Abstract
Background
Sodium-Glucose Cotransporter-2 inhibitors (SGLT2i) represent a deep revolution of the therapeutic approach to heart failure (HF), preventing its insurgence but also improving the management of the disease and slowing its natural progression. To date, few studies have explored the effectiveness of SGLT2i and, in particular, Dapagliflozin in a real-world population. Therefore, in this observational prospective study, we evaluated Dapagliflozin's effectiveness in a real-world HF population categorized in the different hemodynamic profiles.
Methods
From January 2022 to June 2023, we enrolled 240 patients with chronic HF and reduced ejection fraction (HFrEF) on optimal medical therapy, according to 2021 ESC guidelines, that added treatment with Dapagliflozin from the HF Clinics of 6 Italian University Hospitals. Clinical, biochemical, and echocardiographic parameters were collected before and after 6 months of Dapagliflozin introduction. Moreover, the HFrEF population was classified according to hemodynamic profiles (A: SV ≥ 35 ml/m2; E/e′ < 15; B: SV ≥ 35 ml/m2; E/e′ ≥ 15; C: SV < 35 ml/m2; E/e′ < 15; D: SV < 35 ml/m2; E/e′ ≥ 15). Then, we compared the Dapagliflozin population with two retrospective HF cohorts, hereinafter referred to as Guide Line 2012 (GL 2012) group and Guide Line 2016 (GL 2016) group, in accordance with the HF ESC guidelines in force at the time of patients enrolment. Precisely, we evaluated the changes to baseline in clinical, functional, biochemical, and echocardiographic parameters and compared them to the GL 2012 and GL 2016 groups.
Results
Dapagliflozin population (67.18 ± 11.11 years) showed a significant improvement in the echocardiographic and functional parameters (left ventricular ejection fraction [LVEF], LV end-diastolic volume [LVEDV], LVEDV index, stroke volume index [SVi], left atrium volume index [LAVi], filling pressure [E/e′ ratio], tricuspid annular plane systolic excursion [TAPSE], tricuspid annular S′ velocity [RVs’], fractional area change [FAC], inferior vena cava [IVC diameter], pulmonary artery systolic pressure [sPAP], NYHA class, and quality of life) compared to baseline. In particular, TAPSE and right ventricle diameter (RVD1) ameliorate in congestive profiles (B and D); accordingly, the furosemide dose significantly decreased in these profiles. Comparing the three populations, the analysis of echocardiographic parameters (baseline vs follow-up) highlighted a significant decrease of sPAP in the Dapagliflozin population (p < 0.05), while no changes were recorded in the GL 2012 and GL 2016 population. Moreover, at the baseline evaluation, the GL 2012 and 2016 groups needed a higher significant dose of furosemide compared to Dapagliflozin group. Finally, Dapagliflozin patients had significantly fewer rehospitalizations (1.25%) compared with the other two groups (GL 2012 18.89%, p 0.0097; GL 2016 15.32%, p 0.0497).
Conclusions
We demonstrate that Dapagliflozin is rapidly effective in an HFrEF real-world population; furthermore, the more significant effect is recorded in HFrEF patients with a congestive profile (B and D), supporting the introduction of Dapagliflozin in patients with a congestive profile and a worse prognosis. In conclusion, our data suggest evaluating the patient's hemodynamic state beyond LVEF in HFrEF.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-024-02515-5.
Keywords: Dapagliflozin, SGLT2 inhibitors, Cardiac function, Heart failure with reduced ejection fraction, Hemodynamic profile
Introduction
Heart failure (HF) is an ever-evolving medical and economic challenge [1]. However, more and more therapeutic strategies have been implemented to prevent the onset and to improve the prognosis of this complex medical syndrome [2–4]. Current European and American guidelines for HF strongly recommend the use of a relatively new class of drug, the Sodium-Glucose Cotransporter-2 inhibitors (SGLT2i) [5–7]. This class of drugs was initially introduced as an antidiabetic medication. Since DECLARE TIMI [8] and EMPAREG-OUTCOME [9] demonstrated their potential value in diabetic patients at high cardiovascular risk, their role in the treatment of HF has been established. Subsequent large-scale randomized clinical trials, such as DAPA-HF [10], DELIVER [11], EMPEROR-Reduced [12] and EMPEROR-Preserved [13], demonstrated that Dapagliflozin and Empagliflozin, compared to placebo, reduce major adverse cardiovascular events in patients affected by HF, irrespective of left ventricular ejection fraction (LVEF) and diabetic status.
