ABSTRACT
Rationale
Firefighters are at risk for cardiovascular disease due to occupational-related inflammation, oxidative stress, and lifestyle practices. Astaxanthin (AX) possesses anti-inflammatory/antioxidant and purported ergogenic properties. This study examined the impact of supplementing the diet with 12 mg/d AX for four weeks on markers of inflammation, oxidative stress, cardiometabolic health, exercise capacity, and occupation-related performance in career firefighters.
Methods
In a randomized, double-blinded, placebo-controlled, crossover fashion, 15 male career firefighters (34.5 ± 7.4 years; 177.7 ± 7.0 cm; 95.6 ± 12.0 kg; 30.1 ± 2.9 kg/m2; 11.03 ± 6.85 years of service) ingested 12 mg/d of AX (AstaReal®, AstaReal AB, Nacka, SWE) or placebo (PLA) for four weeks while following a standardized resistance training program. After each treatment, testing sessions were completed to assess inflammatory markers, oxidative stress markers, cardiopulmonary exercise capacity, and performance to a fire ground test (FGT) consisting of nine fire suppressive activities. Data were analyzed using general linear model (GLM) analysis with repeated measures. Clinical significance was assessed via mean changes from baseline with 95% confidence intervals.
Results
Analysis of mean percent changes from baseline revealed that AX supplementation lessened the inflammatory response to to performing an incremental maximal exercise test and attenuated increases in interleukin-1β, cortisol, and uric acid in response to performing fire suppressive activities compared to when they ingested PLA. However, most of these effects were within groups rather than between groups. Additionally, there was evidence that AX ingestion increased the ventilatory anaerobic threshold. Four weeks of AX supplementation did not significantly affect fasting markers of oxidative stress, blood lipids, performance during the FGT, general clinical chemistry panels, or self-reported side effects.
Conclusions
Results provide some evidence that AX supplementation may help mediate occupation-related inflammation in response to high-intensity, short-duration exercise in firefighters. More research is warranted to determine if long-term supplementation can improve cardiometabolic risk in this population.
Clinical trial registration
ISRCTN10901752.
KEYWORDS: Firefighting, oxidative stress, antioxidants, tactical athlete
1. Introduction
Firefighting is a physically demanding occupation where unpredictable and dangerous environmental conditions are commonplace. Exposure to occupational-related, environmental stressors (e.g. products of incomplete combustion, smoke inhalation, heat stress) coupled with lifestyle stressors (e.g. disrupted sleep patterns, frequent snacking/feeding habits, alcohol abuse, and psychological stressors, such as family separation, trauma, or dual-stress cognitive challenges) can promote progression of cardiovascular disease (CVD) and lead to premature mortality [1–7]. Firefighting has also been reported to elevate markers of inflammation and oxidative stress [8–13]. For this reason, nutritional interventions that can help manage stress and inflammation or promote cardiometabolic health may benefit firefighters [5].
Astaxanthin is a naturally occurring lipid-soluble carotenoid found in microalgae and marine species [14] that has been purported to possess antioxidant properties and health benefits [15–20]. For example, Yoshida et al. [21] reported that 61 non-obese individuals with mild hyperlipidemia consuming 12 mg/d of astaxanthin for 12 weeks increased high-density lipoprotein cholesterol (HDL, 14.5%) and plasma adiponectin (28%) while reducing triglycerides (−25.2%). Shokri-Mashhadi and colleagues [22] found that type-2 diabetic patients consuming 8 mg/d of astaxanthin for eight weeks reduced interleukin-6 (−9.2%) and the oxidative stress marker malondialdehyde (−31.1%). Moreover, Choi et al. [23] reported that 27 overweight adults ingesting 20 mg/d of astaxanthin for 12 weeks experienced reductions in low-density lipoproteins (LDL, −10.4%), apolipoprotein B (−7.5%), malondialdehyde (−32.7%), and 15-isoprostane F2 (−59%) and an increase in total antioxidant capacity (34.5%). Consequently, it is plausible that astaxanthin supplementation may help firefighters manage oxidative stress, inflammation, and/or blood lipid levels.
There are also exercise and training-related effects of astaxanthin that could enhance firefighter-related performance and/or recovery. In this regard, Brown et al. [24] reported that 12 recreationally trained male cyclists consuming 12 mg/d of astaxanthin for seven days improved fatty acid oxidation (69.2%) during the final stages of a 40-km cycle and time trial performance times (−1.2%). Baralic and associates [25] reported that 40 male soccer players consuming 4 mg/d of astaxanthin for 90 days increased the antioxidants paraoxonase (17.1%) and diazoxon (42%) compared to the placebo trial, highlighting the ability to protect against oxidative damage to lipoproteins and the glycation of HDL [26]. Additionally, these researchers found that astaxanthin supplementation decreased the inflammatory marker and cardiometabolic risk factor C-reactive protein (−12%) during training compared to a 57% increase in the placebo group [27]. Finally, Fleischmann and coworkers [28] reported that 22 healthy males supplementing their diet with 12 mg/d of astaxanthin for 30 days experienced a −11.4% reduction in maximal exercise blood lactate and a −10.9% decrease in post-exercise oxygen uptake (−10.9%) suggesting a greater contribution of oxidative metabolism to incremental maximal exercise and improved recovery. These findings provide evidence that astaxanthin may affect exercise and/or recovery.
This study aimed to assess the effects of ingesting 12 mg/d of astaxanthin for four weeks on markers of oxidative stress, inflammation, cardiometabolic health, exercise capacity, and occupational task-related performance in career firefighters. We hypothesized that astaxanthin supplementation would reduce oxidative stress and inflammation markers while improving blood lipid profiles, exercise capacity, and recovery. The primary outcomes were fasting and exercise markers of inflammation, oxidative stress, and blood lipid profiles. Secondary outcomes were maximal exercise capacity, fire ground test performance, clinical chemistry markers of health and safety, and self-reported symptoms and side effects.
2. Methods
2.1. Experimental design
The study was conducted in a randomized, double-blind, placebo-controlled, crossover manner with the approval of an Institutional Review Board (IRB2020-1379F) in compliance with the Declaration of Helsinki. This clinical trial was registered with the International Standard Randomized Control Number Registry (ISRCTN10901752). Healthy, career male firefighters (24–48 years of age) were recruited to participate in this study. Participants supplemented their diet with a placebo or astaxanthin for four weeks while participating in a standardized training program. A cross-over design was used based on the ability to recruit participants from one firestation, reduce the impact of training and seasonal variations on results, and improve statistical power. Recommended doses were ingested for 4 weeks to assess the impact a consumer might experience if purchasing a bottle of the supplement and to determine if findings would justify conducting a larger and longer clinical trial. Participants performed a series of laboratory-based and occupation-specific tests. Participants observed a two-week washout and repeated the experiment with the alternative treatment. The length of washout was selected assuming that that the primary outcome of astaxanthin would be as a natural anti-inflammatory in which the impact would diminish within a few days after the cessation of supplementation. Figure 1 displays the general experimental design and testing sequences employed.
Figure 1.
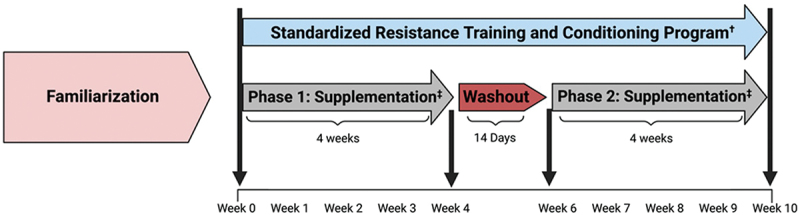
Overview of study design.
2.2. Study participants
Healthy male career firefighters from a cooperating local fire department were recruited to participate in this study. Participants were required to have: 1) no signs, symptoms, or diagnoses of cardiometabolic diseases; 2) no known blood disorders; 3) refrained from ingesting caffeinated supplements, as well as alcohol or nicotine, for 24 hours prior to all testing; 4) refrained from consuming ergogenic aids two weeks prior study initiation; 5) no current or previous (within the last year) musculoskeletal injuries; and 6) no known allergy to the extra virgin olive oil or astaxanthin. Participants had to have been engaged in resistance training (at least twice per week) and moderate-intensity aerobic exercise (at least 150 minutes per week) over the past six months. All participants provided voluntary, written informed consent to participate in the study. Figure 2 presents the study’s Consolidated Standard of Reports Trials (CONSORT) diagram. Twenty-three experienced male firefighters responded to study advertisements and underwent preliminary screening. Of these, three potential participants declined to participate due to scheduling issues. Twenty participants met eligibility criteria, gave voluntary consent to participate in the study, and were familiarized with the study requirements. A total of 15 firefighters who were 34.5 ± 7.4 years old, 177.7 ± 7.0 cm, 95.6 ± 12.0 kg, and 30.1 ± 2.9 kg/m2 body mass index with 11.0 ± 6.9 years of service as a firefighter completed the study. Five participants dropped out after beginning the study due to time and scheduling conflicts.
Figure 2.
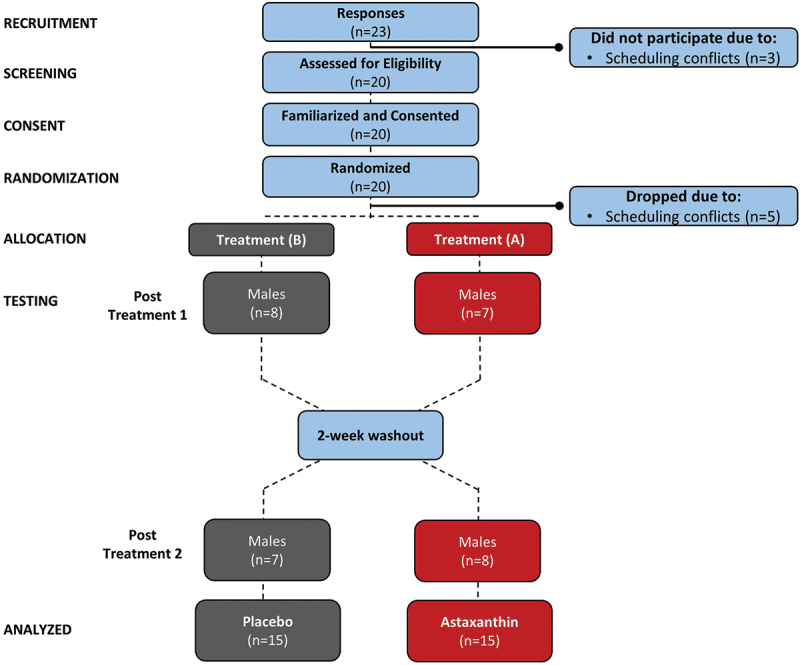
Consolidated standards of reporting trials (CONSORT) diagram.
2.3. Testing sequence
2.3.1. Laboratory-Based Testing
Participants reported to the laboratory between 0500 and 1100 in a fasted state (≥12 hours), returned a 4-day food log, and completed a side effects assessment. The participants rested for six minutes before resting heart rate and blood pressure were recorded. Then, anthropometric data, body composition via a dual-energy x-ray absorptiometry (DEXA), and a pre-exercise fasting blood sample were obtained. The participants performed an incremental maximal cardiopulmonary exercise test to fatigue and then donated a post-exercise blood sample. Figure 3 displays the Day 1 testing sequence.
Figure 3.
