Abstract
The objective of this study was to examine the response of geranium plants to different irrigation levels (100%, 80%, and 60% based on ETo) and Kaolin application rates (0, 100, 200 and 300 ppm) during 2022 and 2023 seasons, at Aly Mobarak Experimental Farm, Horticulture Research Station, located at El-Bustan site, El-Behiera Governorate, Egypt, by using a two-way factorial analysis experimental design. The results revealed that water deficit significantly reduced most studied traits. Irrigation level at 60% based on ETo exhibited poorest performance on growth parameters and decreased fresh yield and essential oil yield by 27.77% 10.73%, respectively as compared with full irrigated plants. However, foliar application of kaolin at 200 and 300 ppm led to increasing biomass accumulation by 28.51, 26.16%, and essential oil yield by 79.51, 89.95%, respectively, as compared with untreated plants grown under the same level of water deficit (60% based on ETo). GC–MS analysis of essential oil showed that water deficit and kaolin application increased geraniol/citronellol ratio and consequently improved oil quality. Results highlight the positive influence of water deficit and kaolin rates on the development and performance of anatomical parameters. Enzymes assay in leaves revealed in an increase superoxide dismutase (SOD) and peroxidase (POD) activities, and decreased in catalase (CAT) activity under water deficit. As for WUE at 60%, followed by 80% based on ETo recorded excellent response for geranium plants which led to more water saving. So, it could be concluded that foliar application of kaolin at 200 and 300 ppm obtained the optimal characteristics of geranium plants under experimental conditions. In particular, essential oil yield and productivity.
Keywords: Anatomy, Enzymes activity, Essential oil, Evapotranspiration for stander crop (ETo), Geranium, Kaolin, Water deficit, WUE
Introduction
Geranium (Pelargonium graveolens) herbaceous plant belongs to the Geraniaceae family and is a considerable medicinal and aromatic plant in Egypt. It is a main source of essential oil used in many aspects, such as perfumery and food processing [1]. The major constituents of this oil are citronellol, geraniol, iso-metone, citronellyl formate, and geraniol formate [2]. Geranium oil quality is controlled by the citronellol and geraniol ratio of C/G (1–3). The lower ratio is an indication of good-quality oil [3, 4].
Water is among the most vital variables influencing growth, yield, and quality of medicinal and aromatic plants since its shortage and scarcity may cause genuine growth hurts and yield loss. Development and essential oil produced from geranium plants are negatively impacted by drought [5]. Several shortages of water affect physiological functions such as leaf development, gas exchange, and carbon fixation at the cellular level [6]. Also, it increases leaf thickness and changes leaf anatomy as well as the arrangement of both palisade and sponge tissue cells with increasing intercellular space [7]. Water stress causes stomatal closure and increases photorespiration, leading to oxidative damage due to the accumulation of reactive oxygen species (ROS) in plants [8]. ROS stress causes disruption of chloroplast leading to chlorophyll loss [9]. Plants have a defense mechanism against ROS through the induction of enzymatic and non-enzymatic antioxidant defense chemicals [10, 11]. These endogenous anti-drought compounds are inadequate to permit pushed crops to withstand water deficits. So, plants need outside application of substances that stimulate resistance [12].
Kaolin can act as a diluting factor in minimizing water stress in this connection [13] reported that kaolin mitigated the harmful effects of combined stress in several ways. Kaolin (an aluminum phyllosilicate), when applied to plants it forms thin nanoparticle films that lower the canopy temperature [14]. Furthermore, the findings demonstrated that foliar spraying kaolin on phaseolus vulgaris L. leaves in conjunction with skipping one DI-vegetative or DI-ripen irrigation had a positive effect on the chemical contents of leaves (N, P, K, chlorophyll, carotenoids, and TSS), plant water status (relative water content (RWC) and membrane stability index (MSI)), pods (N, P, chlorophyll, carotenoids, and TSS), and fruit firmness. which are required for plant growth. Consequently, kaolin enhanced the activities related to photosynthesis [15]. Furthermore, the activity of stress enzymes was regulated by kaolin to give maize plants the best defense against drought stress Furthermore, it has been shown that kaolin enhanced post-harvest quality, yield, color, gas exchange, photosynthetic rate, and net CO2 assimilation in olive, sweet basil, Mentha pulegium, Ocimum basilicum L, walnut, apple, mango, pomegranate, grape, tomato, and phaseolus vulgaris [16].
The present study was carried out to evaluate the role of kaolin rate in mitigating the effect of water deficit on geranium plants grown under sandy soil conditions.
Materials and methods
Experimental site and plant source
Healthy mothers of geranium are grown at Aly Mobarak Experimental Farm, Horticulture Research Station, located in the El-Bustan region, El-Behiera Governorate, Egypt. The farm is located at a latitude of 33°30′ 1.4''N, a longitude of 30°19′ 10.9''E, and an altitude of 21 m above sea level. This survey has been conducted over two consecutive seasons, 2022 and 2023.
Table 1 presents meteorological data collected on site during growth stages on both distinct seasons according to the methods described by [17].
Table 1.
Meteorological data at the site during two seasons
| Month | season 2022 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Feb | Mar | Apr | May | June | July | Aug | Sep | Oct | ||
| Temperature ℃ | Max | 19.00 | 21.00 | 26.50 | 30.94 | 33.05 | 35.69 | 36.00 | 32.33 | 31.00 |
| Min | 6.59 | 8.85 | 11.60 | 16.25 | 20.16 | 21.50 | 21.68 | 19.80 | 18.75 | |
| RH _AVG % | 60.71 | 57.92 | 50.00 | 39.12 | 49.21 | 50.25 | 51.72 | 56.00 | 58.57 | |
| Wind speed (m/sec) | 2.68 | 2.98 | 2.99 | 2.61 | 3.18 | 2.71 | 2.85 | 3.00 | 2.63 | |
| Radiation (MJ m−2) | 14.00 | 18.89 | 24.84 | 26.87 | 28.86 | 28.61 | 26.32 | 22.69 | 17.31 | |
| Et0 mm day−1 | 3.58 | 4.60 | 6.90 | 8.74 | 8.93 | 8.88 | 8.62 | 6.58 | 5.00 | |
| Season 2023 | ||||||||||
| Temperature ℃ | Max | 20.90 | 23.90 | 28.50 | 38.04 | 37.95 | 37.79 | 37.72 | 36.93 | 33.58 |
| Min | 9.15 | 10.93 | 13.78 | 18.15 | 22.64 | 23.76 | 23.68 | 22.68 | 20.25 | |
| RH-AVG% | 64.25 | 60.06 | 50.82 | 41.00 | 51.95 | 52.73 | 53.84 | 57.28 | 60.79 | |
| Wind speed (m/sec) | 2.98 | 3.34 | 3.55 | 3.91 | 3.56 | 3.85 | 3.07 | 3.20 | 3.05 | |
| Radiation (MJ m−2) | 15.70 | 19.95 | 25.50 | 27.77 | 29.24 | 29.77 | 28.10 | 24.11 | 19.01 | |
| Et0 mm day−1 | 3.00 | 4.00 | 5.00 | 6.68 | 7.03 | 7.38 | 6.60 | 6.00 | 3.00 | |
RH AVG% = relative humidity average %, (m/sec) meter per second, (MJ/m2 day−1) = megajoule per square meter and per day and (mm day−1) millimeter per day
Table 2 chemical and physical analyses of the experimental soil were carried out according to the methods described by [18].
Table 2.
Physical and chemical properties of the experimental soil
| Sandy soil | ||
|---|---|---|
| Physical properties | ||
| 1st season | 2nd season | |
| Sand (%) | 91.30 | 90.50 |
| Silt (%) | 4.60 | 5.60 |
| Clay (%) | 4.10 | 3.90 |
| Texture | sandy | Sandy |
| Field Capacity, (%) | 13.30 | 13.60 |
| Wilting Point, (%) | 4.70 | 4.60 |
| Available water, (%) | 8.60 | 9.00 |
| Bulk density (t m−3) | 1.79 | 1.78 |
| Chemical properties | ||
| EC1:5 (dS m−1) | 0.75 | 0.53 |
| pH (1:2.5) | 8.96 | 8.70 |
| Total CaCO3 (%) | 7.00 | 5.66 |
Experimental procedures
On 15th February 2022 and 2023, rooted terminal stem cuttings measuring approximately 10 to 15 cm in height were planted for the first and second seasons, respectively. To prepare the soil, 15 m3/fed. of compost and 300 kg/fed of calcium superphosphate (15.5% P2O5) were incorporated. Potassium application was carried out using potassium sulfate (48% K2O) at 100 kg/fed and nitrogen was applied as ammonium nitrate (33.5% N) at a rate of 600 kg/fed. This methodology involved dissolving soluble fertilizers in the fertilizer tank and applying them by fertigation. The drip irrigation system was used with a flow rate of 4 L/h. Once the plants were established, irrigation treatments were initiated at 2-day intervals. The results of the chemical analysis of irrigation water from the farm well are presented in Table 3.
Table 3.
Chemical analysis of the irrigation water at the experimental site
| pH | Ecw | Soluble anions (meq/l) | Soluble cations (meq/l) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 7.78 | ppm | dS/m | CO3− − | HCO3−− | Cl− | Ca++ | Mg ++ | Na + | K + |
| 1664 | 2.6 | - | 5.20 | 17.20 | 4.00 | 3.60 | 18.01 | 0.32 | |
Irrigation levels (based on % ETO)
100% of ETo (3550 and 3750 m3/fed. in the first and second seasons respectively) as control,
80% of ETo (2840 and 3000 m3/fed. in the first and second seasons respectively) and,
60% of ETo (2130 and 2250 m3/fed. as in the first and second seasons respectively).
Evapotranspiration (ETo) mm/day was calculated by Penman Monteith equation [19] using the climatologically data of El-Bostan area according to Table 1.
