Abstract
Background
In aquaculture, feed additives are widely explored. Among them, Panax ginseng Meyer, a natural herbal remedy, has demonstrated its efficacy in many aquaculture species. However, research regarding Penaeus vannamei shrimp, one of the most significant species in aquaculture, remains limited.
Methods
This study investigates the benefits of P. ginseng for P. vannamei, specifically its effects on growth, innate immunity, and shrimp microbiome. Juvenile P. vannamei were fed commercial feed mixed with red ginseng extract at 5 concentrations (0.00 %, 0.05 %, 0.10 %, 0.50 %, and 1.00 %) for 6 weeks. Body weight was measured on days 21 and 42. On day 42, three shrimp per group were selected for further analysis.
Results
In the growth study, Group 0.10 % displayed significantly improved FBW, WG, SGR, and FCR compared to those in Group 0.00 % on day 42. The qPCR assay showed significantly higher IGF-BP gene expression in Groups 0.05 %, 0.10 %, and 1.00 % compared to Group 0.00 %. In the innate immunity analysis, SOD activity was significantly higher in Groups 0.05 % and 0.50 % compared to that in Group 0.00 %. In the bacterial community analysis, Group 0.10 % exhibited higher Flavobacteriaceae and lower Vibrionaceae at the family level compared to Group 0.00 %. At the genus level, Group 0.10 % showed increased unspecified Flavobacteriaceae and decreased Vibrio compared to Group 0.00 %.
Conclusion
Adding P. ginseng to the feed enhanced growth, immune response, and microbiome composition in P. vannamei. Further research on refining dosage levels and utilizing red ginseng residues could boost commercial productivity and economic benefits in aquaculture practices.
Keywords: Aquaculture, Feed additive, Microbiome analysis, Red ginseng, Shrimp
Graphical abstract
1. Introduction
Aquaculture is one of the most rapidly expanding sectors in global food production, exceeding fisheries in its contributions [1]. Seafood now provides 17 % of global animal proteins, and in some developing countries, it plays a pivotal role by providing over half of the animal proteins [1]. With the rising demand, a pressing need for enhanced growth and efficiency is increasing in aquaculture production. To enhance growth and induce other beneficial effects, feed additives are widely used in aquaculture [2].
Ginseng is a group of herbal remedies derived from various plant species within the Araliaceae family. Among these species, Panax ginseng Meyer is one of the most well-known and extensively studied species, with a rich history of traditional herbal use in Asia, particularly in Korea, for thousands of years. P. ginseng is renowned for its widespread application as a natural remedy to enhance cognitive function [3,4], boost exercise performance [5], and support immune system functions [6]. The pharmacological efficacies of ginseng are derived from a substance called ginsenosides. These compounds comprise several steroid glycosides and saponin triterpene glycosides, and it has been confirmed that Korean ginseng contains 38 ginsenosides [7]. In animal models, ginsenosides have been shown to enhance the phagocytic activity of macrophages [8,9], improve cognitive activity in rodents (rats and mice) [10,11], induce vasodilation [12,13], and increase resistance against exogenous stress factors [14].
The efficacy of P. ginseng as a beneficial feed additive has been demonstrated in various aquaculture species. For instance, utilizing ginseng in the diet of Nile tilapia (Oreochromis niloticus) has yielded significantly higher growth rates [15]. African catfish (Clarias gariepinus), when fed with ginseng additive feed, exhibited significantly increased weight gain and improved feed efficiency [16]. In the case of goldfish (Carassius auratus), where varying concentrations of ginseng extracts in the feed were studied, a correlation between specific growth rate (SGR) and the logarithm of the respective concentrations was found [17].
Pacific whiteleg shrimp, Penaeus vannamei is one of the most significant species in global aquaculture. Despite its significance, the research regarding the effects of P. ginseng on P. vannamei remains limited. Thus, the present study aims to understand the potential beneficial effects of P. ginseng on growth, innate immunity, and gut microbiome of P. vannamei. The efficacy of P. ginseng is evaluated through various methodologies. These methods include calculating growth metrics, such as SGR, feed conversion ratio (FCR), and protein efficiency ratio (PER), examining insulin-like growth factor binding protein (IGF-BP) expression, analyzing the innate immune response biomarkers, phenoloxidase (PO) and superoxide dismutase (SOD) activities, and microbiome profiling. The current study suggests the clinical application of a natural supplement, P. ginseng, and contribute to the aquaculture industry by enhancing shrimp immunity and growth performance, leading to the supply of sustainable and healthy seafood.
2. Materials and methods
2.1. Ethics statement
This study has been approved by the Institutional Animal Care and Use Committee (IACUC) of Kyungpook National University (IACUC approval number 2023-0220). All efforts were made to improve animal welfare and minimize suffering.
2.2. Ginseng extract and experimental shrimp feed
Red ginseng extract (moisture content 40 %) used as an additive in conventional shrimp feed was generously provided by the Korean Society of Ginseng. Red ginseng extract compromises 11 ginsenosides, including 6 major ginsenosides (Rb1, Rb2, Rc, Rd, Re, and Rg1). No bacteria or fungi were detected in the extract. The test report of the ginsenoside content is presented in Table S1. The red ginseng extract was diluted with distilled water into 5 different concentrations: 0.00 %, 0.05 %, 0.10 %, 0.50 %, and 1.00 %. Each ginseng extract concentration was then mixed in a one-to-one weight ratio with the conventional shrimp feed. The nutritional composition of the shrimp feed used in the current study is presented in Table S2. The extract-feed mixture was dried thoroughly and stored in a 4 °C refrigerator until further use.
