Abstract
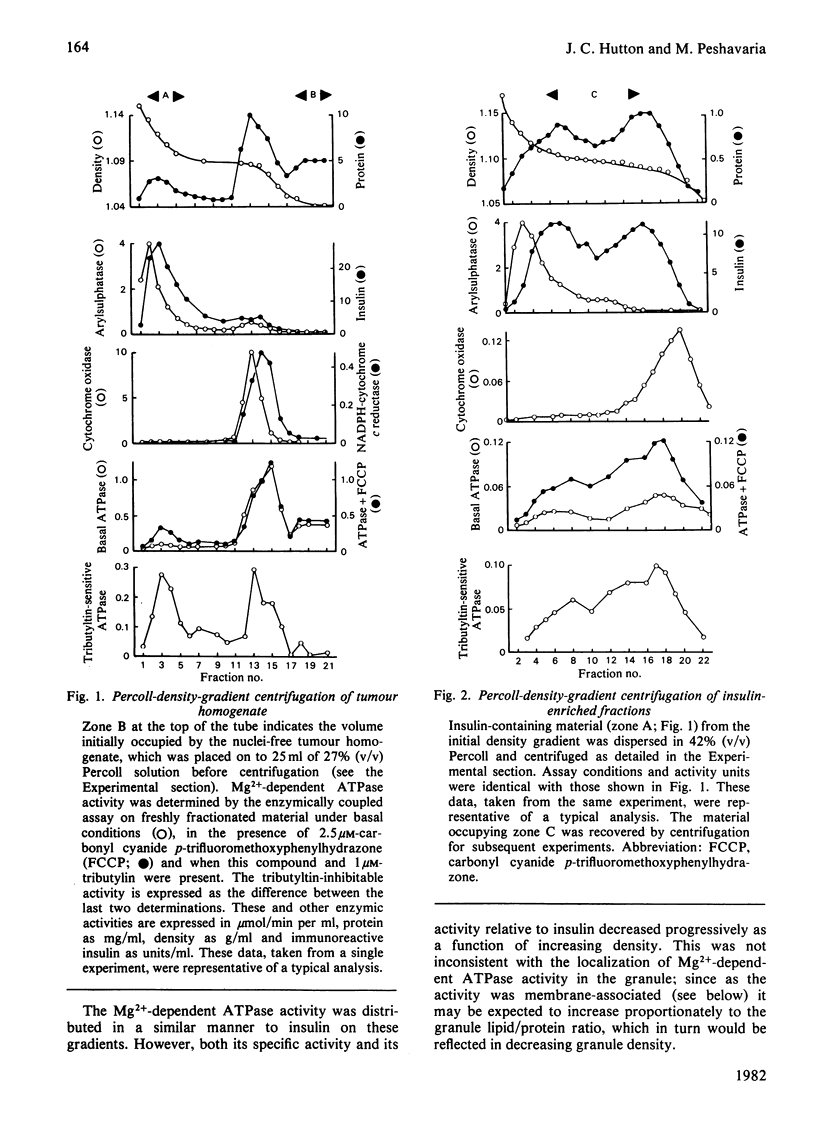
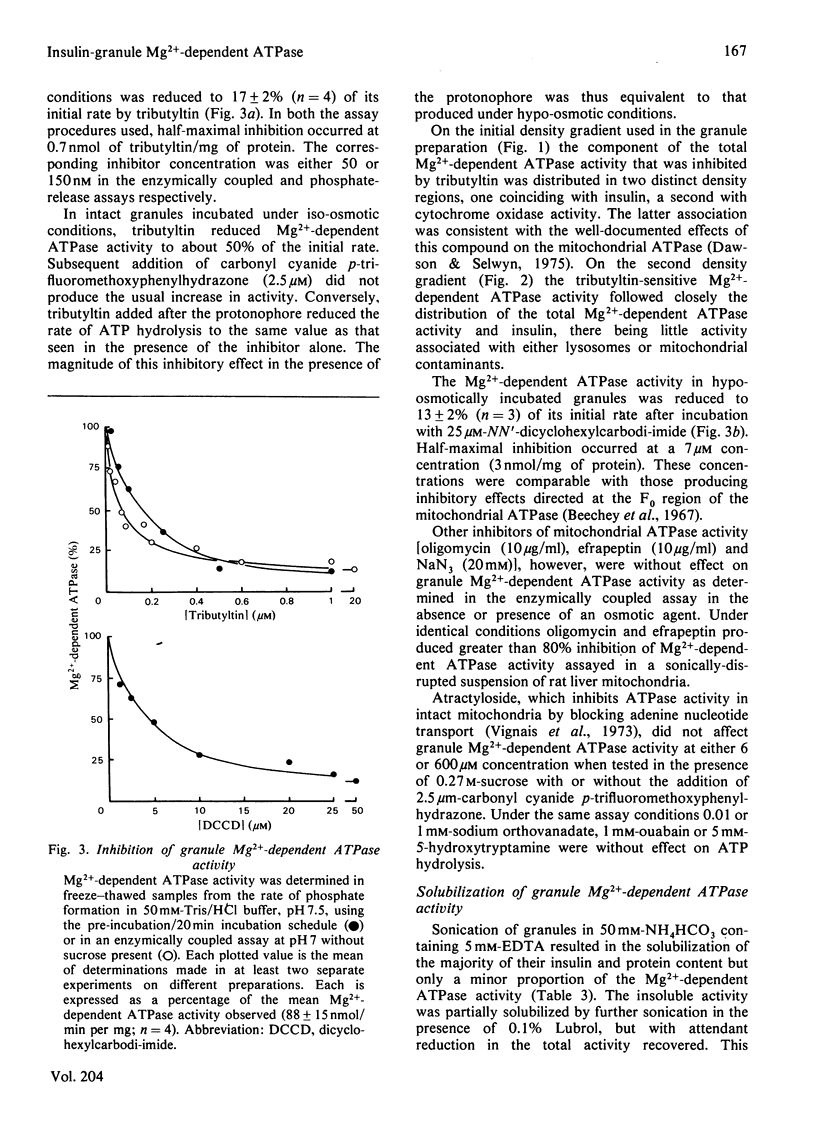
Insulin-secretory granules isolated from a pancreatic islet-cell tumour by centrifugation on Percoll density gradients exhibited a membrane-associated Mg2+-dependent ATPase activity. In granule suspensions incubated in iso-osmotic media, activity was increased 2–3-fold by carbonyl cyanide p-trifluoromethoxyphenylhydrazone, the combination of valinomycin, nigericin and K2SO4 or by the addition of a detergent. Permeant anions also increased Mg2+-dependent ATPase activity under iso-osmotic conditions when combined with K+ and nigericin, or NH4+. It was deduced that a major component of the activity was coupled to the translocation of protons into the granule interior. The granule membrane appeared poorly permeable to H+, K+, NH4+ and SO42− but permeable, in increasing order, to phosphate or acetate, Cl−, I− and SCN−. Like the proton-translocating ATPase of mammalian mitochondria the granule enzyme when membrane-bound was inhibited by up to 85% by tributyltin or NN′-dicyclohexylcarbodi-imide and was solubilized in a tributyltin-insensitive form after extraction with dichloromethane. It was clearly not a mitochondrial contaminant as evidence by the distribution of marker proteins on density gradients. Unlike mitochondrial activity it was insensitive to oligomycin, efrapeptin, atractyloside, azide and oxyanions. Its properties, however, were indistinguishable from those of the proton-translocating ATPase found in the chromaffin granules of the adrenal medulla. Moreover, insulin granules and chromaffin granules exhibited similar levels of activity. This indicated that in spite of the differences in their internal composition, granules from tissues involved in polypeptide and amine hormone secretion possess catalytic components in common. Only a minor role for the ATPase in amine transport in insulin granules was apparent. Rather, its presence here may relate to the process of secretory vesicle morphogenesis or to the exocytotic mechanism.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamsson H., Gylfe E. Demonstration of a proton gradient across the insulin granule membrane. Acta Physiol Scand. 1980 May;109(1):113–114. doi: 10.1111/j.1748-1716.1980.tb06573.x. [DOI] [PubMed] [Google Scholar]
