Abstract
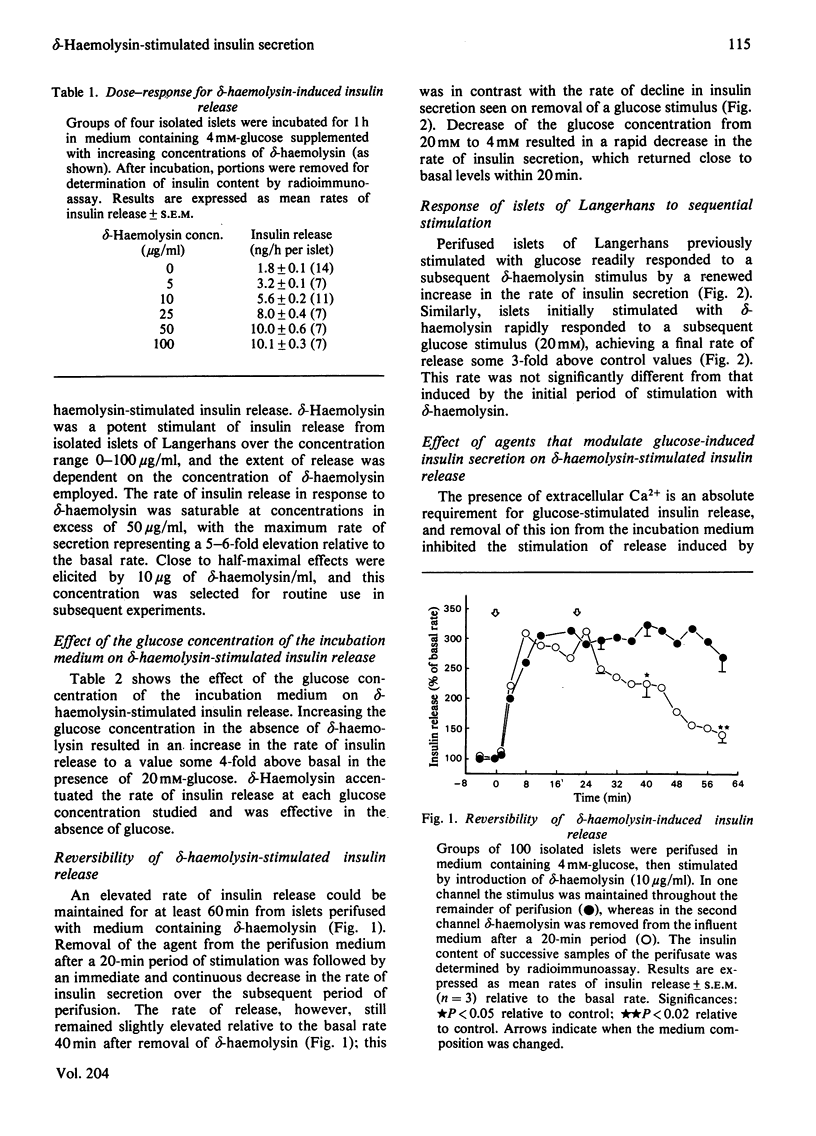
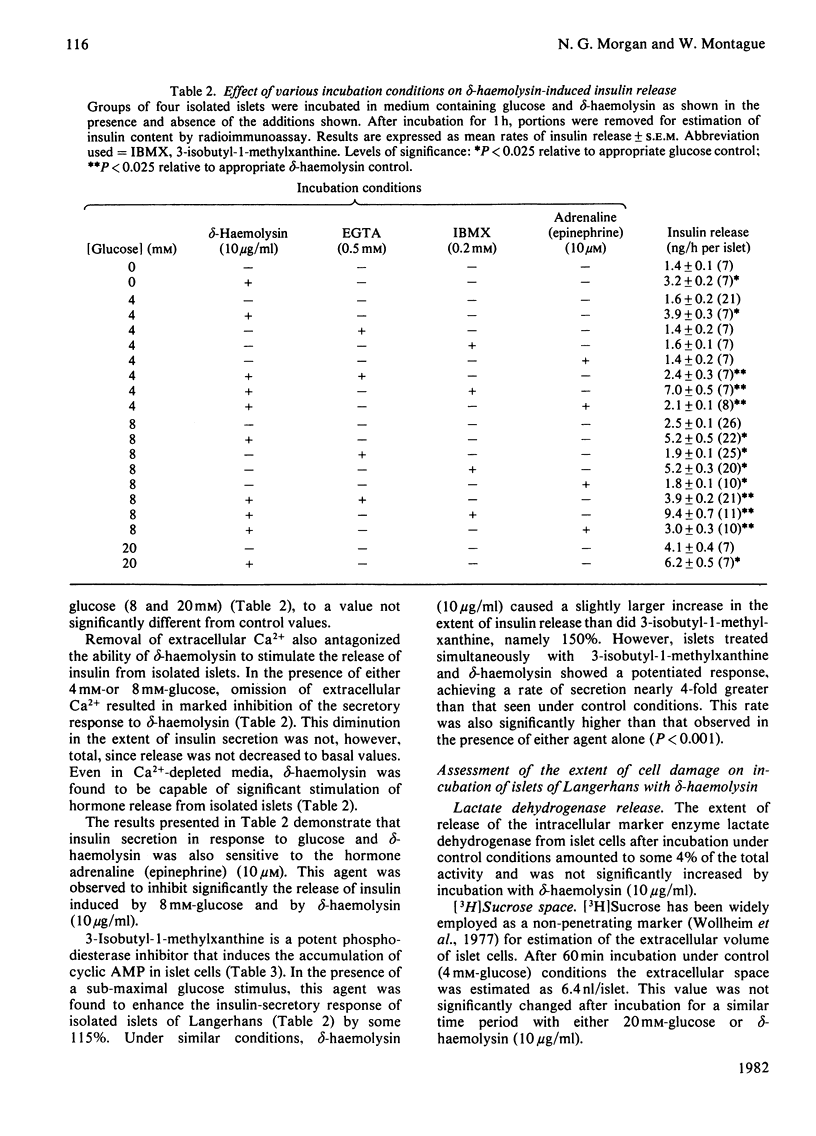
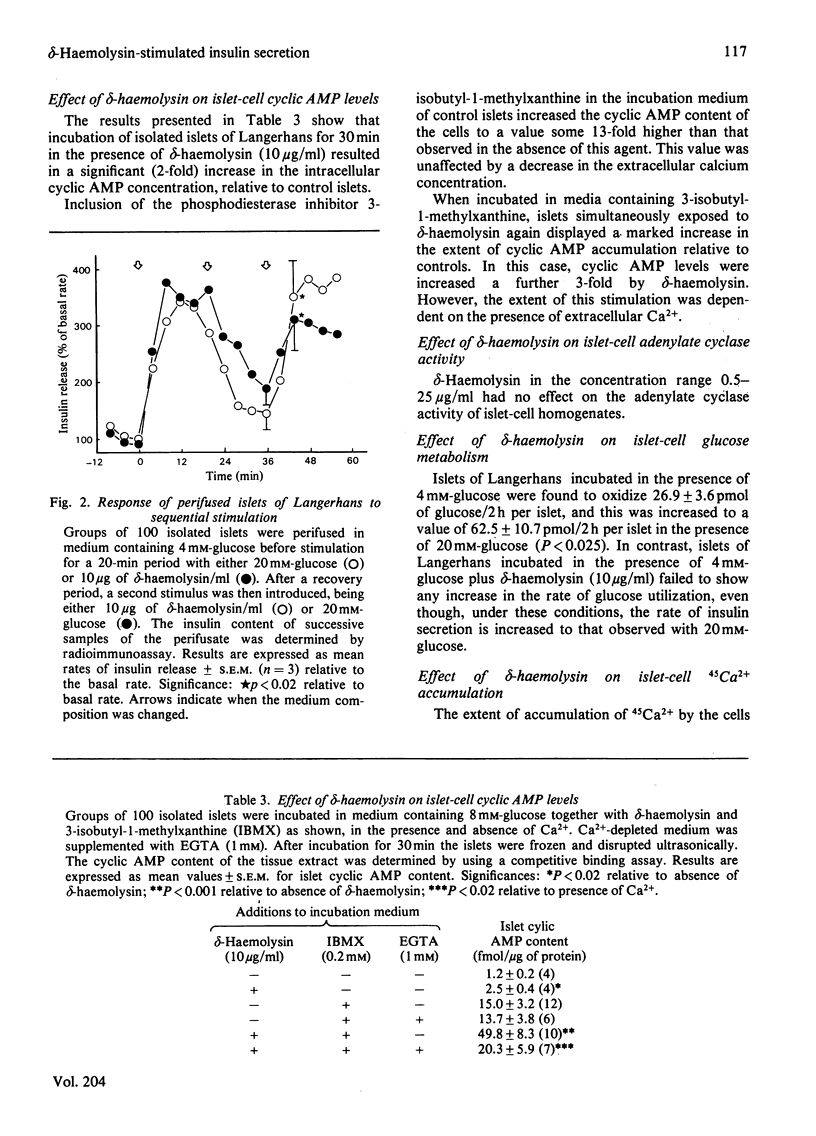
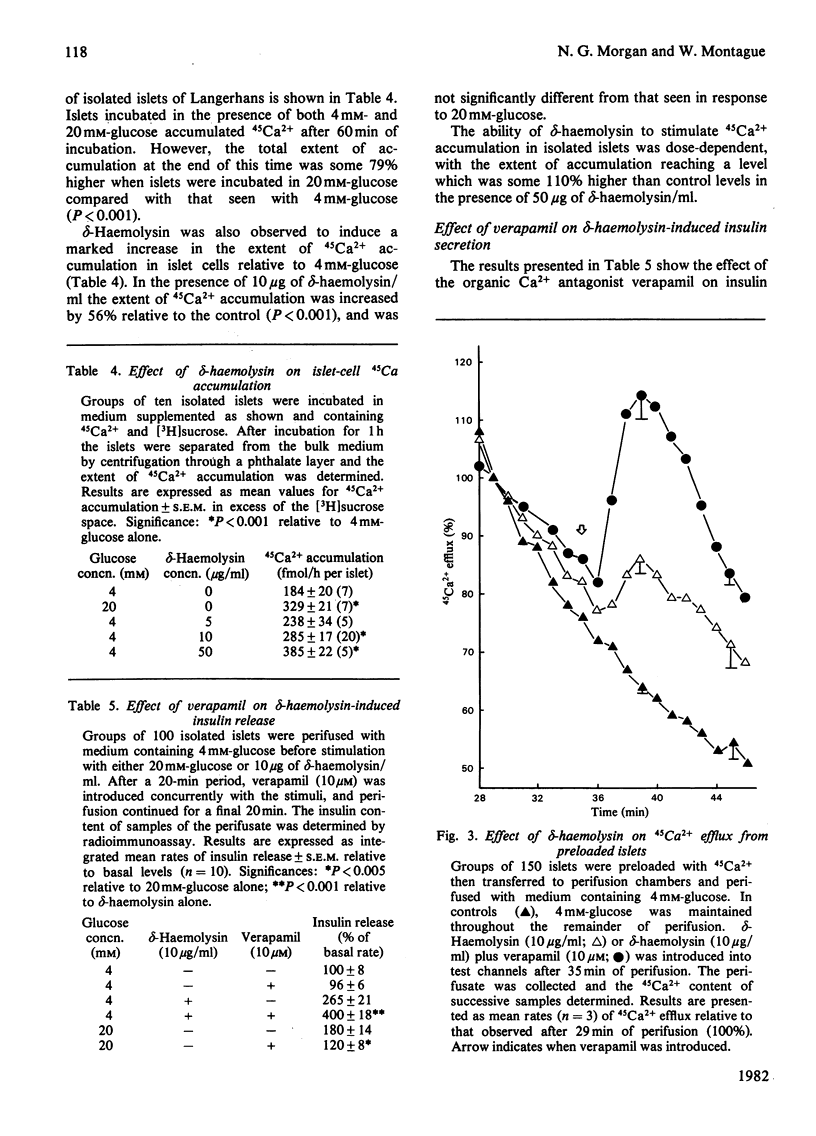
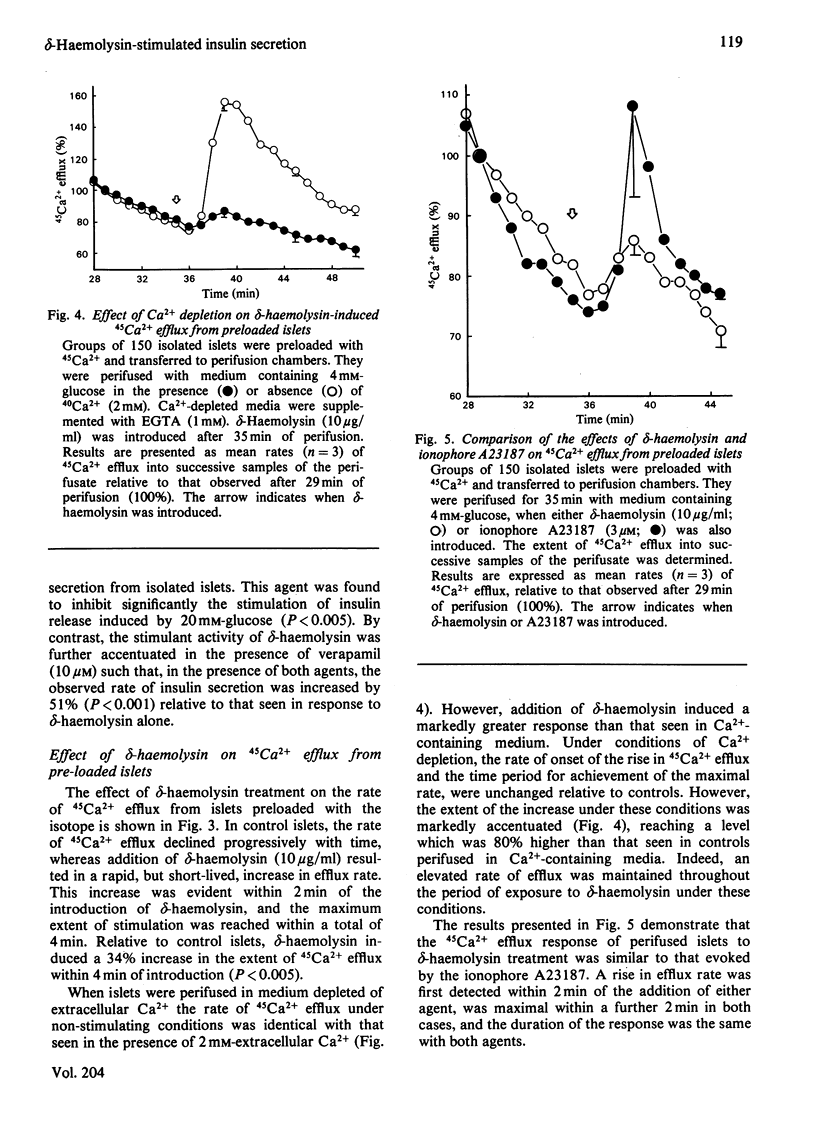
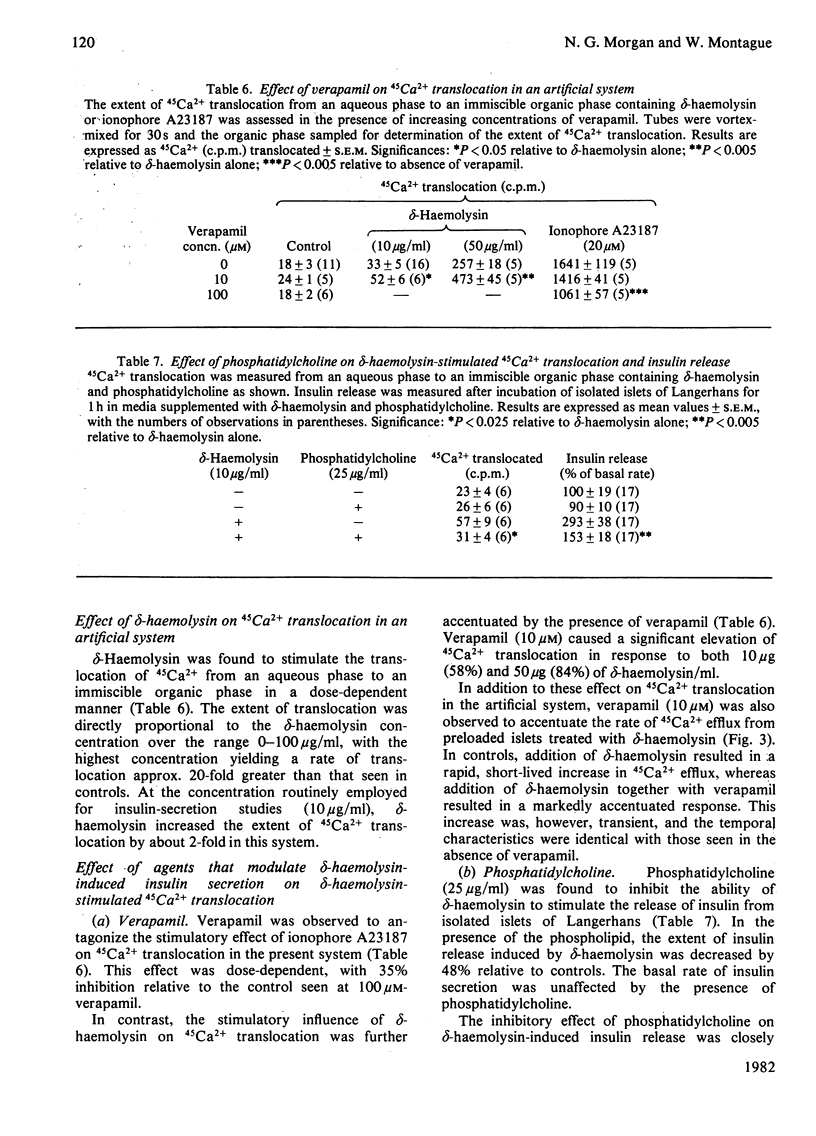
delta-Haemolysin, a small surface-active polypeptide purified from the culture media of Staphylococcus aureus, was observed to stimulate the release of insulin from isolated rat islets of Langerhans. This effect was dose-dependent and saturable, with the half-maximal response elicited by a delta-haemolysin concentration of 10 micrograms/ml. Stimulation of insulin release by delta-haemolysin (10 micrograms/ml) was not dependent on the presence of glucose in the incubation medium, but was augmented by increasing concentrations of the sugar. The release of insulin in response to delta-haemolysin could be inhibited by depletion of extracellular Ca2+ or by adrenaline (epinephrine) (10 microM) and was readily reversible when delta-haemolysin was removed from the medium. In addition, the response was potentiated by incubation with the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (0.2 mM). These observations suggest that delta-haemolysin induced a true activation of the beta-cell secretory mechanism. Stimulation of islets of Langerhans with delta-haemolysin was found to be associated with a modest increase in intracellular cyclic AMP levels, although the adenylate cyclase activity of islet homogenates was not increased by delta-haemolysin. delta-Haemolysin was observed to induce a dose-dependent net accumulation of 45Ca2+ by islet cells and to stimulate the efflux of 45Ca2+ from preloaded islets. The efflux of 45Ca2+ was modest in size and short-lived, but dramatically increased in medium depleted fo 40Ca2+. Incubation in the presence of verapamil augmented delta-haemolysin-induced 45Ca2+ efflux and insulin secretion. delta-Haemolysin was found to be a potent 45Ca2+-translocating ionophore in an artificial system. This response was dose-dependent and could be augmented by verapamil. In addition, phosphatidylcholine (25 micrograms/ml) was found to inhibit both delta-haemolysin induced 45Ca2+ translocation and insulin release in a precisely parallel manner. These studies suggest that the ability of delta-haemolysin to stimulate insulin release may be due, in part, to the facilitation of Ca2+ entry into the beta-cells of islets of Langerhans, mediated directly by an ionophoretic mechanism.