2.3. Feeding trial
Juvenile P. vannamei (N = 225, initial mean body weight 0.32 g) was used for the laboratory growth experiment. The shrimp were divided into 5 groups, namely 0.00 % (referred to as the negative control group), 0.05 %, 0.10 %, 0.50 %, and 1.00 %. Each group corresponded to the 5 different red ginseng extract concentrations to be included in the shrimp’s dietary intake.
The experiment was conducted in triplicate (3 tanks per group), with each tank containing 15 shrimp. The tanks were set up with 22 L of 25 ppt seawater with aeration, and the temperature was maintained at 28 °C. A 50 % water change was done every 4 days. The shrimp in each tank were fed 3 times a day with a total daily feed weight equivalent to 5 % of their body weight (estimated based on the initial body weight and average growth rate of the shrimp). The daily feed weight was re-estimated at day 21, based on the re-measured shrimp body weight. The experimental shrimp were closely monitored daily, and any deceased shrimp were removed immediately. The experiment was conducted for 6 weeks (42 days).
For the growth study, shrimp body weights were measured twice during the experiment: day 21 and day 42. The measured body weight was utilized for calculating final body weight (FBW), weight gain (WG), specific growth rate (SGR), feed conversion ratio (FCR), and protein efficiency ratio (PER). The FBW refers to the mean body weight of the shrimp at the point of weight measurement, and the calculations for WG, SGR, FCR, and PER are as follows:
Weight gain (%) = [(final mean body weight-initial mean body weight)/initial mean body weight] × 100.
Specific growth rate (%) = 100 × [(ln(final body weight)-ln(initial body weight))]/days [18].
Feed conversion ratio = dry feed fed/wet weight gain(g) [19].
Protein efficiency ratio = wet weight gain(g)/total protein fed [20].
After a 6-week feeding trial, 3 representative shrimp from each tank (nine shrimp per experimental group, N = 45) were randomly selected and humanely sacrificed. After the sacrifice, the hepatopancreas, hemolymph, and intestine were harvested from the shrimp for further analysis.
2.4. Quantitative analysis of growth gene expression
The hepatopancreas tissues sampled from the representative shrimp were utilized for assessing growth gene expression levels through a quantitative polymerase chain reaction (qPCR) assay. In particular, IGF-BP gene was selected for the analysis. Initially, RNA was extracted from 50 mg of the hepatopancreas collected from each shrimp using the RNeasy Micro kit (cat. no. 74004; Qiagen, Valencia, CA, USA). Complementary DNA (cDNA) was synthesized from the extracted RNA using AccuPower® RocketScript™ Cycle RT PreMix (cat. no. K-2201; Bioneer). A qPCR assay was then performed on the synthesized cDNA, with the β-actin gene as the reference housekeeping gene, using the StepOnePlus real-time PCR system (ThermoFisher Scientific, Waltham, MA, USA). The primers used for the qPCR in this study are presented in Table 1.
Table 1.
The primers used in the growth gene analysis and microbiome profiling.
| Name | Primer sequence (5′–3′) | Description | Reference |
|---|---|---|---|
| β-actin F | GAGCAACACGGAGTTCGTTGT | Housekeeping gene | [21] |
| β-actin R | CATCACCAACTGGGACGACATGGA | ||
| IGF-BP F | GTGGGCAGGGACCAAATC | IGF-BP expression | [22] |
| IGF-BP R | TCAGTTACCACCAGCGATT | ||
| 16s rRNA_515F | GTGCCAGCMGCCGCGGTAA | 16S rRNA V4 region | [23] |
| 16s rRNA_806R | GGACTACHVGGGTWTCTAAT |
2.5. Biochemical analysis of innate immunity
The innate immunity activity was analyzed by measurement of PO activity based on the method described by Hernandez et al. [24]. Briefly, hemolymph was drawn from the shrimp with a 27-gauge 1 mL syringe containing the anticoagulant Alsever’s solution. The collected hemolymph mixed with Alsever’s solution was then centrifuged at 800×g for 20 min. Afterward, the centrifuged cell pellet was washed with anticoagulant and resuspended in PBS. The 50 μL of resuspension was then incubated with 50 μl trypsin (0.1 mg/mL in cacodylate [CAC] buffer pH 7.0) for 10 min at 25 °C. After the incubation, 50 μL L-3,4-dihydroxyphenylalanine (l-DOPA) (3 mg/ml in CAC buffer pH 7.0) was added, and the mixture was incubated for 10 min at 25 °C. Finally, the absorbance was measured at 425 nm to access the PO activity spectrophotometrically.
The antioxidant activity was analyzed by measuring SOD activity in the hemolymph. The SOD activity was measured with the EZ-SOD assay kit (cat. no. DG-SOD400; DoGenBio, Seoul, South Korea), following the manufacturer’s protocol.
2.6. Microbiome profiling
The analysis of the intestinal microbiome structure in shrimp was also conducted. DNA was extracted from intestines pooled from 3 representative shrimp from each tank, using the DNeasy Blood & Tissue kit (cat. no. 69504; Qiagen, Valencia, CA, USA), and a PCR assay was performed targeting the V4 region of the 16S rRNA gene. The primers used for the PCR assay are presented in Table 1. For targeted amplicon sequencing, amplicons and paired reads of fragments were generated using the Illumina MiSeq system (Illumina, San Diego, CA, USA). Overall data processing and analysis were done using the software platform Quantitative Insights Into Microbial Ecology 2 (QIIME 2) [25]. Amplicon sequence variants (ASVs) were then obtained from the amplicon data. Alpha diversity, a measure of the richness and evenness of the microbiome within a sample, was calculated using the Shannon index and Chao1. Beta diversity, a measure of the similarity or dissimilarity in the microbiome composition between samples, was calculated using the Bray-Curtis distance. Two ordination methods were employed to visualize the beta diversity: metric multidimensional scaling (MDS), also referred to as Principal Coordinates Analysis (PCoA), and non-metric multidimensional scaling (NMDS). In addition, taxonomic analysis was conducted by comparing ASVs to a reference database.
