Abstract
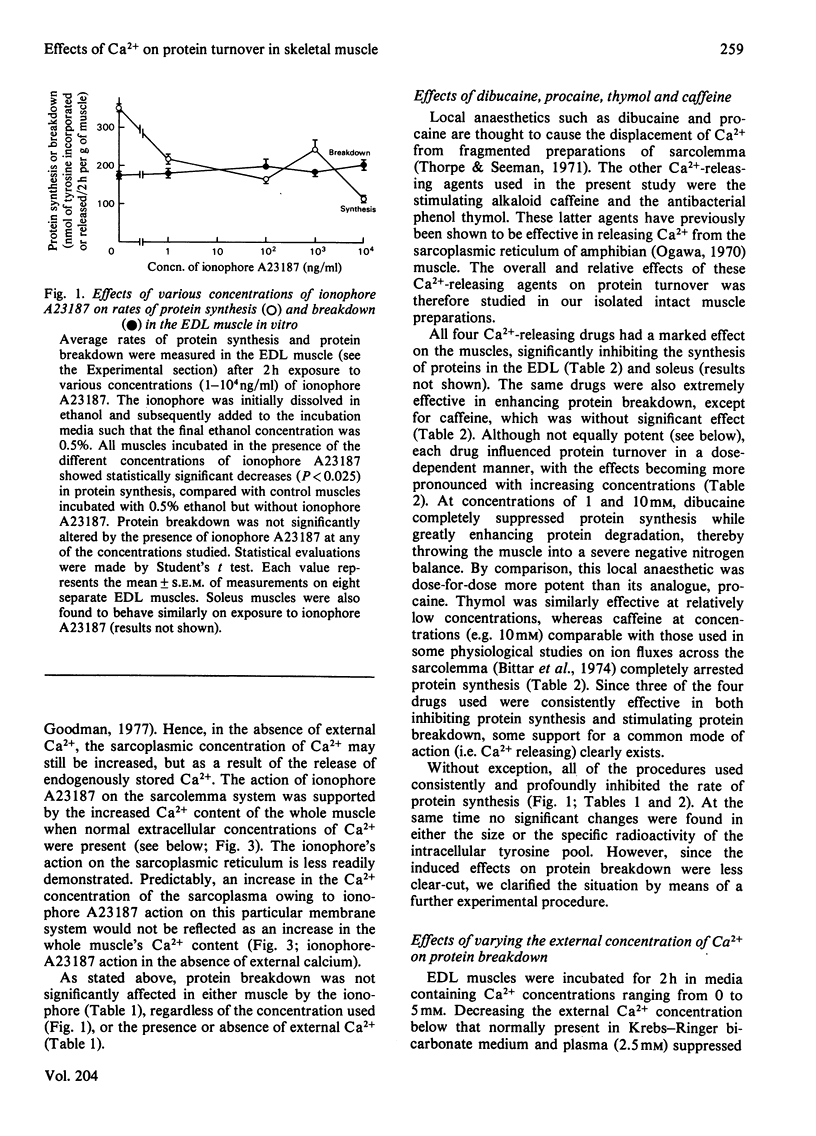
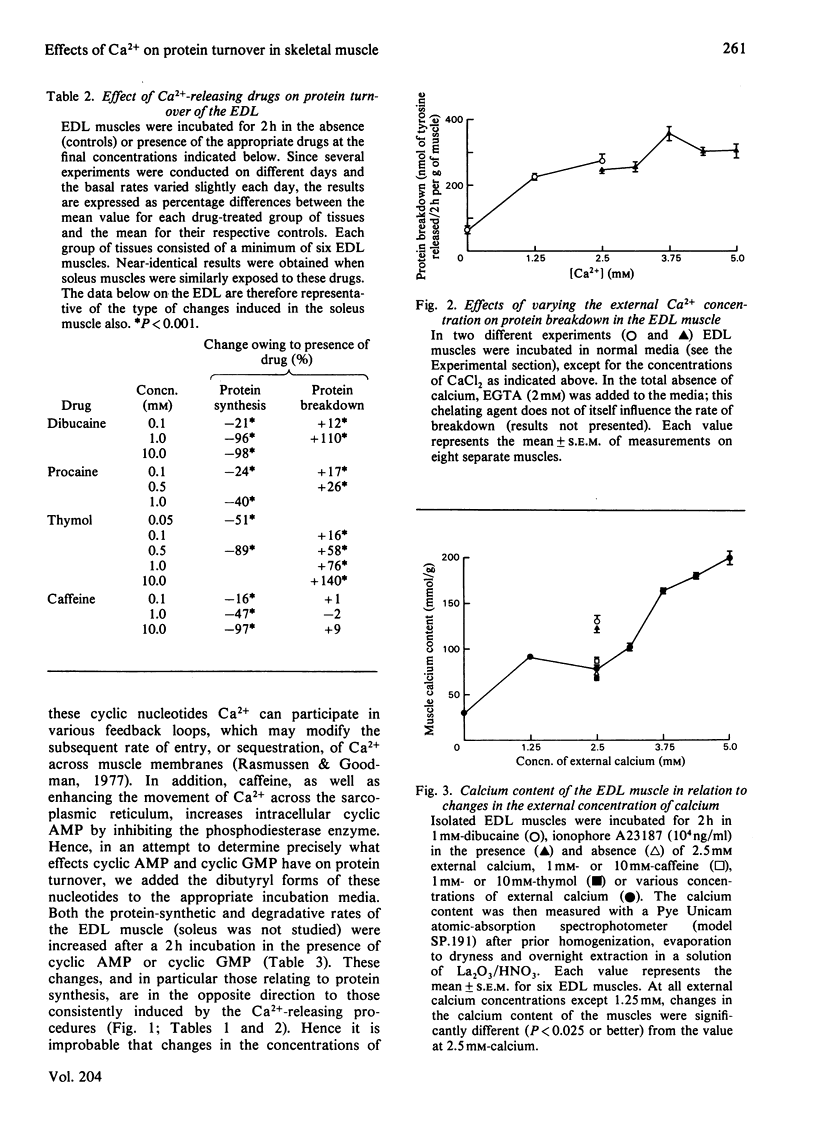
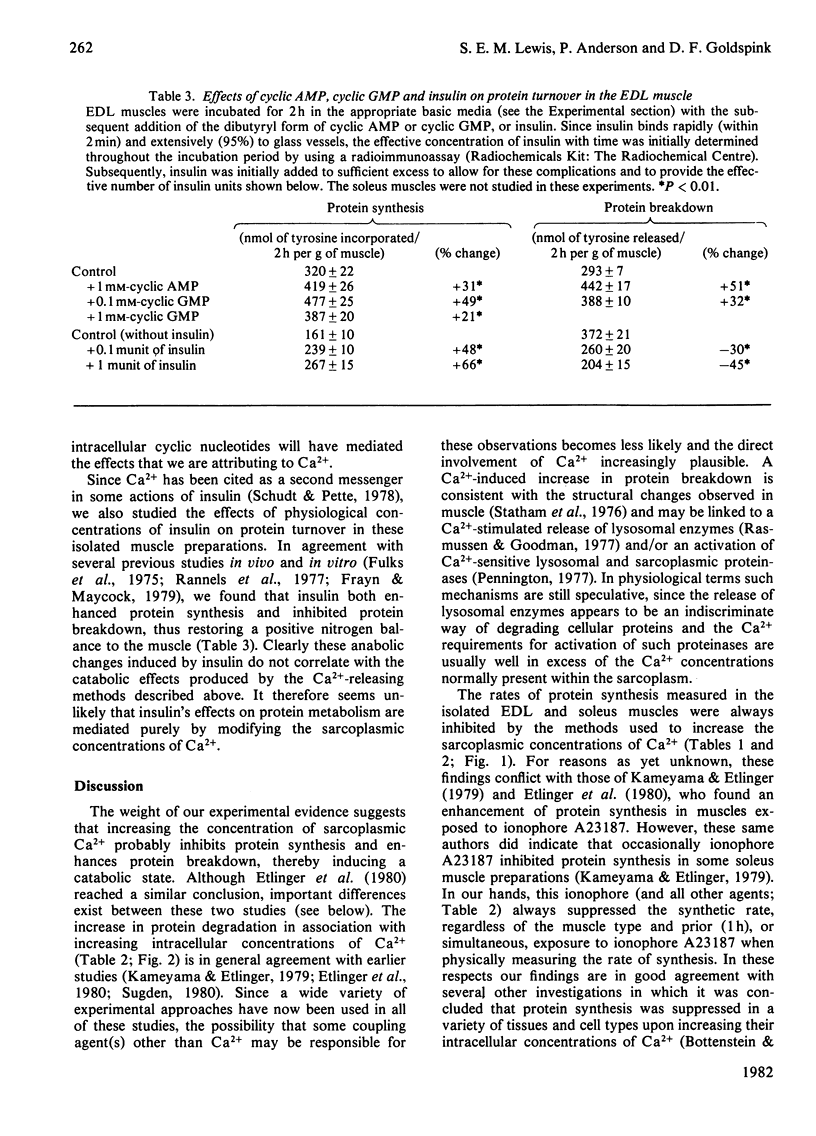
Several experimental procedures were used to increase the intracellular concentration of Ca2+ and determine its effects on protein turnover in isolated extensor digitorum longus and soleus muscle. These methods included the use of ionophore A23187, caffeine, dibucaine, thymol and procaine, all agents known to induce the release of calcium by acting either on the sarcolemma and/or on the sarcoplasmic reticulum. Another approach involved varying the external concentration of Ca2+ in the media in which the muscles were incubated. The changes in muscle Ca2+ concentrations after exposure to the various calcium-releasing agents were in keeping with accepted modes of action of these agents on muscle membranes. The findings suggest that increasing the sarcoplasmic concentration of Ca2+ inhibits protein synthesis and enhances protein breakdown. These catabolic effects of Ca2+ are compared with the changes induced in muscle protein turnover after exposure to insulin or cyclic nucleotides, and in myopathic muscle and situations of work overload. Attention is also drawn to some of the difficulties involved in definitively implicating Ca2+ as a factor involved in the normal regulation of protein turnover.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Blinks J. R. Calcium transients in aequorin-injected frog cardiac muscle. Nature. 1978 Jun 15;273(5663):509–513. doi: 10.1038/273509a0. [DOI] [PubMed] [Google Scholar]
- Ashley C. C., Ridgway E. B. On the relationships between membrane potential, calcium transient and tension in single barnacle muscle fibres. J Physiol. 1970 Jul;209(1):105–130. doi: 10.1113/jphysiol.1970.sp009158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittar E. E., Hift H., Huddart H., Tong E. The effects of caffeine on sodium transport, membrane potential, mechanical tension and ultrastructure in barnacle muscle fibres. J Physiol. 1974 Oct;242(1):1–34. doi: 10.1113/jphysiol.1974.sp010691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottenstein J. E., de Vellis J. Divalent cation ionophore A23187: a potent protein synthesis inhibitor. Biochem Biophys Res Commun. 1976 Nov 22;73(2):486–493. doi: 10.1016/0006-291x(76)90733-6. [DOI] [PubMed] [Google Scholar]