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft S. J. Glucoreceptor mechanisms and the control of insulin release and biosynthesis. Diabetologia. 1980 Jan;18(1):5–15. doi: 10.1007/BF01228295. [DOI] [PubMed] [Google Scholar]
- Bernheimer A. W. Cytolytic toxins of bacterial origin. The nature and properties of cytolytic proteins are discussed with emphasis on staphylococcal alpha-toxin. Science. 1968 Feb 23;159(3817):847–851. doi: 10.1126/science.159.3817.847. [DOI] [PubMed] [Google Scholar]
- Brown B. L., Albano J. D., Ekins R. P., Sgherzi A. M. A simple and sensitive saturation assay method for the measurement of adenosine 3':5'-cyclic monophosphate. Biochem J. 1971 Feb;121(3):561–562. doi: 10.1042/bj1210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles M. A., Fanska R., Schmid F. G., Forsham P. H., Grodsky G. M. Adenosine 3',5'-monophosphate in pancreatic islets: glucose-induced insulin release. Science. 1973 Feb 9;179(4073):569–571. doi: 10.1126/science.179.4073.569. [DOI] [PubMed] [Google Scholar]
- Charles M. A., Lawecki J., Pictet R., Grodsky G. M. Insulin secretion. Interrelationships of glucose, cyclic adenosine 3:5-monophosphate, and calcium. J Biol Chem. 1975 Aug 10;250(15):6134–6140. [PubMed] [Google Scholar]
- Coore H. G., Randle P. J. Regulation of insulin secretion studied with pieces of rabbit pancreas incubated in vitro. Biochem J. 1964 Oct;93(1):66–78. doi: 10.1042/bj0930066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresto J. C., Dujovne I. L., Castellani P. I., Mitta E. A., De Majo S. F., Foglia V. G. Insulin radioimmunoassay by the charcoal-dextran technique. Diabetologia. 1972 Aug;8(4):292–295. doi: 10.1007/BF01225574. [DOI] [PubMed] [Google Scholar]
- Curry D. L., Bennett L. L., Grodsky G. M. Dynamics of insulin secretion by the perfused rat pancreas. Endocrinology. 1968 Sep;83(3):572–584. doi: 10.1210/endo-83-3-572. [DOI] [PubMed] [Google Scholar]
