Abstract
Background
The International Psoriasis Council (IPC) updated the classification of psoriasis severity to guide clinical decision-making. According to IPC guidelines, patients are considered candidates for systemic therapy when body surface area (BSA) is >10%, when lesions affect special body areas (ie, face, palms, soles, genitals, scalp, or nails), or when topical therapy fails to control symptoms.
Objective
To evaluate patient candidacy for systemic therapy in real-world settings, according to disease severity criteria.
Methods
This cross-sectional study included systemic treatment-naive patients from the CorEvitas Psoriasis Registry who initiated systemic treatment at Registry visits between April 2015 and April 2023. Based on IPC criteria, systemic therapy candidates were identified, and data on demographics and clinical characteristics, including disease severity indicators (ie, BSA and Psoriasis Area Severity Index [PASI] scores) and patient-reported outcome measures, were collected and descriptively summarized.
Results
The analysis included 2739 systemic therapy initiators with plaque psoriasis, of whom 82.7% met IPC criteria for systemic therapy. Of the 2265 systemic therapy candidates, 56.2% had a BSA >10%, 53.2% had a history of psoriasis affecting special areas, and 55.2% had prior but not current use of topical therapy. Notably, 71.0% of candidates for systemic therapy had PASI scores ≤12.
Conclusion
In this large real-world study, most patients with psoriasis who initiated systemic therapy met the IPC disease severity criteria to do so. Disease severity categorization based on PASI scores and BSA percentage alone may not adequately capture all patients who might be candidates for systemic psoriasis treatment.
Clinicaltrials.gov
Keywords: clinical guidelines, cross-sectional studies, disease severity, psoriasis registry, skin and connective tissue diseases
Introduction
Plaque psoriasis is a common, chronic, immune-mediated inflammatory disease that can result in significant physical, mental, and socioeconomic burden.1,2 Over the last decade, advances in systemic treatments have been made to manage moderate to severe psoriasis; however, the definition of moderate to severe psoriasis varies.1,3-5 The Rule of Tens, frequently used in European countries, has classified psoriasis as moderate to severe if the patient has a body surface area (BSA) involvement >10%, a Psoriasis Area and Severity Index (PASI) score >10, or a Dermatology Life Quality Index (DLQI) score >10.4,6 Alternatively, a BSA of 3% or 5% is often used in North America as the minimum threshold for classification of psoriasis as moderate to severe psoriasis.4,7,8 In clinical trials, a PASI score of ≥12 is often used to denote moderate to severe psoriasis.9,10 In real-world practice, a substantial proportion of patients with moderate to severe psoriasis, regardless of varying definitions, continued to receive suboptimal treatment which may impact QoL and treatment outcomes.4,6,11,12 In a US study of female nurses with psoriasis, 87% of study participants with moderate to severe psoriasis were diagnosed by a dermatologist, yet 66% of them were not on systemic therapy. 13 The population-based UPLIFT survey of psoriatic disease outcomes, conducted in North America, Europe, and Japan, found that a substantial proportion of patients with psoriasis (with or without concomitant psoriatic arthritis) were either receiving no treatment for their psoriasis symptoms or were being treated with topical therapy alone. 3 These findings, among others, highlight the need for a more practical and meaningful method of defining psoriasis severity to support the initiation of systemic therapies.
While traditional classification systems categorize psoriasis severity as mild, moderate, or severe based upon objective clinical measures with strict cutoff values, these systems fail to account for lesion location and characteristics, comorbidities, and/or patient preference and satisfaction.1,4,13,14 To address this issue, the International Psoriasis Council (IPC) used a modified Delphi approach to incorporate collective expertise and generate a consensus statement on psoriasis severity and criteria for initiating systemic therapy.4,11 From this exercise, a dichotomous definition of psoriasis severity emerged in which patients were categorized as either candidates for topical therapy or candidates for systemic therapy based on objective numeric thresholds, lesion location(s), and the subjective measure of the patients’ experience. 11 Patients who met at least 1 of the IPC criteria, which included a BSA >10%, disease involving special body areas (ie, the face, palms, soles, genitals, scalp, or nails), and failure on topical therapy, were classified as candidates for systemic therapy. 11 Of note, these criteria are similar to those set forth within the 2009 Spanish Academy of Dermatology and Venereology consensus document and in subsequent updates to Spanish clinical practice guidelines.15,16
This study assessed patient candidacy for systemic psoriasis therapy, according to disease severity as defined by PASI, BSA or DLQI, and the IPC guidelines, using data from the CorEvitas Psoriasis Registry (formerly Corrona Psoriasis Registry).