Water utilization efficiency values were estimated according to [20], as follows:
Water applied (m3) = Whole quantity of irrigation water for each treatment.
Oil Yield (liter) = Sum of total essential oil / fed in 1st and 2nd cuts for each season.
Kaolin application
Geranium plants were subjected to two spraying cycles per cutting with kaolin particle film as a reflective at (Surround WP Crop Protectant, 95% Kaolin, 5% inner ingredients, AL-Goumhoria Co., Egypt). The first application took place during the branching initiation phase, precisely 30 days after transplanting. Subsequently, the second spraying took place at the full branch stage, i.e. 20 days after the first application. Throughout the growing season, plants were treated with different concentration levels, including 0, 100, 200, and 300 ppm.
Harvesting
The aerial parts of the plants were cut at a height of 10–15 cm above the soil surface when they were in full flower. This harvesting process was carried out twice during each season. Specifically, the first cut took place on the 23rd and 22nd of May in both, respectively while, the second took place on the 22nd and 23rd of October in both, respectively.
Data recorded
During each incision, vegetative growth characteristics were documented, including plant height in centimeters, number of branches per plant, leaf thickness in millimeters, and weight grams or plant in a fresh, dry state, measured in grams.
Essential oil productivity
Essential oil extraction
The hydro-distillation method was used to extract the essential oil from freshly harvested leaves, using a Clevenger-type apparatus, following the guidelines established by [21].
Gas chromatography-mass spectrometry (GC–MS)
Gas chromatography and mass spectrometry (GC/MS) techniques were used to carry out the identification and analysis of essential oils. Preliminary identification of the components was performed by comparing their relative retention times and mass spectra with those stored in the NIST and WILLY libraries of the GC/MS system [22, 23].
Anatomical study
Several investigations have been carried out on the anatomical structure of Pelargonium graveolens L. leaves. The microtechnology activities were carried out by Agric. Bot. Department, Faculty of Agriculture, Cairo University, Giza, Egypt. The samples underwent a series of procedures including fixation in formaldehyde, acetic acid and alcohol (F.A.A.) solution for a minimum of 48 h, dehydration and embedding in paraffin wax [24]. Subsequently, the sections, which were cut at a thickness of 15 to 20 microns using a rotary microtome, were stained with crystal violet/erythrosine before being mounted in Canada balsam. The resulting slides were then photomicrograph and observed under a light microscope.
Determination of photosynthetic pigments contents
Pigment content was extracted by using dimethyl sulfoxide (DMSO) solvent [25]. The calculation of chlorophyll a (Chl a) and chlorophyll b (Chl b) was carried out according to the equation proposed by [26]. On the other hand, the determination of the total carotenoid concentration was calculated based on the equation described by [27].
Determination of antioxidant activity of the enzymes
Sample preparation
Leaf sample (1 g) tissue was grind in the cold mortar and pestle with the addition of cold PBS (phosphate buffered saline pH 7.4, (5 – 10 ml) i,e, 50 mM potassium phosphate, pH 7.4. 1 mM EDTA and 1 ml/L Triton X-100) per gram tissue. Centrifuge at 4,000 rpm for 15 min. at 4°C. Remove the supernatant for assay and store on ice. Aliquots of 0.05 ml supernatant samples were taken for the determination of catalase and peroxidase, while 0.1 ml sample was taken for the determination of SOD enzyme [28, 29].
Catalase assay
According to the method of [28], catalase activity was determined.
Supernatant (0.05 ml) + 0.05 ml H2O2 + 0.05 ml of buffer + 100 ml diluted H2O.
Incubate for exactly one minute at 25℃, then add 200 ml of chromogen- inhibitor.
- Incubate at 37℃ for 10 min read at 510 nm. against a blank sample (0.05 ml of buffer instead of plant tissue homogenate.
Peroxidase assay
Supernatant (0.05 ml) + 0.05 ml diluted H2O + 0.05 ml chromogen.
- Incubate for 10 min. at 37℃. Read at 510 nm against blank sample using 50 ml diluted H2O instead of plant sample. The assay was described by [30].
Superoxide dismutase (SOD) assay
Supernatant (0.1 ml) sample + 1 ml working reagent (R1 + R2 + R3 + in the ratio of 10 + 1 + 1 ml) use diluted H2O (100 ml) instead of supernatant.
Mix well and add (R4 = PMS) at 100ml.
Read at 560 nm for 5 min.
R1 = phosphate buffer pH 8.5.
R2 = Nitroblue tetrazolium (NBT).
- R3 = NADH.According to the assay designed by [31].
Statistical analysis
Two-way factorial analysis experimental design, three replicates were included. To analyze the significant differences observed between treatments mean, the statistical program (Statisix 8) was used to perform an analysis of variance (ANOVA). Differences between treatment means were assessed using the least significant difference (L.S.D. 0.05) test, with a probability level of 0.05, as recommended by [32].
Results and discussion
Effect of water deficit and kaolin rates on vegetative characteristics of geranium plants
Data in Table 4 showed the effect of foliar application of kaolin at different concentrations on growth characteristics of geranium plants grown under different water levels at both studied seasons. The results clearly showed that, plant height and shoot number gradually decreased with increasing water deficit. Maximum reduction in this trait was obtained at the water level of 60% based on ETo reduction of plant height by 20.62% and shoot number by 31.80% compared with normal irrigation by 100% based on ETo. This could be demonstrated to dehydration had negative effects on various physiological processes in plants, such as leaf development, gas exchange at the organ level, and carbon fixation at the cellular level. Finally, normal growth and division of cells [6]. Similar results were obtained by [33] on Hot pepper and [10] on sweet basil. On the contrary, leaf thickness increased as water level decreased. It increased by 36.73% on plants irrigated with 60% These results are in agreement with [7] on Camellia oleifera cultivars, those reported that plant leaf thickness is increasing for more water stored is the basis for plant response and adaptation to environmental changes. Furthermore, [42] noted that, the increase in leaf thickness on 'Chemlali' cultivar was due to an increase in the thickness of the spongy parenchyma and upper palisade. That enhanced and facilitated to CO2 fixation and rapid diffusion of CO2 to these sites in plants grown under water deficit as confirmed by anatomical studies.
Table 4.
Effect of Irrigation levels and kaolin rates on vegetative characteristics of geranium (Pelargonium graveolens) plants
| Treatments | First Season (2022) | Second Season (2023) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First cut | Second cut | First cut | Second cut | ||||||||||
| PH (cm) | NB | LT (mm) | PH (cm) | NB | LT (mm) | PH (cm) | NB | LT (mm) | PH (cm) | NB | LT (mm) | ||
|
Irrigation levels (A) |
100% (con.) | 57.5 a | 10.6 a | 0.82 b | 65.8 a | 13.8 a | 0.95 b | 54.3 a | 14.0 a | 0.88 c | 62.8 a | 16.4 a | 0.91 c |
| 80% | 53.7 a | 9.1 b | 0.97 a | 61.2 a | 12.3 a | 0.99 b | 46.4 b | 10.9 b | 0.99 b | 58.3 ab | 12.9 b | 1.10 b | |
| 60% | 43.4 b | 7.6 c | 0.97 a | 53.5 b | 10.2 b | 1.16 a | 42.0 b | 8.0 c | 1.19 a | 52.3 b | 11.5 c | 1.47a | |
|
Kaolin rate (ppm) (B) |
Control | 47.6 b | 7.6 c | 0.82 b | 54.1 c | 10.0 b | 0.75 c | 40.6 b | 9.0 b | 0.75 b | 49.7 c | 11.4 b | 0.87 b |
| 100 | 50.8 ab | 8.6 bc | 0.94 a | 59.6 b | 11.8 ab | 0.95 b | 46.8 a | 11.0 a | 1.1 a | 56.4 bc | 14.1 a | 1.20 a | |
| 200 | 53.7 a | 9.6 ab | 1.02 a | 62.0 ab | 13.0 a | 1.21 a | 51.0 a | 12.2 a | 1.09 a | 60.8 ab | 14.8 a | 1.30 a | |
| 300 | 54.1 a | 10.7 a | 1.00 a | 65.0 a | 13.4 a | 1.23 a | 52.0 a | 11.7 a | 1.11 a | 64.1 a | 14.1 a | 1.26 a | |
|
Interactions (A X B) |
100% + con | 53.7 ab | 9.3 abc | 0.69 f | 60.7 bcd | 12.0abc | 0.65 e | 44.7 de | 10.7 bcd | 0.60 f | 54.0 cd | 13.0 bc | 0.71 f |
| 100% + 100 | 58.3 ab | 11.0 a | 0.83 def | 65.0 ab | 14.0 ab | 0.93 d | 55.3 abc | 15.3 a | 1.01 cde | 60.3 abc | 18.3 a | 1.08 cd | |
| 100% + 200 | 60.3 a | 10.0 ab | 0.88cde | 67.3 ab | 15.0 a | 1.09bc | 58.0 ab | 16.3 a | 0.94 e | 66.7 ab | 19.0 a | 1.06 cde | |
| 100% + 300 | 57.7 ab | 12.0 a | 0.89 bcde | 70.3 a | 14.0 ab | 1.11 b | 59.0 a | 13.7 ab | 0.98 de | 70.0 a | 15.3 b | 0.80 def | |
| 80% + con | 51.0 bc | 7.0 cd | 0.80 ef | 54.7 cde | 10.0 cd | 0.66 e | 42.0 def | 9.0 cde | 0.61 f | 51.0d | 11.3 cd | 0.75 ef | |
| 80% + 100 | 52.0 abc | 8.0 bcd | 0.99 bcd | 60.0 bcd | 11.7abc | 0.96 cd | 46.3 cde | 10.0 cde | 1.07 bcde | 57.0 bc | 12.3 cd | 1.19 c | |