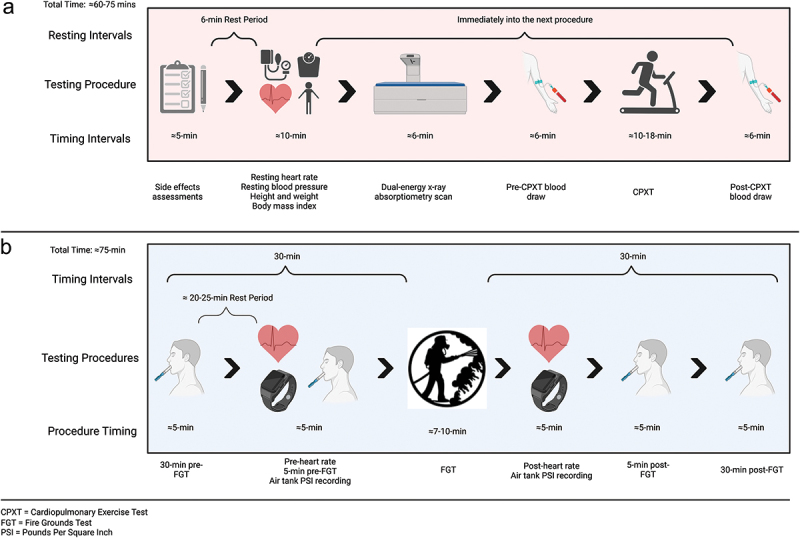
Overview of day one tests performed and sequence.
2.3.2. Occupation-specific testing
Participants reported to the Bryan Fire Department Training Tower site (Bryan, TX, USA) between 0700 and 1100 during a familiarization practice session and following each treatment. The participants completed the fire grounds test (FGT), which consisted of nine occupation-related tasks performed during live fire suppression conditions (see Figure 4). Participants had a monitor strapped to their chest to record their heart rate. Participants were dressed in full personal protective equipment (PPE) and “on air” (i.e. breathing positive pressure oxygen from a self-containing breathing apparatus [SCBA]). Saliva samples were collected 30 minutes and 5 minutes before the FGT and 5 minutes and 30 minutes after the FGT. Table S1 shows the environmental conditions participants experienced during the practice and treatment sessions.
Figure 4.
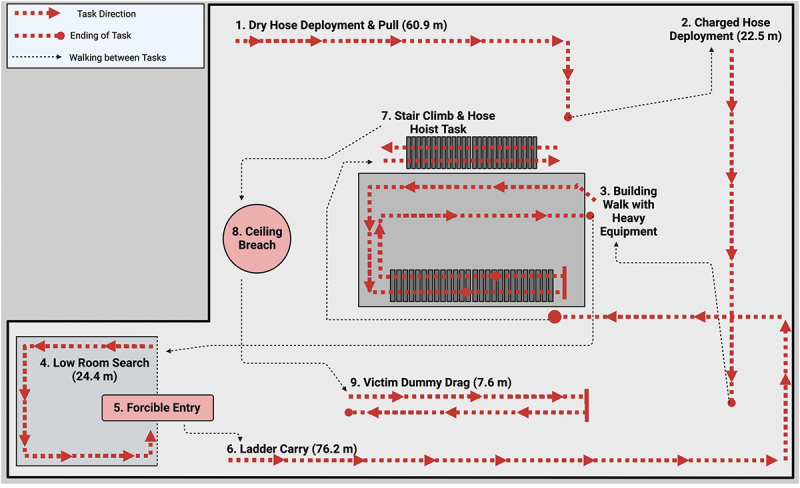
Overview of the fire ground tests and sequence.
2.4. Supplementation protocol
Participants ingested two 416 mg soft gelatin capsules containing extra virgin olive oil and Caramel Powder (Colorant) with 0 mg astaxanthin (PLA) or algal meal of Haematococcus pluvialis (AstaRealⓇ, AstaReal Inc., Moses Lake, WA, USA,), including extra virgin olive oil (AX). The AX capsules contained 6 mg ±15% of high-performance liquid chromatography (HPLC) verified astaxanthin. The PLA (batch 21112001P) and AX (batch UC210760) supplements were prepared, evaluated, and certified for content by the quality manager at AstaReal Inc. (Moses Lake, WA, US). Capsules containing the PLA or AX were packaged into small zip-lock bags labeled “A” or “B” by a colleague not involved in the study who maintained the study code. Participants were instructed to take two capsules of the assigned treatment daily at the same time of day during each treatment phase. Supplement compliance was checked weekly using an online survey and reported to be 100%.
2.5. Standardized resistance training and conditioning program
Participants followed a standardized eight-week strength and conditioning program (see Tables S2a–S2e). Training sessions consisted of a 10-minute cardiovascular warm-up followed by performing a dynamic warm-up and stretching protocol (Table S2d). The resistance training program consisted of weekly workouts focused on core, accessory, circuit training, and cardiovascular power exercises (Table S2b) using standard strength and conditioning equipment (Table S2c) performed on shift following an undulated (i.e. high-volume/low-intensity and low-volume//high-intensity training sessions within the training week/block), non-linear training periodization model with non-circuit, circuit training, and muscle endurance-based workouts (see Table S2e). The muscular endurance portion of the program consisted of circuit-based training with movement patterns mimicking firefighter occupational tasks, while the other components (hypertrophy, strength, and power) focused on compound movements and traditional strength and conditioning exercises. The training session frequency was two sessions per week except for weeks 1, 4, and 7, which consisted of three training sessions for those weeks (see Table S2e). The training intensity, focus, sets and repetitions, and work-to-rest ratios can be viewed in Table S2a. Training compliance was assessed via weekly surveys where participants reported a percentage of the training sessions, they were able to complete for the respective week. Random compliance checks via e-mail or text message were done throughout the study. However, we did not ask participants to record exercise intensities or amounts of weight lifted to calculate training volume. Table S3 shows subjective training compliance data.
2.6. Procedures
2.6.1. Demographics
Body weight and height were evaluated via a Health-O-Meter Professional 500KL (Pelstar LLC, Alsip, IL, USA) self-calibrating digital scale (±0.02 kg). Resting heart rate and blood pressure were measured using a calibrated ConnexSupplemental data for this article can be accessed online at https://doi.org/10.1080/15502783.2024.2427751 ProBP™ 3400 digital blood pressure device (Welch Allyn, Tilburg, NL) using standard procedures [29,30]. Body composition was assessed via a DEXA (Hologic Inc., Waltham, MA, USA using APEX Corporation Software, Pittsburg, PA, USA) [31,32]. Test-retest reliability studies in our lab show CV ranges of 0.31–0.45% for fat-free mass with a mean intraclass correlation (ICC) of 0.98 [33].
2.6.2. Dietary records
All food and energy-containing beverages consumption was standardized via four-day (3 weekdays and 1 weekend day) self-reported dietary records. Participants were instructed to record their dietary intakes on the MyFitnessPal Calorie Counter smartphone app (MyFitnessPal, Inc., Baltimore, MD, USA) or a provided written food log. The participants were instructed to replicate their Phase 1 dietary records for Phase 2 to standardize.
2.6.3. Maximal cardiopulmonary exercise test
Participants completed a symptom-limited incremental maximal cardiopulmonary exercise test (CPXT) on a motorized treadmill (TrackMaster 425, Newton, KS, USA) while wearing an H10 heart rate monitor (Polar Electro Inc., Bethpage, NY, USA) positioned at chest level. The participants were encouraged to exercise to volitional exhaustion using standard test termination criteria [30]. Cardiorespiratory data were collected using a TrueOne 2400 (Parvo Medics, Sandy, UT, USA) metabolic cart. The exercise test consisted of 3-minute stages beginning at 5.5 km/h and a 1% grade, then 7.5 km/h and a 3% grade, followed by an increase of 2% grade every stage thereafter until volitional fatigue [34]. Breath-by-breath data were collected and averaged from 15-sec intervals in the last minute of each stage and used to calculate substrate oxidation rates, minute ventilation (VE), volume of oxygen consumption (VO2), volume of carbon dioxide production (VCO2), and respiratory exchange ratio (RER). The VO2 and VE values of each participant were plotted in MicrosoftⓇ ExcelⓇ (MicrosoftⓇ, Redmond, WA, USA) to establish lines of best-fit [35], which was used to estimate the ventilatory anaerobic threshold (VANT) [36]. At the end of each stage, heart rate and ratings of perceived exertion using Borg’s 6–20 scale [37] were recorded. Oxidation rates were calculated from the averaged VO2 and VCO2 values for fats [1.718 × VO2 − 1.718 × VCO2 (g/min)] and carbohydrates [4.170 × VO2 − 2.965 × VCO2 (g/min)] [38].
2.6.4. Occupation-related fire grounds test
Upon arrival, participants performed a mouth rinse prior to the first saliva sample collection and were briefed on the testing protocol. The participants were instructed to provide a passive drool saliva sample 30 minutes before performing the FGT by tilting their heads and leaning forward. Then, an H10 heart rate monitor (Polar Electro Inc., Bethpage, NY, USA) was attached using a chest strap. Heart rate was determined 5-min before performing the FGT, at the top-of-stairs (following task 7) during the FGT, and immediately following performing the FGT. Heart rate was evaluated in beats per min (bpm) and as a percentage of maximal heart rate determined on the CPXT. Participants then dress in full firefighter personal protection equipment (i.e. turnout gear, hoods, fire helmet, boots, gloves, belts, SCBA harness, etc.) and rest until the next saliva sample collection. Five minutes before performing the FGT, the participants had their pre-FGT heart rate recorded and provided a second passive drool saliva sample (5-min pre-FGT). The participants were then fixed with an air tank and pre-FGT pounds per square inch (PSI) values were recorded from the air tank and extension piece.
The FGT consisted of nine firefighter-related tasks including: 1.) a dry hose deployment and pull; 2.) charged hose deployment; 3.) building walk with heavy equipment; 4.) low room search; 5.) forcible entry; 6.) ladder carry; 7.) stair climb and hose hoist task; 8.) ceiling breach; and 9.) victim dummy drag. Participants were encouraged to complete the FGT as fast as possible. Time-to-complete the FGT was recorded using a Deluxe stopwatch (Model: H-5671; Uline, Pleasant Prairie, WI, USA). The equipment used for the stations was provided by the Bryan Fire Department, and a Keiser sled Force Machine Model 6070 (Keiser Corporation, Fresno, CA, USA) was used to simulate forcible entry. Researchers and safety officers were present for the duration of the testing and monitored the participants throughout the FGT. Immediately after completing the FGT, heart rate, and air tank and air tank extension piece PSI values were recorded. Finally, saliva samples were obtained after 5 and 30 minutes of recovery. Saliva samples were immediately frozen at −20°C and were transported to the laboratory for storage at −80°C for later analysis within 6 hours of collection. The air tank difference in the pre-post air tank and extension recordings of PSI was recorded and used to calculate percent change: [((Post-FGT PSI – Pre-FGT PSI)/Pre-FGT PSI) x 100 (%)]. In addition, work efficiency [1/((Pre-FGT PSI – Post-FGT PSI) x FGT completion time) x 10,000 (lbs./in2/min)] was assessed via the air tank PSI data based on previous work [39].