Methods
This cross-sectional observational study included patients from the CorEvitas Psoriasis Registry (clinicaltrials.gov NCT02707341). 8 As of January 2023, the Registry comprised 263 private and academic clinical sites located across 47 states and provinces in North America. Each participating investigator obtained ethical approval to conduct this noninterventional study, and the study sponsor, CorEvitas, obtained approval and continuing review through a central institutional review board (IntegReview, protocol Corrona-PSO-500). The study was conducted in accordance with the guidelines for Good Pharmacoepidemiology Practice and the International Council for Harmonisation Good Clinical Practice, and all patients provided written informed consent for inclusion in the Registry before enrollment. Patient eligibility and enrollment criteria for the CorEvitas Registry were previously described by McLean et al. 17
The primary outcome of this study was whether patients would be categorized as eligible for treatment with systemic or topical therapy according to the IPC guidelines. Candidates for systemic therapy were those who met ≥1 of the IPC criteria, including BSA >10%, disease involving special body areas (ie, the face, palms, soles, genitals, scalp, or nails), and failure on topical therapy. 11 To align with patient categorization within the CorEvitas Registry, the 3 criteria were amended slightly to include a BSA >10%, history of psoriasis involving special areas (ie, palmoplantar, genitals, scalp, or nails), and previous use of topical therapy with no reported current use.
The study included systemic treatment-naive patients from the CorEvitas Psoriasis Registry who initiated systemic treatment for psoriasis at a Registry visit between April 2015 and April 2023. Patient demographics (ie, age, sex, race, ethnicity, lifestyle, and comorbidity history) and clinical characteristics (ie, BSA percentage, PASI scores, DLQI scores, and Investigator’s Global Assessment [IGA] scale scores) collected at systemic therapy initiation were described for all systemic initiators and for the subgroup of patients identified as systemic treatment candidates based on IPC criteria. Means and standard deviations (SDs) or frequencies and percentages were reported. A Venn diagram was used to visualize the proportion of patients who met criteria for use of systemic therapy.
Results
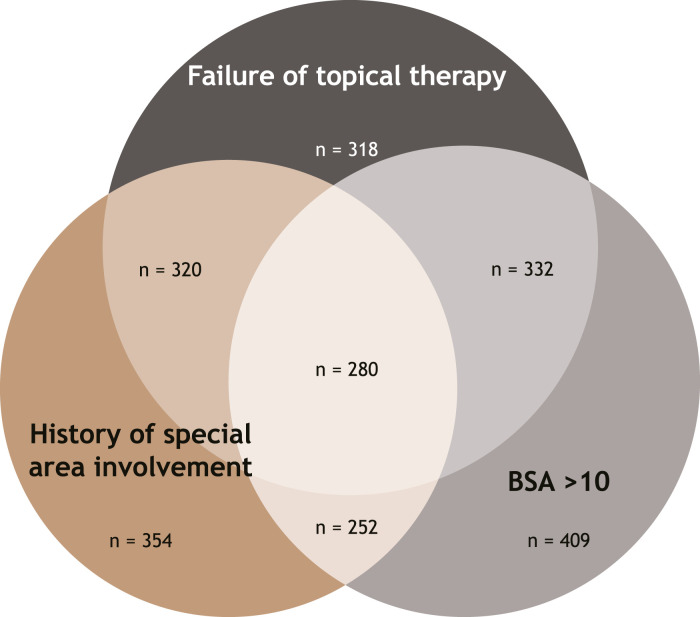
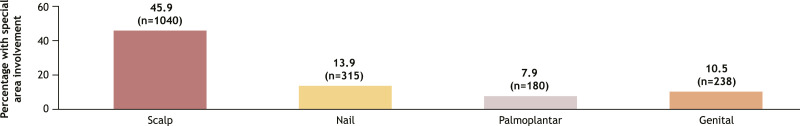
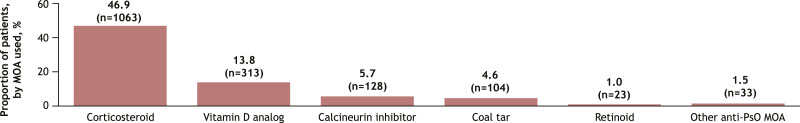