| 80% + 200 | 56.7 ab | 9.3 abc | 1.01 abc | 63.7 abc | 13.0abc | 1.15 b | 48.0 cd | 11.3 bc | 0.95 de | 60.7 abc | 13.0 bc | 1.16 c | |
| 80% + 300 | 55.0 ab | 12.0 a | 1.05 ab | 66.3 ab | 14.3 ab | 1.19 b | 49.3 bcd | 13.3 ab | 1.19 abc | 63.3 abc | 15.0 b | 1.29 c | |
| 60% + con | 38.0 e | 6.3 d | 0.97 f | 47.0 e | 8.0 d | 0.93 d | 35.0 f | 7.3 e | 1.03 bcde | 44.0 d | 10.0 d | 1.15 c | |
| 60% + 100 | 42.0 de | 6.7 cd | 1.01 abc | 53.7 de | 9.7 cd | 0.95 d | 38.7 ef | 7.7 de | 1.21 ab | 52.0 cd | 11.7 cd | 1.35 bc | |
| 60% + 200 | 44.0 cde | 9.3 abc | 1.16 a | 55.0 cde | 11.0bcd | 1.38 a | 47.0 cde | 9.0 cde | 1.37 a | 55.0 bcd | 12.3 cd | 1.67 ab | |
| 60% + 300 | 49.7 bcd | 8.0 bcd | 1.05 ab | 58.3 bcd | 12.0 bc | 1.38 a | 47.4cde | 8.0 de | 1.15 bcd | 58.0 abc | 12.0 cd | 1.71 a | |
| L.S.D (0.05) = | |||||||||||||
| A | 4.5 | 1.4 | 0.08 | 4.7 | 1.7 | 0.07 | 4.6 | 1.6 | 0.10 | 6.3 | 1.3 | 0.16 | |
| B | 5.2 | 1.6 | 0.96 | 4.4 | 2.0 | 0.08 | 5.3 | 1.8 | 0.12 | 7.2 | 1.5 | 0.19 | |
| AXB | 8.9 | 2.7 | 0.17 | 9.4 | 3.3 | 0.13 | 9.2 | 3.0 | 0.20 | 12.5 | 2.5 | 0.32 | |
Different letters within columns indicate significant differences (P < 0.05) of variation
Irr. Irrigation levels (% of ETo), K. Kaolin rates (ppm), PH Plant height(cm), NB Number of shoots, LT Leaf thickness (mm)
Foliar application led to a significant increase in all studied characteristics. Kaolin rate at 300 ppm enhanced plant height by 30.55%, shoot number by 26.38%, and leaf thickness by 29.25% as compared with the untreated plants grown under the same irrigation level at 60% based on ETo in both 1st and 2nd cut during both seasons. These results are in agreement with the findings at [34] on Physalis peruviana kaolin application enhanced plant height, total dry mass in water-stressed cape gooseberry plants, and lowered leaf temperature. This is due to the foliar application of kaolin on Cucurbita pepo L. plant increasing the moisture status of treated plants during water deficit conditions [35]. Furthermore, kaolin increases the absorption of essential elements, such as potassium, phosphorus, and nitrogen, which are required for plant growth. Consequently, kaolin enhanced the activities related to plant productivity [15] on Maize plants.
Effect of water deficit and kaolin rates on geranium yield
Data illustrated in Table 5 showed the effect of irrigation treatments and spraying with kaolin on fresh herb yield/plant (g), dry weight (g), and yield of fresh herb (ton)/fed. Data showed that, water deficit had a negative effect on biomass accumulation similar trends to vegetative growth parameters. Precisely, water deficit at 60% based on ETo led to a decrease in yield/fed. by 27.77% in plants grown under 1st season conditions compared with full irrigated plants. These negative results could be attributed to all growth characteristics and biochemical processes resulting from the water stress disorders such as prevention of water and photo-assimilate translocation, photosynthetic capacity, and nutrient take-up [36] and [37] on tomato. Furthermore, this decay may be clarified by a diminish in the development of leaf cells or indeed by a lower rate of cell division in the plant, which in turn causes a decrease in dry matter and the generation of plant yield [38] and [39] on sweet pepper (Capsicum annuum L.)
Table 5.
Effect of Irrigation levels and kaolin rates on yield of geranium (Pelargonium graveolens) plants
| First Season (2022) | Second Season (2023) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | First cut | Second cut | First cut | Second cut | |||||||||
| FW (g) | DW (g) | FW (ton/f.) | FW (g) | DW (g) | FW (ton/f.) | FW (g) | DW (g) | FW (ton/f.) | FW (g) | DW (g) | FW (ton/f.) | ||
|
Irr (A) |
100% (con.) | 784.7 a | 130.6 a | 16.5 a | 1030.9 a | 167.5 a | 21.7 a | 819.1 a | 131.0 a | 17.2 a | 1012.5 a | 167.5 a | 21.3 a |
| 80% | 675.0 b | 118.4 a | 14.2 b | 843.8 b | 148.0 b | 17.7 b | 592.5 b | 103.2 b | 12.4 b | 849.0 b | 148.7 ab | 17.8 b | |
| 60% | 581.0 c | 107.9 a | 12.2 c | 716.2 c | 134.6 b | 15.3 c | 488.0 c | 89.7 b | 10.3 c | 691.7 c | 127.6 b | 14.0 c | |
|
K (B) |
Control | 616.6 c | 89.8 c | 13.0 c | 770.8 b | 112.0 c | 16.2 b | 558.3 c | 80.9 c | 11.7 c | 772.3 b | 112.2 c | 16.2 b |
| 100 | 649.1bc | 110.9bc | 13.6 bc | 811.3 b | 138.5 b | 17.0 b | 597.0 bc | 102.0 b | 12.5 bc | 805.6 ab | 137.5 bc | 16.2 b | |
| 200 | 734.4 a | 134.4 ab | 15.4 a | 904.6 a | 168.2 a | 19.3 a | 658.3 ab | 119.5 a | 13.8 ab | 910.8 a | 166.3 ab | 19.1 a | |
| 300 | 720.9 ab | 140.7 a | 15.1 ab | 967.8 a | 181.5 a | 20.3 a | 719.2 a | 129.6 a | 15.1 a | 915.6 a | 175.6 a | 19.2 a | |
|
Interactions (A X B) |
100% + con | 722.5 bc | 93.6bcd | 15.2bc | 903.1 b | 116.9 e | 19.0 b | 698.6 cd | 92.2defg | 14.7bcd | 903.1 bc | 116.9 cd | 19.0bc |
| 100% + 100 | 729.2abc | 125.7abcd | 15.3abc | 911.4 b | 157.1 cd | 19.1 b | 786.6 bc | 134.3 ab | 16.5 bc | 911.4 bc | 157.2 abc | 19.1 bc | |
| 100% + 200 | 874.4 a | 155.2 a | 18.4 a | 1092.9 a | 194.0 ab | 23.0 a | 834.6 b | 148.2 a | 17.5 ab | 1092.9ab | 193.2 ab | 23.0 ab | |
| 100% + 300 | 812.8 ab | 147.9 a | 17.1 ab | 1216.0 a | 201.1 a | 25.5 a | 956.5 a | 149.4 a | 20.1 a | 1142.7 a | 202.6 a | 24.0 a | |
| 80% + con | 621.9cde | 93.4bcd | 13.1cde | 777.4bcd | 116.8 e | 16.3 bcd | 565.1 e | 84.0efg | 11.9 de | 798.7cde | 119.8 cd | 16.8 cd | |
| 80% + 100 | 678.2bcd | 116.5abcd | 14.2bcd | 847.8 bc | 145.6 d | 17.8 bc | 573.7 e | 98.5cdef | 12.1 de | 847.2 cd | 145.6 bcd | 17.8 c | |
| 80% + 200 | 689.7 bc | 123.8abcd | 14.5bc | 862.2 bc | 154.9 cd | 18.1 bc | 585.5 de | 104.4 de | 12.3 d | 862.2 c | 154.6abc | 18.1 c | |
| 80% + 300 | 710.3bc | 139.8 ab | 14.9 bc | 887.8 bc | 174.8 abc | 18.6 bc | 645.8 de | 125.9abc | 13.6 cd | 887.8 c | 174.8 ab | 18.6 c | |
| 60% + con | 505.5 e | 82.6 d | 10.6 e | 631.8 e | 102.3 e | 13.3 e | 411.1 f | 66.5 g | 8.6 f | 615.2 e | 99.9 d | 12.9 de | |
| 60% + 100 | 539.8 de | 90.4 cd | 11.3 de | 674.8 de | 112.9 e | 14.2 de | 430.8 f | 73.2 fg | 9.1ef | 658.1 de | 109.7 cd | 11.7 e | |
| 60% + 200 | 639.0cde | 124.2abcd | 13.4cde | 758.7cde | 155.7 cd | 16.8cde | 554.8 e | 105.8cde | 11.7def | 777.4cde | 151.3abcd | 16.3 cd | |
| 60% + 300 | 639.5cde | 134.6abc | 13.4cde | 799.4bcd | 167.6bcd | 16.8bcd | 555.2 e | 113.5bcd | 11.7def | 716.2cde | 149.3 bcd | 15.0 cde | |
| L.S.D (0.05) = | |||||||||||||
| A | 72.8 | 24.0 | 1.5 | 67.7 | 13.7 | 1.4 | 58.1 | 14.2 | 1.59 | 98.7 | 26.3 | 2.1 | |
| B | 84.1 | 27.7 | 1.8 | 78.2 | 15.8 | 1.6 | 67.1 | 16.3 | 1.83 | 114.0 | 30.3 | 2.4 | |
| AXB | 145.6 | 48.0 | 3.1 | 135.4 | 27.4 | 2.9 | 116.2 | 28.3 | 3.18 | 197.4 | 52.6 | 4.2 | |
Different letters within columns indicate significant differences (P < 0.05) of variation
Irr. Irrigation levels (% of ETo), K. Kaolin rates (ppm), FW Fresh weight (g/plant), DW Dry weight (g/plant), FW (ton/fed) Fresh weight (ton/fed)
Foliar application of kaolin tended to have a significant effect on biomass accumulation as well as dry matter of geranium plant and alleviate the adverse effects of water deficit, corresponding to an increase by 61.61% on plant dry matter and by 26.26% on plant yield treated by kaolin at 300ppm rate grown under higher water deficit. These findings align with earlier research [35] which noted that numerous plants generate less total leaf area at severe water stress. This is due to the reduced rate of cell division and development under osmotic stress and the reduction of turgor pressure and leaf loss results from the generation of ethylene and ABA. kaolin foliar application increased the moisture status, which led to enhanced cell division, nitrogen metabolism, enzymatic activity, and protein content of treated plants relative to untreated plants during water stress conditions. Also [40], Found that use kaolin spraying on Zea mays L. at intervals to reduce water stress and enhance plant nutrient uptake. The substantial increase in yield and economics over alternative mulching materials can be attributed to the improved physical condition of the soil, which favorably increased nutrient uptake by the crop by supplying a sufficient amount of N, P, and K through a steady and slow rate of nutrient release. Applying anti-transpirants improved metabolic, enzymatic, and protein synthesis under drought stress, which may have improved the harvest index by preserving relative plant hydration and lowering transpiration water loss. Plant water potential was increased during flower development by applying kaolin and coir pith. By reducing transpiration loss and increasing plant water potential during flower development, kaolin increased maize output. In a semi-arid area, coir pith mulching produced a greater grain yield than all other mulches combined.