2.7. Statistical analysis
The data were expressed as mean ± standard deviation (SD) and analyzed using repeated measures of variance. The Tukey test was used to test for differences among means for which the analysis of variance (ANOVA) indicated a significant (P < 0.05) F-ratio.
3. Results
3.1. Feeding trial and growth metrics calculation
During the 6-week (42 days) feeding trial, all shrimp appeared to be in good health, exhibiting normal general behaviors, such as feeding and swimming. The daily mortality rate showed no significant difference among all 5 experimental groups throughout the 6-week test period. Shrimp body weight was measured on week 3 (day 21) and week 6 (day 42). In week 3, Groups 0.10 % and 1.00 % exhibited significantly (P < 0.05) improved FBW, WG, SGR, FCR, and PER of the shrimp, compared to those of Group 0.00 %. In week 6, Group 0.10 % showed significantly (P < 0.05) improved FBW, WG, SGR, and FCR of the shrimp, compared to those of Group 0.00 %, whereas the PER showed no significant variance between experimental groups. The FBW, WG, SGR, FCR, PER values, and the survival rate of P. vannamei after 3 and 6 weeks of the experiment are presented in Tables 2A and 2B, respectively.
Table 2.
The FBW, WG, SGR, FCR, PER values, and the survival rate of P. vannamei after (A) 3 weeks and (B) 6 weeks of the experiment. 2A.2B.
| Group | FBW | WG | SGR | FCR | PER | Survival (%) |
|---|---|---|---|---|---|---|
| 0.00 % | 0.77 ± 0.05bc | 141 ± 14.5bc | 4.40 ± 0.30bc | 1.22 ± 0.12ab | 2.07 ± 0.22bc | 95.6 ± 3.85 |
| 0.05 % | 0.85 ± 0.00ab | 165 ± 1.38ab | 4.88 ± 0.03ab | 1.03 ± 0.01bc | 2.42 ± 0.03ab | 91.1 ± 10.2 |
| 0.10 % | 0.90 ± 0.04a | 180 ± 11.5a | 5.15 ± 0.21a | 0.95 ± 0.07c | 2.64 ± 0.19a | 91.1 ± 3.85 |
| 0.50 % | 0.75 ± 0.05c | 135 ± 15.5c | 4.26 ± 0.33c | 1.28 ± 0.15a | 1.95 ± 0.24c | 95.6 ± 7.70 |
| 1.00 % | 0.87 ± 0.06a | 171 ± 19.2a | 4.98 ± 0.36a | 1.00 ± 0.13c | 2.52 ± 0.30a | 91.1 ± 3.85 |
| Group | FBW | WG | SGR | FCR | PER | Survival (%) |
|---|---|---|---|---|---|---|
| 0.00 % | 1.75 ± 0.19b | 448 ± 58.6b | 4.14 ± 0.26b | 1.11 ± 0.13ab | 2.27 ± 0.28 | 82.2 ± 10.2 |
| 0.05 % | 1.83 ± 0.04b | 471 ± 12.6ab | 4.25 ± 0.05ab | 1.10 ± 0.08ab | 2.28 ± 0.17 | 73.3 ± 6.67 |
| 0.10 % | 2.02 ± 0.10a | 529 ± 32.7a | 4.48 ± 0.13a | 1.06 ± 0.12c | 2.37 ± 0.26 | 75.6 ± 3.85 |
| 0.50 % | 1.64 ± 0.02b | 413 ± 5.67b | 3.99 ± 0.03b | 1.27 ± 0.13a | 1.96 ± 0.21 | 86.4 ± 10.2 |
| 1.00 % | 1.76 ± 0.06b | 449 ± 22.1b | 4.15 ± 0.10b | 1.17 ± 0.05ab | 2.13 ± 0.10 | 82.2 ± 7.70 |
1) Values are the mean of triplicate groups and are presented as mean ± SD.
2) Values with different superscripts in the same column are significantly different (P < 0.05).
3) The lack of superscript letters indicates no significant differences among treatments.
3.2. Quantitative analysis of growth gene expression
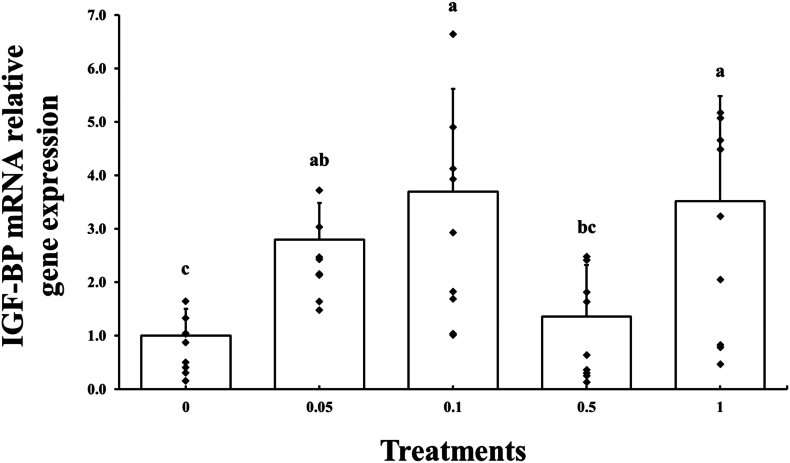
The hepatopancreas was collected from 9 shrimp per experimental group, totaling 45 shrimp from 5 different experimental groups. RNA extraction from the hepatopancreas was followed by cDNA synthesis and then subjected to qPCR analysis targeting the IGF-BP gene. In week 6, the IGF-BP expression was significantly (P < 0.05) higher in Groups 0.05 %, 0.10 %, and 1.00 %, compared to that of Group 0.00 %. The relative IGF-PB gene expression is presented in Fig. 1.