The analysis included a total of 2739 patients with plaque psoriasis who initiated systemic therapy, of whom 2265 (82.7%) were classified as candidates for systemic therapy based on the IPC guidelines (Figure 1). Among candidates for systemic therapy who initiated therapy, 12.4% (n = 280) met all 3 IPC criteria, while 47.7% (n = 1081) met only 1 eligibility criterion. Similarly, 56.2% of candidates for systemic therapy had a BSA >10%, 53.2% had a history of psoriasis affecting special areas, and 45.9% had a history of scalp psoriasis (Figure 2). More than half (55.2%; n = 1250) had a history of topical therapy use, and 46.9% (n = 1063) had previously used a corticosteroid (Figure 3).
Figure 1.
Systemic Therapy Candidates, Based on IPC Guidelines (n = 2265). BSA, body surface area; IPC, International Psoriasis Council.
Figure 2.
History of Special Area Psoriasis Involvement, by Specific Areaa (n = 2265). aCategories were not mutually exclusive.
Figure 3.
Topical Treatments for Psoriasis, by Mechanism of Actiona (n = 2265). aCategories are not mutually exclusive. Denominator for percentages is for patients who previously had used a topical therapy. Patients using a dual MOA topical treatment contributed to the count of each of the MOAs for that topical treatment. MOA, mechanism of action; PsO, psoriasis.
Among systemic therapy initiators who were classified as candidates for systemic therapy, 48.9% (n = 1107) were female and 77.8% (n = 1751) were White; the mean age was 48.4 years (Table 1). At the time of systemic therapy initiation, most patients (92.8%; n = 2102) had a BSA of ≥3%, which is indicative of moderate to severe psoriasis, and a psoriasis duration of <10 years (63.2%; n = 1420; mean, 9.9 years). Notably, 71.0% of candidates for systemic therapy had PASI scores ≤12, including 32.3% with PASI scores of ≤5 and 38.7% with PASI scores between 5 and 12. Similarly, 43.8% had a BSA of ≤10% at the time of systemic therapy initiation. A moderate to severe impact on QoL (ie, DLQI >5) was reported in 65.1% (n = 1469) of systemic therapy candidates. Approximately 38.9% (n = 571) of patients with BSA ≤10% who were classified as systemic therapy candidates also had a DLQI score >5 (mean DLQI, 7.8). Additionally, 64.5% of systemic therapy candidates with a PASI score <12 also had a DLQI score >5. The mean DLQI score (SD) among patients with a history of special area involvement who had a BSA ≤10% was 7.8 (5.4). Mean PASI and IGA scores were 10.0 (8.4) and 3.0 (0.7), respectively. Most systemic therapy candidates (89.0%; n = 2016) initiated treatment with a biologic agent that inhibits tumor necrosis factor or interleukin (IL)-17, IL-23, or IL-12/23. 18
Table 1.