2.6.5. Blood collection and analysis
Participants donated about 20 mL of fasted (≥12 hours) blood before and after performing and incremental maximal exercise test. Venous blood was collected into 2 × 8.5 mL serum separation tubes (SST) and 1 × 4 mL K2 EDTA tubes (Becton, Dickinson and Company, Franklin Lakes, New Jersey) using standard procedures [40]. After sitting at room temperature for 15-min, the SST samples were centrifuged at 3500 G for 10-min in a refrigerated (4ºC) benchtop Thermo Scientific Heraeus MegaFuge 40 R Centrifuge (Thermo Electron North America LLC, West Palm Beach, FL, USA). Serum samples were aliquoted from one SST and stored at −80ºC in polypropylene microcentrifuge tubes (Eppendorf North America, Inc., Hauppauge, NY, USA) for later analysis, while the other SST tube was sent with the K2 EDTA tube to a commercial lab (Clinical Pathology Labs Inc., Austin, TX) for analysis. An automated multichannel hematology analyzer method with platelet counts and 5-part differential determination was used to analyze whole blood samples. Serum samples were analyzed using a Roche Cobas Gen 2 enzymatic/colorimetric analyzer (Roche Diagnostics International AG, Rotkreuz, Switzerland). Test-retest reliability of performing these assays ranged from 2% to 6%.
Commercially available enzyme-linked immunosorbent assay (ELISA) kits were used to determine serum concentrations of adiponectin (R&D Systems Inc., Minneapolis, MN, USA), advanced glycated end products (AGEs; Cell Bio Labs, San Diego, CA, USA), and advanced oxidation protein products (AOPP; OxiSelect, Cell Bio Labs, San Diego, CA, USA). Absorbance was determined using an Epoch 2 colorimetric plate reader (BioTek, Winooski, VT, USA). Assays wash steps using an automated plate washer (BioTek, Winooski, VT, USA). Serum cytokine concentrations of granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α), and inflammatory interleukins (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-8, and IL-10 were measured in duplicate using a multiplex, magnetic bead-based Luminex™ MagPlex magnetic microsphere assays (Thermo Fisher Scientific, Waltham, MA, USA) with a Luminex MagPix instrument (Luminex Corporation, Austin, TX, USA), given its previous validity and reliability [41–43]. The inter-assay and intra-assay variation for the serum cytokine Luminex assays typically ranges between 2.2–17.5% and 3.3–9.8% in our laboratory, respectively.
2.6.6. Saliva analysis
Saliva analysis involved thawing frozen samples and centrifuging at 4ºC for 15-min at 1500 × G using a refrigerated (4°C) Thermo Scientific Heraeus MegaFuge 40 R Centrifuge (Thermo Electron North America LLC, West Palm Beach, FL, USA). Saliva samples were analyzed for cortisol (Catalog#: 1–3002), uric acid (Catalog#: 1–3802), and IL-1β (Catalog#: 1–3902) using commercially available kits (Salimetrics LLC., Carlsbad, CA, USA). Salivary uric acid required incubation in which a standard laboratory incubator was used (Thermo Fisher Scientific, Waltham, MA, USA). Absorbance was determined using an Epoch 2 colorimetric plate reader (BioTek, Winooski, VT, USA). An automated plate washer was used (BioTek, Winooski, VT, USA) for wash steps. Intra-assay and inter-assay CVs for these assays are typically between 4.5% to 7.2% [44].
2.6.7. Side Effects Assessment
Participants ranked the frequency (F) and severity (S) of experienced symptoms or side effects (i.e. dizziness, headache, tachycardia, heart skipping/palpitations, shortness of breath, nervousness, blurred vision), if any, using (0) none; (1) minimal, 1 to 2/week; (2) slight, 3 to 4/week; (3) F: occasional, 5 to 6/week, S: moderate; (4) F: frequent, 7 to 8/week, S: severe; or (5) F: severe ≥ 9/week, S: very severe. Test to test the variability of performing this survey yielded mean CVs in the range of 1.2 to 2.6 and a mean ICC range of 0.59 to 0.88 for individual items on the survey [45].
2.6.8. Statistical Analysis
Data were analyzed by the IBMⓇ Version 29 SPSSⓇ statistical analysis software (IBM Corp., Armonk, NY, USA). We determined the sample size based on our prior research evaluating the effects of nutritional interventions on exercise performance and markers of inflammation [33,46,47] and oxidative stress [46–51] which reported significant effects in several related markers, with sample sizes ranging from 10 -to 16 per group in parallel-arm-designed studies. Additionally, we evaluated the sample sizes used in studies conducted on firefighters that assessed these markers [9,34,35] and astaxanthin supplementation on inflammatory markers [52,53], markers of oxidative stress [25,52,54], and blood lipids [25] that had 10–20 per group in parallel-arm designed studies and 13 per group in a crossover designed study [53]. We also considered reported effect sizes of related literature and used the reported means, standard deviations, and statistically significant mean differences reported in these studies to calculate power, assuming an 80% power with a 5%–10% standard deviation to the mean and a 5%–10% improvement in primary outcomes. This analysis generally revealed that a sample size of 12–20 per group in a parallel-arm study with one treatment or a sample size of 10–15 in a crossover-design was sufficiently powered to detect significant differences between treatment groups. General linear model analysis of variance (ANOVA) using a mixed model was used to analyze the data where between-subjects effects were evaluated as separate groups and within-subjects effects over time were evaluated using repeated measures. Sphericity was assessed using Mauchly’s test, while skewness and kurtosis statistics assessed normality. The Wilks’ Lambda and Greenhouse-Geisser univariate correction tests were used to assess Time and Group x Time interaction effects to adjust for F-value inflation if the assumption of sphericity was violated. Fisher’s Least Significant difference (LSD) tests and 95% upper and lower confidence intervals (CIs) at preplanned contrasts of interest were used to assess pairwise comparisons of means and post-hoc tests. Additional tests to correct for multiple comparisons were not employed since the Greenhouse-Geisser correction was used to adjusts for F-value inflation [55,56]. The probability of type I error (p-level) was set at 0.05 or less. Statistical trends were noted when p-values >0.05 to < 0.10 were observed. Partial Eta squared (ηp2) values were used to assess effect size where values of 0.01 represented a small effect, 0.06 represented a medium effect, and 0.14 represented a large effect size [57]. The clinical significance of the findings was evaluated by assessing mean changes or percentage mean changes from baseline or PLA with 95% confidence intervals (CI). Means and 95% CIs entirely above or below baseline or PLA were considered statistically and clinically significant [58]. We did not use measurement error or consider clinician perspectives regarding minimally meaningful clinical changes to assess clinical significance. However, test-retest reliability CVs are reported for readers to consider. Data are means ± standard deviations (SD) or mean percent changes from baseline (mean change [LL, UL]). Missing data (298 of 6,570 data points; ≈4%) were replaced using the series means for numerical data [59] while responses to categorical survey questions (i.e. ordinal data) were replaced using the most frequent response or value method [60]. Both methods have been reported to be appropriate and valid methods of replacing missing data [59,60]. Our statistical approach provides a comprehensive and transparent statistical analysis that goes beyond assessing statistical differences using p-levels by reporting effect sizes and pairwise comparisons of contrasts of interest to reduce likelihood of type II statistical error and help researchers decide whether additional research with larger populations is warranted [61–63] and the clinical significance of findings by assessing percent changes from baseline with 95% CIs [58,61–66].
3. Results
3.1. Demographics
Table S4 presents participant demographic and resting hemodynamic data. No significant differences were observed between treatments in demographic markers. Collectively, participants were 34.5 ± 7.4 years old, 177.7 ± 7.0 cm, 95.6 ± 12.0 kg, 30.1 ± 2.9 kg/m2, and 22.7 ± 4.5% body fat. Participants had normal resting heart rate (63 ± 11 bpm), systolic blood pressure (126.2 ± 11.7 mmHg), and diastolic blood pressure (75.5 ± 6.8 mmHg).
3.2. Primary outcome variables
3.2.1. Markers of inflammation
Table S5 shows the pre- and post-maximal exercise inflammatory and cytokine response to the treatments. GLM analysis revealed no significant overall Wilk’s Lambda treatment x time effect (p = 0.243, = 0.428, large effect) for the panel of 10 inflammatory cytokines. Univariate analysis found no significant treatment x time effects for IL-1β, IL-6, and IL-8 pro-inflammatory cytokines, the anti-inflammatory cytokine IL-4, or white cell stimulator Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF). The anti-inflammatory cytokine IL-10 approached a significant treatment x time effect (p = 0.099, np2 = 0.094, medium effect) with changes significantly increased from baseline with PLA treatment (0.21 pg/mL [0.028, 0.384], p = 0.025) while IL-10 was unchanged with AX (−0.003 pg/mL [−0.181, 0.175], p = 0.970). Similarly, pairwise analysis revealed that IL-2 (regulator or inflammatory homeostasis), IL-5 (B cell growth stimulator of immunoglobulins), and proinflammatory markers IFN-γ and TNF-α significantly increased in response to maximal exercise with PLA treatment but not AX treatment. The only inflammatory cytokine that tended to increase in response to AX treatment was IL-4, which is an anti-inflammatory cytokine (0.56 pg/mL [−0.013, 1.128], p = 0.055). This is more clearly seen in Figure 5, which presents the mean percent change from baseline with 95% CIs for the blood inflammatory cytokines. Analysis of percent mean change from baseline indicated that the inflammatory cytokines IL-1β (16.4% [0.8, 32.0], p = 0.040), IL-2 (13.1% [0.8, 25.4], p = 0.038), IL-5 (5.2% [0.3, 9.9], p = 0.037), IL-6 (6.9% [−0.94, 14.7], p = 0.082), IL-10 (8.8% [1.6, 16.0], p = 0.018), GM-CSF (14.7% [0.6, 28.9], p = 0.041), IFN-γ (9.9% [0.5, 19.2], p = 0.039), and TNF-α (18.8% [6.1, 31.5], p = 0.005) increased above baseline with PLA treatment while only the anti-inflammatory cytokine IL-4 (4.7% [−0.4, 9.8], p = 0.068) and pro-inflammatory marker TNF-α (10.9% [−1.7, 23.7], p = 0.086) tended to increase above baseline with AX treatment. These findings suggest that AX supplementation lessened the inflammatory and immune response to incremental maximal exercise.
Figure 5.
