curium Luminescence through an Antenna Protein To Investigate Biological Actinide Transport Mechanisms. J. Am. Chem. Soc. 2013, 135, 2676–2683. 10.1021/ja310957f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanson A.; Ferrand M.; Funke H.; Hennig C.; Moisy P.; Solari P. L.; Vidaud C.; Den Auwer C. The Role of Transferrin in Actinide(IV) Uptake: Comparison with Iron(III), Chemistry – A. European Journal 2010, 16, 1378–1387. 10.1002/chem.200901209. [DOI] [PubMed] [Google Scholar]
- Morris D. E.; Hobart D. E.; Palmer P. D.; Haire R. G.; Peterson J. R. Voltammetric Investigation of the Berkelium(IV/III) Couple in Concentrated Aqueous Carbonate Solutions. Radiochim. Acta 1990, 49, 125–134. 10.1524/ract.1990.49.3.125. [DOI] [Google Scholar]
- Piro N. A.; Robinson J. R.; Walsh P. J.; Schelter E. J. The electrochemical behavior of cerium(III/IV) complexes: Thermodynamics, kinetics and applications in synthesis. Coord. Chem. Rev. 2014, 260, 21–36. 10.1016/j.ccr.2013.08.034. [DOI] [Google Scholar]
- Deblonde G.J.-P.; Ricano A.; Abergel R. J. Ultra-selective ligand-driven separation of strategic actinides. Nat. Commun. 2019, 10, 2438. 10.1038/s41467-019-10240-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver M. A.; Cary S. K.; Garza A. J.; Baumbach R. E.; Arico A. A.; Galmin G. A.; Chen K.-W.; Johnson J. A.; Wang J. C.; Clark R. J.; Chemey A.; Eaton T. M.; Marsh M. L.; Seidler K.; Galley S. S.; van de Burgt L.; Gray A. L.; Hobart D. E.; Hanson K.; Van Cleve S. M.; Gendron F.; Autschbach J.; Scuseria G. E.; Maron L.; Speldrich M.; Kögerler P.; Celis-Barros C.; Páez-Hernández D.; Arratia-Pérez R.; Ruf M.; Albrecht-Schmitt T. E. Electronic Structure and Properties of Berkelium Iodates. J. Am. Chem. Soc. 2017, 139, 13361–13375. 10.1021/jacs.7b05569. [DOI] [PubMed] [Google Scholar]
- Cross J. N.; Villa E. M.; Wang S.; Diwu J.; Polinski M. J.; Albrecht-Schmitt T. E. Syntheses, Structures, and Spectroscopic Properties of Plutonium and Americium Phosphites and the Redetermination of the Ionic Radii of Pu(III) and Am(III). Inorg. Chem. 2012, 51, 8419–8424. 10.1021/ic300958z. [DOI] [PubMed] [Google Scholar]
- Colliard I.; Lee J. R. I.; Colla C. A.; Mason H. E.; Sawvel A. M.; Zavarin M.; Nyman M.; Deblonde G.J.-P. Polyoxometalates as ligands to synthesize, isolate and characterize compounds of rare isotopes on the microgram scale. Nat. Chem. 2022, 14, 1357–1366. 10.1038/s41557-022-01018-8. [DOI] [PubMed] [Google Scholar]
- Heller A.; Barkleit A.; Foerstendorf H.; Tsushima S.; Heim K.; Bernhard G. Curium(III) citrate speciation in biological systems: a europium(III) assisted spectroscopic and quantum chemical study. Dalton Trans. 2012, 41, 13969–13983. 10.1039/c2dt31480k. [DOI] [PubMed] [Google Scholar]
- Leguay S.; Vercouter T.; Topin S.; Aupiais J.; Guillaumont D.; Miguirditchian M.; Moisy P.; Le Naour C. New Insights into Formation of Trivalent Actinides Complexes with DTPA. Inorg. Chem. 2012, 51, 12638–12649. 10.1021/ic3011019. [DOI] [PubMed] [Google Scholar]
- Tian G.; Shuh D. K. A spectrophotometric study of Am(III) complexation with nitrate in aqueous solution at elevated temperatures. Dalton Trans. 2014, 43, 14565–14569. 10.1039/C4DT01183J. [DOI] [PubMed] [Google Scholar]