Essential oil productivity
Effect of water deficit and kaolin rates on oil content and geranium oil yield
Data in Table 6 showed that, essential oil (E.O.%) contents was increased in geranium herb as plant was subjected to water deficit. Maximum increased obtained in herb as plant irrigated with 60% based on ETo by 23.08% followed by plant irrigated with 80% based on ETo increased by 8.26% compared with regular irrigation. Similar findings were noted by [41], on Thymus daenensis. and [42] on (Coriandrum Sativum L.). On contrary, the lowest essential oil yield/fed. obtained from plants which irrigated with 60% based on ETo reduction by 10.73% followed by 80% based on ETo reduction by 8.20% compared with plants which irrigated with 100% based on ETo [43] on thymus plants and [10] on Sweet basil plants, mentioned that, the decrease in biomass productivity was probably the reason for decrease the essential oil yield during the water deficit.
Table 6.
Effect of Irrigation Levels and kaolin concentrations on essential oil contents of geranium (Pelargonium graveolens) plants
| First Season (2022) | Second Season (2023) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | First cut | Second cut | First cut | Second cut | |||||||||
| E.O. % | oil/p (ml) | oil/F (L) | E.O. % | oil/p (ml) | oil/F (L) | E.O. % | oil/p (ml) | oil/F (L) | E.O. % | oil/p (ml) | oil/F (L) | ||
|
Irr (A) |
100% (con.) | 0.14 b | 1.139 a | 22.93 a | 0.155 b | 1.575 a | 28.86 a | 0.14 c | 1.12 a | 23.48 a | 0.16 c | 1.53 a | 29.50 a |
| 80% | 0.15 b | 1.052 a | 22.01 a | 0.169 b | 1.435 ab | 27.18 a | 0.16 b | 0.93 b | 19.55 b | 0.17 b | 1.43 a | 27.75 a | |
| 60% | 0.18 a | 1.036 a | 21.87 a | 0.190 a | 1.395 b | 26.26 a | 0.18 a | 0.89 b | 18.67 b | 0.20 a | 1.40 a | 26.83 a | |
|
K (B) |
Control | 0.13 b | 0.799 b | 16.89 b | 0.146 c | 1.119 c | 20.57 c | 0.14 b | 0.77 b | 16.26 b | 0.16 b | 1.12 b | 21.97 b |
| 100 | 0.15 b | 0.932 b | 19.54 b | 0.163 bc | 1.319 b | 24.75 b | 0.15 b | 0.88 b | 18.52 b | 0.16 b | 1.31 b | 24.45 b | |
| 200 | 0.17 a | 1.247 a | 25.19 a | 0.182 ab | 1.664 a | 31.28 a | 0.17 a | 1.10 a | 23.01 a | 0.19 a | 1.66 a | 32.56 a | |
| 300 | 0.19 a | 1.324 a | 27.47 a | 0.192 a | 1.771 a | 33.12 a | 0.18 a | 1.17 a | 24.49 a | 0.20 a | 1.72 a | 33.12 a | |
|
Interactions (A X B) |
100% + con | 0.12 d | 0.863 de | 18.21 de | 0.137 e | 1.260defg | 23.54def | 0.13 e | 0.92 bc | 19.37 bc | 0.14 e | 1.26 def | 23.95 de |
| 100% + 100 | 0.13 cd | 0.970 de | 20.43 cde | 0.150 cde | 1.367cdef | 25.87cde | 0.14 de | 1.10 ab | 23.08 ab | 0.15 de | 1.35 cde | 26.04bcd | |
| 100% + 200 | 0.16bc | 1.367 a | 25.71 abc | 0.157 cde | 1.790 ab | 32.61 ab | 0.15cde | 1.26 a | 26.53 a | 0.16 cde | 1.79 a | 33.91 a | |
| 100% + 300 | 0.17 bc | 1.357 a | 27.36 a | 0.167bcde | 1.883 a | 33.41 a | 0.15 cde | 1.19 a | 24.95 a | 0.17 cde | 1.73 ab | 34.08 a | |
| 80% + con | 0.13 cd | 0.827 de | 17.46 de | 0.140 de | 1.087 fg | 19.94 f | 0.15cde | 0.84 cd | 17.57 cd | 0.16 de | 1.09ef | 22.27 de | |
| 80% + 100 | 0.15 bcd | 1.020 cd | 21.13 bcde | 0.167 bcde | 1.440 cde | 27.26bcd | 0.15 cde | 0.87 bcd | 18.34bcd | 0.17 cde | 1.42 bcde | 25.61cde | |
| 80% + 200 | 0.14 cd | 1.080bcd | 22.60 abcd | 0.177 bc | 1.557 bcd | 29.68abc | 0.16cde | 0.92 bc | 19.39 bc | 0.18 cd | 1.55 abcd | 30.94abc | |
| 80% + 300 | 0.18 ab | 1.280abc | 26.84 ab | 0.193 ab | 1.657 abc | 31.85 ab | 0.18bc | 1.09 ab | 22.89 ab | 0.19 bc | 1.66 abc | 32.19 ab | |
| 60% + con | 0.14 cd | 0.707 e | 15.00 e | 0.160 bcde | 1.010 g | 18.23 f | 0.14 de | 0.56 e | 11.83 e | 0.17 cde | 1.01 f | 19.70 e | |
| 60% + 100 | 0.15 bcd | 0.807 de | 17.06 de | 0.173 bcd | 1.150 efg | 21.33 ef | 0.16 cd | 0.67 de | 14.14 de | 0.16 cde | 1.14 ef | 21.71 de | |
| 60% + 200 | 0.20 a | 1.293abc | 27.25 ab | 0.213 a | 1.647 abc | 31.55 ab | 0.20 ab | 1.10 ab | 23.10 ab | 0.22 ab | 1.65 abc | 32.83 a | |
| 60% + 300 | 0.21 a | 1.337 ab | 28.02 a | 0.217 a | 1.773 ab | 34.11 a | 0.13 e | 1.22 a | 25.62 a | 0.23 a | 1.77 a | 33.08 a | |
|
L.S.D (0.05) = A B AXB |
0.02 0.02 0.04 |
0.138 0.159 0.276 |
3.07 3.55 6.15 |
0.018 0.021 0.036 |
0.163 0.188 0.325 |
2.78 3.21 5.56 |
0.02 0.09 0.03 |
0.12 0.14 0.25 |
2.60 3.20 5.20 |
0.02 0.02 0.03 |
0.17 0.20 0.34 |
3.17 3.66 6.33 |
|
Different letters within columns indicate significant differences (P < 0.05) of variation
Irr. Irrigation levels (% of ETo), K. Kaolin rates (ppm), E.O. % Essential oil content, oil/p Oil Content /plant (ml), oil/F Oil yield /Fed. (L.)
Foliar application of kaolin rates on geranium plants, increased E.O. content oil content per plant (ml) and oil yield per fed. (L) significantly increased at the level of 200 and 300 ppm in both two-level water deficits. The highest increase by 57.14% was obtained at 300 ppm kaolin rate on plants grown under water deficit 60% based on ETo compared with untreated plants in the same level water deficit. Moreover, yield per fed. (L) had significantly augmented increased. It increased by 117.86% compared with untreated plant grown under water deficit 60% based on ETo. These findings align with earlier research at [44] spraying kaolin on Touriga-Nacional contributed to the increased values of esters, alcohols, and volatile phenols compared with control. Also, these results are in agreement with those obtained by [45] on sweet basil who found spraying kaolin gave a significant increase in the oil percentage, oil content per plant and per fed. and harmony with [46] on Ocimum basilicum L. noted that, spraying with kaolin led to increased oil production. and increased the main components of the essential oil of basil.
Effect of water deficit and kaolin rates on oil chemical composition of Pelargoniumgraveolens plants
Table 7 presents the physiological reactions of the constituents present in geranium oil, which are affected by water deficit and application of kaolin. Total of thirteen main constituents were identified through the use of gas chromatography and mass spectrometry (G.C.M.) in geranium oil.
Table 7.
