Abstract
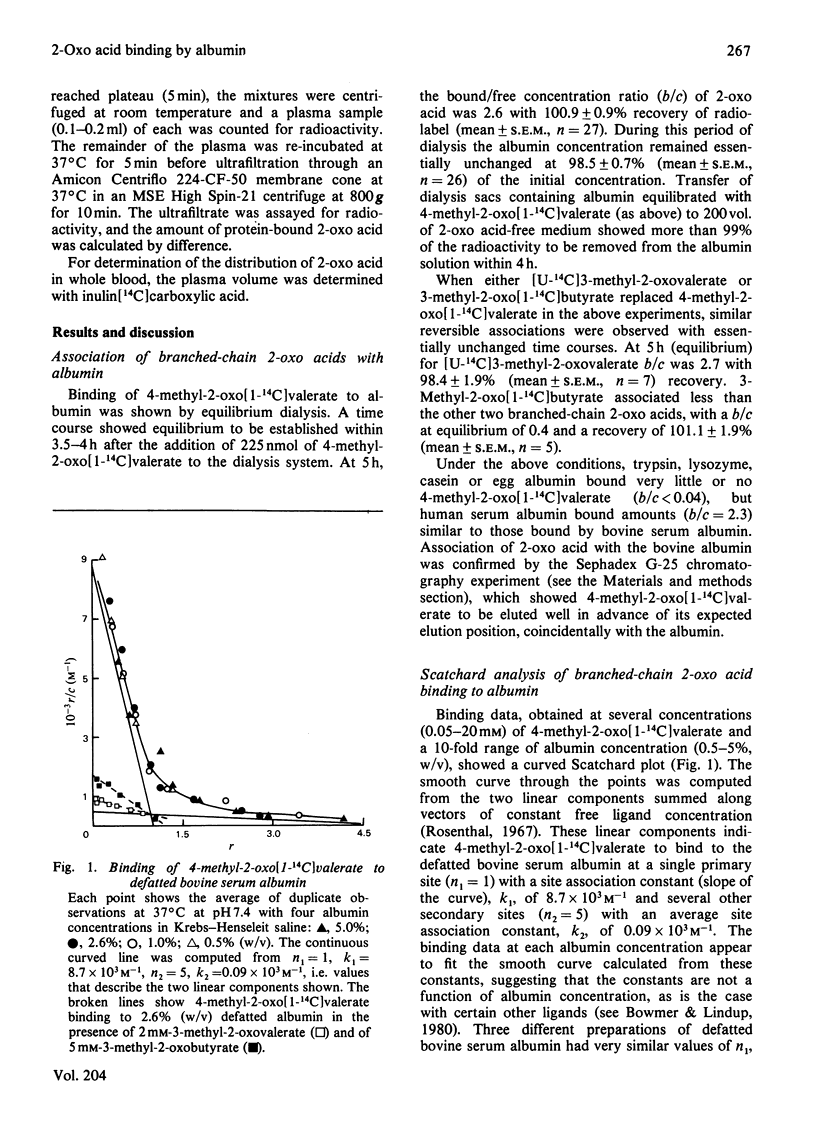
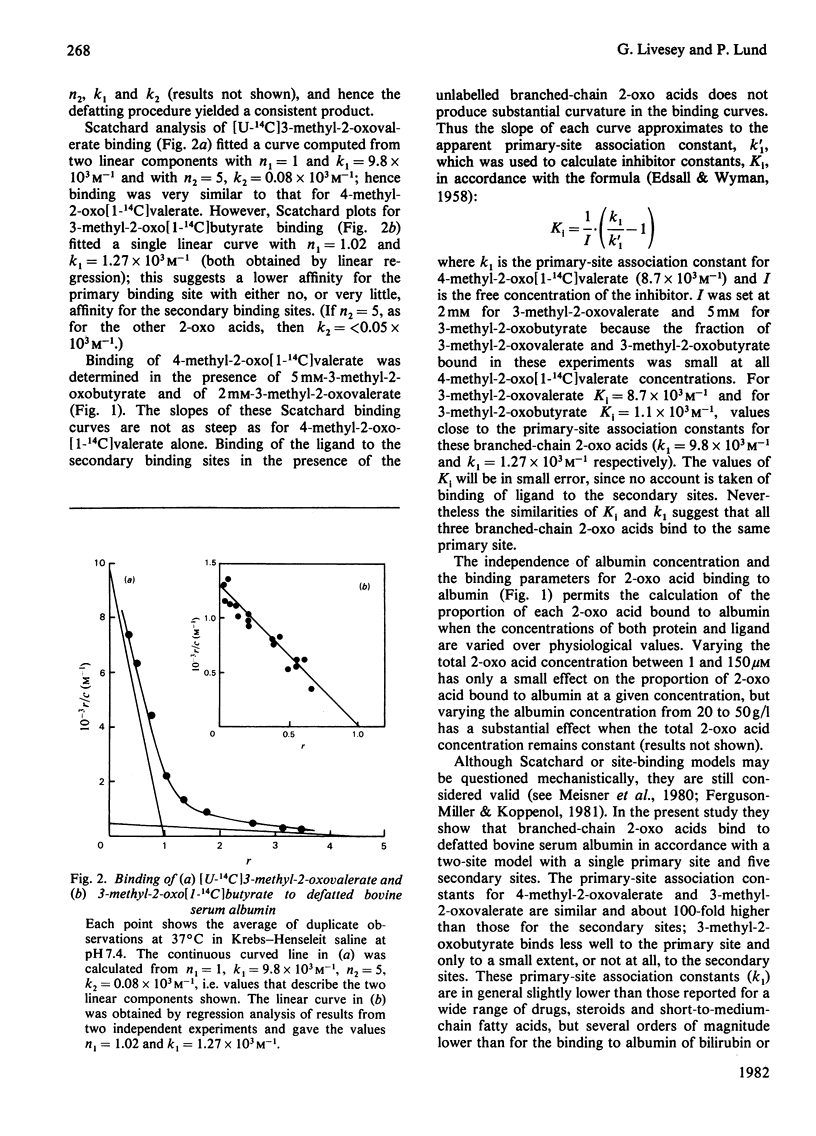
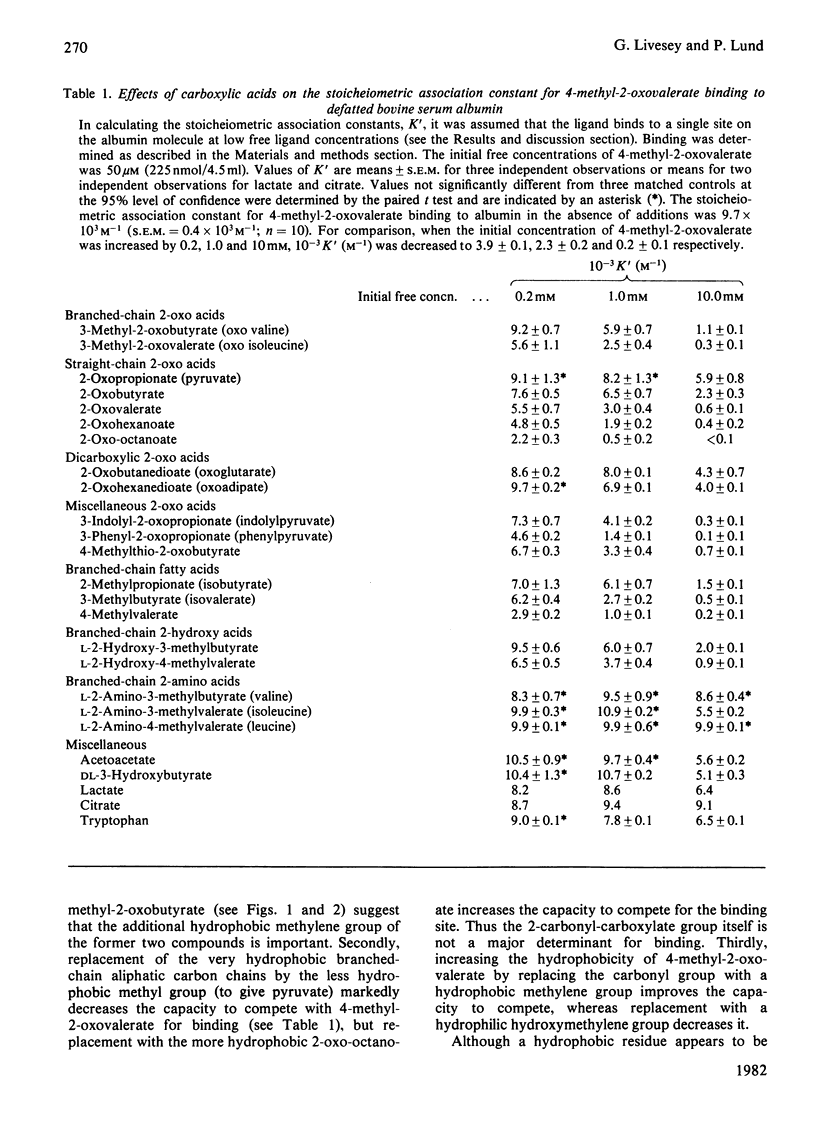
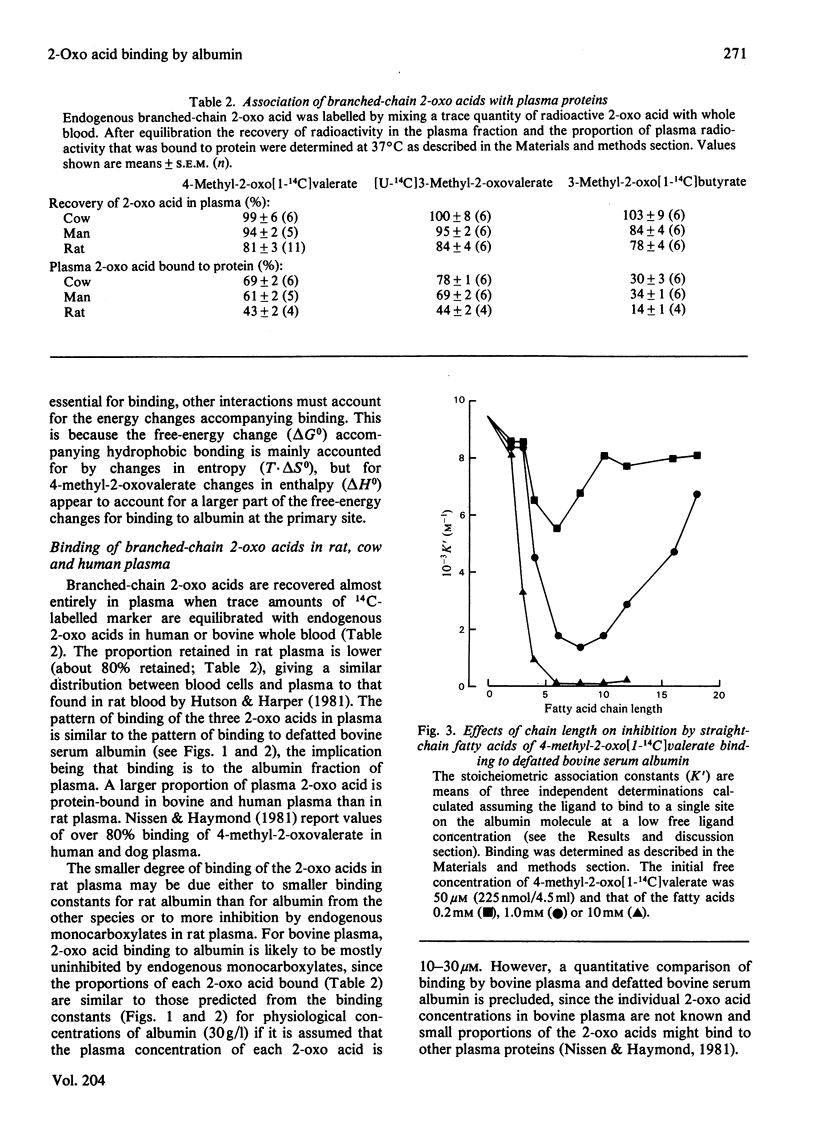
1. Binding of branched-chain 2-oxo acids to defatted bovine serum albumin was shown by gel chromatography and equilibrium dialysis. 2. Equilibrium-dialysis data suggest a two-side model for binding in Krebs-Henseleit saline at 37 degrees C with n1 = 1 and n2 = 5. Site association constants were: 4-methyl-2-oxovalerate, k1 = 8.7 x 10(3) M-1, k2 = 0.09 x 10(3) M-1; 3-methyl-2-oxovalerate, k1 = 9.8 x 10(3) M-1, k2 = 0.08 x 10(3) M-1; 3-methyl-2-oxobutyrate, k1 = 1.27 x 10(3) M-1, k2 = less than 0.05 x 10(3) M-1. 3. Binding of 4-methyl-2-oxovalerate to defatted albumin in a phosphate-buffered saline, pH 7.4, gave the following thermodynamic parameters: primary site delta H0(1) = -28.6kJ . mol-1 and delta S0(1) = -15.2J . mol-1 . K-1 (delta G0(1) = -24.0kJ . mol-1 at 37 degrees C) and secondary sites delta H0(2) = -25.4kJ . mol-1 and delta S0(2) = -46.1J . mol-1 . K-1 (delta G0(1) = -11.2kJ . mol-1 at 37 degrees C). Thus binding at both sites is temperature-dependent and increases with decreasing temperature. 