- Grimes T. S.; Heathman C. R.; Jansone-Popova S.; Bryantsev V. S.; Goverapet Srinivasan S.; Nakase M.; Zalupski P. R. Thermodynamic, Spectroscopic, and Computational Studies of f-Element Complexation by N-Hydroxyethyl-diethylenetriamine-N,N′,N″,N″-tetraacetic Acid. Inorg. Chem. 2017, 56, 1722–1733. 10.1021/acs.inorgchem.6b02897. [DOI] [PubMed] [Google Scholar]
- Deblonde G.J.-P.; Mattocks J. A.; Wang H.; Gale E. M.; Kersting A. B.; Zavarin M.; Cotruvo J. A. Characterization of Americium and Curium Complexes with the Protein Lanmodulin: A Potential Macromolecular Mechanism for Actinide Mobility in the Environment. J. Am. Chem. Soc. 2021, 143, 15769–15783. 10.1021/jacs.1c07103. [DOI] [PubMed] [Google Scholar]
- Ferrier M. G.; Batista E. R.; Berg J. M.; Birnbaum E. R.; Cross J. N.; Engle J. W.; Pierre H. S. L.; Kozimor S. A.; Pacheco J. S. L.; Stein B. W.; Stieber S. C. E.; Wilson J. J. Spectroscopic and computational investigation of actinium coordination chemistry. Nat. Commun. 2016, 7, 12312. 10.1038/ncomms12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrier M. G.; Stein B. W.; Batista E. R.; Berg J. M.; Birnbaum E. R.; Engle J. W.; John K. D.; Kozimor S. A.; Lezama Pacheco J. S.; Redman L. N. Synthesis and Characterization of the Actinium Aquo Ion. ACS Cent. Sci. 2017, 3, 176–185. 10.1021/acscentsci.6b00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auwer C. D.; Llorens I.; Moisy P.; Vidaud C.; Goudard F.; Barbot C.; Solari P. L.; Funke H. Actinide uptake by transferrin and ferritin metalloproteins. Radiochim. Acta 2005, 93, 699–703. 10.1524/ract.2005.93.11.699. [DOI] [Google Scholar]
- Revel R.; Den Auwer C.; Madic C.; David F.; Fourest B.; Hubert S.; Le Du J.-F.; Morss L. R. First Investigation on the L Edges of the 249Cf Aquo Ion by X-ray Absorption Spectroscopy. Inorg. Chem. 1999, 38, 4139–4141. 10.1021/ic990214l. [DOI] [Google Scholar]
- Meinrath G.; Kim J. I. The Carbonate Complexation of the Am(III) Ion. Radiochim. Acta 1991, 52–53, 29–34. 10.1524/ract.1991.5253.1.29. [DOI] [Google Scholar]
- NlTSCHE H.; Standifer E. M. Americium(lll) Carbonate Complexation in Aqueous Perchlorate Solution. Radiochim. Acta 1989, 46, 185–190. 10.1524/ract.1989.46.4.185. [DOI] [Google Scholar]
- Vercouter T.; Vitorge P.; Amekraz B.; Giffaut E.; Hubert S.; Moulin C. Stabilities of the Aqueous Complexes Cm(CO3)33- and Am(CO3)33- in the Temperature Range 10–70 °C. Inorg. Chem. 2005, 44, 5833–5843. 10.1021/ic050214n. [DOI] [PubMed] [Google Scholar]
- Janicki R.; Lindqvist-Reis P. Eu(III) and Cm(III) tetracarbonates – in the quest for the limiting species in solution. Dalton Trans. 2018, 47, 2393–2405. 10.1039/C7DT04836J. [DOI] [PubMed] [Google Scholar]
- Li X.-B.; Wu Q.-Y.; Wang C.-Z.; Lan J.-H.; Ning S.-Y.; Wei Y.-Z. Theoretical study on structures of Am(III) carbonate complexes. J. Radioanal Nucl. Chem. 2020, 325, 527–535. 10.1007/s10967-020-07254-x. [DOI] [Google Scholar]