- Apps D. K., Glover L. A. Isolation and characterization of magnesium adenosinetriphosphatase from the chromaffin granule membrane. FEBS Lett. 1978 Jan 15;85(2):254–258. doi: 10.1016/0014-5793(78)80467-0. [DOI] [PubMed] [Google Scholar]
- Apps D. K., Pryde J. G., Sutton R., Phillips J. H. Inhibition of adenosine triphosphatase, 5-hydroxytryptamine transport and proton-translocation activities of resealed chromaffin-granule 'ghosts'. Biochem J. 1980 Aug 15;190(2):273–282. doi: 10.1042/bj1900273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps D. K., Schatz G. An adenosine triphosphatase isolated from chromaffin-granulate membranes is closely similar to F1-adenosine triphosphatase of mitochondria. Eur J Biochem. 1979 Oct 15;100(2):411–419. doi: 10.1111/j.1432-1033.1979.tb04184.x. [DOI] [PubMed] [Google Scholar]
- BANKS P. THE ADENOSINE-TRIPHOSPHATASE ACTIVITY OF ADRENAL CHROMAFFIN GRANULES. Biochem J. 1965 May;95:490–496. doi: 10.1042/bj0950490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashford C. L., Casey R. P., Radda G. K., Ritchie G. A. Energy-coupling in adrenal chromaffin granules. Neuroscience. 1976;1(5):399–412. doi: 10.1016/0306-4522(76)90133-0. [DOI] [PubMed] [Google Scholar]
- Beechey R. B., Hubbard S. A., Linnett P. E., Mitchell A. D., Munn E. A. A simple and rapid method for the preparation of adenosine triphosphatase from submitochondrial particles. Biochem J. 1975 Jun;148(3):533–537. doi: 10.1042/bj1480533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beechey R. B., Roberton A. M., Holloway C. T., Knight I. G. The properties of dicyclohexylcarbodiimide as an inhibitor of oxidative phosphorylation. Biochemistry. 1967 Dec;6(12):3867–3879. doi: 10.1021/bi00864a033. [DOI] [PubMed] [Google Scholar]
- Buckland R. M., Radda G. K., Wakefield L. M. The role of phospholipids in the modulation of enzyme activities in the chromaffin granule membrane. Biochim Biophys Acta. 1981 May 6;643(2):363–375. doi: 10.1016/0005-2736(81)90081-x. [DOI] [PubMed] [Google Scholar]
- COOPERSTEIN S. J., LAZAROW A. A microspectrophotometric method for the determination of cytochrome oxidase. J Biol Chem. 1951 Apr;189(2):665–670. [PubMed] [Google Scholar]