- Clausen T., Martin B. R. The effect of insulin on the washout of [45Ca]calcium from adipocytes and soleus muscle of the rat. Biochem J. 1977 Apr 15;164(1):251–255. doi: 10.1042/bj1640251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S. Calcium ions and muscle contraction. Nature. 1972 Nov 24;240(5378):217–218. doi: 10.1038/240217a0. [DOI] [PubMed] [Google Scholar]
- Emery A. E., Burt D. Intracellular calcium and pathogenesis and antenatal diagnosis of Duchenne muscular dystrophy. Br Med J. 1980 Feb 9;280(6211):355–357. doi: 10.1136/bmj.280.6211.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frayn K. N., Maycock P. F. Regulation of protein metabolism by a physiological concentration of insulin in mouse soleus and extensor digitorum longus muscles. Effects of starvation and scald injury. Biochem J. 1979 Nov 15;184(2):323–330. doi: 10.1042/bj1840323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulks R. M., Li J. B., Goldberg A. L. Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. J Biol Chem. 1975 Jan 10;250(1):290–298. [PubMed] [Google Scholar]
- Goldspink D. F. The influence of immobilization and stretch on protein turnover of rat skeletal muscle. J Physiol. 1977 Jan;264(1):267–282. doi: 10.1113/jphysiol.1977.sp011667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink D. F. The influence of passive stretch on the growth and protein turnover of the denervated extensor digitorum longus muscle. Biochem J. 1978 Aug 15;174(2):595–602. doi: 10.1042/bj1740595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionasescu V., Zellweger H., Conway T. W. Ribosomal protein synthesis in Duchenne muscular dystrophy. Arch Biochem Biophys. 1971 May;144(1):51–58. doi: 10.1016/0003-9861(71)90453-x. [DOI] [PubMed] [Google Scholar]