- Albrecht-Schmitt T. E.; Hobart D. E.; Páez-Hernández D.; Celis-Barros C. Theoretical examination of covalency in berkelium(IV) carbonate complexes. Int. J. Quantum Chem. 2020, 120, e26254 10.1002/qua.26254. [DOI] [Google Scholar]
- Abraham F.; Arab-Chapelet B.; Rivenet M.; Tamain C.; Grandjean S. Actinide oxalates, solid state structures and applications. Coord. Chem. Rev. 2014, 266–267, 28–68. 10.1016/j.ccr.2013.08.036. [DOI] [Google Scholar]
- Kim H.-K.; Jeong K.; Cho H.-R.; Jung E. C.; Kwak K.; Cha W. Spectroscopic speciation of aqueous Am(III)–oxalate complexes. Dalton Trans. 2019, 48, 10023–10032. 10.1039/C9DT01087D. [DOI] [PubMed] [Google Scholar]
- Arteaga A.; Nicholas A. D.; Ducati L. C.; Autschbach J.; Surbella R. G. I. Americium Oxalate: An Experimental and Computational Investigation of Metal–Ligand Bonding. Inorg. Chem. 2023, 62, 4814–4822. 10.1021/acs.inorgchem.2c03976. [DOI] [PubMed] [Google Scholar]
- Raditzky B.; Sachs S.; Schmeide K.; Barkleit A.; Geipel G.; Bernhard G. Spectroscopic study of americium(III) complexes with nitrogen containing organic model ligands. Polyhedron 2013, 65, 244–251. 10.1016/j.poly.2013.08.047. [DOI] [Google Scholar]
- Peters A. J.; Hamilton-Taylor J.; Tipping E. Americium Binding to Humic Acid. Environ. Sci. Technol. 2001, 35, 3495–3500. 10.1021/es000295g. [DOI] [PubMed] [Google Scholar]
- Sachs S.; Bernhard G. Influence of humic acids on the actinide migration in the environment: suitable humic acid model substances and their application in studies with uranium—a review. J. Radioanal Nucl. Chem. 2011, 290, 17–29. 10.1007/s10967-011-1084-0. [DOI] [Google Scholar]
- Zhang Y. J.; Bryan N. D.; Livens F. R.; Jones M. N. Selectivity in the complexation of actinides by humic substances. Environ. Pollut. 1997, 96, 361–367. 10.1016/S0269-7491(97)00041-9. [DOI] [PubMed] [Google Scholar]
- Reiller P. E.; Evans N. D. M.; Szabó G. Complexation parameters for the actinides(IV)-humic acid system: a search for consistency and application to laboratory and field observations. Radiochim. Acta 2008, 96, 345–358. 10.1524/ract.2008.1500. [DOI] [Google Scholar]
- Monsallier J.-M.; Choppin G. R. Influence of humic acid size on actinide complexation. Radiochim. Acta 2003, 91, 135–140. 10.1524/ract.91.3.135.19980. [DOI] [Google Scholar]
- Kim J. I.; Buckau G.; Bryant E.; Klenze R. Complexation of Americium(III) with Humic Acid. Radiochim. Acta 1989, 48, 135–144. 10.1524/ract.1989.48.34.135. [DOI] [Google Scholar]
- Xue S.; Miao Z.; Gao M.; Wan K. Structural analysis of lignite-derived humic acid and its microscopic interactions with heavy metal ions in aqueous solution. Science of The Total Environment 2023, 897, 165385 10.1016/j.scitotenv.2023.165385. [DOI] [PubMed] [Google Scholar]
- Lee H.; Coulon F.; Wagland S. T. The influence of humic acid on metal(loid)s leaching in landfill leachate for enhancing landfill mining. Science of The Total Environment 2023, 896, 165250 10.1016/j.scitotenv.2023.165250. [DOI] [PubMed] [Google Scholar]
- Moll H.; Glorius M.; Bernhard G. Curium(III) Complexation with Desferrioxamine B (DFO) Investigated Using Fluorescence Spectroscopy. BCSJ. 2008, 81, 857–862. 10.1246/bcsj.81.857. [DOI] [Google Scholar]