The use of SGLT2i in clinical practice has become widespread soon, as their efficacy is associated with an excellent safety profile and pleiotropic effects [14–16]. Moreover, this class of drugs has shown to be effective independently of LVEF. However, there are still few real-world studies, and the patients enrolled in trials often do not reflect those who come daily to cardiologist’s observations. Consequently, in this prospective observational study, the primary aim is to evaluate how Dapagliflozin affects clinical, biochemical, echocardiographic, and functional parameters in a real-world HF with reduced ejection fraction (HFrEF) population in different hemodynamic settings, with respect to baseline. Indeed, although LVEF classification is the most used for therapeutic choices, it does not consider the hemodynamic state of the patients, and it is based on a single variable parameter that is not representative of the dynamic evolution of the disease. Clinically, patients with HF may be stratified according to the Forrester’s classification, into four heamodynamic profiles depending on the peripheral perfusion (cold to warm) and the pulmonary congestion states (wet to dry) [17, 18]; however, echocardiography is an excellent noninvasive hemodynamic tool [19]. Therefore, we applied a well-specified echocardiographic defined haemodynamic classification [19–21] in a real-world HFrEF population, gaining four profiles from noninvasive data, and evaluated the benefits and efficacy of Dapagliflozin in the different groups.
Furthermore, trials on SGLT2i usually compared these drugs to placebo, and only a small percentage of patients had angiotensin receptor-neprilysin inhibitor (ARNI) on board [22]. Consequently, in the second part of our study, we compared Dapagliflozin population and two other HFrEF populations (GL 2012 and GL 2016) on optimal medical therapy (OMT) according to the guidelines in force at the time of evaluation, deriving from a previous study by our center [23], avoiding any crossover. Precisely, we decided not to compare the dapagliflozin population with the non-SGLT2i contemporary population, because the rate of administration of dapagliflozin in our clinic is high and approximately 90% of our HFrEF patients take this drug and the effects of adding dapagliflozin to therapy are already known.
Materials and methods
In this open-label, prospective, observational clinical study, we included 240 patients receiving Dapagliflozin in addition to standard OMT [6], from the HF Clinics of 6 Italian University Hospitals (University Hospital of Salerno, Riuniti Hospital of Foggia, Monaldi Hospital and Federico II University of Naples, San Carlo Hospital of Potenza, and University of Messina). All investigations were carried out according to the principles of the Helsinki Declaration, and approved by local ethics committees for human research (prot. SCCE N° 0019840), and informed consent was obtained from all participants. To be included in the study, participants had to meet the following criteria: (i) have an age of at least 18 years; (ii) have an HFrEF diagnosis, according to 2021 European Society of Cardiology (ESC) guidelines [6]. To specifically evaluate the effects of Dapagliflozin at 6 months of follow-up and avoid potential biases from other therapy or procedure known to improve cardiac function, we excluded patients subjected to (i) CRT in the previous 6 months and/or (ii) introduction of any of the four pillars in the 2 weeks before enrolment or during the follow-up. Also, we excluded patients with (iii) history of malignancy; (iv) severe hepatic impairment; (v) estimated Glomerular Filtration Rate (eGFR) < 25 ml/min/1.73 m2, accordingly with the possibility of prescribing this drug in Italy; (vi) echocardiographic images of low quality. On the contrary, we did not exclude patients with severe valvular disease, to reflect real world HFrEF populations. At enrolment, Dapagliflozin was introduced in therapy. The population enrolled was on OMT at the maximum tolerated dose. Parameters were collected on a predefined computerized data sheet, including demographic parameters, medical history, clinical data and pharmacological treatments, laboratory, electrocardiographic, and echocardiographic data. Clinical follow-ups were periodically performed in our HF outpatient clinics. Baseline features and clinical outcomes were reassessed in all patients at 6 months from enrolment. Moreover, the health-related quality of life (HRQoL) was evaluated at baseline and at 6 months, using the Italian adaptation of the EuroQol Group (EQ-5D). The presence of chronic kidney disease (CKD) was defined by a glomerular filtration rate of less than 60 mL/min per 1.73 m2 (using the last serum creatinine value available at the time of enrolment and CKD-EPI equation). The ischaemic aetiology of the HF syndrome was defined as a previous history of myocardial infarction and/or prior revascularization through percutaneous and/or surgical procedures. Precisely, we have evaluated changes from baseline in clinical, biochemical and echocardiographic parameters in our real-world population, with particular attention to different hemodynamic profiles. Subsequently, to assess differences in outcome between the pre-and post-SGLT2i era, the study population was compared with two retrospective HF cohorts (GL 2016 group and GL 2012 group), on OMT in accordance with 2016 [24] and 2012 ESC HF Guidelines [25]. Precisely, the GL 2016 cohort was enrolled from June 2016 to December 2017, while the GL 2012 was enrolled between January 2014 to May 2016 (before introduction of ARNI in clinical practice). Indeed, GL 2012 patients were treated with the standard HF drugs (including ACEi or ARBs) up-titrated to the maximum tolerated dose, as recommended by 2012 HF ESC guidelines. These patients were matched with the Dapagliflozin group for age and sex. All the patients were stable, and not hospitalized.