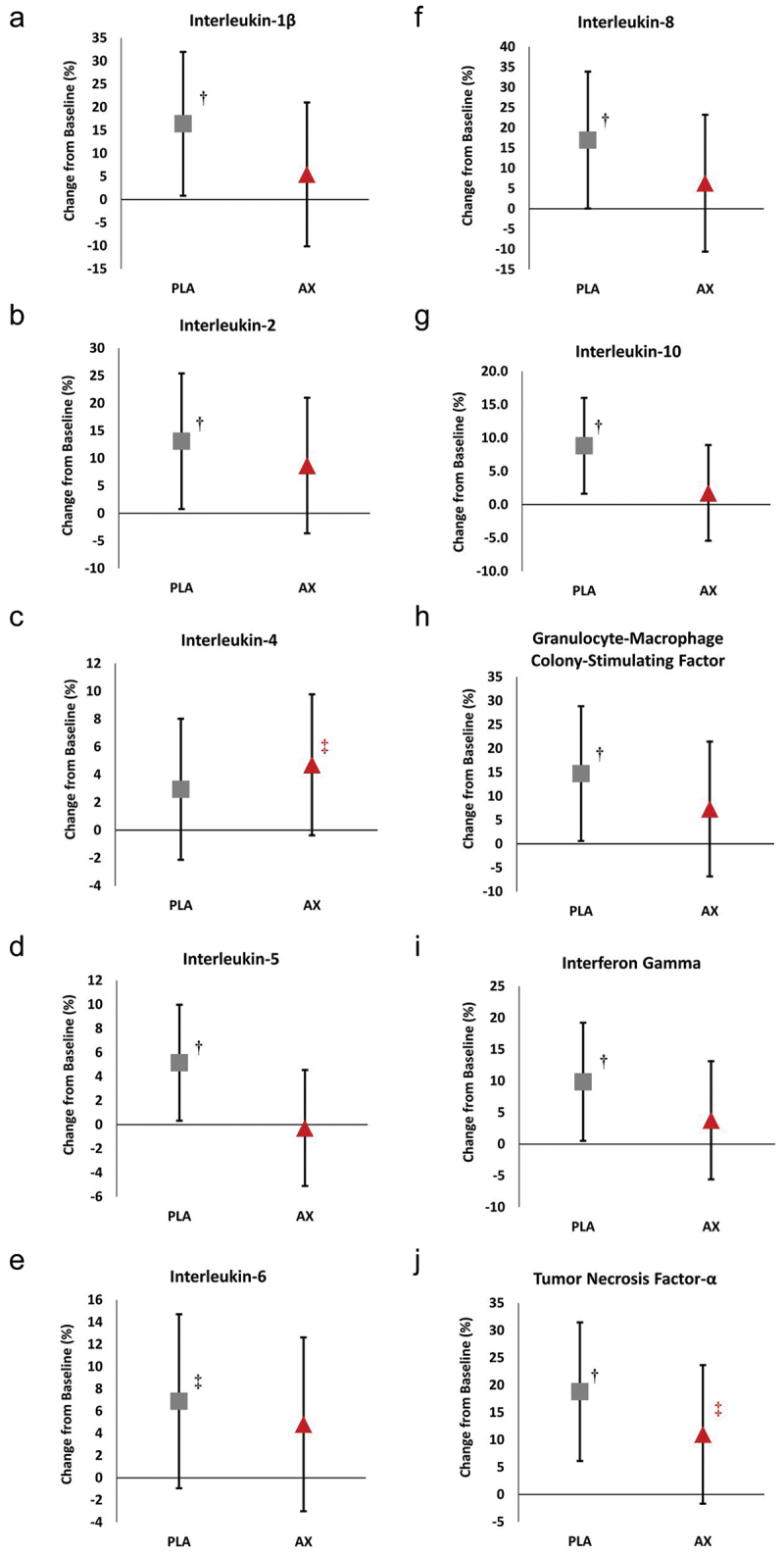
Percent change in blood inflammatory cytokine response to incremental maximal exercise. Data are represented as percent differences from baseline with means ±95% confidence intervals. PLA = placebo, AX = astaxanthin. † represents p < 0.05 difference from baseline. ‡ represents p > 0.05 to p < 0.10 difference from baseline.
Table S6 presents the salivary inflammatory and stress markers evaluated prior to and during the FGT while Figure 6 shows the percent changes observed from baseline in these markers. Overall GLM Wilk’s Lambda analysis revealed a time (p < 0.001, np2 = 0.304, large effect) but no treatment x treatment effects (p = 0.918, np2 = 0.015, small effect) on salivary IL-1β, cortisol, and uric acid levels. Likewise, univariate analysis showed significant time but no treatment x time interaction effects. However, analysis of the percent mean change from baseline with 95% CIs indicated that the exercised-induced increases in IL-1β, cortisol, and uric acid were attenuated to a greater degree with AX treatment compared to PLA treatment. For example, Post-5 IL-1β, cortisol, and uric acid levels increased above baseline with PLA treatment but not with AX treatment and Post-30 FGT IL-1β tended to be higher with PLA treatment (124% [−5, 253], p = 0.059). These findings provide some evidence that AX ingestion may lessen the inflammatory effects of intense exercise by delaying a clinically significant increase in these markers. However, only one data point tended to be different between groups.
Figure 6.
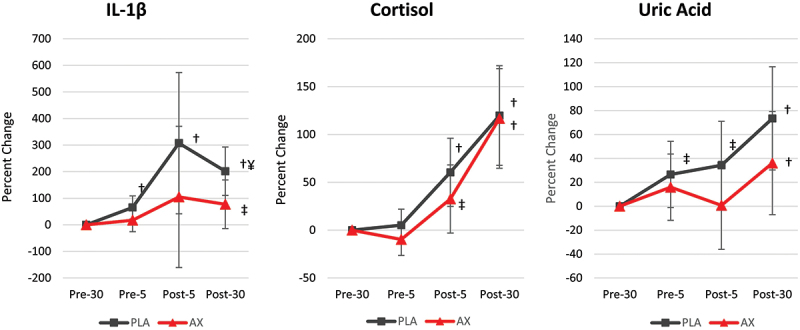
Percent change in salivary inflammatory and oxidative stress markers evaluated during the fire ground test. Data are represented as means ±95% confidence intervals. PLA = placebo, AX = astaxanthin. ‡ represents p > 0.05 to p < 0.10 difference from baseline. ¥ represents p > 0.05 to p < 0.10 difference between PLA and AX treatments.
3.2.2. Markers of oxidative stress
Table S7 presents fasting markers of oxidative stress evaluated in this study. Overall GLM analysis revealed no significant treatment effect (p<0.427, np2 = 0.100, medium effect) for AOPP, AGEs, and adiponectin. Pairwise analysis showed no statistically significant differences between treatments for AOPP (p = 0.530, np2 = 0.014, small effect) or adiponectin (p = 0.836, np2 = 0.002, small effect). However, AGEs concentrations tended to differ between treatments (p = 0.098, np2 = 0.095, medium effect) with AGEs values tending to be higher after AX treatment compared to PLA (0.357 µg/ml [−0.07, 0.783], p = 0.098). Figure 7 presents the percent change from PLA treatment values with 95% CIs for the oxidative stress markers analyzed. Although not statistically significant, analysis of the mean percent change from PLA also indicated that AGEs tended to be higher after 4 weeks of AX supplementation (8.7% [−0.5, 17.9], p = 0.063).
Figure 7.
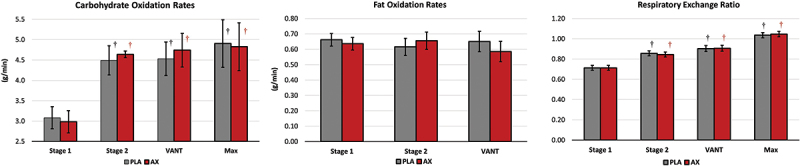
Carbohydrate and fat oxidation rates and respiratory exchange ratio values observed during the incremental maximal exercise test. Data are represented as means ±95% confidence intervals. PLA = placebo, AX = astaxanthin. ‡ represents p > 0.05 to p < 0.10 difference from baseline.
3.2.3. Blood lipids
Table 1 presents pre- and post-CPXT blood lipid responses to four weeks of PLA and AX supplementation. Overall GLM analysis revealed a significant Wilk’s Lambda time effect (p < 0.001, ηp2 = 0.905) with no significant treatment x time effects (p = 0.958, ηp2 = 0.059). Univariate analysis revealed that total cholesterol, triglycerides, HDL, LDL, Non-HDL cholesterol, and very low-density lipoprotein (VLDL) significantly increased in response to incremental maximal exercise while the LDL/HDL ratio and total cholesterol/HDL ratio were unaffected. Moreover, no significant treatment x time interactions were observed. Interestingly though, while not statistically significantly different, fasting total cholesterol, triglycerides, LDL, non-HDL cholesterol, and VLDL lipid values were lower and displayed a more favorable lipid profile with AX treatment.
Table 1.
Blood lipid response to maximal exercise.
| Time Point | Mean | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Treatment | n | PRE | POST | (SEM) | Effect | p-Value | ηp2 |
| Total Cholesterol | PLA | 15 | 207.3 ± 41.6 | 226.6 ± 44.2† | 217.0 ± 9.7 | T | 0.000 | 0.870 |
| (mg/dL) | AX | 15 | 197.8 ± 29.9 | 217.9 ± 33.2† | 207.8 ± 9.7 | G x T | 0.783 | 0.003 |
| Total | 30 | 202.6 ± 35.9 | 222.2 ± 38.6 | 212.4 ± 6.8 | ||||
| Triglycerides | PLA | 15 | 130.9 ± 70.0 | 144.7 ± 67.2† | 137.8 ± 14.7 | T | 0.000 | 0.558 |
| (mg/dL) | AX | 15 | 117.7 ± 40.9 | 132.5 ± 45.0† | 125.1 ± 14.7 | G x T | 0.827 | 0.002 |
| Total | 30 | 124.3 ± 56.7 | 138.6 ± 56.5 | 131.4 ± 10.4 | ||||
| HDL Cholesterol | PLA | 15 | 48.3 ± 7.8 | 53.4 ± 8.3† | 50.9 ± 2.2 | T | 0.000 | 0.862 |
| (mg/dL) | AX | 15 | 47.1 ± 8.4 | 51.9 ± 9.3† | 49.5 ± 2.2 | G x T | 0.724 | 0.005 |
| Total | 30 | 47.7 ± 8.0 | 52.6 ± 8.7 | 50.2 ± 1.5 | ||||
| LDL Cholesterol | PLA | 15 | 134.0 ± 35.6 | 145.7 ± 38.5† | 139.9 ± 8.6 | T | 0.000 | 0.819 |
| (mg/dL) | AX | 15 | 127.7 ± 28.3 | 140.5 ± 30.3† | 134.1 ± 8.6 | G x T | 0.628 | 0.008 |
| Total | 30 | 130.8 ± 31.7 | 143.1 ± 34.2 | 137.0 ± 6.1 | ||||
| Non-HDL Cholesterol | PLA | 15 | 159.0 ± 41.9 | 173.2 ± 45.3† | 166.1 ± 10.0 | T | 0.000 | 0.835 |
| (mg/dL) | AX | 15 | 150.7 ± 32.3 | 166.0 ± 35.3† | 158.4 ± 10.0 | G x T | 0.670 | 0.007 |
| Total | 30 | 154.9 ± 37.0 | 169.6 ± 40.1 | 162.2 ± 7.1 | ||||
| VLDL Cholesterol | PLA | 15 | 25.0 ± 9.6 | 27.5 ± 10.4† | 26.2 ± 2.1 | T | 0.000 | 0.567 |
| (mg/dL) | AX | 15 | 23.1 ± 5.9 | 25.5 ± 6.7† | 24.3 ± 2.1 | G x T | 1.000 | 0.000 |
| Total | 30 | 24.0 ± 7.9 | 26.5 ± 8.6 | 25.3 ± 1.5 | ||||
| LDL/HDL Ratio | PLA | 15 | 2.86 ± 0.89 | 2.81 ± 0.88 | 2.84 ± 0.23 | T | 0.118 | 0.085 |
| AX | 15 | 2.83 ± 0.91 | 2.82 ± 0.87 | 2.82 ± 0.23 | G x T | 0.407 | 0.025 | |
| Total | 30 | 2.85 ± 0.88 | 2.82 ± 0.86 | 2.83 ± 0.16 | ||||
| Total Cholesterol/HDL Ratio | PLA | 15 | 4.40 ± 1.09 | 4.35 ± 1.08 | 4.37 ± 0.27 | T | 0.137 | 0.077 |
| AX | 15 | 4.34 ± 1.05 | 4.33 ± 1.01 | 4.34 ± 0.27 | G x T | 0.396 | 0.026 | |
| Total | 30 | 4.37 ± 1.05 | 4.34 ± 1.03 | 4.36 ± 0.19 |
Data are expressed as means ± standard deviations for the placebo (PLA) and astaxanthin (AX) treatments. HDL = high-Density Lipoprotein; LDL = Low-Density Lipoprotein; VLDL = Very-Low Density Lipoprotein. Data were analyzed using a multivariate and univariate General Linear Model with repeated measures. P-levels, with partial ETA squared (ηp2) effect size, were reported. General Linear Model analysis revealed a significant overall Wilk’s Lambda for Time (p < 0.001, ηp2 = 0.905); however, no significant Treatment x Time (p = 0.958, ηp2 = 0.059) effect. Greenhouse-Geisser univariate p-levels are listed for time (T) and treatment x time (G x T) interaction effects. Significance was determined via pairwise comparison, with LSD posthoc adjustment, indicated as differences from baseline: † = p < 0.05 [‡ = p > 0.05 to p < 0.10]. ηp2 effect size values of 0.01–0.05 = small, 0.06–0.13 = medium, and > 0.14 = large.