- Moll H.; Johnsson A.; Schäfer M.; Pedersen K.; Budzikiewicz H.; Bernhard G. Curium(III) complexation with pyoverdins secreted by a groundwater strain of Pseudomonas fluorescens. Biometals 2008, 21, 219–228. 10.1007/s10534-007-9111-x. [DOI] [PubMed] [Google Scholar]
- Glorius M.; Moll H.; Bernhard G. Complexation of curium(III) with hydroxamic acids investigated by time-resolved laser-induced fluorescence spectroscopy. Polyhedron 2008, 27, 2113–2118. 10.1016/j.poly.2008.04.002. [DOI] [Google Scholar]
- Boukhalfa H.; Reilly S. D.; Neu M. P. Complexation of Pu(IV) with the Natural Siderophore Desferrioxamine B and the Redox Properties of Pu(IV)(siderophore) Complexes. Inorg. Chem. 2007, 46, 1018–1026. 10.1021/ic061544q. [DOI] [PubMed] [Google Scholar]
- Deblonde G.J.-P.; Kersting A. B.; Zavarin M. Open questions on the environmental chemistry of radionuclides. Commun. Chem. 2020, 3, 1–5. 10.1038/s42004-020-00418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao V. K.; Shahani C. J.; Rao C. L. Studies on the Phosphate Complexes of Actinium and Lanthanum. Radiochim. Acta 1970, 14, 31–34. 10.1524/ract.1970.14.1.31. [DOI] [Google Scholar]
- Rao V. K.; Mahajan G. R.; Natarajan P. R. Phosphate Complexation of Americium(III). Radiochim. Acta 1986, 40, 145–150. 10.1524/ract.1986.40.3.145. [DOI] [Google Scholar]
- Moll H.; Brendler V.; Bernhard G. Aqueous curium(III) phosphate species characterized by time-resolved laser-induced fluorescence spectroscopy. Radiochim. Acta 2011, 99, 775–782. 10.1524/ract.2011.1878. [DOI] [Google Scholar]
- Jordan N.; Demnitz M.; Lösch H.; Starke S.; Brendler V.; Huittinen N. Complexation of Trivalent Lanthanides (Eu) and Actinides (Cm) with Aqueous Phosphates at Elevated Temperatures. Inorg. Chem. 2018, 57, 7015–7024. 10.1021/acs.inorgchem.8b00647. [DOI] [PubMed] [Google Scholar]
- Sekine T.; Sakairi M. Studies of Actinium(III) in Various Solutions. III. Actinium (III) Complexes with Oxalate, Sulfate, Chloride, and Thiocyanate Ions in Perchlorate Media. BCSJ. 1969, 42, 2712–2713. 10.1246/bcsj.42.2712. [DOI] [Google Scholar]
- Fröhlich D. R.; Skerencak-Frech A.; Panak P. J. A spectroscopic study on the formation of Cm(III) acetate complexes at elevated temperatures. Dalton Trans. 2014, 43, 3958–3965. 10.1039/c3dt52989d. [DOI] [PubMed] [Google Scholar]
- Skerencak-Frech A.; Trumm M.; Fröhlich D. R.; Panak P. J. Coordination and Thermodynamics of Trivalent Curium with Malonate at Increased Temperatures: A Spectroscopic and Quantum Chemical Study. Inorg. Chem. 2017, 56, 10172–10180. 10.1021/acs.inorgchem.7b00694. [DOI] [PubMed] [Google Scholar]
- Fröhlich D. R.; Skerencak-Frech A.; Morkos M.-L. K.; Panak P. J. A spectroscopic study of Cm(III) complexation with propionate in saline solutions at variable temperatures. New J. Chem. 2013, 37, 1520–1528. 10.1039/c3nj00109a. [DOI] [Google Scholar]
- Nguyen L.V.Complexation studies of actinides (U, Pu, Am) with linear polyaminocarboxylate ligands and siderochelates. Ph.D. Thesis, Institute of Molecular Chemistry of the University of Burgundy (ICMUB), Dijon, 2010. http://www.theses.fr/2010DIJOS052 (accessed July 3, 2021). [Google Scholar]