Fig. 1.
The relative gene expression of insulin-like growth factor binding protein (IGF-BP) mRNA after 6 weeks of experimental feeding. Bars with different letters are significantly different (P < 0.05).
3.3. Biochemical analysis of innate immunity
Extracted hemolymphs were subjected to innate immunity measurement, particularly PO and SOD activity. In week 6, the PO activity showed no significant variance between experimental groups, whereas Groups 0.05 % and 0.50 % showed significantly (P < 0.05) improved SOD activity compared to that of Group 0.00 %. The PO and SOD activities of P. vannamei after 6 weeks of the experiment are presented in Table 3.
Table 3.
The PO and SOD activity of P. vannamei measured spectrophotometrically after 6 weeks of the experiment.
| Group | PO | SOD |
|---|---|---|
| 0.00 % | 0.122 ± 0.01 | 80.7 ± 8.08b |
| 0.05 % | 0.134 ± 0.01 | 92.6 ± 4.71a |
| 0.10 % | 0.130 ± 0.01 | 87.5 ± 3.21ab |
| 0.50 % | 0.131 ± 0.00 | 92.4 ± 3.16a |
| 1.00 % | 0.124 ± 0.01 | 87.0 ± 5.98ab |
1) Values are the mean of triplicates and are presented as mean ± SD.
2) Values with different superscripts in the same column are significantly different (P < 0.05).
3) The lack of superscript letters indicates no significant differences among treatments.
3.4. Microbiome analysis
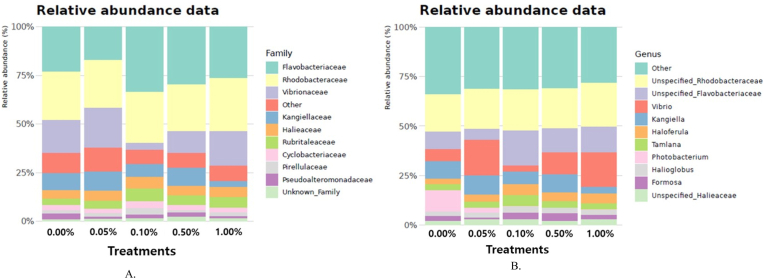
At the family level in the taxonomic analysis of the bacterial community structure, Group 0.10 % displayed a significantly higher Flavobacteriaceae abundance and a lower Vibrionaceae abundance, compared to that of Group 0.00 %. At the genus level, Group 0.10 % exhibited a significantly higher abundance of unspecified Flavobacteriaceae and a lower Vibrio abundance, compared to that of Group 0.00 %. The other bacteria showed no significant difference between the experimental groups at both family and genus levels. The bacterial community structure of the family and genus levels is presented in Fig. 2A and B. The alpha analysis indicated slight differences in both richness and evenness at the genus level of the microbiome between treatment groups, though these distinctions were not statistically significant (data not shown). The beta analysis also showed that the diversity of the bacterial genera between treatment groups was not statistically significant (data not shown).
Fig. 2.
The bacterial community structure of the (A) family and (B) genus level.
4. Discussion
In aquaculture, growth study holds particular interest, as growth affects overall yield. Growth is estimated using various growth metric calculations. Among them, SGR, which indicates an additive change in weight per time [18], is well-established and frequently employed in numerous publications [[26], [27], [28]]. Other metrics, such as FCR, which measures the efficiency of animals converting feed into food products [19], and PER, which calculates the efficiency of utilizing dietary protein for body weight gain [20], are also widely employed in fisheries literature. The IGF-BP gene expression is also frequently employed in growth studies [29,30]. IGF-BP regulates insulin-like growth factor (IGF), a key modulator that influences diverse physiological processes [31,32]. In crustaceans, numerous studies have illustrated the multiple functions of IGF-BP and its homologs, encompassing androgenic hormone modulation [33,34], ovarian development [35,36], immune response [37,38], and growth regulations [39]. Thus, the present study primarily aims to demonstrate the efficacy of P. ginseng as a feed additive on P. vannamei, utilizing the aforementioned measurements.
In aquaculture, feed additives are actively explored for enhancing growth. These additives comprise various features, including probiotics, prebiotics, synbiotics, organic acids, and medicinal herbs [40]. Among the natural herbal remedies, P. ginseng has demonstrated its efficacy in many other aquaculture species, such as Nile tilapia (Oreochromis niloticus) [15], African catfish (Clarias gariepinus) [16], and goldfish (Carassius auratus) [17]. In the present study with shrimp, adding ginseng to the feed significantly improved the overall growth metrics of both Groups 0.10 % and Group 1.00 % in week 3, including a higher PER, and Group 0.10 % in week 6, while having no significant effect on the PER. The results suggest that an optimal concentration of the ginseng additive in feed potentially enhances the overall growth of the shrimp. Also, in the early stages of feeding, ginseng may improve the PER, and although this effect may diminish over time, it does not compromise the protein quality in the feed. In addition, the IGF-BP analysis result aligns with the aforementioned growth metrics calculations, as the experimental groups showing significantly higher SGR (Groups 0.10 % and 1.00 %) also showed significantly higher IGF-BP gene expression compared to that of Group 0.00 %. Group 0.50 % showed no statistical significance compared to the negative control groups.