4. Inhibition studies suggest that 4-methyl-2-oxovalerate may associate with defatted albumin at a binding site for medium-chain fatty acids. 5. Binding of the 2-oxo acids in bovine, rat and human plasma follows a similar pattern to binding to defatted albumin. The proportion bound in bovine and human plasma is much higher than in rat plasma. 6. Binding to plasma protein, and not active transport, explains the high concentration of branched-chain 2-oxo acids leaving rat skeletal muscle relative to the concentration within the tissue, but does not explain the 2-oxo acid concentration gradient between plasma and liver.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowmer C. J., Lindup W. E. Inverse dependence of binding constants upon albumin concentration. Results for L-tryptophan and three anionic dyes. Biochim Biophys Acta. 1980 Jul 24;624(1):260–270. doi: 10.1016/0005-2795(80)90245-7. [DOI] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Hutson S. M., Harper A. E. Blood and tissue branched-chain amino and alpha-keto acid concentrations: effect of diet, starvation, and disease. Am J Clin Nutr. 1981 Feb;34(2):173–183. doi: 10.1093/ajcn/34.2.173. [DOI] [PubMed] [Google Scholar]
- Lipsett D., Madras B. K., Wurtman R. J., Munro H. N. Serum tryptophan level after carbohydrate ingestion: selective decline in non-albumin-bound tryptophan coincident with reduction in serum free fatty acids. Life Sci II. 1973 Jan 22;12(2):57–64. doi: 10.1016/0024-3205(73)90027-1. [DOI] [PubMed] [Google Scholar]
- Livesey G., Lund P. Enzymic determination of branched-chain amino acids and 2-oxoacids in rat tissues. Transfer of 2-oxoacids from skeletal muscle to liver in vivo. Biochem J. 1980 Jun 15;188(3):705–713. doi: 10.1042/bj1880705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMENAMY R. H., ONCLEY J. L. The specific binding of L-tryptophan to serum albumin. J Biol Chem. 1958 Dec;233(6):1436–1447. [PubMed] [Google Scholar]
- Meisner H., Stair J., Neet K. Quantitative assessment of the competitive binding of anionic ligands to albumin. Mol Pharmacol. 1980 Sep;18(2):230–236. [PubMed] [Google Scholar]
- Odessey R., Goldberg A. L. Leucine degradation in cell-free extracts of skeletal muscle. Biochem J. 1979 Feb 15;178(2):475–489. doi: 10.1042/bj1780475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal H. E. A graphic method for the determination and presentation of binding parameters in a complex system. Anal Biochem. 1967 Sep;20(3):525–532. doi: 10.1016/0003-2697(67)90297-7. [DOI] [PubMed] [Google Scholar]
- Spector A. A. Fatty acid binding to plasma albumin. J Lipid Res. 1975 May;16(3):165–179. [PubMed] [Google Scholar]
- WILLIAMSON D. H., MELLANBY J., KREBS H. A. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walser M., Lund P., Ruderman N. B., Coulter A. W. Synthesis of essential amino acids from their alpha-keto analogues by perfused rat liver and muscle. J Clin Invest. 1973 Nov;52(11):2865–2877. doi: 10.1172/JCI107483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wosilait W. D., Ryan M. P. Multiple competitive displacement interactions involving human serum albumin, anticoagulants, oleic acid and various drugs. Gen Pharmacol. 1980;11(4):387–394. doi: 10.1016/0306-3623(80)90104-4. [DOI] [PubMed] [Google Scholar]