- Creff G.; Zurita C.; Jeanson A.; Carle G.; Vidaud C.; Auwer C. D. What do we know about actinides-proteins interactions?. Radiochim. Acta 2019, 107, 993–1009. 10.1515/ract-2019-3120. [DOI] [Google Scholar]
- Zurita C.; Tsushima S.; Bresson C.; Cortes M. G.; Solari P. L.; Jeanson A.; Creff G.; Auwer C. D. How Does Iron Storage Protein Ferritin Interact with Plutonium (and Thorium)?, Chemistry – A. European Journal 2021, 27, 2393–2401. 10.1002/chem.202003653. [DOI] [PubMed] [Google Scholar]
- Wirth R.; Taylor D. M.; Duffield J. Identification of transferrin as the principal neptunium-binding protein in the blood serum of rats. International Journal of Nuclear Medicine and Biology 1985, 12, 327–330. 10.1016/0047-0740(85)90188-3. [DOI] [PubMed] [Google Scholar]
- Duffield J. R.; Taylor D. M. A spectroscopic study on the binding of plutonium(IV) and its chemical analogues to transferrin. Inorg. Chim. Acta 1987, 140, 365–367. 10.1016/S0020-1693(00)81125-1. [DOI] [Google Scholar]
- Jensen M. P.; Gorman-Lewis D.; Aryal B.; Paunesku T.; Vogt S.; Rickert P. G.; Seifert S.; Lai B.; Woloschak G. E.; Soderholm L. An iron-dependent and transferrin-mediated cellular uptake pathway for plutonium. Nat. Chem. Biol. 2011, 7, 560–565. 10.1038/nchembio.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deblonde G.J.-P.; Sturzbecher-Hoehne M.; Mason A. B.; Abergel R. J. Receptor recognition of transferrin bound to lanthanides and actinides: a discriminating step in cellular acquisition of f-block metals. Metallomics 2013, 5, 619–626. 10.1039/c3mt20237b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer N.; Fröhlich D. R.; Panak P. J. Interaction of Cm(III) and Am(III) with human serum transferrin studied by time-resolved laser fluorescence and EXAFS spectroscopy. Dalton Trans. 2014, 43, 6689–6700. 10.1039/C3DT53371A. [DOI] [PubMed] [Google Scholar]
- Bauer N.; Smith V. C.; MacGillivray R. T. A.; Panak P. J. Complexation of Cm(III) with the recombinant N-lobe of human serum transferrin studied by time-resolved laser fluorescence spectroscopy (TRLFS). Dalton Trans. 2015, 44, 1850–1857. 10.1039/C4DT03403A. [DOI] [PubMed] [Google Scholar]
- Bauer N.; Panak P. J. Influence of carbonate on the complexation of Cm(III) with human serum transferrin studied by time-resolved laser fluorescence spectroscopy (TRLFS). New J. Chem. 2015, 39, 1375–1381. 10.1039/C4NJ01877J. [DOI] [PubMed] [Google Scholar]
- Sauge-Merle S.; Lemaire D.; Evans R. W.; Berthomieu C.; Aupiais J. Revisiting binding of plutonium to transferrin by CE-ICP-MS. Dalton Trans. 2017, 46, 1389–1396. 10.1039/C6DT04336D. [DOI] [PubMed] [Google Scholar]
- Basset C.; Averseng O.; Ferron P.-J.; Richaud N.; Hagège A.; Pible O.; Vidaud C. Revision of the Biodistribution of Uranyl in Serum: Is Fetuin-A the Major Protein Target?. Chem. Res. Toxicol. 2013, 26, 645–653. 10.1021/tx400048u. [DOI] [PubMed] [Google Scholar]
- Younes A.; Creff G.; Beccia M. R.; Moisy P.; Roques J.; Aupiais J.; Hennig C.; Solari P. L.; Auwer C. D.; Vidaud C. Is hydroxypyridonate 3,4,3-LI(1,2-HOPO) a good competitor of fetuin for uranyl metabolism?. Metallomics 2019, 11, 496–507. 10.1039/C8MT00272J. [DOI] [PubMed] [Google Scholar]