Antioxidant enzymes are frequently employed as biomarkers of innate immune responses [41]. SOD plays a role in this process by converting reactive oxygen species (ROS) into hydrogen peroxide [41], facilitating its passage through membranes. However, while effective against exogenous antigens with high microbicidal properties, ROS also poses potential side effects, causing oxidative damage to endogenous biomolecules. SOD regulates the innate immune response and the enzymatic antioxidant defense system, which shields biomolecules from oxidative damage induced by free radicals. In P. vannamei, prior investigations have demonstrated that exogenous stimulation leads to elevated SOD levels [42], an indicative sign of innate immunity activation. In the present study, after administrating red ginseng extract to shrimp, Groups 0.05 % and 0.50 % exhibited significant upregulation of the SOD gene compared to that of Group 0.00 %, suggesting a boost in the immune system. The PO activity has also been used as a parameter in invertebrate immune studies [43]. Activated PO is released from hemocytes and regulates melanin synthesis, thereby promoting pathogen melanization [44]. Interestingly, there was no significant variance in PO activity among the experimental groups and the negative control group. It is plausible to suggest that the shrimp’s immune system did not perceive the ginseng additive as a foreign pathogen. Moreover, this also negates the possibility that the higher activity of SOD was a result of ginseng-induced exogenous stimulation.
The term “microbiome” refers to the sum of the microbes and their genomic elements in a particular environment [44], and a close relationship between microorganisms and their host has been revealed in numerous studies [[45], [46], [47]]. In the present study, after administrating red ginseng extract to shrimp, Group 0.10 % exhibited a significantly reduced Vibrio abundance at both the family and genus level in the taxonomic analysis, showing gut microbiota alteration. The longstanding objective in shrimp aquaculture has been to minimize Vibrio presence, given its association with severe acute disease. Acute hepatopancreatic necrosis disease, a variant of vibriosis caused by toxin gene-carrying Vibrio, is notorious for its rapid onset and high mortality rate [48]. Hence, the decrease in the Vibrio bacterial load in the microbiome is a positive development. However, it remains unclear whether the distinct relative abundance of Vibrio is a direct outcome of the efficacy of the ginseng additive, a result of an upregulated immune system, or other unclarified factors. In addition, Flavobacteriaceae is prevalent throughout the growth stage of shrimp, forming the intestinal core microbiome of P. vannamei, and is considered to be minimally pathogenic to shrimp [49]. Although there is uncertainty in the microbiome alteration, this seems to pose no apparent concerns.
One issue surrounding the use of red ginseng extract is its cost, particularly its application in conventional shrimp aquaculture, which raises concerns about pricing. A potential solution is utilizing red ginseng residues, a byproduct produced during the extraction process. The residue contains valuable components, including carbohydrates, indispensable amino acids, dietary fibers, micronutrients, and a significant amount of ginsenoside [50,51]. Over 1000 tons of red ginseng residues are produced annually in Korea; however, these remnants are currently treated as waste and are burned or deposited in landfills [52]. Harnessing these residues not only has positive implications for recycling environmental resources but also holds the potential for creating high-value products. Moreover, the carbohydrates in the red ginseng residue may have another positive effect. Biofloc technology is a technique for enhancing water quality by producing high levels of heterotrophic bacteria. It is readily induced by adding carbohydrates to feed [53]. Carbohydrates are an energy source for microbial organisms, which immobilize nitrogenous waste products. The red ginseng residue contains approximately 70 % carbohydrates and is expected to induce biofloc [53]. Other aforementioned positive effects of ginsenosides are also expected.
Additionally, as ginseng is a natural environmental remedy, it is unlikely to possess toxic effects, as demonstrated in the present study. A comparison between Group 0.00 % and the experimental groups showed no significant difference in the survival rates, implying that ginseng does not pose notable toxicity to shrimp. This finding aligns with prior research, which demonstrated that ginseng supplements have numerous biological activities in P. vannamei, including an upregulated immune system, without apparent side effects [54].
The present study demonstrated the efficacy of P. ginseng in enhancing various aspects of P. vannamei, including growth, innate immunity, and gut microbiome. Specifically, after 6 weeks of experimental feeding ginseng to shrimp, Group 0.10 % exhibited a significant growth enhancement compared to the negative control group. In addition, Group 0.50 % showed improved SOD activation, while Group 0.10 % showed beneficial alteration in the gut microbiome, compared to that of the negative control group. These results did not exhibit a logarithmic relation with the concentration of red ginseng extract. Prior studies examining the effects of ginseng at varying dosages on aquaculture species have similarly indicated that the efficacy of ginseng does not consistently correspond with dosage levels [16,55]. In a study with olive flounder (Paralichthys olivaceus), after 8 weeks of ginseng supplement, growth parameters, including SGR and feeding efficiency ratio, significantly decreased in higher ginseng concentrations [55]. Additionally, the lysozyme activity was highest in the lowest ginseng concentration [55]. These prior studies indicate that finding optimal concentration is vital. Therefore, further investigation is required to refine the red ginseng extract dilution level and pinpoint the optimal concentration that addresses all conditions. Feed additives are readily accessible in aquaculture and have demonstrated their efficacy via oral administration. Successful commercialization promises the potential to enhance productivity in aquaculture while also utilizing the valuable traditional Korean resource, P. ginseng.