- Davis B., Lazarus N. R. Insulin release from mouse islets. Effect of glucose and hormones on adenylate cyclase. Biochem J. 1972 Sep;129(2):373–379. doi: 10.1042/bj1290373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P. M., Matthews E. K. Electrical activity in pancreatic islet cells: effect of ions. J Physiol. 1970 Sep;210(2):265–275. doi: 10.1113/jphysiol.1970.sp009208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean R. T., Hylton W., Allison A. C. Induction of macrophage lysosomal enzyme secretion by agents acting at the plasma membrane. Exp Cell Biol. 1979;47(6):454–462. doi: 10.1159/000162963. [DOI] [PubMed] [Google Scholar]
- Durkin J. P., Shier W. T. Staphylococcal delta toxin stimulates endogenous phospholipase A2 activity and prostaglandin synthesis in fibroblasts. Biochim Biophys Acta. 1981 Feb 23;663(2):467–479. doi: 10.1016/0005-2760(81)90175-2. [DOI] [PubMed] [Google Scholar]
- Ewart R. B., Kornfeld S., Kipnis D. M. Effect of lectins on hormone release from isolated rat islets of langerhans. Diabetes. 1975 Aug;24(8):705–714. doi: 10.2337/diab.24.8.705. [DOI] [PubMed] [Google Scholar]
- Fitton J. E., Dell A., Shaw W. V. The amino acid sequence of the delta haemolysin of Staphylococcus aureus. FEBS Lett. 1980 Jun 30;115(2):209–212. doi: 10.1016/0014-5793(80)81170-7. [DOI] [PubMed] [Google Scholar]
- Flatt P. R., Gylfe E., Hellman B. Thulium binding to the pancreatic beta-cell membrane. Endocrinology. 1981 Jun;108(6):2258–2263. doi: 10.1210/endo-108-6-2258. [DOI] [PubMed] [Google Scholar]
- Frankel B. J., Imagawa W. T., O'Connor M. D., Lundquist I., Kromhout J. A., Fanska R. E., Grodsky G. M. Glucose-stimulated 45Calcium efflux from isolated rat pancreatic islets. J Clin Invest. 1978 Sep;62(3):525–531. doi: 10.1172/JCI109156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green I. C., Perrin D., Pedley K. C., Leslie R. D., Pyke D. A. Effect of enkephalins and morphine on insulin secretion from isolated rat islets. Diabetologia. 1980 Aug;19(2):158–161. doi: 10.1007/BF00421864. [DOI] [PubMed] [Google Scholar]
- Grill V., Cerasi E. Effect of hexoses and mannoheptulose on cyclic AMP accumulation and insulin secretion in rat pancreatic islets. Biochim Biophys Acta. 1976 Jun 23;437(1):36–50. doi: 10.1016/0304-4165(76)90345-7. [DOI] [PubMed] [Google Scholar]