- Kameyama T., Etlinger J. D. Calcium-dependent regulation of protein synthesis and degradation in muscle. Nature. 1979 May 24;279(5711):344–346. doi: 10.1038/279344a0. [DOI] [PubMed] [Google Scholar]
- Kanagasuntheram P., Lim S. C. Calcium-dependent inhibition of protein synthesis in rat parotid gland. Biochem J. 1978 Oct 15;176(1):23–29. doi: 10.1042/bj1760023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent G. J., Sparrow M. P., Millward D. J. Turnover of muscle protein in the fowl. Changes in rates of protein synthesis and breakdown during hypertrophy of the anterior and posterior latissimus dorsi muscles. Biochem J. 1978 Nov 15;176(2):407–417. doi: 10.1042/bj1760407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet L. E., Exton J. H., Park C. R. Control of gluconeogenesis from amino acids in the perfused rat liver. J Biol Chem. 1969 Oct 25;244(20):5713–5723. [PubMed] [Google Scholar]
- Morkin E., Kimata S., Skillman J. J. Myosin synthesis and degradation during development of cardiac hypertrophy in the rabbit. Circ Res. 1972 Jun;30(6):690–702. doi: 10.1161/01.res.30.6.690. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Hall P. F. Inhibition of steroidogenic response to corticotropin in mouse adrenal tumor cells (Y-1) by the ionophore A23187. Role of protein biosynthesis. Biochim Biophys Acta. 1978 Aug 17;542(2):330–339. doi: 10.1016/0304-4165(78)90028-4. [DOI] [PubMed] [Google Scholar]
- Ogawa Y. Some properties of fragmented frog sarcoplasmic reticulum with particular reference to its response to caffeine. J Biochem. 1970 May;67(5):667–683. doi: 10.1093/oxfordjournals.jbchem.a129295. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Goodman D. B. Relationships between calcium and cyclic nucleotides in cell activation. Physiol Rev. 1977 Jul;57(3):421–509. doi: 10.1152/physrev.1977.57.3.421. [DOI] [PubMed] [Google Scholar]
- Reed P. W., Lardy H. A. A23187: a divalent cation ionophore. J Biol Chem. 1972 Nov 10;247(21):6970–6977. [PubMed] [Google Scholar]
- SIMON E. J., GROSS C. S., LESSELL I. M. Turnover of muscle and liver proteins in mice with hereditary muscular dystrophy. Arch Biochem Biophys. 1962 Jan;96:41–46. doi: 10.1016/0003-9861(62)90447-2. [DOI] [PubMed] [Google Scholar]