Patient Demographics and Baseline Clinical Characteristics.
| Parameter | Candidates for Systemic Therapy Based on IPC guidelines a (n = 2265) |
|---|---|
| Age, mean (SD), years | 48.4 (16.2) |
| Female, n (%) | 1107 (48.9) |
| Race, n (%) | |
| White | 1751 (77.8) |
| Black | 118 (5.2) |
| Asian | 183 (8.1) |
| Other | 198 (8.8) |
| Hispanic ethnicity, n (%) | 303 (13.6) |
| Geographic region, n (%) | |
| Northeast | 659 (29.1) |
| Midwest | 423 (18.7) |
| South | 648 (28.6) |
| West | 304 (13.4) |
| Canada | 231 (10.2) |
| Smoking history, n (%) | |
| Never | 1191 (53.3) |
| Current alcohol use, n (%) | |
| None/occasional | 1126 (53.3) |
| Body mass index, n (%) | |
| Underweight/normal (<18.5-24.9) | 516 (23.3) |
| Overweight (25.0-29.9) | 682 (30.8) |
| Obese (≥30.0) | 1015 (45.9) |
| History of comorbidities, n (%) b | 1058 (46.8) |
| History of anxiety or depression, n (%) | 586 (25.9) |
| PsO duration, mean (SD), years | 9.9 (12.4) |
| PsO disease duration, n (%), years | |
| <10 | 1420 (63.2) |
| 10-20 | 440 (19.6) |
| >20 | 386 (17.2) |
| BSA (% involvement), mean (SD) | 17.4 (15.8) |
| BSA, n (%) | |
| 0%–<3% | 163 (7.2) |
| 3%–10% | 829 (36.6) |
| >10%–100% | 1273 (56.2) |
| PASI score 0-72, mean (SD) | 10.0 (8.4) |
| PASI score, n (%) | |
| 0-5 | 731 (32.3) |
| >5-12 | 877 (38.7) |
| >12-72 | 656 (29.0) |
| IGA score, mean (SD) | 3.0 (0.7) |
| DLQI score >5, n (%) | 1469 (65.1) |
| Initiating therapy, n (%) | |
| Nonbiologic/small molecule | 249 (11.0) |
| Biologic/biosimilar | 2016 (89.0) |
BSA, body surface area; DLQI, Dermatology Life Quality Index; IGA, Investigator’s Global Assessment; PASI, Psoriasis Area and Severity Index; PsO, psoriasis; SD, standard deviation.
aAll patients initiated a systemic therapy, and total sample does not represent all patients with psoriasis.
bIncludes cancer (excluding nonmelanoma skin cancer), cardiovascular disease, cerebrovascular disease, hypertension, hyperlipidemia, diabetes mellitus, hepatic events, gastrointestinal perforation, peptic ulcer, inflammatory bowel disease events, and other gastrointestinal disorders.
Discussion
Relying solely on objective measures to guide treatment decisions is often fraught with issues that may lead to the undertreatment of patients. Although many current guidelines are based on objective threshold measures to gauge eligibility for advanced treatment with systemic therapies, a multidisciplinary, collaborative approach may be best suited to standardize severity assessment and to elevate standards of care in psoriasis treatment. This study, therefore, aimed to evaluate the assessment of eligibility for systemic therapy according to IPC guidelines for real-world patients who initiated systemic therapy for psoriasis.
Among patients in this study, 82.7% met IPC criteria 11 and would be classified as candidates for systemic therapy. If assessed using the Rule of Tens4,6 (ie, BSA >10%, PASI score >10, or DLQI score >10), many of these patients would be classified as having mild psoriasis and be prescribed topical therapy only.12,19 These findings are consistent with those of a National Psoriasis Foundation survey that found that 20% to 30% of patients with moderate to severe psoriasis were being treated solely with topical therapy. 14 Consequently, broader adoption of IPC guidelines by stakeholders, including physicians, researchers, clinical guideline makers, payers, and policymakers, may substantially impact patient eligibility for treatment.