Echocardiographic methods are reported in supplementary file.
Haemodynamic classification
By combining E/e′ ratio and SV from baseline echocardiography, patients were classified into four haemodynamic profiles [19–21]:
Profile A: normal-flow and normal-pressure (SV ≥ 35 ml/m2; E/e′ < 15);
Profile B: normal-flow, high-pressure (SV ≥ 35 ml/m2; E/e′ ≥ 15);
Profile C: low-flow, normal-pressure (SV < 35 ml/m2; E/e′ < 15);
Profile D: low-flow, high-pressure (SV < 35 ml/m2; E/e′ ≥ 15).
LV stroke volume (SV) and E/e′ were derived from echocardiographic measurement. LV stroke volume was calculated as the product of the cross-sectional area of the left ventricular outflow tract (LVOT) and time-velocity integral (VTI) at LVOT derived by pulsed-wave Doppler; precisely, this method was used to avoid overestimation of systemic output that occurs when 2D-derived stroke volume (difference between LVEDV and LVESV) is applied in patients with significant mitral regurgitation..
Statistical analysis
Continuous variables were reported as mean ± standard deviation. Numbers and percentages were used for categorical variables. The Shapiro–Wilk test was used to check for normal distribution. Student t-test was used to calculate statistical significance between means, while the chi-square and Fisher’s exact tests were used to asses statistical significance between categorical parameters (baseline vs. 6 months follow-up). Repeated measures analysis of variance (ANOVA) test was performed to assess changes in clinical, biochemical, echocardiographic parameters and medications data was employed to detect differences among the three study groups (Dapagliflozin vs GL 2016 vs. GL 2012). Precisely, to assess the difference between two groups we used Sidak post-hoc analysis. For all tests, a p-value < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS software version 23.0 (SPSS Inc., Chicago, Illinois), while the graphs were created using GraphPad Prism 6 (GraphPad Software, Boston, MA 02110).
Results
The results are structured as follows: the first section presents the total Dapagliflozin population features; the second section reports the results based on hemodynamic profiles in the Dapagliflozin population; finally, the third section concludes with the comparison of the Dapagliflozin population with GL 2012 and GL 2016 groups.