- Chick W. L., Warren S., Chute R. N., Like A. A., Lauris V., Kitchen K. C. A transplantable insulinoma in the rat. Proc Natl Acad Sci U S A. 1977 Feb;74(2):628–632. doi: 10.1073/pnas.74.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coore H. G., Hellman B., Pihl E., Täljedal I. B. Physicochemical characteristics of insulin secretion granules. Biochem J. 1969 Jan;111(1):107–113. doi: 10.1042/bj1110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A. P., Selwyn M. J. The action of tributyltin on energy coupling in coupling-factor-deficient submitochondrial particles. Biochem J. 1975 Nov;152(2):333–339. doi: 10.1042/bj1520333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie J. A., Gibson F., Cox G. B. Membrane adenosine triphosphatases of prokaryotic cells. Annu Rev Biochem. 1979;48:103–131. doi: 10.1146/annurev.bi.48.070179.000535. [DOI] [PubMed] [Google Scholar]
- Ekholm R., Ericson L. E., Lundquist I. Monoamines in the pancreatic islets of the mouse. Subcellular localization of 5-hydroxytryptamine by electron microscopic autoradiography. Diabetologia. 1971 Oct;7(5):339–348. doi: 10.1007/BF01219468. [DOI] [PubMed] [Google Scholar]
- Fillingame R. H. The proton-translocating pumps of oxidative phosphorylation. Annu Rev Biochem. 1980;49:1079–1113. doi: 10.1146/annurev.bi.49.070180.005243. [DOI] [PubMed] [Google Scholar]
- Flatmark T., Terland O., Helle K. B. Electron carriers of the bovine adrenal chromaffin granules. Biochim Biophys Acta. 1971 Jan 12;226(1):9–19. doi: 10.1016/0005-2728(71)90173-3. [DOI] [PubMed] [Google Scholar]
- Hellman B., Lernmark A., Sehlin J., Täljedal I. B. Transport and storage of 5-hydroxytryptamine in pancreatic -cells. Biochem Pharmacol. 1972 Mar 1;21(5):695–706. doi: 10.1016/0006-2952(72)90062-7. [DOI] [PubMed] [Google Scholar]
- Howell S. L., Young D. A., Lacy P. E. Isolation and properties of secretory granules from rat islets of Langerhans. 3. Studies of the stability of the isolated beta granules. J Cell Biol. 1969 Apr;41(1):167–176. doi: 10.1083/jcb.41.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa Y., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. 8. Properties of a factor conferring oligomycin sensitivity on mitochondrial adenosine triphosphatase. J Biol Chem. 1966 May 25;241(10):2461–2466. [PubMed] [Google Scholar]