Effect of irrigation levels and kaolin rates on essential oil constituents of Pelargonium graveolens (Average of two seasons 2022 and 2023)
| Identified constituents (%) | Water levels based on ETo | |||||
|---|---|---|---|---|---|---|
| 100% | 80% | 60% | ||||
| Control | Kaolin | Control | Kaolin | Control | Kaolin | |
| α-pinene | 0.71 | 0.24 | 0.41 | 0.30 | 0.24 | 0.16 |
| Limonene | 3.65 | 4.12 | 4.65 | 2.42 | 0.75 | 0.77 |
| L-linalool | 0.70 | 2.06 | 0.76 | 1.11 | 1.06 | 0.88 |
| Nerol | 9.08 | nd | 5.24 | nd | nd | 6.23 |
| Rose oxide | 8.12 | 0.10 | 1.18 | 7.17 | 5.01 | 0.98 |
| Citronellol | 28.95 | 27.90 | 21.90 | 23.50 | 25.76 | 24.69 |
| Geraniol | 11.41 | 13.72 | 14.42 | 17.41 | 20.99 | 23.45 |
| Isogeraniol | 2.00 | 1.15 | 5.82 | 2.32 | 2.13 | 2.79 |
| Citral | 1.15 | 1.60 | 2.79 | 3.64 | 0.20 | 1.67 |
| Citronellyl formate | 1.94 | 7.20 | 3.67 | 2.89 | 2.30 | 2.98 |
| Geranyl acetate | 0.55 | 0.05 | 3.75 | 3.44 | 1.55 | 1.79 |
| 10-epi-ҫ-eudesmol | 12.44 | 9.08 | 12.30 | 11.82 | 14.87 | 11.00 |
| Geranyl tiglate | 1.27 | 1.32 | 2.99 | 5.09 | 2.90 | 4.76 |
| C/G ratio | 2.54 | 2.03 | 1.52 | 1.35 | 1.23 | 1.05 |
Kaolin Rates at 300 ppm
nd Not detected, C Citronellol G geraniol
The composition of geranium oil was significantly affected by the foliar application of kaolin. At every irrigation level, the relative proportions of different monoterpenes were altered. Deficit level and kaolin application rate had a substantial impact on reactions involving oxygenated monoterpenes, including geraniol, isogeraniol, citronellol, citronellylformate, citral, linalool, and geranyl acetate. Based on the combined effects of irrigation and kaolin levels, oxygenated constituents appeared as the most significant category among the oil constituents, accounting for between 47.60% and 60.20%. The ratios of geraniol to citronellol indicate the quality of geranium oil. Both of these compounds are known to be able to change into one another, with geraniol serving as a precursor to citronellol. This understanding was backed by earlier research by [4] and [47]. An essential metric is the C/G ratio, which has a range of 1 to 3. A ratio closer to 1 is frequently linked to a higher-quality product and yields the highest quality [4]. It's interesting to note that the current study discovered that higher water stress led to a lowest C/G ratio by decreasing the amount of citronellol and increasing geraniol. Nevertheless, this reduction was only noticed in the presence of 300 ppm kaolin.
The findings are in harmony with study by [48], who found that the chemical composition essential oil was altered by water stress circumstances that favor the development of secondary plant metabolites like essential oils [49]. Also discovered that there was a relationship between the frequency of irrigation and the composition of essential oils, with geraniol, the primary volatile component, declining as the percentage of water depletion increased.
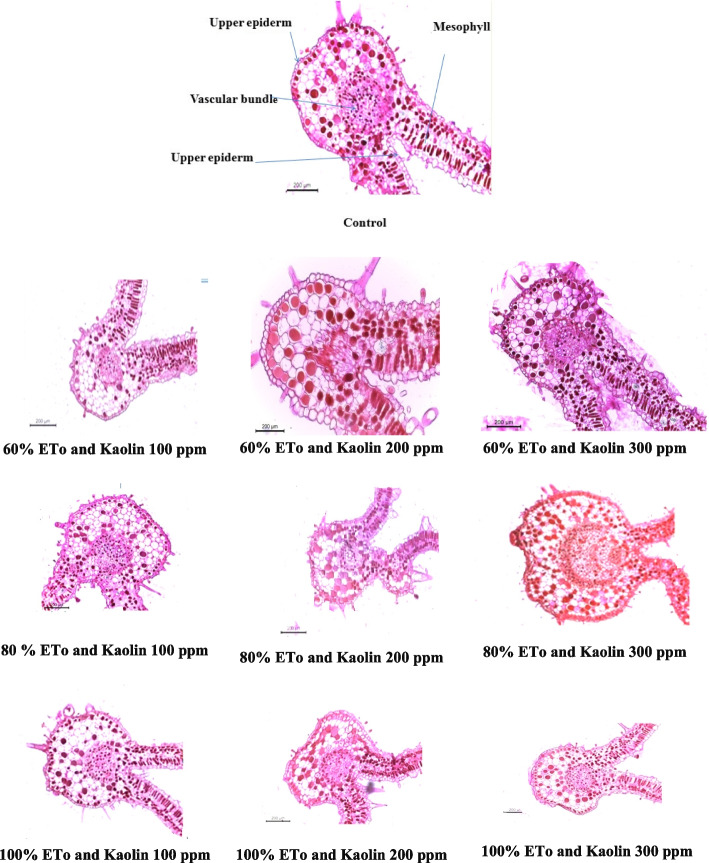
Anatomical study
The measurement of the anatomical parameters of Pelargonium graveolensL sand. Leaves subjected to drought deficit and kaolin treatment are shown in Fig. 1 and Table 8. The results indicate that the thickness of the upper epidermis showed a significant increase compared to the control group. More precisely, at 60% and 200 it reaches 53.7%. In contrast, the thickness of the lower epidermis only increased by 28.7% in treatment of 60% and 200 ppm.
Fig. 1.
Transverse sections of Pelargonium graveolens leaf treated with draught and kaolin (10X)
Table 8.
Effect of irrigation levels and kaolin rates on anatomical parameters of Pelargonium graveolens leaves (mm)
| Characters (mm) | Water levels based on ETo | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 100 | 80 | 60 | ||||||||
| Kaolin rates (ppm) | ||||||||||
| Control | 100 | 200 | 300 | 100 | 200 | 300 | 100 | 200 | 300 | |
| Upper epidermis thickness | 23.784 | 26.918 | 36.570 | 15.833 | 30.940 | 26.735 | 20.610 | 31.249 | 38.745 | 23.076 |
| Lower epidermisthickness | 45.264 | 25.672 | 29.530 | 15.413 | 27.424 | 21.891 | 22.700 | 22.022 | 58..272 | 44.731 |
| Palisade tissue thickness | 52.888 | 55.680 | 54.223 | 48.678 | 92.508 | 37.208 | 59.492 | 72.238 | 108.679 | 57.760 |
| Spongy tissue thickness | 120.045 | 156.439 | 155.532 | 70.400 | 111.901 | 94.670 | 116.138 | 192.979 | 198.618 | 106.817 |
| Mesophyll thickness | 172.933 | 62.945 | 101.955 | 137.673 | 212.875 | 132.344 | 190.717 | 75.966 | 307.297 | 195.067 |
| Midvein vascular bundle | ||||||||||
| Width | 239.631 | 288.736 | 297.787 | 241.664 | 744.510 | 179.936 | 348.257 | 219.993 | 289.898 | 289.213 |
| Length | 268.365 | 251.619 | 242.135 | 222.243 | 683.667 | 194.076 | 308.018 | 180.018 | 364.592 | 347.932 |
mm Millimeters
Under the influence of irrigation conditions and different concentrations of kaolin, the palisade and sponge fabrics containing mesophyll showed significant improvement. In particular, at a kaolin concentration of 80% and under irrigation, the palisade fabric showed a remarkable improvement of 23%. Similarly, terry cloth showed a remarkable improvement of 77.7% when exposed to 100 ppm kaolin concentration and irrigation conditions. These results highlight the positive influence of irrigation and specific kaolin concentrations on the development and performance of mesophyll-containing tissues.
The width and length of the vascular bundle experienced a substantial increase of 210.6% and 154.7%, respectively, when subjected to concentrations of 80% and 100 ppm, as observed in studies conducted by [50] on Triticum aestivum [51] on Solanum lycopersicum L. Application of anti-transpirant under water deficit conditions resulted in improvement of all histological parameters. The use of three different anti-transpirant, at lower and higher concentrations, under water deficit conditions led to an increase in the thickness of the wheat leaf blade. This increase was attributed to thickening of the mesophyll tissue and vascular bundle, as well as a significant reduction in stomata opening at the upper and lower epidermis, compared to the control group [52] observed that anti transpirant do not cause permanent damage to the stomata mechanism and have specific effects on guard cells, without affecting other cells.
The aforementioned results provided evidence for the importance of kaolin, as discussed by [53, 54]. This function encompasses alterations of crucial morphological, physiological and biochemical processes through enhancement of radiation reflection. Initially, kaolin prevents the accumulation of thermal charge, thereby decreasing transpiration, while maintaining relatively high stomatal conductance.
Drought deficit has been shown to have obvious effects on the histological composition of plants, as shown in various experiments. Leaves of 'Chemlali' cultivar showed a significant increase in the thickness of spongy parenchyma and upper palisade under water deficit. The thicker palisade parenchyma in leaves may indicate to more CO2 fixation sites, while the thicker spongy parenchyma may facilitate more rapid diffusion of CO2 to these sites [55, 56] suggest that a decrease in water content in the plant body promotes cell wall strengthening and reduces turgor pressure. In the study [57], in agreement to negative impact of draught to plant tissue that led to decrease in lamina thickness and drought stress may decrease vessel diameter ris considered as an adaptation mechanism that protect plants from water deficiency caused by leaf transpiration and affects the conductance of CO2 diffusion that play acrucial role to avoid cavitation. Moreover, reductions in metaxylem diameter and vascular bundle size are normally in plant exposed to drought stress.