In this study, 53.2% of patients had a history of psoriasis involving a special body area and were eligible for systemic therapy based on this IPC guideline criterion. Although psoriasis in special areas contributes to high disease burden and significantly impacts patient QoL, traditional classification systems would most likely classify these patients as having mild disease, especially if the BSA percent involvement or PASI score was <10.12,19-21 Moreover, a large proportion of patients in this study reported a moderate or greater impact on QoL based on DLQI scores, despite having psoriasis to an extent that would be classified as mild based on BSA percent involvement. Based on clinical guidelines, these patients would typically be undertreated using topical therapy.12,19,20 Therefore, despite the widespread use of BSA percentages and PASI scores, these tools alone are inadequate for quantifying disease severity and for guiding treatment decisions when psoriasis lesions are located across smaller surface areas.12,19-21 In two systematic reviews of psoriasis severity classification, Goldbari et al 12 and Robinson et al 21 showed that PASI scores become less sensitive to change with BSA involvement <10%. Similarly, Yang et al 19 noted that patients with lower PASI scores were more likely to be treated with topical therapies, even when DLQI scores indicate the presence of severe disease.
This study used the CorEvitas Psoriasis Registry, a large, well-established, longitudinal registry that collects real-world data on psoriasis treatment from both patients and clinicians, along with a wide range of demographic, disease, and QoL data. This Registry provides a unique resource with a large sample size and longitudinal follow-up for real-world patients with psoriasis in the United States and Canada and lends strength to this study. However, some limitations do exist. The criteria for systemic therapy initiation vary by country. The CorEvitas Registry primarily enrolls patients from the United States, with some patients from Canada, which may limit the generalizability of study findings to patients in other geographic regions. Additionally, in some countries with publicly funded national insurance systems, patient access to systemic treatment is restricted by stringent criteria based on BSA or PASI.5,20,22 As such, future research is needed to assess systemic treatment utilization based on IPC guidelines in other countries. Furthermore, some misclassifications may have occurred when operationalizing the term “failure of topical therapy”; this is unlikely to have greatly impacted study findings. Finally, the Registry does not collect reasons for discontinuation of topical therapy, nor does it report whether a special body area was the target of a particular therapy.
Conclusions
Most patients who initiated systemic therapy in this large, real-world study met the IPC criteria to do so. High PASI scores (≥10) or BSA (≥10%) do not adequately capture all patients in North America who may be candidates for systemic therapy. Use of such rigid criteria by physicians, payers, or formulary decision makers may therefore limit patient access to appropriate treatment and may ultimately affect disease burden and patients’ QoL.
Acknowledgements
Medical writing and editorial assistance was provided by Michon Jackson, PhD, of Peloton Advantage, an OPEN Health company, and funded by Bristol Myers Squibb. The CorEvitas Psoriasis Registry was developed in collaboration with the National Psoriasis Foundation.
Footnotes