3.3. Secondary outcome variables
3.3.1. Exercise performance
Table 2 presents results from the incremental maximal cardiopulmonary exercise test. GLM analysis revealed an overall Wilk’s Lambda revealed a significant treatment effect (p = 0.013, np2 = 0.478, large effect) with univariate treatment effects observed in VANT expressed in L/min (PLA 2.67 ± 0.38, AX 3.03 ± 0.56, L/min, p < 0.06, np2 = 0.121, medium effect) and as a percentage of peak oxygen uptake (PLA 66.7 ± 4.7%, AX 73.8 ± 4.7%, p < 0.001, np2 = 0.315, large effect). Figure 7 presents carbohydrate and fat oxidation rates and RER values observed at stage 1, stage 2, VANT, or maximal effort. Carbohydrate oxidation and RER values increased during the incremental maximal exercise test. However, no significant differences were observed between treatments in carbohydrate and fat oxidation or RER values.
Table 2.
Maximal exercise test results.
| Variable | Treatment | n | Mean | p-Value | ηp2 |
|---|---|---|---|---|---|
| Relative Peak Oxygen Uptake | PLA | 15 | 42.4 ± 5.8 | 0.818 | 0.002 |
| (ml/kg/min) | AX | 15 | 43.0 ± 6.5 | ||
| Total | 30 | 42.7 ± 6.0 | |||
| Absolute Peak Oxygen Uptake | PLA | 15 | 4.03 ± 0.59 | 0.839 | 0.002 |
| (L/min) | AX | 15 | 4.07 ± 0.60 | ||
| Total | 30 | 4.05 ± 0.58 | |||
| Ventilatory Threshold | PLA | 15 | 2.67 ± 0.38 | 0.060 | 0.121 |
| (L/min) | AX | 15 | 3.03 ± 0.56 | ||
| Total | 30 | 2.85 ± 0.51 | |||
| Ventilatory Threshold | PLA | 15 | 66.7 ± 4.7 | 0.001 | 0.315 |
| (%) | AX | 15 | 73.8 ± 4.7 | ||
| Total | 30 | 70.2 ± 6.5 | |||
| Time to Exhaustion | PLA | 15 | 14.72 ± 3.74 | 0.862 | 0.001 |
| (min) | AX | 15 | 14.96 ± 3.82 | ||
| Total | 30 | 14.84 ± 3.72 | |||
| Time to VANT | PLA | 15 | 7.92 ± 3.04 | 0.518 | 0.015 |
| (min) | AX | 15 | 8.66 ± 3.11 | ||
| Total | 30 | 8.29 ± 3.05 |
Data are expressed as means ± standard deviations for the placebo (PLA) and astaxanthin (AX) treatments. VANT = Ventilatory anaerobic threshold. Data were analyzed using a multivariate and univariate General Linear Model. P-levels, with partial ETA squared (ηp2) effect size, were reported. General Linear Model analysis revealed a significant overall Wilk’s Lambda for treatment (p = 0.013, ηp2 = 0.478) effect. ηp2 effect size values of 0.01–0.05 = small, 0.06–0.13 = medium, and > 0.14 = large.
Table 3 overviews performance-related variables assessed during the FGT. GLM overall Wilks’ Lambda analysis revealed no significant treatment effects (p = 0.873, np2 = 0.206) for the FGT performance variables. Pairwise analysis also showed no differences between treatments in time-to-completion, air tank difference (PSI), air extension piece difference (PSI), work efficiency, pre-FGT heart rate, top-of-stair heart rate, immediate post-FGT heart rate, peak heart rate during the FGT, or average heart rate during the FGT. Interestingly, although not statistically significant, participants took fewer hits on the Keiser Sled simulated door breach with AX treatment (−1.9 hits, p = 0.127, np2 = 0.081, medium effect).
Table 3.
Fire ground test performance data.
| Variable | Treatment | n | Mean | p-Value | ηp2 |
|---|---|---|---|---|---|
| Time-to-completion | PLA | 15 | 9.34 ± 1.42 | 0.966 | 0.000 |
| (min) | AX | 15 | 9.31 ± 1.91 | ||
| Total | 30 | 9.32 ± 1.66 | |||
| Air Tank Difference | PLA | 15 | 2,400 ± 382 | 0.829 | 0.002 |
| (PSI) | AX | 15 | 2,430 ± 373 | ||
| Total | 30 | 2,415 ± 371 | |||
| Air Extension Piece Difference | PLA | 15 | 2,343 ± 399 | 0.496 | 0.017 |
| (PSI) | AX | 15 | 2,437 ± 339 | ||
| Total | 30 | 2,390 ± 367 | |||
| Work Efficiency | PLA | 15 | 0.47 ± 0.14 | 0.986 | 0.000 |
| (lbs/in2/min) | AX | 15 | 0.48 ± 0.14 | ||
| Total | 30 | 0.47 ± 0.13 | |||
| Pre-FGT Heart Rate | PLA | 15 | 93.95 ± 19.31 | 0.817 | 0.002 |
| (bpm) | AX | 15 | 92.60 ± 11.51 | ||
| Total | 30 | 93.28 ± 15.63 | |||
| Post-FGT Heart Rate | PLA | 15 | 176.4 ± 11.3 | 1.000 | 0.000 |
| (bpm) | AX | 15 | 176.4 ± 7.6 | ||
| Total | 30 | 176.4 ± 9.5 | |||
| Top-of Stair Heart Rate | PLA | 15 | 179.3 ± 9.3 | 0.919 | 0.000 |
| (bpm) | AX | 15 | 178.9 ± 9.2 | ||
| Total | 30 | 179.1 ± 9.1 | |||
| Average Heart Rate | PLA | 15 | 158.8 ± 11.5 | 0.404 | 0.025 |
| (bpm) | AX | 15 | 154.9 ± 13.5 | ||
| Total | 30 | 156.8 ± 12.5 | |||
| Maximal Heart Rate | PLA | 15 | 181.3 ± 10.0 | 1.000 | 0.000 |
| (bpm) | AX | 15 | 181.3 ± 8.6 | ||
| Total | 30 | 181.3 ± 9.2 | |||
| Keiser Sled | PLA | 15 | 17.00 ± 3.95 | 0.127 | 0.081 |
| (# of hits) | AX | 15 | 15.13 ± 2.36 | ||
| Total | 30 | 16.07 ± 3.33 |
Data are expressed as means ± standard deviations for the placebo (PLA) and astaxanthin (AX) treatments. FGT = Fire Ground Test; PSI = Pounds Per Square Inch. Data were analyzed using a multivariate and univariate General Linear Model. P-levels, with partial ETA squared (ηp2) effect size, were reported. General Linear Model analysis revealed a nonsignificant overall Wilk’s Lambda for treatment (p = 0.873, ηp2 = 0.206) effect. ηp2 effect size values of 0.01–0.05 = small, 0.06–0.13 = medium, and > 0.14 = large.
3.4. General health markers
Tables S8–S10 present pre- and post-CPXT liver function biomarkers, serum glucose, electrolytes, and renal function biomarkers, and whole blood red and white cell counts with percent differential results, respectively. Although some time effects were observed, no significant overall or univariate treatment x time interaction effects were observed in pre- and post-CPXT serum markers of liver function (p = 0.815, ηp2 = 0.140, large effect), renal function (p = 0.983, ηp2 = 0.120, medium effect), or whole blood red and white cell complete blood counts (p = 0.871, ηp2 = 0.336, large effect). All values were within normal clinical ranges for active individuals.
3.5. Side effects assessment
Table S11 shows a Chi-square analysis of symptom frequency and severity values observed following the four weeks for each treatment. No significant differences were observed between treatments in perceptions of the frequency or severity of dizziness, headaches, tachycardia, heart palpitations, dyspnea, nervousness, blurred vision, or other side effects.
4. Discussion
The main findings of this study were that astaxanthin supplementation (12 mg/d for four weeks) blunted some markers of inflammation and oxidative stress in response to an incremental maximal exercise test and firefighter occupational tasks, as well as attenuated VANT. However, four weeks of astaxanthin supplementation did not statistically significantly affect fasting oxidative stress markers, lipid profiles, or occupational performance. These findings provide some evidence that astaxanthin may help mediate inflammation and oxidative stress in response to high-intensity, short-duration exercise in firefighters. The following provides a more detailed assessment of these findings in the context of related work.
4.1. Primary outcomes
4.1.1. Markers of inflammation
Several studies have demonstrated that astaxanthin can reduce inflammation [22,67,68]. For example, Shokri-Mashhadi et al. [22] demonstrated that 44 type-2 diabetic patients consuming 8 mg/d for eight weeks of astaxanthin reduced IL-6 (−9.2%). Chan et al. [67] also demonstrated that 54 type-2 diabetic patients consuming 12 mg/d of astaxanthin for eight weeks reduced C-reactive protein (−23.5%) and IL-6 (−24.5%). Interestingly, other reports have noted no statistically significant effects of astaxanthin supplementation inflammatory biomarkers [53,69]. For instance, Coombes and colleagues [69] demonstrated that 58 patients undergoing renal transplantation who consumed 12 mg/d of astaxanthin or placebo for 12 months experienced non-statistically significantly different changes in the inflammatory marker pentraxin-3 (PLA: 50.6% increase; AX: 11.0% decrease). Furthermore, a recent study by Waldman et al. [53] found that 13 resistance-trained males consuming 12 mg/d of astaxanthin for four weeks did not impact the IL-6 and C-reactive protein inflammatory markers. Researchers have purported that higher doses of astaxanthin (12 mg/d) for extended supplementation periods (12 weeks or more) may be needed to induce more consistent and favorable outcomes on resting markers of inflammation [70,71]. For example, Satoh et al. [71] reported some dose-related benefits of astaxanthin supplementation on fasting glucose and triglycerides in participants at risk of metabolic syndrome when supplementing the diet with 4, 8, and 20 mg/d of astaxanthin for 4 weeks and cognitive benefits when supplementing the diet with 12 mg/d for 12-weeks.
Our findings demonstrate that the inflammatory response to an incremental maximal exercise test was blunted following the astaxanthin treatment compared to the PLA. In particular, post-exercise mean percent change from baseline values for IL-1β, IL-2, IL-5, IL-10, IFN-γ, and TNF-α were significantly elevated following the PLA but not astaxanthin treatments. These findings suggest that astaxanthin may lessen the inflammatory response to intense exercise, which aligns with previous reports showing a blunting in the inflammatory response to strenuous exercise bouts (e.g. lower C-reactive protein concentrations post 90-d astaxanthin supplementation) [27]. Considering the antioxidant and anti-inflammatory potential, future research should consider assessing the impact of astaxanthin supplementation over longer periods, particularly in older and less fit firefighters. We also evaluated the effects of occupation-related fire-suppressive activities on oxidative stress markers. It is well established that firefighting exposes the operator to several stressors that increase pro-inflammatory activity. Our study demonstrates a blunted inflammatory response to the FGT following the astaxanthin treatment compared to the PLA. Salivary IL-1β values increased above baseline at 5-min pre-FGT, 5-min post-FGT, and 30-min post-FGT following the PLA, whereas IL-1β values were only increased above baseline following the astaxanthin treatment at 30-min post-FGT. In addition, salivary cortisol increased above baseline at 5-min post-FGT and 30-min post-FGT following the PLA, whereas salivary cortisol values only increased above baseline following the astaxanthin treatment at 30-min post-FGT. Salivary cortisol is a common marker of physiological stress [6] and has been shown to increase when environmental factors (i.e. heat stress) are introduced [9,72]. These findings support contentions that astaxanthin may attenuate the normal increases in inflammatory responses to intense exercise. However, only Post-30 IL-1β values tended to differ between groups. Together, these findings provide promising real-world information about the utility of astaxanthin in lessening the physiological stress and inflammatory response to fire-suppressive activities.