Data statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
CRediT authorship contribution statement
Bumkeun Kim: Conception and design of study, Acquisition of data, Formal analysis, Interpretation of data, Writing – original draft. Hye Jin Jeon: Acquisition of data, Formal analysis, Interpretation of data. Man Hee Rhee: Conception and design of study, Formal analysis, Interpretation of data. Ji Hyung Kim: Writing – original draft, Revising the manuscript critically for important intellectual content. Jee Eun Han: Formal analysis, Interpretation of data, Revising the manuscript critically for important intellectual content.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was supported by the 2022 grant from the Korean Society of Ginseng, and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF2019R1C1C1006212 and NRF-2022R1I1A3066435). Graphic abstract designed by BZZRINCANTATION, andinur, and Freepik from Flaticon.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2024.06.002.
Contributor Information
Ji Hyung Kim, Email: kzh81@gachon.ac.kr.
Jee Eun Han, Email: jehan@knu.ac.kr.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.FAO . FAO; 2022. The state of world fisheries and aquaculture 2022. [Google Scholar]
- 2.Dawood M.A.O., Koshio S., Esteban M.Á. Beneficial roles of feed additives as immunostimulants in aquaculture: a review. Rev Aquacult. 2017 Aug 18;10(4):950–974. [Google Scholar]
- 3.Lee S.T., Chu K., Sim J.Y., Heo J.H., Kim M. Panax ginseng enhances cognitive performance in Alzheimer disease. Alzheimer Dis Assoc Disord. 2008 Jul;22(3):222–226. doi: 10.1097/WAD.0b013e31816c92e6. [DOI] [PubMed] [Google Scholar]
- 4.Reay J.L., Kennedy D.O., Scholey A.B. Single doses of Panax ginseng (G115) reduce Blood glucose levels and improve cognitive performance during sustained mental activity. J Psychopharmacol. 2005;19(4):357–365. doi: 10.1177/0269881105053286. https://pubmed.ncbi.nlm.nih.gov/15982990/ (Oxford, England) [Internet] [DOI] [PubMed] [Google Scholar]
- 5.Ma G.D., Chiu C.H., Hsu Y.J., Hou C.W., Chen Y.M., Huang C.C. Changbai Mountain ginseng (Panax ginseng C.A. Mey) extract supplementation improves exercise performance and energy utilization and decreases fatigue-associated parameters in mice. Molecules. 2017 Feb 5;22(2):237. doi: 10.3390/molecules22020237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang S.W., Min H.Y. Ginseng, the “immunity boost”: the effects of Panax ginseng on immune system. J Ginseng Res [Internet] 2012;36(4):354–368. doi: 10.5142/jgr.2012.36.4.354. http://koreascience.or.kr/article/JAKO201229664766672.page Oct 15 [cited 2019 May 26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Botanical Characteristics C.H.O.I.K. Pharmacological effects and medicinal components of Korean Panax ginseng C A meyer. Acta Pharmacol Sin. 2008 Sep;29(9):1109–1118. doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 8.Shin J.Y., Song J.Y., Yun Y.S., Yang H.O., Rhee D.K., Pyo S. Immunostimulating effects of acidic polysaccharides extract of Panax ginseng on macrophage function. Immunopharmacol Immunotoxicol. 2002 Jan;24(3):469–482. doi: 10.1081/iph-120014730. [DOI] [PubMed] [Google Scholar]
- 9.Scaglione F., Ferrara F., Dugnani S., Falchi M., Santoro G., Fraschini F. Immunomodulatory effects of two extracts of Panax ginseng C.A. Meyer. PubMed. 1990 Jan 1;16(10):537–542. [PubMed] [Google Scholar]
- 10.Park C.H., Park S.K., Seung T.W., Jin D.E., Guo T., Heo H.J. Effect of ginseng (Panax ginseng) berry EtOAc fraction on cognitive impairment in C57BL/6 mice under high-fat diet inducement. Evid base Compl Alternative Med. 2015;2015:1–10. doi: 10.1155/2015/316527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nitta H., Matsumoto K., Shimizu M., Ni X., Watanabe H. Panax ginseng extract improves the scopolamine-induced disruption of 8-arm radial maze performance in rats. Biol Pharm Bull. 1995;18(10):1439–1442. doi: 10.1248/bpb.18.1439. [DOI] [PubMed] [Google Scholar]
- 12.Byeong Hwa Jeon, Kim Cuk Seong, Kim H.S., Park J.B., Nam Ki Yeul, Seok Jong Chang. Effect of Korean red ginseng on Blood pressure and nitric oxide production. PubMed. 2000 Dec 1;21(12):1095–1100. [PubMed] [Google Scholar]
- 13.Kim N.D., Kang S.Y., Schini V.B. Ginsenosides evoke endothelium-dependent vascular relaxation in rat aorta. Gen Pharmacol Vasc Syst. 1994 Oct;25(6):1071–1077. doi: 10.1016/0306-3623(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 14.Rai D., Bhatia G., Sen T., Palit G. Anti-stress effects of ginkgo biloba and Panax ginseng: a comparative study. J Pharmacol Sci. 2003;93(4):458–464. doi: 10.1254/jphs.93.458. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed M.M., Mohammed A.T., Farag M.R., Hassan M.A., Mawed S.A., Alagawany Mahmoud, et al. Dietary supplementation of nile Tilapia (Oreochromis niloticus) with Panax ginseng essential oil: positive impact on animal health and productive performance, and mitigating effects on atrazine- induced toxicity. Front Mar Sci. 2022 Jun 30;9 [Google Scholar]
- 16.Mehrim A.I., Refaey M.M., Hassan Zaki MA., Zenhom O.A. Ginseng® as a reproductive enhancer agent for African catfish, Clarias gariepinus (Burchell, 1822) Fish Physiol Biochem. 2021 Nov 27;48(1):15–32. doi: 10.1007/s10695-021-00969-y. [DOI] [PubMed] [Google Scholar]
- 17.Değirmencioğlu T. Possibilities of using ginseng in diets of goldfish. Vet Zootech. 2022;80(2) [Google Scholar]
- 18.Houde ED. Growth rates, rations and cohort consumption of marine fish larvae in relation to prey concentrations. Rapp et procès-verbaux des réunions, 1981;178, 441-453.