- Grodsky G. M., Bennett L. L. Cation requirements for insulin secretion in the isolated perfused pancreas. Diabetes. 1966 Dec;15(12):910–913. doi: 10.2337/diab.15.12.910. [DOI] [PubMed] [Google Scholar]
- Hahn H. J., Hellman B., Lernmark A., Täljedal I. B. Veränderung der Insulinsekretion isolierter Langerhans'scher Inseln durch Behandlung mit Neuraminidase. Acta Biol Med Ger. 1974;32(4):375–383. [PubMed] [Google Scholar]
- Heatley N. G. A new method for the preparation and some properties of staphylococcal delta-haemolysin. J Gen Microbiol. 1971 Dec;69(2):269–278. doi: 10.1099/00221287-69-2-269. [DOI] [PubMed] [Google Scholar]
- Hedeskov C. J. Mechanism of glucose-induced insulin secretion. Physiol Rev. 1980 Apr;60(2):442–509. doi: 10.1152/physrev.1980.60.2.442. [DOI] [PubMed] [Google Scholar]
- Hellman B., Idahl L. A., Lernmark A., Sehlin J., Täljedal I. B. Stimulation and inhibition of insulin release by an amino-reactive probe of plasma membrane. J Membr Biol. 1973 Dec 31;14(2):135–142. doi: 10.1007/BF01868074. [DOI] [PubMed] [Google Scholar]
- Hellman B., Idahl L. A., Lernmark A., Täljedal I. B. The pancreatic beta-cell recognition of insulin secretagogues. XIII. Effects of sulphydryl reagents on cyclic AMP. Biochim Biophys Acta. 1974 Nov 4;372(1):127–134. doi: 10.1016/0304-4165(74)90079-8. [DOI] [PubMed] [Google Scholar]
- Hellman B., Idahl L. A., Lernmark A., Täljedal I. B. The pancreatic beta-cell recognition of insulin secretagogues: does cyclic AMP mediate the effect of glucose? Proc Natl Acad Sci U S A. 1974 Sep;71(9):3405–3409. doi: 10.1073/pnas.71.9.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman B., Sehlin J., Söderberg M., Täljedal I. B. The mechanisms of action of chloromercuribenzene-p-sulphonic acid as insulin secretagogue: fluxes of calcium, sodium and rubidium in islets exposed to mercurial and a membrane-active antagonist. J Physiol. 1975 Nov;252(3):701–712. doi: 10.1113/jphysiol.1975.sp011166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman B., Sehlin J., Täljedal I. B. Calcium uptake by pancreatic -cells as measured with the aid of 45 Ca and mannitol- 3 H. Am J Physiol. 1971 Dec;221(6):1795–1801. doi: 10.1152/ajplegacy.1971.221.6.1795. [DOI] [PubMed] [Google Scholar]