- Vidaud C.; Miccoli L.; Brulfert F.; Aupiais J. Fetuin exhibits a strong affinity for plutonium and may facilitate its accumulation in the skeleton. Sci. Rep 2019, 9, 17584. 10.1038/s41598-019-53770-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M.; Kumar A.; Kumar M.; Pandey B. N. The interaction of human serum albumin with selected lanthanide and actinide ions: Binding affinities, protein unfolding and conformational changes. Biochimie 2016, 123, 117–129. 10.1016/j.biochi.2016.01.012. [DOI] [PubMed] [Google Scholar]
- Drobot B.; Schmidt M.; Mochizuki Y.; Abe T.; Okuwaki K.; Brulfert F.; Falke S.; Samsonov S. A.; Komeiji Y.; Betzel C.; Stumpf T.; Raff J.; Tsushima S. Cm3+/Eu3+ induced structural, mechanistic and functional implications for calmodulin. Phys. Chem. Chem. Phys. 2019, 21, 21213–21222. 10.1039/C9CP03750K. [DOI] [PubMed] [Google Scholar]
- Allred B. E.; Rupert P. B.; Gauny S. S.; An D. D.; Ralston C. Y.; Sturzbecher-Hoehne M.; Strong R. K.; Abergel R. J. Siderocalin-mediated recognition, sensitization, and cellular uptake of actinides. Proc. Natl. Acad. Sci. U.S.A. 2015, 112, 10342–10347. 10.1073/pnas.1508902112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L.; Basset C.; Averseng O.; Quéméneur E.; Hagège A.; Vidaud C. Characterization of UO22+ binding to osteopontin, a highly phosphorylated protein: insights into potential mechanisms of uranyl accumulation in bones†. Metallomics 2014, 6, 166–176. 10.1039/C3MT00269A. [DOI] [PubMed] [Google Scholar]
- Szyrwiel Ł.; Liauchuk V.; Chavatte L.; Lobinski R. In vitro induction and proteomics characterisation of a uranyl–protein interaction network in bovine serum†. Metallomics 2015, 7, 1604–1611. 10.1039/C5MT00207A. [DOI] [PubMed] [Google Scholar]
- Barkleit A.; Heller A.; Ikeda-Ohno A.; Bernhard G. Interaction of europium and curium with alpha-amylase. Dalton Trans. 2016, 45, 8724–8733. 10.1039/C5DT04790K. [DOI] [PubMed] [Google Scholar]
- Barkleit A.; Wilke C.; Heller A.; Stumpf T.; Ikeda-Ohno A. Trivalent f-elements in human saliva: a comprehensive speciation study by time-resolved laser-induced fluorescence spectroscopy and thermodynamic calculations. Dalton Trans. 2017, 46, 1593–1605. 10.1039/C6DT03726G. [DOI] [PubMed] [Google Scholar]
- Barkleit A.; Hennig C.; Ikeda-Ohno A. Interaction of Uranium(VI) with α-Amylase and Its Implication for Enzyme Activity. Chem. Res. Toxicol. 2018, 31, 1032–1041. 10.1021/acs.chemrestox.8b00106. [DOI] [PubMed] [Google Scholar]
- Wally J.; Halbrooks P. J.; Vonrhein C.; Rould M. A.; Everse S. J.; Mason A. B.; Buchanan S. K. The Crystal Structure of Iron-free Human Serum Transferrin Provides Insight into Inter-lobe Communication and Receptor Binding *. J. Biol. Chem. 2006, 281, 24934–24944. 10.1074/jbc.M604592200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansoborlo E.; Prat O.; Moisy P.; Den Auwer C.; Guilbaud P.; Carriere M.; Gouget B.; Duffield J.; Doizi D.; Vercouter T.; Moulin C.; Moulin V. Actinide speciation in relation to biological processes. Biochimie 2006, 88, 1605–1618. 