- 19.Fry J.P., Mailloux N.A., Love D.C., Milli M.C., Cao L. Feed conversion efficiency in aquaculture: do we measure it correctly? Environ Res Lett. 2018 Feb 1;13(2) [Google Scholar]
- 20.Chapman D.G., Castillo R., Campbell J.A. Evaluation of protein in food: 1. A method for determination of protein efficiency ratios. Can J Biochem Physiol. 1959 Jan 1;37(1):679–686. [PubMed] [Google Scholar]
- 21.Rahimnejad S., Yuan X., Wang L., Lu K., Song K., Zhang C. Chitooligosaccharide supplementation in low-fish meal diets for pacific white shrimp (Litopenaeus vannamei): effects on growth, innate immunity, gut histology, and immune-related genes expression. Fish Shellfish Immunol. 2018 Sep;80:405–415. doi: 10.1016/j.fsi.2018.06.025. [DOI] [PubMed] [Google Scholar]
- 22.Wang X., Li E., Xu Z., Li T., Xu C., Chen L. Molecular response of carbohydrate metabolism to dietary carbohydrate and acute low salinity stress in pacific white leg shrimp Litopenaeus vannamei. Turk J Fish Aquat Sci. 2017;17(1) [Google Scholar]
- 23.dos Santos H.R.M., Argolo C.S., Argôlo-Filho R.C., Loguercio L.L. A 16S rDNA PCR-based theoretical to actual delta approach on culturable mock communities revealed severe losses of diversity information. BMC Microbiol. 2019 Apr 8;19(1) doi: 10.1186/s12866-019-1446-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernández-López J., Gollas-Galván T., Vargas-Albores F. Activation of the prophenoloxidase system of the Brown shrimp Penaeus californiensis holmes. Comparative biochemistry and physiology Part C: pharmacology. Toxicol Endocrinol. 1996;113(1):61–66. [Google Scholar]
- 25.Bolyen E., Rideout J.R., Dillon M.R., Bokulich N.A., Abnet C.C., Al-Ghalith G.A., Caporaso J.G. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37(8):852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shoup D.E., Michaletz P.H. American Fisheries Society; Bethesda, Maryland: 2017. Growth estimation: summarization. Age and growth of fishes: principles and techniques; pp. 233–264. [Google Scholar]
- 27.Lugert V., Thaller G., Tetens J., Schulz C., Krieter J. A review on fish growth calculation: multiple functions in fish production and their specific application. Rev Aquacult. 2014 Aug 25;8(1):30–42. [Google Scholar]
- 28.Cook J.T., McNiven M.A., Richardson G.F., Sutterlin A.M. Growth rate, body composition and feed digestibility/conversion of hrowth-enhanced transgenic atlantic salmon (Salmo salar) Aquaculture. 2000 Aug;188(1–2):15–32. [Google Scholar]
- 29.Kawaguchi K., Kaneko N., Fukuda M., Nakano Y., Kimura S., Hara A., et al. Responses of insulin-like growth factor (IGF)-I and two IGF-binding protein-1 subtypes to fasting and Re-feeding, and their relationships with individual growth rates in yearling masu salmon (Oncorhynchus masou) Comp Biochem Physiol Mol Integr Physiol. 2013 Jun 1;165(2):191–198. doi: 10.1016/j.cbpa.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 30.Peterson B.C., Small B.C., Bosworth B.G. Effects of bovine growth hormone (Posilac®) on growth performance, body composition, and IGFBPs in two strains of channel catfish. Aquaculture. 2004 Apr;232(1–4):651–663. [Google Scholar]
- 31.Li M., Bureau D.P., King W.A., Leatherland J.F. The actions of in ovo cortisol on egg fertility, embryo development and the expression of growth-related genes in rainbow trout embryos, and the growth performance of juveniles. Mol Reprod Dev. 2010 Sep 20;77(10):922–931. doi: 10.1002/mrd.21239. [DOI] [PubMed] [Google Scholar]
- 32.Mommsen T.P., Vijayan M.M., Moon T.W. Cortisol in teleosts: dynamics, mechanisms of action, and metabolic regulation. Rev Fish Biol Fish. 1999;9:211–268. [Google Scholar]
- 33.Li F., Bai H., Xiong Y., Fu H., Jiang S., Jiang F., et al. Molecular characterization of insulin-like androgenic hland hormone-binding protein gene from the oriental river prawn Macrobrachium nipponense and investigation of its transcriptional relationship with the insulin-like androgenic gland hormone gene. Gen Comp Endocrinol. 2015 May 1;216:152–160. doi: 10.1016/j.ygcen.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Rosen O., Weil S., Manor R., Roth Z., Khalaila I., Sagi A. A crayfish insulin-like-binding protein. J Biol Chem. 2013 Aug;288(31):22289–22298. doi: 10.1074/jbc.M113.484279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang X., Ye H., Sook Chung J. The presence of an insulin-like androgenic gland factor (IAG) and insulin-like peptide binding protein (ILPBP) in the ovary of the blue crab, Callinectes sapidus and their roles in ovarian development. Gen Comp Endocrinol. 2017 Aug 1;249:64–70. doi: 10.1016/j.ygcen.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Huang X., Ye H., Feng B., Huang H. Insights into insulin-like peptide system in invertebrates from studies on IGF binding domain-containing proteins in the female mud crab. Scylla Paramamosain. 2015 Nov 1;416:36–45. doi: 10.1016/j.mce.