Author Contributions: Access to the study data was limited to CorEvitas, and CorEvitas statisticians completed all analyses. BS, YZ, AS, EB, and JZ contributed to the study design. AS, AB, TE, and JZ participated in the collection and assembly of data. AS, AB, TE, EB, and ML contributed to data analysis. All authors contributed to the interpretation of the results and to the drafting, critical review, and revision of the manuscript, with the support of a medical writer provided by Bristol Myers Squibb. All authors granted approval of the final manuscript for submission.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Bruce Strober serves as a consultant, investigator, and/or speaker for AbbVie, Almirall, Amgen, Arcutis, Arena, Aristea, Asana, Boehringer Ingelheim, Bristol Myers Squibb, Cara Therapeutics, Connect Biopharma, CorEvitas Psoriasis Registry, Dermavant, Dermira, Equillium Bio, GSK, Immunic Therapeutics, Janssen, Leo Pharma, Lilly, Maruho, Meiji Seika Pharma, Mindera Health, Novartis, Ortho Dermatologics, Pfizer, Regeneron, Sanofi Genzyme, Sun Pharma, UCB, Ventyx Biosciences, and vTv Therapeutics. Yichen Zhong, Eugene Balagula, and Joe Zhuo are employees and shareholders of Bristol Myers Squibb. Adam Sima, Alicia Beeghly, and Thomas Eckmann are employees of CorEvitas, LLC. Mark Lebwohl has received research funding on behalf of Mount Sinai from AbbVie, Amgen, Arcutis, AstraZeneca, Avotres, Boehringer Ingelheim, Cara Therapeutics, Dermavant, Incyte, Inozyme, Janssen, Lilly, Ortho Dermatologics, Regeneron, Sanofi-Regeneron, Takeda, and UCB; and has received consulting fees from Almirall, AltruBio, AnaptysBio, Arcutis, AstraZeneca, Avotres, Boehringer Ingelheim, Brickell Biotech (Fresh Tracks Therapeutics), Bristol Myers Squibb, Castle Biosciences, Celltrion, CorEvitas, Dermavant, EPI Health (Novan), Evommune, Forte Biosciences, Galderma, Genentech, Incyte, Inozyme, Leo Pharma, Meiji Seika Pharma, Mindera Health, Pfizer, Sanofi-Regeneron, Seanergy, Strata Skin Sciences, Takeda, Trevi Therapeutics, and Verrica.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by CorEvitas, LLC and the analysis was funded by Bristol Myers Squibb. Access to study data was limited to CorEvitas and CorEvitas statisticians completed all the analysis; all authors contributed to the interpretation of the results. CorEvitas has been supported through contracted subscriptions in the last two years by AbbVie, Amgen, Inc., Arena, Boehringer Ingelheim, Bristol Myers Squibb, Chugai, Eli Lilly and Company, Genentech, GSK, Janssen Pharmaceuticals, Inc., LEO Pharma, Novartis, Ortho Dermatologics, Pfizer, Inc., Sun Pharmaceutical Industries Ltd., and UCB S.A.
Ethical Statement
Ethical Approval
Ethical approval for this study was obtained by each participating investigator to conduct this noninterventional study, and the study sponsor, CorEvitas, obtained approval and continuing review through a central institutional review board (IntegReview; protocol Corrona-PSO-500). The study was conducted in accordance with the guidelines for Good Pharmacoepidemiology Practice and the International Council for Harmonisation Good Clinical Practice.
Informed Consent
All patients provided written informed consent before study participation.
ORCID iD
Bruce Strober https://orcid.org/0000-0002-8394-2057
References
- 1.Michalek IM, Loring B, Malte S. Global report on psoriasis. 2016. https://apps.who.int/iris/bitstream/handle/10665/204417/9789241565189_eng.pdf.psoriasis?sequence=1, Accessed 23 January 2024.
- 2.Damiani G, Bragazzi NL, Karimkhani Aksut C, et al. The global, regional, and national burden of psoriasis: results and insights from the Global Burden of Disease 2019 study. Front Med. 2021;8:743180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lebwohl M, Langley RG, Paul C, et al. Evolution of patient perceptions of psoriatic disease: results from the Understanding Psoriatic Disease Leveraging Insights for Treatment (UPLIFT) survey. Dermatol Ther. 2022;12(1):61-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303(1):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicolescu AC, Ionescu MA, Constantin MM, et al. Psoriasis management challenges regarding difficult-to-treat areas: therapeutic decision and effectiveness. Life (Basel, Switzerland). 2022;12(12):2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finlay AY. Current severe psoriasis and the rule of tens. Br J Dermatol. 2005;152(5):861-867. [DOI] [PubMed] [Google Scholar]
- 7.Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029-1072. [DOI] [PubMed] [Google Scholar]