4.1.2. Markers of oxidative stress
Several studies have demonstrated that astaxanthin can function as an antioxidant. For example, Shokri-Mashhadi et al. [22] demonstrated that 44 type-2 diabetic patients consuming 8 mg/d for eight weeks of astaxanthin reduced fasting malondialdehyde (31.1%) compared to a placebo. In addition, Choi and colleagues [73] demonstrated that low (5 mg/d) and high (20 mg/d) doses of astaxanthin decreased malondialdehyde (Low: 34.7%; High: 35.3%) and isoprostane (Low: 64.8%; High: 64.6%) with concomitant increases in plasma superoxide dismutase (Low: 93.9%; High: 93.8%;) and total antioxidant capacity (Low: 22.2%; High: 26.2%) among 27 overweight participants supplementing for 12 weeks. Kim et al. [74] also showed that various doses of astaxanthin (5, 20, or 40 mg/d for 3 weeks) decreased plasma malondialdehyde (5 mg/d: 32.1%; 20 mg/d: 30.3%; 40 mg/d: 60.6%) and isoprostane (5 mg/d: 87.4%; 20 mg/d: 93.1%; 40 mg/d: 94.8%) while increased superoxide dismutase (5 mg/d: 66.7%; 20 mg/d: 250%; 40 mg/d: 120%) and total antioxidant capacity (5 mg/d: 53.8%; 20 mg/d: 66.7%; 40 mg/d: 56.7%) among 39 healthy smokers supplementing for three weeks.
In the present study, we did not find that astaxanthin supplementation (12 mg/d for 4 weeks) improved fasting AOPP, AGEs, or adiponectin levels compared to ingesting the placebo for four weeks. It is plausible that a longer duration study is needed as previous studies have employed longer-duration supplementation periods (i.e. 8–12 weeks) and found improvements in oxidative stress markers. We did note a trend toward an increase in fasting AGEs with AX. AGEs are formed endogenously by condensing carbonyl groups from sugars and free amine groups from proteins, lipids, and nucleic acids and can be obtained from the diet [75]. AGEs also interact with their cellular receptors that help mediate inflammatory and oxidative stress pathways [75]. An increase in fasting AGEs has been associated with chronic disease pathology and exercise training has been suggested as a way to manage elevated AGEs in patients with human immunodeficiency virus [76]. However, the effects of acute exercise on AGE production with or without antioxidant and/or anti-inflammatory nutrients or medications are unknown. A transient increase may reflect an increased influence of AX ingestion on oxidative stress and/or inflammatory cell signaling. However, the etiology of this increase remains to be determined. Additionally, it should be noted that the firefighters assessed in this study were healthy and did not have elevated oxidative stress markers at baseline. Interestingly, we did see some evidence that astaxanthin reduced oxidative stress in response to performing the FGT. In this regard, salivary uric acid levels, used as a cost-effective, noninvasive method to assess oxidative stress [77], were blunted with astaxanthin supplementation during and following the fire-suppressive exercise assessment. These findings provide some evidence that astaxanthin can serve as an antioxidant in fire-suppressive activities. However, more research is needed to determine whether long-term supplementation may affect fasting oxidative stress markers, including AGEs.
4.1.3. Blood lipids
Several human trials have demonstrated that astaxanthin can improve blood lipid profiles. For instance, Chan et al. [67] demonstrated that 54 type-2 diabetic patients consuming either a low dose (6 mg/d) or a high dose (12 mg/d) of astaxanthin for eight weeks experienced reductions in triglycerides (6 mg/d: 4.5%; 12 mg/d: 17.6%) and LDL (6 mg/d: 6.5%; 12 mg/d: 8.8%). The 12 mg/d astaxanthin group also demonstrated lower total cholesterol levels than baseline (6.4%). Yoshida et al. [21] also showed that 61 non-obese individuals with mild hyperlipidemia consuming 6 and 12 mg/d of astaxanthin for 12 weeks increased HDL (10.6% and 15.4%, respectively), while 12 and 18 mg/d of astaxanthin for 12 weeks reduced triglycerides (25.2% and 23.8%, respectively). Lastly, albeit without statistical significance, Saito et al. [78] demonstrated that 20 healthy participants supplementing their diet with 12 mg/d of astaxanthin for four weeks reduced triglyceride concentrations by 15%. Additionally, Ciaraldi and colleagues [79] reported that 12 mg/d of astaxanthin supplementation for 12-weeks decreased low-density lipoprotein, total cholesterol, fibrinogen, L-selectin, and fetuin-A while tending to improve insulin-stimulated whole body glucose disposal. Our findings did not reveal improvements in blood lipids, which may be attributed to the short study duration. Several studies have employed more than 8-weeks of supplementation with 12 mg/d of astaxanthin is needed to observe positive effects on blood lipids [21,23,67]. In addition, the study populations showing benefit of astaxanthin on blood lipids were patient populations [21,23,67], while the firefighter cohort of our study were healthy and had blood lipid profiles within normal ranges. Consequently, it is also possible that astaxanthin supplementation may have a greater impact on blood lipids when they are elevated. Nevertheless, future research is warranted to assess the potential cardiometabolic benefit of astaxanthin among firefighters with higher blood lipids and based on risk stratification.
4.2. Secondary outcomes
4.2.1. Maximal exercise capacity
Astaxanthin has been purported to impact substrate oxidation rates, recovery, and cardiovascular/metabolic response to exercise. For instance, Fleischmann et al. [28] demonstrated that 22 healthy males supplementing with 12 mg/d of astaxanthin for 30 days lowered post-exercise blood lactate levels by 11.4%. In addition, Brown et al. [24] demonstrated that 12 recreationally trained male cyclists consuming 12 mg/d of astaxanthin for 7 days improved fatty acid oxidation rates. However, Wika et al. [80] demonstrated that 19 overweight males and females consuming 12 mg/d for four weeks of astaxanthin did not experience favorable changes in fatty acid oxidation rates. In addition, Res et al. [81] also reported no effect on substrate oxidation rates with 32 young, well-trained male cyclists consuming 20 mg/d of astaxanthin for four weeks. Lastly, McAllister et al. [82] demonstrated that 14 healthy college-aged males consuming 6 mg/d of astaxanthin for four weeks did not favorably impact fatty acid oxidation rates. The authors postulated that 12 mg/d for four or more weeks may be needed to induce changes in substrate oxidation. Although our study did not find an effect on substrate oxidation rates for the cardiopulmonary exercise test, we did find an improvement in absolute and relative VANT, as well as time to VANT. The etiology of this finding is unclear. Speculatively, if astaxanthin affects inflammation and/or perceptions of pain, it could promote greater training adaptations. Additionally, since firefighters do intense exercise while wearing masks and attached to oxygen tanks, there may be a training effect on respiratory muscles thereby enhancing ventilation and/or on respiratory buffering which could affect VANT. There is also some evidence that astaxanthin may affect fat oxidation. If the percentage of fat oxidation is increased, it could attenuate how long it takes to begin hyperventilating and thereby affect VANT. However, more research is needed to replicate these findings, assess potential mechanisms, and determine whether astaxanthin supplementation may affect endurance exercise capacity at anaerobic threshold intensities.
4.2.2. Occupation-related performance
The FGT used in this study comprises a series of firefighting suppressive activities performed at near-maximal effort for over nine minutes. Astaxanthin did not improve FGT performance times or affect exercise heart rate, the on-tank oxygen needed to perform the FGT, or work efficiency. The only potentially ergogenic benefit observed was in the Keiser Sled number of hits, which simulates door breaching; however, this finding was not statistically significantly different between treatments (PLA: 17.0 ± 3.95; AX: 15.1 ± 2.36, p = 0.127, np2 = 0.081, medium effect). Nevertheless, the time to perform the FGT was not affected. It should also be noted that since it took some time to turn off air tanks after mask removal and air may leak around the mask during intense exercise, air tank and piece differences may have been affected. Therefore, these data should be viewed as descriptive. Further work is needed to assess the impact of nutritional interventions on air tank utilization and the economy.
4.3. Safety
Our findings demonstrate that astaxanthin does not negatively affect whole blood cell count, liver function, renal function biomarkers, or self-perceived side effects. These findings are consistent with other studies reporting that astaxanthin is well-tolerated at the dosages studied.
4.4. Limitations and future directions
The present study is limited by several factors. First, coordinating testing sessions and scheduling around shiftwork/mandatory overtime shifts can be difficult. The participants may have experienced a high call volume during their shift before testing, influencing the performance outcomes. Future research should consider recording non-emergency/emergency response call volumes better to assess shiftwork’s impact on health and performance outcomes. Second, our study only involved four weeks of supplementation. While this represented the typical amount and length of supplementation of taking a commercial supplement, a longer supplementation duration is likely needed to induce favorable health and performance outcomes. Third, we did not obtain a pre-supplementation baseline blood sample. Even though the study was conducted in a crossover manner, it is difficult to know how four weeks of astaxanthin supplementation affected fasting markers of cardiometabolic health. Fourth, air tank and piece differences may have been affected by air leaking around the mask and time to turn off the tanks and should therefore only be viewed as a descriptive estimate of air use. Fifth, while we monitored training compliance and participants completed a standardized training program, we did not record exercise intensities or amount of weight lifted to determine training volume. It is possible that astaxanthin may have allowed for more training to occur compared to the placebo. Lastly, the participants of the present study were considered trained. Future work should assess the long-term effects of astaxanthin on unhealthy firefighters during more consistent training on cardiometabolic health outcomes.
5. Conclusion
Four weeks of astaxanthin supplementation (12 mg/d) attenuated VANT and appeared to blunt the clinically significant increase in some markers of inflammatory and oxidative stress during training, in response to an incremental maximal exercise test, and during the fire ground test. However, most changes observed were from assessing the clinical significance of changes from baseline within groups rather than between groups. Astaxanthin supplementation had no statistically or clinically-significant effects on cardiometabolic health markers and performance measures. These findings provide some evidence that astaxanthin supplementation may help mediate occupation-related inflammation and oxidative stress response to high-intensity, short-duration exercise in firefighters. However, more research is needed to determine if long-term supplementation may have a greater impact in this population.
Supplementary Material
Acknowledgments
The authors would like to thank the individuals of the Bryan, Texas Fire Department including Jordan Gallagher and others who aided the study. The authors also thank Dr. Matt J. McAllister and Courtney C. Dillard at the Texas State University for assisting in blood and saliva analysis. Lastly, the authors would like to thank the Texas A&M University Tactical Athlete Research Unit undergraduate students Georgia Reed and Lucas Gallegos for helping with data collection.
Funding Statement
This study was funded as a doctoral student research grant by AstaReal® (Burlington, NJ, USA) to Dr. Kreider and the Exercise and Sport Nutrition Laboratory. The funding agency was not involved in the data collection, analysis, interpretation, or preparation of this paper. AstaReal® (Burlington, NJ, USA) [2201149].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contributions
Conceptualization: RBK & DEG; project management: DEG, RJS, CJR, and RBK; data collection: DEG, BLD, SEJ, KEW, ML, CY, JK, DX, VM, LE, and RJS; sample analysis: DEG, SEJ, DLB, and RJS. Data analysis: DEG, RJS, and RBK; writing – preparation of the manuscript: DEG and RBK; writing – review and editing the manuscript: all authors; funding acquisition: RBK. All authors have read and agreed to the published version of the manuscript.