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 37.Gai Y., Wang L.L., Song L., Zhao J., Qiu L., Xing K. A putative endocrine factor SIBD (single insulin binding domain protein) involved in immune response of Chinese mitten crab eriocheir sinensis. 2010 Jan 1;28(1):10–17. doi: 10.1016/j.fsi.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 38.Castellanos M., Jiménez-Vega F., Vargas-Albores F. Single IB domain (SIBD) protein from Litopenaeus vannamei, a novel member for the IGFBP family. Comp Biochem Physiol Genom Proteonomics. 2008 Dec 1;3(4):270–274. doi: 10.1016/j.cbd.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Gutiérrez A., Nieto J., Pozo F., Stern S., Schoofs L. Effect of insulin/IGF-I like peptides on glucose metabolism in the white shrimp Penaeus vannamei. Gen Comp Endocrinol. 2007 Aug 1;153(1–3):170–175. doi: 10.1016/j.ygcen.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 40.Dawood M.A.O., Koshio S., Esteban M.Á. Beneficial roles of feed additives as immunostimulants in aquaculture: a review. Rev Aquacult. 2017 Aug 18;10(4):950–974. [Google Scholar]
- 41.Knight J.A. Free radicals, antioxidants, and the immune system. Ann Clin Lab Sci. 2000;30(2):145–158. [PubMed] [Google Scholar]
- 42.Campa-Córdova A.I., Hernández-Saavedra N.Y., Ascencio F. Superoxide dismutase as modulator of immune function in American white shrimp (Litopenaeus vannamei) Comp Biochem Physiol C Toxicol Pharmacol. 2002 Dec 1;133(4):557–565. doi: 10.1016/s1532-0456(02)00125-4. [DOI] [PubMed] [Google Scholar]
- 43.Huang J., Yang Y., Wang A. Reconsideration of phenoloxidase activity determination in white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2010 Jan;28(1):240–244. doi: 10.1016/j.fsi.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 44.Cerenius L, Söderhäll K. Immune properties of invertebrate phenoloxidases. Dev Comp Immunol. 2021 Sep;122:104098.[44] Berg G, Rybakova D, Fischer D, Cernava T, Vergès MCC, Charles T, et al. Correction to: Microbiome Definition Re-visited: Old Concepts and New Challenges. Microbiome. 2020 Aug 20;8(1):1-22. [DOI] [PMC free article] [PubMed]
- 45.Simon J.C., Marchesi J.R., Mougel C., Selosse M.A. Host-microbiota interactions: from holobiont theory to analysis. Microbiome. 2019 Jan 11;7(1) doi: 10.1186/s40168-019-0619-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Theis K.R., Dheilly N.M., Klassen J.L., Brucker R.M., Baines J.F., Bosch T.C.G., et al. Getting the hologenome concept right: an eco-evolutionary framework for hosts and their microbiomes. Gilbert J.A., editor. mSystems. 2016 Apr 26;1(2) doi: 10.1128/mSystems.00028-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zilber-Rosenberg I., Rosenberg E. Role of microorganisms in the evolution of animals and plants: the hologenome theory of evolution. FEMS (Fed Eur Microbiol Soc) Microbiol Rev. 2008 Aug;32(5):723–735. doi: 10.1111/j.1574-6976.2008.00123.x. [DOI] [PubMed] [Google Scholar]
- 48.Tran L., Nunan L., Redman R., Mohney L., Pantoja C., Fitzsimmons K., et al. Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting penaeid shrimp. Dise Aquat Org [Internet] 2013;105(1):45–55. doi: 10.3354/dao02621. https://www.int-res.com/articles/dao2013/105/d105p045.pdf [DOI] [PubMed] [Google Scholar]
- 49.Huang Z., Li X., Wang L., Shao Z. Changes in the intestinal bacterial community during the growth of white shrimp,Litopenaeus vannamei. Aquacult Res. 2014 Oct 28;47(6):1737–1746. doi: 10.1111/are.12628. [DOI] [Google Scholar]
- 50.Kim D.C., In M.J. Production of hydrolyzed red ginseng residue and its application to lactic acid bacteria cultivation. J Ginseng Res. 2010 Dec 29;34(4):321–326. [Google Scholar]
- 51.Park S.H., Kim W.J. Study of hongsambak for medicinal foods applications-nutritional composition, antioxidants contents and antioxidative activity- J Physiol Pathol Korean Med. 2006 Jan 1;20(2):449–454. [Google Scholar]
- 52.Myung-Han Park, Hyun-Joo Sohn, Byeong-Seon Jeon, Na-Mi K., Park Chae-Kyu, An-Kyun K., et al. Studies on flavor components and organoleptic properties in roasted red ginseng marc. J Ginseng Res. 1999 Jan 1;23(4):211–216. [Google Scholar]
- 53.Avnimelech Yoram, World Aquaculture Society . La World Aquaculture Society; Baton Rouge: 2015. Biofloc technology a practical guide book. [Google Scholar]
- 54.Liu X., Xi Q., Yang L., Li H., Jiang Q., Shu G., et al. The effect of dietary Panax ginseng polysaccharide extract on the immune responses in white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2011 Feb 1;30(2):495–500. doi: 10.1016/j.fsi.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 55.Choi I.-C., Kim K.-T., Bang I.-C., Kwon M.-G., Lee J.-H., Lee B.-I., et al. Effects of dietary inclusion of red ginseng byproduct on growth, body composition, serum chemistry, and lysozyme activity in juvenile olive flounder (Paralichthys olivaceus) Fisher Aquat Sci. 2010 Dec 31;13(4):300–307. doi: 10.5657/fas.2010.13.4.300. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.