- 8.Strober B, Karki C, Mason M, et al. Characterization of disease burden, comorbidities, and treatment use in a large, us-based cohort: results from the corrona psoriasis registry. J Am Acad Dermatol. 2018;78(2):323-332. [DOI] [PubMed] [Google Scholar]
- 9.Reich K, Gooderham M, Green L, et al. The efficacy and safety of apremilast, etanercept and placebo in patients with moderate-to-severe plaque psoriasis: 52-week results from a phase IIIb, randomized, placebo-controlled trial (LIBERATE). J Eur Acad Dermatol Venereol. 2017;31(3):507-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gooderham M, Pinter A, Ferris LK, et al. Long-term, durable, absolute Psoriasis Area and Severity Index and health-related quality of life improvements with risankizumab treatment: a post hoc integrated analysis of patients with moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2022;36(6):855-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strober B, Ryan C, van de Kerkhof P, et al. Recategorization of psoriasis severity: Delphi consensus from the International Psoriasis Council. J Am Acad Dermatol. 2020;82(1):117-122. [DOI] [PubMed] [Google Scholar]
- 12.Golbari NM, van der Walt JM, Blauvelt A, Ryan C, van de Kerkhof P, Kimball AB. Psoriasis severity: commonly used clinical thresholds may not adequately convey patient impact. J Eur Acad Dermatol Venereol. 2021;35(2):417-421. [DOI] [PubMed] [Google Scholar]
- 13.Tajalli M, Li T, Drucker AM, Qureshi AA, Cho E. A description of treatment patterns of psoriasis by medical providers and disease severity in US women. J Psoriasis Psoriatic Arthritis. 2021;6(1):45-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armstrong AW, Robertson AD, Wu J, Schupp C, Lebwohl MG. Undertreatment, treatment trends, and treatment dissatisfaction among patients with psoriasis and psoriatic arthritis in the United States: findings from the National Psoriasis Foundation surveys, 2003-2011. JAMA Dermatol. 2013;149(10):1180-1185. [DOI] [PubMed] [Google Scholar]
- 15.Puig L, Bordas X, Carrascosa JM, et al. Consensus document on the evaluation and treatment of moderate-to-severe psoriasis. Spanish psoriasis group of the Spanish Academy of Dermatology and Venereology. Actas Dermosifiliogr. 2009;100(4):277-286. [PubMed] [Google Scholar]
- 16.Puig L, Carrascosa JM, Carretero G, et al. Spanish evidence-based guidelines on the treatment of psoriasis with biologic agents, 2013. Part 1: on efficacy and choice of treatment. Spanish psoriasis group of the Spanish Academy of Dermatology and Venereology. Actas Dermosifiliogr. 2013;104(8):694-709. [DOI] [PubMed] [Google Scholar]
- 17.McLean RR, Sima AP, Beaty S, et al. Durability of near-complete skin clearance in patients with psoriasis using systemic biologic therapies: real-world evidence from the CorEvitas Psoriasis Registry. Dermatol Ther. 2023;13(11):2753-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Angelo S, Tramontano G, Gilio M, Leccese P, Olivieri I. Review of the treatment of psoriatic arthritis with biological agents: choice of drug for initial therapy and switch therapy for non-responders. Open Access Rheumatol. 2017;9:21-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J, Hu K, Li X, et al. Psoriatic foot involvement is the most significant contributor to the inconsistency between PASI and DLQI: a retrospective study from China. Clin Cosmet Invest Dermatol. 2023;16:443-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merola JF, Qureshi A, Husni ME. Underdiagnosed and undertreated psoriasis: nuances of treating psoriasis affecting the scalp, face, intertriginous areas, genitals, hands, feet, and nails. Dermatol Ther. 2018;31(3):e12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson A, Kardos M, Kimball AB. Physician global assessment (PGA) and Psoriasis Area and Severity Index (PASI): why do both? A systematic analysis of randomized controlled trials of biologic agents for moderate to severe plaque psoriasis. J Am Acad Dermatol. 2012;66(3):369-375. [DOI] [PubMed] [Google Scholar]
- 22.Mrowietz U, Steinz K, Gerdes S. Psoriasis: to treat or to manage? Exp Dermatol. 2014;23(10):705-709. [DOI] [PubMed] [Google Scholar]