Effect of water deficit and kaolin rates on photosynthetic pigment and carotenoid content
Chlorophyll a (6.93 mg/g FW) and chlorophyll b (3.00 mg/g FW) concentrations recorded the value highest in plants irrigated with 100% of ETo combined with the highest rate of kaolin (300 ppm), (Table 9). Furthermore, when 100% of ETo was combined with the treatment of kaolin at 200 ppm, a notable level of kaolin carotenoid content (4.44 mg/g FW) was noted. Conversely, the lowest levels of kaolin (100 ppm) in combination with 60% ETo irrigation resulted in the lowest quantities of chlorophyll a (1.31 mg/g FW) and chlorophyll b (0.08 mg/g FW). Furthermore, the combination of 100 ppm spraying kaolin and 80% of ETo irrigation resulted the lowest concentration of carotenoid (0.35 mg/g FW). The geranium plant experienced a decrease in chlorophyll and carotenoid content due to the suppression or degradation of chlorophyll biosynthesis caused by water deficit. As the duration of drought deficit in plants prolonged, the pigment content decreased significantly. However, the application of kaolin had a contrasting effect, leading to a significant increase in the chlorophyll and carotenoid content of the leaves. This finding is consistent with previous studies conducted on walnut [56] and grapevine [57]. On Loss of chlorophyll as a result of photo oxidation and subsequent oxidative damage is one effect of drought stress [58]. According to [15] on maize plant decrease in chlorophyll content, photosynthetic, net photosynthetic rate, and transpiration rate can be viewed as a sign of oxidative stress. while, kaolin led to increased photosynthetic pigments. This shows that the plants are more resilient to drought stress, which could lead to a higher energy efficiency in photosynthetic process. Same result as indicated by [14] on Corylus avellana. According to [59] on Amaranthus tricolor's pigment content gradually decreased as drought stress increased, which is in line with our observations. Similar decreases in pigment concentration were also noted by [60] in peanuts and by [9] in Mentha pulegium.
Table 9.
Effect of irrigation levels and kaolin rates on chlorophyll (a, b) and carotenoid concentration (mg g-1 F.W.) of Pelargonium graveolens plant
| Pigment | Water levels based on ETo | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 | 80 | 60 | ||||||||||
| Kaolin rates (ppm) | ||||||||||||
| 0.0 | 100 | 200 | 300 | 100 | 200 | 300 | 100 | 200 | 300 | |||
| Total Chl | 7.06 ± 0.80e | 14.36 ± 4.21b | 14.42 ± 3.33b | 17.15 ± 3.34a | 5.78 ± 1.34ee | 9.97 ± 2.76c | 12.92 ± 3.46d | 1.74 ± 1.01 g | 4.61 ± 1.66f | 4.82 ± 1.78f | ||
| Chl a | 5.32 ± 3.07b | 5.22 ± 3.01b | 6.81 ± 3.93a | 7.09 ± 4.09a | 2.53 ± 1.46de | 3.34 ± 1.93 cd | 4.07 ± 2.35c | 1.31 ± 0.76f | 1.73 ± 1.00ef | 2.97 ± 1.72d | ||
| Chl b | 2.07 ± 0.04b | 1.72 ± 0.12c | 2.24 ± 0.12b | 3.00 ± 0.29a | 0.34 ± 0.02de | 0.35 ± 0.02de | 0.48 ± 0.02d | 0.08 ± 0.01e | 0.09 ± 0.01e | 0.3 ± 0.06de | ||
| Caro | 0.71 ± 0.06e | 3.40 ± 0.01b | 4.44 ± 0.02a | 2.86 ± 0.02c | 0.35 ± 0.01e | 4.22 ± 0.34a | 2.40 ± 0.23 cd | 2.40 ± 0.12 cd | 2.31 ± 0.17d | 2.77 ± 0.17 cd | ||
Data are the means ± SE of three different experiments with three replicated measurements; different letters within a column indicate significant differences (P < 0.05) of variation
Chl. Chlorophyll, Chl a Chlorophyll a, Chl b Chlorophyll b, Caro. Carotenoid. (mg g−1 F.W.)
Effect of irrigation levels and kaolin rates on antioxidant enzymes activities
Table 10 presents the results of the investigation into the impact of water deficit treatments and different levels of kaolin on metabolizing enzymes. The study focused on leaves of Pelargonium graveolens, specifically analyzing the activity levels of SOD, POD and CAT in its leaves. The results indicated a notable increase in SOD and POD activities, while CAT activity showed a decline under drought treatment. However, the negative effects of water deficit were mitigated by the application of kaolin to the plant.
Table 10.
Effect of irrigation levels and kaolin rates on antioxidant enzymes activities (U/g/ FW/hour) of Pelargonium graveolens plant
| enzymes | Water levelsbased on ETo | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 | 80 | 60 | ||||||||||
| Kaolin rates (ppm) | ||||||||||||
| 0.0 | 100 | 200 | 300 | 100 | 200 | 300 | 100 | 200 | 300 | |||
| SOD | 0.3 ± 0.03def | 0.38 ± 0.02cde | 0.23 ± 0.01ef | 0.15 ± 0.03f | 1.28 ± 0.03a | 0.49 ± 0.02c | 0.45 ± 0.03 cd | 1.35 ± 0.09a | 1.05 ± 0.03b | 0.9 ± 0.12b | ||
| CAT | 0.05 ± 0.01de | 0.5 ± 0.06b | 0.92 ± 0.12a | 0.95 ± 0.03a | 0.15 ± 0.02cde | 0.17 ± 0.01 cd | 0.23 ± 0.06c | 0.02 ± 0.01e | 0.11 ± 0.01 cde | 0.17 ± 0.02 cd | ||
| POD | 0.18 ± 0.01 cd | 0.19 ± 0.02 cd | 0.18 ± 0.02 cd | 0.13 ± 0.02d | 0.25 ± 0.03bc | 0.21 ± 0.01 cd | 0.2 ± 0.2 cd | 0.34 ± 0.02a | 0.31 ± 0.06ab | 0.3 ± 0.03ab | ||
Data are the means ± SE of three different experiments with three replicated measurements; different letters within rows indicate significant differences (P < 0.05) of variation
SOD Superoxide dismutase, POD Hydrogen peroxidase, CAT Catalase
In Table 10 droughtness of soil preferentially increased the activities of superoxide dismutase (SOD) and peroxidase (POD), which increased the rate from 0.3 to 1.35 and from18 to 34 U/g/F.W./hour, respectively, while catalase activity (CAT) declined. These results are in complete agreement with those reported by [61] mention that, the upregulation of antioxidant enzymes represents an important marker for drought stress. In the cell, the production and scavenging of ROS is strictly controlled by an efficient and versatile scavenging system. The antioxidant defense system comprises enzymes such as including CAT, and SOD. While [62] found that on Oryza sativa L. CAT and SOD were an important enzyme used to eliminate H2O2. With the prolongation of drought time, observed that the CAT activity increased in leaves. While POD activity increased at the beginning of the drought and then seemed to enter a platform stage. The same results were absolved at [63] on wheat.
The result obtained that foliar application of kaolin increases the activity of antioxidant enzymes. The results are in agreement with [64], from studies on apple kaolin helps plants withstand environmental stress by stimulating their enzyme systems and preserving their cell membrane. spraying kaolin on apple leaves enhanced the activity of antioxidant enzymes such as glutathione reductase, superoxide dismutase, catalase, and ascorbate peroxidase during drought stress. And in align with [14], who indicated that. kaolin applications on hazelnut increased proline and antioxidant enzymes. Conversely, there was a decrease in protein concentration, H2O2 level, and lipid peroxidation.
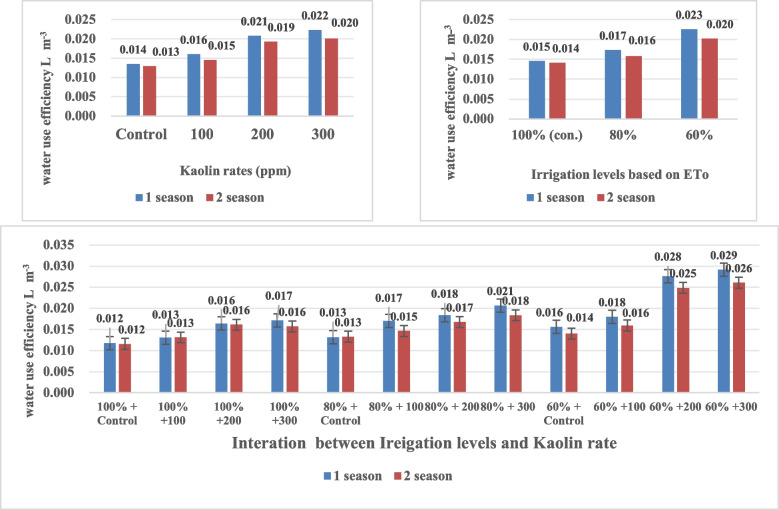
Effect of irrigation levels and kaolin rates on water use efficiency (WUE)
Results of geranium WUE are affected by water deficit and kaolin rate as shown in Fig. 2. The highest values of WUE were obtained by 60% of ETo (0.23 and 0.20 L/m3) in 1st and 2nd seasons, respectively. These results are in agreement with [65] who mentioned that both WUE and yield can be improved under drought deficit.
Fig. 2.
Effect of irrigation levels and Kaolin rate on water use efficiency in total oil yield of Pelargonium graveolens during two season
Kaolin at rates of 200 and 300 ppm showed an improving WUE due to reducing losses of water through evaporation by closed leaves stomata during different growth stages. According to, improved transportation of all soluble substances leads to better growth and yield. The same results were obtained at [34] on gooseberry plants.
Conclusion
It concluded that geranium plant growth characteristics, fresh herb yield/fed, and essential oil yield/fed gradually decreased as the water deficit increased. Comparing fresh herb yield/fed and essential oil yield/fed to regular irrigation, the biggest decreases occurred at a water level of 60% based on ETo, which was 27.77% and 0.73%, respectively. However, when the water level dropped, plants that were irrigated 60% of the time according to ETo showed increases in leaf thickness and E.O.%. of 36.73% and 23.08%, respectively. According to the findings, kaolin foliar application at 200 and 300 ppm may help treat water deficit disorders by improving anatomical features. Compared to the untreated plant group, the upper epidermis' thickness considerably increased. More precisely, at 60% and 200, it reaches 53.7%. Additionally, the thickness of the lower epidermis increased by 28.7%. It has been shown that irrigation and specific kaolin concentrations improve the development and functionality of mesophyll tissues. Furthermore, kaolin dramatically raises the leaves' carotenoid and chlorophyll contents. And kaolin makes antioxidant enzymes more active. Additionally, when compared to untreated plants grown under a 60% water deficit based on ETo, kaolin spraying enhances the quality of essential oil by increasing geraniol at the expense of citronellol. This results in a 35% increase in water use efficiency and a yield/feed of essential oils of over 117.86%.