- Howell S. L., Whitfield M. Cytochemical localization of adenyl cyclase activity in rat islets of Langerhans. J Histochem Cytochem. 1972 Nov;20(11):873–879. doi: 10.1177/20.11.873. [DOI] [PubMed] [Google Scholar]
- Ismail N. A., Montague W. Effects of guanosine on insulin secretion and adenylyl and guanylyl cyclase activities of isolated rat islets of Langerhans. Biochim Biophys Acta. 1977 Jul 21;498(1):325–330. doi: 10.1016/0304-4165(77)90270-7. [DOI] [PubMed] [Google Scholar]
- Kanatsuna T., Lernmark A., Rubenstein A. H., Steiner D. F. Block in insulin release from column-perifused pancreatic beta-cells induced by islet cell surface antibodies and complement. Diabetes. 1981 Mar;30(3):231–234. doi: 10.2337/diab.30.3.231. [DOI] [PubMed] [Google Scholar]
- Kapral F. A. Inhibition of Staphylococcus aureus delta hemolysin by phospholipids. Proc Soc Exp Biol Med. 1972 Nov;141(2):519–521. doi: 10.3181/00379727-141-36812. [DOI] [PubMed] [Google Scholar]
- Katada T., Ui M. Islet-activating protein. Enhanced insulin secretion and cyclic AMP accumulation in pancreatic islets due to activation of native calcium ionophores. J Biol Chem. 1979 Jan 25;254(2):469–479. [PubMed] [Google Scholar]
- Kohlhardt M., Bauer B., Krause H., Fleckenstein A. Differentiation of the transmembrane Na and Ca channels in mammalian cardiac fibres by the use of specific inhibitors. Pflugers Arch. 1972;335(4):309–322. doi: 10.1007/BF00586221. [DOI] [PubMed] [Google Scholar]
- Krause U., Puchinger H., Wacker A. Inhibition of glucose-induced insulin secretion in trypsin-treated islets of Langerhans. Horm Metab Res. 1973 Sep;5(5):325–329. doi: 10.1055/s-0028-1093936. [DOI] [PubMed] [Google Scholar]
- Kreger A. S., Bernheimer A. W. Disruption of bacterial protoplasts and spheroplasts by staphylococcal delta hemolysin. Infect Immun. 1971 Apr;3(4):603–605. doi: 10.1128/iai.3.4.603-605.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macchi I. A., Zeytinoğlu F. N., Beaser S. B. Reversible inhibition by hyaluronidase of the insulinotropic action of tolbutamide in isolated hamster pancreatic islets. Proc Soc Exp Biol Med. 1974 Feb;145(2):500–503. doi: 10.3181/00379727-145-37839. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Devis G., Somers G. Inhibition by verapamil of ionophore-mediated calcium translocation. Experientia. 1977 Aug 15;33(8):1035–1036. doi: 10.1007/BF01945953. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Herchuelz A., Devis G., Somers G., Boschero A. C., Hutton J. C., Kawazu S., Sener A., Atwater I. J., Duncan G. Regulation of calcium fluxes and their regulatory roles in pancreatic islets. Ann N Y Acad Sci. 1978 Apr 28;307:562–582. doi: 10.1111/j.1749-6632.1978.tb41982.x. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Kawazu S., Herchuelz A., Hutton J. C., Somers G., Devis G., Sener A. The stimulus secretion coupling of glucose-induced insulin release. Arch Biochem Biophys. 1979 Apr 15;194(1):49–62. doi: 10.1016/0003-9861(79)90594-0. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Sener A., Devis G., Somers G. Calcium-antagonists and islet function. V. Effect of R33711. Horm Metab Res. 1976 Nov;8(6):434–438. doi: 10.1055/s-0028-1093608. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Sener A., Mahy M. The stimulus-secretion coupling of glucose-induced insulin release. Sorbitol metabolism in isolated islets. Eur J Biochem. 1974 Sep 1;47(2):365–370. doi: 10.1111/j.1432-1033.1974.tb03701.x. [DOI] [PubMed] [Google Scholar]
- McDaniel M. L., Anderson S., Fink J., Roth C., Lacy P. E. Effect of alloxan on permeability and hexose transport in rat pancreatic islets. Endocrinology. 1975 Jul;97(1):68–75. doi: 10.1210/endo-97-1-68. [DOI] [PubMed] [Google Scholar]
- Milner R. D., Hales C. N. The role of calcium and magnesium in insulin secretion from rabbit pancreas studied in vitro. Diabetologia. 1967 Mar;3(1):47–49. doi: 10.1007/BF01269910. [DOI] [PubMed] [Google Scholar]
- Montague W., Parkin E. N. Changes in membrane lipids of the beta-cell during insulin secretion. Horm Metab Res Suppl. 1980;Suppl 10:153–157. [PubMed] [Google Scholar]
- Montague W., Taylor K. W. Pentitols and insulin release by isolated rat islets of Langerhans. Biochem J. 1968 Sep;109(3):333–339. doi: 10.1042/bj1090333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan N. G., Fitzhugh A. J., Montague W. The effect of staphylococcal delta-haemolysin on the secretory activity of the pancreatic beta-cell. Biosci Rep. 1981 Feb;1(2):135–140. doi: 10.1007/BF01117010. [DOI] [PubMed] [Google Scholar]