- Lardy H., Reed P., Lin C. H. Antibiotic inhibitors of mitochondrial ATP synthesis. Fed Proc. 1975 Jul;34(8):1707–1710. [PubMed] [Google Scholar]
- Njus D., Radda G. K. Bioenergetic processes in chromaffin granules a new perspective on some old problems. Biochim Biophys Acta. 1978 Mar 10;463(3-4):219–244. doi: 10.1016/0304-4173(78)90001-0. [DOI] [PubMed] [Google Scholar]
- Owman C., Håkanson R., Sundler F. Occurrence and function of amines in endocrine cells producing polypeptide hormones. Fed Proc. 1973 Jul;32(7):1785–1791. [PubMed] [Google Scholar]
- Pollard H. B., Pazoles C. J., Creutz C. E., Zinder O. The chromaffin granule and possible mechanisms of exocytosis. Int Rev Cytol. 1979;58:159–197. doi: 10.1016/s0074-7696(08)61475-8. [DOI] [PubMed] [Google Scholar]
- ROY A. B. The sulphatase of ox liver. I. The complex nature of the enzyme. Biochem J. 1953 Jan;53(1):12–15. doi: 10.1042/bj0530012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Sopwith A. M., Hutton J. C., Naber S. P., Chick W. L., Hales C. N. Insulin secretion by a transplantable rat islet cell tumour. Diabetologia. 1981 Sep;21(3):224–229. doi: 10.1007/BF00252658. [DOI] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifaró J. M., Warner M. Membranes of adrenal chromaffin granules. Solubilization and partial characterization of the Mg ++ -dependent adenosine triphosphatase. Mol Pharmacol. 1972 Mar;8(2):159–169. [PubMed] [Google Scholar]
- Vignais P. V., Vignais P. M., Defaye G. Adenosine diphosphate translocation in mitochondria. Nature of the receptor site for carboxyatractyloside (gummiferin). Biochemistry. 1973 Apr 10;12(8):1508–1519. doi: 10.1021/bi00732a007. [DOI] [PubMed] [Google Scholar]
- Wright P. H., Makulu D. R., Malaisse W. J., Roberts N. M., Yu P. L. A method for the immunoassay of insulin. Diabetes. 1968 Sep;17(9):537–546. doi: 10.2337/diab.17.9.537. [DOI] [PubMed] [Google Scholar]
- Zern R. T., Bird J. L., Feldman J. M. Effect of increased pancreatic islet norepinephrine, dopamine and serotonin concentration on insulin secretion in the golden hamster. Diabetologia. 1980 Apr;18(4):341–346. doi: 10.1007/BF00251017. [DOI] [PubMed] [Google Scholar]