Recommendation
Apply water deficit treatments one month before harvest and from the flower bud’s initiation phase to avoid a decrease in the vegetative yield and benefit from the increase in the oil percentage and quality resulting from the water deficit.
Suggestions to the future
We need to conduct further research using kaolin in a different form (nano form), study it at the molecular level to understand gene expression, use the photosynthesis parameters determined by the Licor system, and apply it to other stressors.
Acknowledgements
The research facilities were made possible with the assistance of the agricultural Research center, Giza, Egypt, National Authority for Remote Sensing and Space Sciences, Cairo, Egypt, and Cairo University, Giza, Egypt. The authors express their gratitude towards these entities for their support.
Authors’ contributions
Eman F. AbuEl-Leil: “Conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing—original draft preparation, writing—review and editing, visualization, supervision, project administration, funding acquisition”, Mohamed A. E. Abdel Rahaman: “Conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing—original draft preparation, writing—review and editing, visualization, supervision, project administration, funding acquisition”, and S. F. Desoukey: “Conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing—original draft preparation, writing—review and editing, visualization, supervision, project administration, funding acquisition”. All authors have read and agreed to the published version of the manuscript.”
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research received no external funding.
Data availability
Data can be made available upon reasonable request from the corresponding author (M.A.E.A.) maekaoud@gmail.com.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.El-Tahawey MA, Gharib AH, El Sayed AA. Stability and variability of the herb and volatile oil traits in geranium (Pelargonium graveolens L.). Hortscience Journal of Suez Canal University. 2020;9(1):49–56. 10.21608/HJSC.2020.156295.
- 2.Eiasu BK, Dyafta V, Araya HT. Effect of leaf age on essential oil yield and composition in rose-scented geranium. Hortscience. 2022;57(12):1524–1528. 10.21273/HORTSCI15048-20.
- 3.Calamai A, Palchetti E, Masoni A, Marini L, Chiaramonti D, Dibari C, Brilli L. The Influabbiience of Biochar and Solid Digestate on Rose-Scented Geranium (Pelargonium graveolens L'Hér.) Productivity and Essential Oil Quality. Agronomy. 2019;9(5):260. 10.3390/agronomy9050260.
- 4.Palchetti E, Calamai A, Valenzi E, Vecchio V. Pelargonio (Pelargonium graveolens). In Oli e Grassi, 1st ed.; Edagricole New Business Media: Milano, Italy, (2019). ISBN 978–88–506–5564–9.
- 5.Mazrou RM, Hassan FA, Mansour MMF, Moussa MM. Melatonin Enhanced Drought Stress Tolerance and Productivity of Pelargonium graveolens L. (Herit) by Regulating Physiological and Biochemical Responses. Horticulturae. 2023;9(11):1222. 10.3390/horticulturae9111222.
- 6.Münchinger IK, Hajek P, Akdogan B, Caicoya AT, Kunert N. Leaf thermal tolerance and sensitivity of temperate tree species are correlated with leaf physiological and functional drought resistance traits. J Forestry Res. 2023;34(1):63–76. 10.1007/s11676-022-01594-y.
- 7.Shu HY, Liu YY, Zhang XY, Tan XF, Li Z. Effects of drought stress on the physiology, photosynthesis, and anatomical structure of container and bareroot plants of two Camellia oleifera cultivars. This work is licensed under a CC BY 4.0 license. 2024. 10.21203/rs.3.rs-4376858/v1.
- 8.Iqbal MS, Singh AK, Ansari MI. Effect of Drought Stress on Crop Production. In New Frontiers in Stress Management for Durable Agriculture; Rakshit, A., Singh, H.B., Singh, A.K., Singh, U.S., Fraceto, L., Eds.; Springer: Singapore. 2020:35–47. 10.1007/978-981-15-1322-0_3.
- 9.Ulusu F, Tümer K, Ulusu Y. Antioxidant responses to drought stress in pennyroyal (Mentha pulegium) J Scientific Rep-A, no. 051, pp. 26–48. 2022. https://dergipark.org.tr/en/pub/jsr-a/issue/73021/1146859.
- 10.Farouk S, Omar MM. Sweet basil growth, physiological and ultrastructural modification, and oxidative defense system under water deficit and silicon forms treatment. J. Plant Growth Regul. 2020;39:1307–1331. 10.1007/s00344-020-10071-x.
- 11.Shehzad MA, Nawaz F, Ahmad F, Ahmad N, Masood S. Protective effect of potassium and chitosan supply on growth, physiological processes, and anti-oxidative machinery in sunflower (Helianthus annuus) under drought stress. Ecotoxicol Environ Saf. 2020;187: 109841. 10.1016/j.ecoenv.2019.109841. [DOI] [PubMed] [Google Scholar]
- 12.Farouk S, Al-Huqail AA. Sodium nitroprusside application regulates antioxidant capacity, improves phytopharmaceutical production, and essential oil yield of marjoram herb under drought. Ind Crops Prod. 2020;158:113034. 10.1016/j.indcrop.2020.113034.
- 13.Terán F, Vives-Peris V, López-Climent MF, Gómez-Cadenas A, Pérez-Clemente RM. Palliative effects of kaolin on citrus plants under controlled stress conditions of high temperature and high light intensity. J Plant Growth Reg. 2024;1–14. 10.1007/s00344-023-11103-y.
- 14.Khavari M, Fatahi R, Zamani Z. Salicylic acid and kaolin effects on pomological, physiological, and phytochemical characters of hazelnut (Corylus avellana) at warm summer condition. Sci Rep. 2021;11(1):4568. 10.1038/s41598-021-83790-0. [DOI] [PMC free article] [PubMed]
- 15.Semida W, Emara A, Ghoneim IM, Barakat MA. Kaolin foliar application enhanced physiological functions and pods quality of (phaseolus vulgaris L.) under deficit irrigation regimes. Labyrinth: Fayoum J Sci Interdisciplin Stud. 2023;1(1):84–94. 10.21608/ifjsis.2023.302858.
- 16.Al-Mokadem AZ, Sheta MH, Mancy AG, Hussein HAA, Kenawy SK, Sofy AR, Abu-Shahba MS, Mahdy HM, Sofy MR, Al Bakry AF, Agha MS. Synergistic effects of Kaolin and Silicon Nanoparticles for ameliorating deficit irrigation stress in maize plants by upregulating antioxidant Defense systems. Plants. 2023;12(11):2221. 10.3390/plants12112221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapman HD, Pratt PF. Methods of analysis for soils, plants, and water. Univ. California, Berkeley, CA, USA. 1961:309. 10.2136/sssaj1963.03615995002700010004x.
- 18.Israelson OW, Hansen VE. Irrigation principles and practices. Third edition, John Wiley and Sons, Inc., New York, USA. 1962:448. 10.2136/sssaj1963.03615995002700020010x.
- 19.FAO. Physical and chemical methods of soil and water analysis. Soils Bull. No. 10, FAO, Rome, Italy. 1970. 10.5555/19711901105.
- 20.Allam MN, Abdel-Azim R. Review and analysis of water use efficiency and water productivity in Egypt. Options Méditerranéennes, Series B. 2007;(57). http://om.ciheam.org/article.php?IDPDF=800780.
- 21.ASTA. Official lAnalyticai methods of the American Spice Trade Association, ASTA. Engeleweed Cliffs. 1985;1:33–17.
- 22.Hoftman, E. ‘Chromotography, Reinhild.’, Corp., 2nd Ed., 1967; pp. 208–515.
- 23.Bunzen J, Guichard N, Labbr P, Prevot J, Trenchant J. In Practical Manual of Gas Chromatography. (J. Ttenchant, Ed.). El-Seivier, Publ. Comp., Amesterdam, Netherland. 1969.
- 24.Sass JS. Botanical Microtechnique: 2nd Edition, the Iowa State College Press building, Ames, Iowa. 1951; pp.228.
- 25.Wellburn AR. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolutions. J Plant Physiol. 1994;144:307–13. 10.1016/S0176-1617(11)81192-2. [Google Scholar]
- 26.Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed]
- 27.Witham FH, Blaydes DF, Devlin RM. Experiments in plant physiology. Van Nostrand Reinhold Co, New York. 1971.
- 28.Aebi H. Catalase in Vitro Methods Enzymology. 1984;105:121–6. 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 29.Fossati P, Prencipe L, Berti G. Use of 3,5-Dichloro-2-Hydroxybenzenesulfonic Acid/4-Aminophenazone Chromogenic System in Direct Enzymic Assay of Uric Acid in Serum and Urine. Clin Chem. 1980;26:227–31. 10.1093/clinchem/26.2.227. [PubMed] [Google Scholar]
- 30.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169. https://www.translationalres.com/article/0022-2143(67)90076-5. [PubMed]
- 31.Nishikimi M, Roa NA, Yogi K. The Occurrence of Superoxide Anion in the Reaction of Reduced Phenazine Methosulfate and Molecular Oxygen. Biochemical Biophysical Research Communications. 1972;46:849–54. 10.1016/S0006-291X(72)80218-3. [DOI] [PubMed] [Google Scholar]
- 32.Steel RG, Torrie JH. Principles and procedures of statistics. A biometrical approach. McGraw. Hill Book Company, USA. 1980;633 p.10.5555/19810721475.