- Nolte F. S., Kapral F. A. Binding of radiolabeled Staphylococcus aureus delta-toxin to human erythrocytes. Infect Immun. 1981 Mar;31(3):1086–1093. doi: 10.1128/iai.31.3.1086-1093.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., Kapral F. A. Increased cyclic adenosine 3',5'-monophosphate content in guinea pig ileum after exposure to Staphylococcus aureus delta-toxin. Infect Immun. 1976 Jan;13(1):152–162. doi: 10.1128/iai.13.1.152-162.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., McClung H. J., Kapral F. A. Increased tissue conductance and ion transport in guinea pig ileum after exposure to Staphylococcus aureus delta-toxin in vitro. Infect Immun. 1978 Jul;21(1):102–113. doi: 10.1128/iai.21.1.102-113.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer D. R., Lardy H. A. Ionophore A23187: the effect of H+ concentration on complex formation with divalent and monovalent cations and the demonstration of K+ transport in mitochondria mediated by A23187. Biochemistry. 1976 Mar 9;15(5):935–943. doi: 10.1021/bi00650a001. [DOI] [PubMed] [Google Scholar]
- Rogolsky M. Nonenteric toxins of Staphylococcus aureus. Microbiol Rev. 1979 Sep;43(3):320–360. doi: 10.1128/mr.43.3.320-360.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sando H., Akanuma Y., Kosaka K. Effect of sodium desoxycholate and phospholipase C on insulin secretion. Horm Metab Res. 1977 Jan;9(1):94–94. doi: 10.1055/s-0028-1095548. [DOI] [PubMed] [Google Scholar]
- Sharp G. W. The adenylate cyclase-cyclic AMP system in islets of Langerhans and its role in the control of insulin release. Diabetologia. 1979 May;16(5):287–296. doi: 10.1007/BF01223617. [DOI] [PubMed] [Google Scholar]
- Sharp G. W., Wiedenkeller D. E., Kaelin D., Siegel E. G., Wollheim C. B. Stimulation of adenylate cyclase by Ca2+ and calmodulin in rat islets of langerhans: explanation for the glucose-induced increase in cyclic AMP levels. Diabetes. 1980 Jan;29(1):74–77. doi: 10.2337/diab.29.1.74. [DOI] [PubMed] [Google Scholar]