- Samaha F. J., Gergely J. Biochemical abnormalities of the sarcoplasmic reticulum in muscular dystrophy. N Engl J Med. 1969 Jan 23;280(4):184–188. doi: 10.1056/NEJM196901232800403. [DOI] [PubMed] [Google Scholar]
- Schudt C., Pette D. Ca2+ -ions as coupling agents in enzymatic differentiation and carbohydrate metabolism of cultured muscle cells. Adv Enzyme Regul. 1977 Oct 3;16:121–139. doi: 10.1016/0065-2571(78)90070-5. [DOI] [PubMed] [Google Scholar]
- Srivastava U. Biochemical changes in progressive muscular dystrophy. 8. Protein synthesis in skeletal muscle of mouse as a function of muscular dystrophy. Arch Biochem Biophys. 1969 Dec;135(1):237–243. doi: 10.1016/0003-9861(69)90535-9. [DOI] [PubMed] [Google Scholar]
- Statham H. E., Duncan C. J., Smith J. L. The effect of the ionophore A23187 on the ultrastructure and electrophysiological properties of frog skeletal muscle. Cell Tissue Res. 1976 Oct 6;173(2):193–209. doi: 10.1007/BF00221375. [DOI] [PubMed] [Google Scholar]
- Sugden P. H. The effects of calcium ions, ionophore A23187 and inhibition of energy metabolism on protein degradation in the rat diaphragm and epitrochlearis muscles in vitro. Biochem J. 1980 Sep 15;190(3):593–603. doi: 10.1042/bj1900593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi A., Schotland D. L., Rowland L. P. Sarcoplasmic reticulum in Duchenne muscular dystrophy. Arch Neurol. 1973 Jun;28(6):380–384. doi: 10.1001/archneur.1973.00490240040006. [DOI] [PubMed] [Google Scholar]
- Thorpe W. R., Seeman P. The site of action of caffeine and procaine in skeletal muscle. J Pharmacol Exp Ther. 1971 Nov;179(2):324–330. [PubMed] [Google Scholar]
- WAALKES T. P., UDENFRIEND S. A fluorometric method for the estimation of tyrosine in plasma and tissues. J Lab Clin Med. 1957 Nov;50(5):733–736. [PubMed] [Google Scholar]
- Yaffe D. Cellular aspects of muscle differentiation in vitro. Curr Top Dev Biol. 1969;4:37–77. doi: 10.1016/s0070-2153(08)60480-9. [DOI] [PubMed] [Google Scholar]