- 33.Ahmed AF, Yu H, Yang X, Jiang W. Deficit irrigation affects growth, yield, vitamin C content, and irrigation water use efficiency of hot peppers grown in soilless culture. Hortscience. 2014;49(6):722–728. 10.21273/HORTSCI.49.6.722.
- 34.Segura-Monroy S, Uribe-Vallejo A, Ramirez-Godoy A, Restrepo-Diaz H. Effect of Kaolin application on growth, water use efficiency, and leaf epidermis characteristics of Physallis peruviana seedlings under two irrigation regimes. J Agri Sci Technol. 2015;17(6):1585–1596. http://jast.modares.ac.ir/files/jast/user_files_749497/archive_global-A-23-1000-1894-6979cc1.
- 35.Khalili M, Nejatzadeh F. Effect of deficit irrigation and kaolin clay on yield and yield components of pumpkin (Cucurbita pepo L.). S N Applied Sciences 2021;3:1–11. 10.1007/s42452-021-04536-1.
- 36.Blum A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant, Cell Environ. 2017;40(1):4–10. 10.1111/pce.12800. [DOI] [PubMed] [Google Scholar]
- 37.Siddiqui MH, Alamri S, Alsubaie QD, Ali HM, Khan MN, Al-Ghamdi A, Ibrahim AA, Alsadon A. Exogenous nitric oxide alleviates sulfur deficiency induced oxidative damage in tomato seedlings. Nitric Oxide. 2020;94:95–107. 10.1016/j.niox.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Hong-Bo Shao ABD, Li-Y E, Chu D, Cheruth AJC, Chang-Xing Z. Water-deficit stress-induced anatomical changes in higher plants. H.-B. Shao et al. / C. R. Biologies. 2008;331:215–225. 10.1016/j.crvi.2008.01.002. [DOI] [PubMed]
- 39.Sayyari M, Ghanbari F. Effects of super absorbent polymer A200 on the growth, yield, and some physiological responses in sweet pepper (Capsicum annuum L.) under various irrigation regimes. Intl. J. Agr. Food Res. 2012;1:1–11. 10.24102/ijafr.v1i1.123.
- 40.Reddy DVB, Ramesh N, Manimaran S, Thangavel P. Effect of organic mulches and foliar spray of kaolin on NPK uptake in enhancing yield and economics of dry land maize (Zea mays L.). J Appl Nat Sci. 2023;15(1):116–119. 10.31018/jans.v15i1.4192.
- 41.Bahreininejad B, Razmjoo J, Mirza M. Influence of water stress on morpho-physiological and phytochemical traits in Thymus daenensis. Intl. J. Plant Prod. 2013;7:155–166. https://ijpp.gau.ac.ir/article_927_7275c0f18373026df9480f943ccca109.
- 42.Afshari M, Pazoki A, Sadeghipour O. Foliar‐applied silicon and its nanoparticles stimulate physio‐chemical changes to improve growth, yield and active constituents of coriander (Coriandrum Sativum L.) Essential oil under different irrigation regimes. Silicon. 2021;13:4177–4188. 10.21203/rs.3.rs-176146/v1.
- 43.Mohasseli V, Sadeghi S. Exogenously applied sodium nitroprusside improves physiological attributes and essential oil yield of two drought susceptible and resistant specie of thymus under reduced irrigation. Ind Crops Prod. 2019;130:130–6. 10.1016/j.indcrop.2018.12.058. [Google Scholar]
- 44.Dinis LT, Pereira S, Fraga I, Rocha SM, Costa C, Martins C, Vilela A, Arrobas M, Moutinho-Pereira J. Kaolin foliar spray induces positive modifications in volatile compounds and fruit quality of Touriga-Nacional red wine. OENO One. 2024;58(2). 10.20870/oeno-one.2024.58.2.7945.
- 45.Rania MRK, Al-Azzony EAA. Kaolin and irrigation intervals affect growth and essential oil of sweet basil. Middle East J Appl Sci. 2019;10(04):792–802. 10.36632/mejas/2020.10.4.70.
- 46.Rania M, Khater. Effect of hydrogel and antitranspirants treatments on the productivity of sweet basil (Ocimum basilicum L.) plant. Egyptian J. Desert Res. 2015;65(2):193–214. https://ejdr.journals.ekb.eg/article_5950_3ff9f67943aaac752ab08106af4491e1.
- 47.Saxena G, Rahman L, Verma PC, Banerjee S, Kumar S. Field performance of somaclones of rose scented geranium (Pelargonium graveolensL’Her Ex Ait.) for evaluation of their essential oil yield and composition. Ind. Crops Prod. 2008;27:86–90. 10.1016/j.indcrop.2007.08.001.
- 48.Simon JE, Reiss BO, Joly RJ, Charles DJ. Water stress induced alteration in essential oil content of sweet basil. J Essential Oil Res. 1992;1:71–5. 10.1080/10412905.1992.9698013. [Google Scholar]
- 49.Metwally SA, Shaheen MS, Mohamed SL, Abou-Leila BH. Effect of different water regimes interacted with zinc on growth and chemical composition of Geranium. World J Agri Sci. 2013;9(5):389–396. 10.5829/idosi.wjas.2013.9.5.14065.
- 50.Desoky EM, Tohamy MR, Eisa GS, EL- Sarkassy. Effect of some anti-transpirant substances on growth, yield and flag leaf structure of wheat plant (Triticum aestivum L.) Grown under water stress conditions. Zagazig J. Agric. Res. 2013;40(2):16.
- 51.Abd Allah A. Impacts of kaolin and Pinoline foliar application on growth, yield and water use efficiency of tomato (Solanum lycopersicum L.) grown under water deficit: A comparative study. J Saud Soc Agri Sci. 2019;18(3):256–268. 10.1016/j.jssas.2017.08.001.
- 52.Rao G, Babu M, Sravan V, Sindhuja MA. Review on-influence of Antitranspirants (ATs) in vegetable crops. Int. J. Pure App. Biosci. 2018;6(3):394–399. IJPAB-SPE-2018–6–3–394–399.
- 53.Brito C, Dinis L, Moutinho-Pereira J, Correia C. Kaolin, an emerging tool to alleviate the effects of abiotic stresses on crop performance. Sci Hortic. 2019;250:310–6. 10.1016/j.scienta.2019.02.070. [Google Scholar]
- 54.Guleria V, Shweta. Anti-transpirants: an effective approach to mitigate the stress in field crops. Int J Curr Microbiol App Sci. 2020;9(5):1671–1678. 10.20546/ijcmas.2020.905.188.
- 55.Taratima W, Ritmaha T, Jongrungklang N, Maneerattanarungroj P. Leaf anatomical adaptation under early drought stress of sugarcane cultivars–KKU-1999–02 and KKU-1999–03. Acta Agrobotanica. 2021;74(1). 10.5586/aa.7419.
- 56.Gharaghani A, Javarzari AM, Vahdati K. Kaolin particle film alleviates adverse effects of light and heat stresses and improves nut and kernel quality in Persian walnut. Sci Hortic. 2018;239:35–40. 10.1016/j.scienta.2018.05.024. [Google Scholar]
- 57.Dinis L-T, Bernardo S, Conde A, Pimentel D, Ferreira H, Félix L, Geros H, Correia C, Moutinho-Pereira J. Kaolin exogenous application boosts antioxidant capacity and phenolic content in berries and leaves of grapevine under summer stress. J. Plant Physiol. 2016;191:45–53. 10.1016/j.jplph.2015.12.005. [DOI] [PubMed]
- 58.Karimi S, Tavallali V. Interactive effects of soil salinity and boron on growth, mineral composition and CO2 assimilation of pistachio seedlings. Acta Physiol. Plant. 2017;39(11):242. 10.1007/s11738-017-2545-z.
- 59.Sarker U, Oba S. Drought stress effects on growth, ROS markers, compatible solutes, phenolics, flavonoids, and antioxidant activity in Amaranthus tricolor. Appl Biochem Biotechnol. 2018;186(4):999–1016. 10.1007/s12010-018-2784-5. [DOI] [PubMed]
- 60.Meher Shivakrishna P, Reddy KA, Rao DM. Effect of PEG-6000 imposed drought stress on RNA content, relative water content (RWC), and chlorophyll content in peanut leaves and roots. Saud J Biol Sci. 2018;25(2):285. 10.1016/j.sjbs.2017.04.008. [DOI] [PMC free article] [PubMed]
- 61.Laxa M, Liebthal M, Telman W, Chibani K, Dietz K. The role of the plant antioxidant system in drought tolerance. Antioxidants. 2019;8:94. 10.3390/antiox8040094. [DOI] [PMC free article] [PubMed]
- 62.Wang X, Liu H, Yu F, Hu B, Jia Y, Sha H, Zhao H. Diferential activity of the antioxidant defence system and alterations in the accumulation of osmolyte and reactive oxygen species under drought stress and recovery in rice (Oryza sativa L.) tillering. 2019. 10.1038/s41598-019-44958-x. [DOI] [PMC free article] [PubMed]
- 63.Bhardwaj RD, Singh N, Sharma A, Joshi R, Srivastava P. Hydrogen peroxide regulates antioxidant responses and redox-related proteins in drought-stressed wheat seedlings. Physiol Mol Biol Plants. 2021;27(1):151–163. 10.1007/s12298-021-00937-z. [DOI] [PMC free article] [PubMed]
- 64.Faghih S, Zamani Z, Fatahi R, Omidi M. Influence of kaolin application on most important fruit and leaf characteristics of two apple cultivars under sustained deficit irrigation. Biol Res. 2021;54. 10.1186/s40659-020-00325-z. [DOI] [PMC free article] [PubMed]
- 65.Yu L, Gao X, Zhao X. Global synthesis of the impact of droughts on crops’ water-use efficiency (WUE): Towards both high WUE and productivity. Agri Syst. 2020;177:102723. 10.1016/j.agsy.2019.102723.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be made available upon reasonable request from the corresponding author (M.A.E.A.) maekaoud@gmail.com.