Competing interests
The authors of this paper have not received any financial remuneration for preparing or reviewing this paper. DEG and RBK have participated as unpaid members of the AstaReal® Sports Nutrition Network, which includes several research groups that have researched astaxanthin to discuss their findings and plan additional research efforts. RBK has conducted industry-sponsored research, received financial support for presenting research on dietary supplements at scientific and industry-sponsored scientific conferences, and has served as an expert witness on cases related to supplementation. BLD, SEJ, KEW, ML, CY, JK, DX, VM, LE, RJS, CJR, and SEM have no competing interests.
Ethics approval and consent to participate
This study was reviewed and approved by Texas A&M University’s Institutional Review Board (IRB2020-1379F) in compliance with the Declaration of Helsinki.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15502783.2024.2427751
References
- 1.Superko HR, Momary KM, Pendyala LK, et al. Firefighters, heart disease, and aspects of insulin resistance: the FEMA Firefighter heart disease prevention study. J Occup Environ Med. 2011. Jul;53(7):758–27. doi: 10.1097/JOM.0b013e31821f64c3 [DOI] [PubMed] [Google Scholar]
- 2.Soteriades ES, Smith DL, Tsismenakis AJ, et al. Cardiovascular disease in US firefighters: a systematic review. Cardiol Rev. 2011;19(4):202–215. doi: 10.1097/CRD.0b013e318215c105 [DOI] [PubMed] [Google Scholar]
- 3.Burris JC, Werner CM, Woolf K.. The relationship between dietary intake and dietary-focused lifestyle interventions on risk factors associated with cardiovascular disease in firefighters. Curr Nutr Rep. 2022. Jun;11(2):206–224. doi: 10.1007/s13668-022-00406-3 [DOI] [PubMed] [Google Scholar]
- 4.Bode ED, Mathias KC, Stewart DF, et al. Cardiovascular disease risk factors by BMI and Age in United States firefighters. Obesity (Silver Spring). 2021. Jul;29(7):1186–1194. doi: 10.1002/oby.23175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzalez DE, McAllister MJ, Waldman HS, et al. International society of sports nutrition position stand: tactical athlete nutrition. J Int Soc Sports Nutr. 2022;19(1):267–315. doi: 10.1080/15502783.2022.2086017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang CJ, Webb HE, Zourdos MC, et al. Cardiovascular reactivity, stress, and physical activity. Front Physiol. 2013 Nov 7;4:314. doi: 10.3389/fphys.2013.00314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith DL, Horn GP, Goldstein E, et al. Firefighter fatalities and injuries: the role of heat stress and PPE. 2008. https://www.ideals.illinois.edu/items/9075 [Google Scholar]
- 8.McAllister MJ, Basham SA, Smith JW, et al. Effects of environmental heat and antioxidant ingestion on blood markers of oxidative stress in professional firefighters performing structural fire exercises. J Occup Environ Med. 2018. Nov;60(11):e595–e601. doi: 10.1097/JOM.0000000000001452 [DOI] [PubMed] [Google Scholar]
- 9.McAllister MJ, Gonzalez AE, Waldman HS.. Time restricted feeding reduces inflammation and cortisol response to a firegrounds test in professional firefighters. J Occup Environ Med. 2021 May 1;63(5):441–447. doi: 10.1097/JOM.0000000000002169 [DOI] [PubMed] [Google Scholar]
- 10.Walker A, Keene T, Argus C, et al. Immune and inflammatory responses of Australian firefighters after repeated exposures to the heat. Ergonomics. 2015;58(12):2032–2039. doi: 10.1080/00140139.2015.1051596 [DOI] [PubMed] [Google Scholar]
- 11.Wolkow A, Aisbett B, Jefferies S, et al. Effect of heat exposure and simulated physical firefighting work on acute inflammatory and cortisol responses. Ann Work Expo Health. 2017 Jun 1;61(5):600–603. doi: 10.1093/annweh/wxx029 [DOI] [PubMed] [Google Scholar]
- 12.Hunter AL, Shah AS, Langrish JP, et al. Fire simulation and cardiovascular health in firefighters. Circulation. 2017 Apr 4;135(14):1284–1295. doi: 10.1161/CIRCULATIONAHA.116.025711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orysiak J, Młynarczyk M, Piec R, et al. Lifestyle and environmental factors may induce airway and systemic inflammation in firefighters. Environ Sci Pollut Res Int. 2022. Oct;29(49):73741–73768. doi: 10.1007/s11356-022-22479-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambati RR, Phang SM, Ravi S, et al. Astaxanthin: sources, extraction, stability, biological activities and its commercial applications–a review. Mar Drugs. 2014 Jan 7;12(1):128–152. doi: 10.3390/md12010128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pereira CPM, Souza ACR, Vasconcelos AR, et al. Antioxidant and anti‑inflammatory mechanisms of action of astaxanthin in cardiovascular diseases (review). Int J Mol Med. 2021. Jan;47(1):37–48. doi: 10.3892/ijmm.2020.4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Guo C, Wu J. Astaxanthin in liver health and disease: a potential therapeutic agent. Drug Des Devel Ther. 2020;14:2275–2285. doi: 10.2147/DDDT.S230749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohandel Z, Farkhondeh T, Aschner M, et al. Anti-inflammatory action of astaxanthin and its use in the treatment of various diseases. Biomed Pharmacother. 2022. Jan;145:112179. doi: 10.1016/j.biopha.2021.112179 [DOI] [PubMed] [Google Scholar]
- 18.Chang MX, Xiong F. Astaxanthin and its effects in inflammatory responses and inflammation-associated diseases: recent advances and future directions. Molecules. 2020 Nov 16;25(22):5342. doi: 10.3390/molecules25225342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao Y, Yang L, Qiao X, et al. Dietary astaxanthin: an excellent carotenoid with multiple health benefits. Crit Rev Food Sci Nutr. 2021. Sep;28:1–27. [DOI] [PubMed] [Google Scholar]
- 20.Alugoju P, Krishna Swamy VKD, Anthikapalli NVA, et al. Health benefits of astaxanthin against age-related diseases of multiple organs: a comprehensive review. Crit Rev Food Sci Nutr. 2022. Jun;16:1–66. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida H, Yanai H, Ito K, et al. Administration of natural astaxanthin increases serum hdl-cholesterol and adiponectin in subjects with mild hyperlipidemia. Atherosclerosis. 2010. Apr;209(2):520–523. doi: 10.1016/j.atherosclerosis.2009.10.012 [DOI] [PubMed] [Google Scholar]
- 22.Shokri-Mashhadi N, Tahmasebi M, Mohammadi-Asl J, et al. The antioxidant and anti-inflammatory effects of astaxanthin supplementation on the expression of miR-146a and miR-126 in patients with type 2 diabetes mellitus: a randomised, double-blind, placebo-controlled clinical trial. Int J Clin Pract. 2021. May;75(5):e14022. doi: 10.1111/ijcp.14022 [DOI] [PubMed] [Google Scholar]
- 23.Choi HD, Youn YK, Shin WG. Positive effects of astaxanthin on lipid profiles and oxidative stress in overweight subjects. Plant Foods Hum Nutr. 2011. Nov;66(4):363–369. doi: 10.1007/s11130-011-0258-9 [DOI] [PubMed] [Google Scholar]
- 24.Brown DR, Warner AR, Deb SK, et al. The effect of astaxanthin supplementation on performance and fat oxidation during a 40 km cycling time trial. J Sci Med Sport. 2021;24(1):92–97. doi: 10.1016/j.jsams.2020.06.017 [DOI] [PubMed] [Google Scholar]
- 25.Baralic I, Djordjevic B, Dikic N, et al. Effect of astaxanthin supplementation on paraoxonase 1 activities and oxidative stress status in young soccer players. Phytother Res. 2013. Oct;27(10):1536–1542. doi: 10.1002/ptr.4898 [DOI] [PubMed] [Google Scholar]
- 26.Ferretti G, Bacchetti T, Moroni C, et al. Paraoxonase activity in high-density lipoproteins: a comparison between healthy and obese females. J Clin Endocrinol Metab. 2005. Mar;90(3):1728–1733. doi: 10.1210/jc.2004-0486 [DOI] [PubMed] [Google Scholar]
- 27.Baralic I, Andjelkovic M, Djordjevic B, et al. Effect of astaxanthin supplementation on salivary IgA, oxidative stress, and inflammation in young soccer players. Evidence-Based Complementary And Alternative Med. 2015; 2015.2015:1–9. doi: 10.1155/2015/783761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleischmann C, Horowitz M, Yanovich R, et al. Asthaxanthin improves aerobic exercise recovery without affecting heat tolerance in humans. Front Sports Act Living. 2019;1:17. doi: 10.3389/fspor.2019.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferguson B, Stern PJ. ACSM’s guidelines for exercise testing and prescription 9th Ed. J Can Chiropr Assoc. 2014;2014;58(4):377–383. [PMC free article] [PubMed] [Google Scholar]
- 30.Bayles MP. ACSM’s exercise testing and prescription. Philadelphia, PA USA: Lippincott Williams & Wilkins; 2023. [Google Scholar]
- 31.Klesges RC, Ward KD, Shelton ML, et al. Changes in bone mineral content in male athletes. Mechanisms of action and intervention effects. JAMA. 1996 Jul 17;276(3):226–230. doi: 10.1001/jama.1996.03540030060033 [DOI] [PubMed] [Google Scholar]
- 32.Lohman TG, Harris M, Teixeira PJ, et al. Assessing body composition and changes in body composition: another look at dual‐energy x‐ray absorptiometry. Ann N Y Acad Sci. 2000;904(1):45–54. doi: 10.1111/j.1749-6632.2000.tb06420.x [DOI] [PubMed] [Google Scholar]
- 33.Magrans-Courtney T, Wilborn C, Rasmussen C, et al. Effects of diet type and supplementation of glucosamine, chondroitin, and MSM on body composition, functional status, and markers of health in women with knee osteoarthritis initiating a resistance-based exercise and weight loss program. J Int Soc Sports Nutr. 2011 Jun 20;8(1):8. doi: 10.1186/1550-2783-8-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McAllister MJ, Gonzalez AE, Waldman HS. Impact of time restricted feeding on markers of cardiometabolic health and oxidative stress in resistance-trained firefighters. J Strength Cond Res. 2022 Sep 1;36(9):2515–2522. doi: 10.1519/JSC.0000000000003860 [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez AE, Waldman HS, Abel MG, et al. Impact of time restricted feeding on fitness variables in professional resistance trained firefighters. J Occup Environ Med. 2021 Apr 1;63(4):343–349. doi: 10.1097/JOM.0000000000002144 [DOI] [PubMed] [Google Scholar]
- 36.Fairshter RD, Salness K, Walters J, et al. Relationships between minute ventilation, oxygen uptake, and time during incremental exercise. Respiration. 1987;51(3):223–231. doi: 10.1159/000195205 [DOI] [PubMed] [Google Scholar]
- 37.Borg GA. Perceived exertion: a note on “history” and methods. Med Sci Sports. 1973. Summer;5(2):90–93. doi: 10.1249/00005768-197300520-00017 [DOI] [PubMed] [Google Scholar]
- 38.Brouwer E. On simple formulae for calculating the heat expenditure and the quantities of carbohydrate and fat oxidized in metabolism of men and animals, from gaseous exchange (oxygen intake and carbonic acid output) and urine-N. Acta Physiol Pharmacol Neerl. 1957;6:795–802. [PubMed] [Google Scholar]