- Somers G., Devis G., van Obberghen E., Malaisse W. J. Calcium-antagonists and islet function. VI. Effects of barium. Pflugers Arch. 1976 Sep 3;365(1):21–28. doi: 10.1007/BF00583624. [DOI] [PubMed] [Google Scholar]
- Straub F. B. Crystalline lactic dehydrogenase from heart muscle. Biochem J. 1940 Apr;34(4):483–486. doi: 10.1042/bj0340483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamarit-Rodriguez J. Different effects of the ionophore A-23187 and D-glucose on 45Ca2+ fluxes in isolated islets of ob/ob-mice. Acta Physiol Scand. 1978 Aug;103(4):379–383. doi: 10.1111/j.1748-1716.1978.tb06231.x. [DOI] [PubMed] [Google Scholar]
- Thelestam M., Möllby R., Wadström T. Effects of staphylococcal alpha-, beta-, delta-, and gamma-hemolysins on human diploid fibroblasts and HeLa cells: evaluation of a new quantitative as say for measuring cell damage. Infect Immun. 1973 Dec;8(6):938–946. doi: 10.1128/iai.8.6.938-946.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Täljedal I. B. Polarization of chlorotetracycline fluorescence in pancreatic islet cells and its response to calcium ions and D-glucose. Biochem J. 1979 Jan 15;178(1):187–193. doi: 10.1042/bj1780187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa K., Weinstein I. B., Shaw W. V. Staphylococcal delta-hemolysin inhibits cellular binding of epidermal growth factor and induces arachidonic acid release. Biochem Biophys Res Commun. 1980 May 30;94(2):625–629. doi: 10.1016/0006-291x(80)91278-4. [DOI] [PubMed] [Google Scholar]
- Valverde I., Vandermeers A., Anjaneyulu R., Malaisse W. J. Calmodulin activation of adenylate cyclase in pancreatic islets. Science. 1979 Oct 12;206(4415):225–227. doi: 10.1126/science.225798. [DOI] [PubMed] [Google Scholar]
- Wollheim C. B., Kikuchi M., Renold A. E., Sharp G. W. Somatostatin- and epinephrine-induced modifications of 45Ca++ fluxes and insulin release in rat pancreatic islets maintained in tissue culture. J Clin Invest. 1977 Nov;60(5):1165–1173. doi: 10.1172/JCI108869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawalich W. S., Karl R. C., Ferrendelli J. A., Matschinsky F. M. Factors governing glucose induced elevation of cyclic 3'5' AMP levels in pancreatic islets. Diabetologia. 1975 Jun;11(3):231–235. doi: 10.1007/BF00422327. [DOI] [PubMed] [Google Scholar]