10.1016/j.biochi.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Williams J.; Moreton K. The distribution of iron between the metal-binding sites of transferrin human serum. Biochem. J. 1980, 185, 483–488. 10.1042/bj1850483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipperfield A. R.; Taylor D. M. Binding of Plutonium and Americium to Bone Glycoproteins. Nature 1968, 219, 609–610. 10.1038/219609a0. [DOI] [PubMed] [Google Scholar]
- Chipperfield A. R.; Taylor D. M. The Binding of Americium and Plutonium to Bone Glycoproteins. Eur. J. Biochem. 1970, 17, 581–585. 10.1111/j.1432-1033.1970.tb01203.x. [DOI] [PubMed] [Google Scholar]
- Durbin P.W.Actinides in Animals and Man. In The Chemistry of the Actinide and Transactinide Elements; Morss L.R.; Edelstein N.M.; Fuger J., Eds.; Springer Netherlands, Dordrecht, 2006: pp 3339–3440. [Google Scholar]
- Taylor D. M. The bioinorganic chemistry of actinides in blood. J. Alloys Compd. 1998, 271–273, 6–10. 10.1016/S0925-8388(98)00014-0. [DOI] [Google Scholar]
- Boocock G.; Popplewell D. S. In vitro Distribution of Americium in Human Blood Serum Proteins. Nature 1966, 210, 1283–1284. 10.1038/2101283a0. [DOI] [PubMed] [Google Scholar]
- Turner G. A.; Taylor D. M. The Transport of Plutonium, Americium and Curium in the Blood of Rats. Phys. Med. Biol. 1968, 13, 535–546. 10.1088/0031-9155/13/4/304. [DOI] [PubMed] [Google Scholar]
- Kratochwil C.; Bruchertseifer F.; Rathke H.; Bronzel M.; Apostolidis C.; Weichert W.; Haberkorn U.; Giesel F.L.; Morgenstern A. Targeted Alpha Therapy of mCRPC with 225Actinium-PSMA-617: Dosimetry estimate and empirical dose finding. J. Nucl. Med. 2017, 58, 1624. 10.2967/jnumed.117.191395. [DOI] [PubMed] [Google Scholar]
- Kratochwil C.; Bruchertseifer F.; Rathke H.; Hohenfellner M.; Giesel F. L.; Haberkorn U.; Morgenstern A. Targeted α-Therapy of Metastatic Castration-Resistant Prostate Cancer with 225Ac-PSMA-617: Swimmer-Plot Analysis Suggests Efficacy Regarding Duration of Tumor Control. J. Nucl. Med. 2018, 59, 795–802. 10.2967/jnumed.117.203539. [DOI] [PubMed] [Google Scholar]
- Deblonde G.J.-P.; Mattocks J. A.; Dong Z.; Wooddy P. T.; Cotruvo J. A.; Zavarin M. Capturing an elusive but critical element: Natural protein enables actinium chemistry, Science. Advances 2021, 7, eabk0273. 10.1126/sciadv.abk0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattocks J. A.; Cotruvo J. A.; Deblonde G.J.-P. Engineering lanmodulin’s selectivity for actinides over lanthanides by controlling solvent coordination and second-sphere interactions. Chem. Sci. 2022, 13, 6054–6066. 10.1039/D2SC01261H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz D. H.; Holmes M. A.; Borregaard N.; Bluhm M. E.; Raymond K. N.; Strong R. K. The Neutrophil Lipocalin NGAL Is a Bacteriostatic Agent that Interferes with Siderophore-Mediated Iron Acquisition. Mol. Cell 2002, 10, 1033–1043. 10.1016/S1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- Özçubukçu S.; Mandal K.; Wegner S.; Jensen M. P.; He C. Selective Recognition of Americium by Peptide-Based Reagents. Inorg. Chem. 2011, 50, 7937–7939. 10.1021/ic201094e. [DOI] [PubMed] [Google Scholar]