- 39.Norris MS, McAllister M, Gonzalez AE, et al. Predictors of work efficiency in structural firefighters. J Occup Environ Med. 2021 Jul 1;63(7):622–628. doi: 10.1097/JOM.0000000000002197 [DOI] [PubMed] [Google Scholar]
- 40.Organization WH. WHO guidelines on drawing blood: best practices in phlebotomy. World Health Organ. 2010. [PubMed] [Google Scholar]
- 41.Litteljohn D, Hayley S. Cytokines as potential biomarkers for Parkinson’s disease: a multiplex approach. Psychoneuroimmunology: Methods And Protocol. 2012:121–144. [DOI] [PubMed] [Google Scholar]
- 42.Jacobson JW, Oliver KG, Weiss C, et al. Analysis of individual data from bead‐based assays (“bead arrays”). Cytometry Part A. 2006;69(5):384–390. doi: 10.1002/cyto.a.20293 [DOI] [PubMed] [Google Scholar]
- 43.Dunbar SA, Hoffmeyer MR. Microsphere-based multiplex immunoassays: development and applications using Luminex® xMAP® technology. The immunoassay handbook: theory and applications of ligand binding, ELISA and related techniques. 2013:157 doi: 10.1007/978-3-031-28012-2_26 [DOI] [Google Scholar]
- 44.McAllister MJ, Martaindale MH, Rentería LI. Active Shooter training drill increases blood and salivary markers of stress. Int J Environ Res Public Health. 2020 Jul 13;17(14):5042. doi: 10.3390/ijerph17145042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grubic TJ, Sowinski RJ, Nevares BE, et al. Comparison of ingesting a food bar containing whey protein and isomalto-oligosaccharides to carbohydrate on performance and recovery from an acute bout of resistance-exercise and sprint conditioning: an open label, randomized, counterbalanced, crossover pilot study. J Int Soc Sports Nutr. 2019 Aug 13;16(1):34. doi: 10.1186/s12970-019-0301-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levers K, Dalton R, Galvan E, et al. Effects of powdered Montmorency tart cherry supplementation on an acute bout of intense lower body strength exercise in resistance trained males. J Int Soc Sports Nutr. 2015;12(1):41. doi: 10.1186/s12970-015-0102-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levers K, Dalton R, Galvan E, et al. Effects of powdered Montmorency tart cherry supplementation on acute endurance exercise performance in aerobically trained individuals. J Int Soc Sports Nutr. 2016;13(1):22. doi: 10.1186/s12970-016-0133-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cooke M, Iosia M, Buford T, et al. Effects of acute and 14-day coenzyme Q10 supplementation on exercise performance in both trained and untrained individuals. J Int Soc Sports Nutr. 2008 Mar 4;5(1):8. doi: 10.1186/1550-2783-5-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kerksick CM, Kreider RB, Willoughby DS. Intramuscular adaptations to eccentric exercise and antioxidant supplementation. Amino Acids. 2010. Jun;39(1):219–232. doi: 10.1007/s00726-009-0432-7 [DOI] [PubMed] [Google Scholar]
- 50.Kim H, Simbo SY, Fang C, et al. Acai (Euterpe oleracea Mart.) beverage consumption improves biomarkers for inflammation but not glucose- or lipid-metabolism in individuals with metabolic syndrome in a randomized, double-blinded, placebo-controlled clinical trial. Food Funct. 2018 Jun 20;9(6):3097–3103. doi: 10.1039/C8FO00595H [DOI] [PubMed] [Google Scholar]
- 51.Koozehchian MS, Daneshfar A, Fallah E, et al. Effects of nine weeks L-Carnitine supplementation on exercise performance, anaerobic power, and exercise-induced oxidative stress in resistance-trained males. J Exerc Nutrition Biochem. 2018 Dec 31;22(4):7–19. doi: 10.20463/jenb.2018.0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baralic I, Andjelkovic M, Djordjevic B, et al. Effect of astaxanthin supplementation on salivary IgA, oxidative stress, and inflammation in young soccer players. Evid Based Complement Alternat Med. 2015; 20152015:1–9. doi: 10.1155/2015/783761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waldman HS, Bryant AR, Parten AL, et al. Astaxanthin supplementation does not affect markers of muscle damage or inflammation after an exercise-induced muscle damage protocol in resistance-trained males. J Strength Cond Res. 2023 Jan 18;37(7):e413–e421. doi: 10.1519/JSC.0000000000004408 [DOI] [PubMed] [Google Scholar]
- 54.Djordjevic B, Baralic I, Kotur-Stevuljevic J, et al. Effect of astaxanthin supplementation on muscle damage and oxidative stress markers in elite young soccer players. J Sports Med Phys Fitness. 2012. Aug;52(4):382–392. [PubMed] [Google Scholar]
- 55.Perneger TV. What’s wrong with Bonferroni adjustments. BMJ. 1998 Apr 18;316(7139):1236–1238. doi: 10.1136/bmj.316.7139.1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990. Jan;1(1):43–46. doi: 10.1097/00001648-199001000-00010 [DOI] [PubMed] [Google Scholar]
- 57.Cohen J. Statistical power analysis for the behavioral sciences. Lawrence Erlbaum. New York, NY, USA: Hillsdale (NJ); 1988. p. 75–108. [Google Scholar]
- 58.Page P. Beyond statistical significance: clinical interpretation of rehabilitation research literature. Int J Sports Phys Ther. 2014;9(5):726. [PMC free article] [PubMed] [Google Scholar]
- 59.Kang H. The prevention and handling of the missing data. Korean J Anesthesiol. 2013. May;64(5):402–406. doi: 10.4097/kjae.2013.64.5.402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quintero M, L A. Missing data imputation for ordinal data. Int J Comput Appl. 2018;181(5):10–16. doi: 10.5120/ijca2018917522 [DOI] [Google Scholar]
- 61.Earnest CP, Roberts BM, Harnish CR, et al. Reporting characteristics in sports nutrition. Sports. 2018;6(4):139. doi: 10.3390/sports6040139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hopkins WG, Marshall SW, Batterham AM, et al. Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc. 2009. Jan;41(1):3–13. doi: 10.1249/MSS.0b013e31818cb278 [DOI] [PubMed] [Google Scholar]
- 63.Drinkwater E. Applications of confidence limits and effect sizes in sport research. The Open Sports Sci J. 2008;1(1):3–4. doi: 10.2174/1875399X00801010003 [DOI] [Google Scholar]
- 64.Grabowski B. ”P < 0.05” might not mean what you think: American statistical association clarifies P values. J Natl Cancer Inst. 2016. Aug;108(8):djw194. doi: 10.1093/jnci/djw194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharma H. Statistical significance or clinical significance? A researcher’s dilemma for appropriate interpretation of research results. Saudi J Anaesth. 2021. Oct-Dec;15(4):431–434. doi: 10.4103/sja.sja_158_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sullivan GM, Feinn R. Using effect size-or why the P value is not enough. J Grad Med Educ. 2012. Sep;4(3):279–282. doi: 10.4300/JGME-D-12-00156.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.K-C C, S-C C, P-C C. Astaxanthin attenuated thrombotic risk factors in type 2 diabetic patients. J Funct Foods. 2019;53:22–27. doi: 10.1016/j.jff.2018.12.012 [DOI] [Google Scholar]
- 68.Park JS, Chyun JH, Kim YK, et al. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr Metab (Lond). 2010;7(1):1–10. doi: 10.1186/1743-7075-7-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Coombes JS, Sharman JE, Fassett RG. Astaxanthin has no effect on arterial stiffness, oxidative stress, or inflammation in renal transplant recipients: a randomized controlled trial (the XANTHIN trial). Am J Clin Nutr. 2016;103(1):283–289. doi: 10.3945/ajcn.115.115477 [DOI] [PubMed] [Google Scholar]
- 70.Xia W, Tang N, Kord-Varkaneh H, et al. The effects of astaxanthin supplementation on obesity, blood pressure, CRP, glycemic biomarkers, and lipid profile: a meta-analysis of randomized controlled trials. Pharmacol Res. 20202020/11/01/;161:105113. doi: 10.1016/j.phrs.2020.105113 [DOI] [PubMed] [Google Scholar]
- 71.Satoh A, Tsuji S, Okada Y, et al. Preliminary clinical evaluation of toxicity and efficacy of a New Astaxanthin-rich Haematococcus pluvialis extract. J Clin Biochem Nutr. 2009. May;44(3):280–284. doi: 10.3164/jcbn.08-238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gonzalez DE, Waldman H, McAllister MJ. The metabolic and physiological demands of a simulated fire ground test versus a live-fire training evolution in professional firefighters. Int J Exercise Sci. 2023;16(7):230–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Choi HD, Kim JH, Chang MJ, et al. Effects of astaxanthin on oxidative stress in overweight and obese adults. Phytotherapy Res. 2011;25(12):1813–1818. doi: 10.1002/ptr.3494 [DOI] [PubMed] [Google Scholar]
- 74.Kim JH, Chang MJ, Choi HD, et al. Protective effects of haematococcus astaxanthin on oxidative stress in healthy smokers. J Med Food. 2011. Nov;14(11):1469–1475. doi: 10.1089/jmf.2011.1626 [DOI] [PubMed] [Google Scholar]
- 75.Twarda-Clapa A, Olczak A, Białkowska AM, et al. Advanced glycation end-products (AGEs): formation, chemistry, classification, receptors, and diseases related to AGEs. Cells. 2022 Apr 12;11(8):1312. doi: 10.3390/cells11081312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rodrigues KL, Borges JP, Lopes GO, et al. Influence of physical exercise on advanced glycation end products levels in patients living with the human immunodeficiency virus. Front Physiol. 2018;9:1641. doi: 10.3389/fphys.2018.01641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jaiswal A, Madaan S, Acharya N, et al. Salivary uric acid: a noninvasive wonder for clinicians? Cureus. 2021. Nov;13(11):e19649. doi: 10.7759/cureus.19649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saito M, Yoshida K, Saito W, et al. Astaxanthin increases choroidal blood flow velocity. Graefes Arch Clin Exp Ophthalmol. 2012. Feb;250(2):239–245. doi: 10.1007/s00417-011-1843-1 [DOI] [PubMed] [Google Scholar]
- 79.Ciaraldi TP, Boeder SC, Mudaliar SR, et al. Astaxanthin, a natural antioxidant, lowers cholesterol and markers of cardiovascular risk in individuals with prediabetes and dyslipidaemia. Diabetes Obes Metab. 2023. Jul;25(7):1985–1994. doi: 10.1111/dom.15070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wika AA, Reason KW, Green JM, et al. Astaxanthin reduces heart rate and carbohydrate oxidation rates during exercise in overweight individuals. Int J Exerc Sci. 2023;16(2):252–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Res PT, Cermak NM, Stinkens R, et al. Astaxanthin supplementation does not augment fat use or improve endurance performance. Med Sci Sports Exerc. 2013;45(6):1158–1165. doi: 10.1249/MSS.0b013e31827fddc4 [DOI] [PubMed] [Google Scholar]
- 82.McAllister MJ, Mettler JA, Patek K, et al. Astaxanthin supplementation increases glutathione concentrations but does not impact fat oxidation during exercise in active young men. Int J Sport Nutr Exerc Metab. 2022 Jan 1;32(1):8–15. doi: 10.1123/ijsnem.2021-0138 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