- Bao G.; Clifton M.; Hoette T. M.; Mori K.; Deng S.-X.; Qiu A.; Viltard M.; Williams D.; Paragas N.; Leete T.; Kulkarni R.; Li X.; Lee B.; Kalandadze A.; Ratner A. J.; Pizarro J. C.; Schmidt-Ott K. M.; Landry D. W.; Raymond K. N.; Strong R. K.; Barasch J. Iron traffics in circulation bound to a siderocalin (Ngal)–catechol complex. Nat. Chem. Biol. 2010, 6, 602–609. 10.1038/nchembio.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotruvo J.A.Jr.; Featherston E. R.; Mattocks J. A.; Ho J. V.; Laremore T. N. Lanmodulin: A Highly Selective Lanthanide-Binding Protein from a Lanthanide-Utilizing Bacterium. J. Am. Chem. Soc. 2018, 140, 15056–15061. 10.1021/jacs.8b09842. [DOI] [PubMed] [Google Scholar]
- Deblonde G.J.-P.; Morrison K.; Mattocks J.A.Jr.; Cotruvo J. A.; Zavarin M.; Kersting A. B. Impact of a Biological Chelator, Lanmodulin, on Minor Actinide Aqueous Speciation and Transport in the Environment. Environ. Sci. Technol. 2023, 57, 20830–20843. 10.1021/acs.est.3c06033. [DOI] [PubMed] [Google Scholar]
- Nitz M.; Sherawat M.; Franz K. J.; Peisach E.; Allen K. N.; Imperiali B. Structural Origin of the High Affinity of a Chemically Evolved Lanthanide-Binding Peptide. Angew. Chem., Int. Ed. 2004, 43, 3682–3685. 10.1002/anie.200460028. [DOI] [PubMed] [Google Scholar]
- Deblonde G.J.-P.; Mattocks J. A.; Park D. M.; Reed D. W.; Cotruvo J. A.; Jiao Y. Selective and Efficient Biomacromolecular Extraction of Rare-Earth Elements using Lanmodulin. Inorg. Chem. 2020, 59, 11855–11867. 10.1021/acs.inorgchem.0c01303. [DOI] [PubMed] [Google Scholar]
- Dong Z.; Mattocks J. A.; Deblonde G.J.-P.; Hu D.; Jiao Y.; Cotruvo J. A.; Park D. M. Bridging Hydrometallurgy and Biochemistry: A Protein-Based Process for Recovery and Separation of Rare Earth Elements. ACS Cent. Sci. 2021, 7, 1798–1808. 10.1021/acscentsci.1c00724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattocks J. A.; Jung J. J.; Lin C.-Y.; Dong Z.; Yennawar N. H.; Featherston E. R.; Kang-Yun C. S.; Hamilton T. A.; Park D. M.; Boal A. K.; Cotruvo J. A. Enhanced rare-earth separation with a metal-sensitive lanmodulin dimer. Nature 2023, 618, 87–93. 10.1038/s41586-023-05945-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersting A. B. Plutonium Transport in the Environment. Inorg. Chem. 2013, 52, 3533–3546. 10.1021/ic3018908. [DOI] [PubMed] [Google Scholar]
- Zhao P.; Zavarin M.; Leif R. N.; Powell B. A.; Singleton M. J.; Lindvall R. E.; Kersting A. B. Mobilization of actinides by dissolved organic compounds at the Nevada Test Site. Appl. Geochem. 2011, 26, 308–318. 10.1016/j.apgeochem.2010.12.004. [DOI] [Google Scholar]
- Sgouros G.; Bodei L.; McDevitt M. R.; Nedrow J. R. Radiopharmaceutical therapy in cancer: clinical advances and challenges. Nat. Rev. Drug Discov 2020, 19, 589–608. 10.1038/s41573-020-0073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan B.; Davis A.; Bak P.. The radiopharmaceutical renaissance: radiating hope in cancer therapy. Biopharma Dealmakers; 2024. 10.1038/d43747-024-00014-w. [DOI] [Google Scholar]