Total Dapagliflozin population features
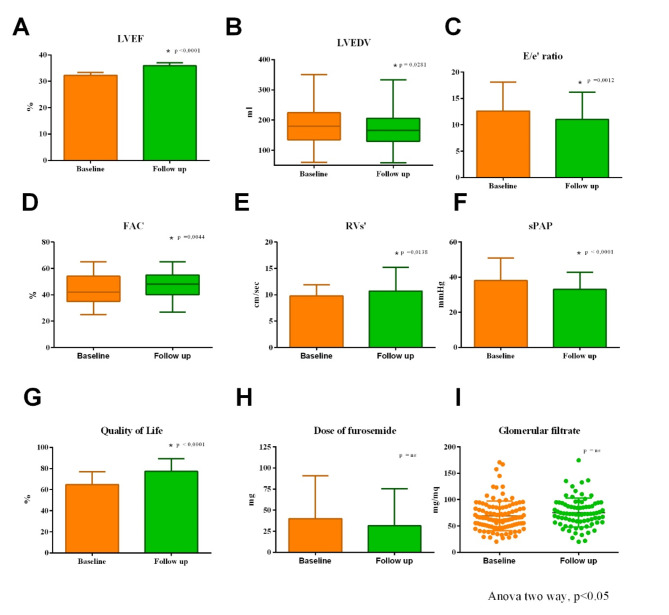
No patients were lost at follow-up or needed to interrupt the treatment with Dapagliflozin. Demographic data, comorbidities, and medications of the study population (67.18 ± 11.11 years; 81.50% male) are reported in Tables 1 and 1S. Likewise, clinical and biochemical data of the Dapagliflozin population are reported in Table 2. Precisely, systolic and diastolic blood pressure and heart rate did not change significantly from baseline to follow-up evaluations (Table 2). Moreover, as shown in Table 2, the values of hemoglobin, creatinine, and glomerular filtrate rate did not significantly change at follow-up. As showed in Table 2 and Fig. 1, from baseline to follow-up the following echocardiographic parameters significantly improved: LVEF, LVEDV, LVEDV index (LVEDVi), SV index (SVi), LA volume index (LAVi), E/e′ ratio, TAPSE, RVs’, FAC, IVC diameter and sPAP. Instead, there were no relevant modifications for SV and RVD1. Furthermore, QoL significantly improved at follow-up (p < 0.0001) (Table 2), while the dose of furosemide did not significantly change from basal to follow-up (Fig. 1). Finally, at 6 months follow-up, we observed no death.
Table 1.
Baseline parameters of 3 groups: Dapagliflozin, GL 2016 and GL 2012 group.
| Variables | Dapagliflozin (n = 240) | GL2016 (n = 111) | GL2012 n (n = 90) | Dapagliflozin vs GL2016 | Dapagliflozin vs GL2012 | GL2016 vs GL2012 |
|---|---|---|---|---|---|---|
| Demographic data | ||||||
| Age, years | 67.18 ± 11.11 | 67.10 ± 11.65 | 67.00 ± 11.20 | 0.99 | 1 | 1 |
| BSA, m2 | 1.90 ± 0.19 | 1.92 ± 0.18 | 1.90 ± 0.20 | 1 | 1 | 1 |
| Male, n (%) | 196 (81.50) | 90 (81.08) | 73 (81.11) | 1 | 1 | 1 |
| Comorbidities | ||||||
| Hypertension, n (%) | 169 (70.42) | 88 (79.28) | 51 (56.70) | 0.081 | 0.018 | 0.0004 |
| Dyslipidemia, n (%) | 165 (68.75) | 85 (76.58) | 63 (70.00) | 0.132 | 0.829 | 0.295 |
| Smoke, n (%) | 55 (22.90) | 31 (27.93) | 33 (36.70) | 0.513 | 0.020 | 0.149 |
| Previous smoke, n (%) | 83 (34.58) | 12 (10.81) | 24 (26.70) | 0.000002 | 0.172 | 0.003 |
| Obesity, n (%) | 57 (23.75) | 43 (38.74) | 35 (38.90) | 0.003 | 0.006 | 0.982 |
| Diabetes NID, n (%) | 61 (25.42) | 32 (28.83) | 35 (38.90) | 0.501 | 0.016 | 0.133 |
| Diabetes ID, n (%) | 34 (14.16) | – | – | – | – | – |
| Chronic renal failure, n (%) | 51 (21.25) | 54 (48.63) | 49 (54.40) | 0.0000001 | 0.016 | 0 |
| Atrial fibrillation, n (%) | 86 (35.84) | 34 (30.63) | 22 (24.70) | 0.340 | 0.333 | 0.051 |
| COPD, n (%) | 67 (27.91) | 32 (28.83) | 21 (23.30) | 0.860 | 0.403 | 0.381 |
| Anemia, n (%) | 37 (15.40) | 30 (27.03) | 25 (27.80) | 0.010 | 0.010 | 0.906 |
| Chronic coronary syndrome, n (%) | 88 (36.67) | 58 (52.25) | 60 (66.70) | 0.050 | 0.0000005 | 0.039 |
| Previous ICD implantation, n (%) | 101 (42.00) | 41 (36.94) | 46 (51.10) | 0.362 | 0.142 | 0.051 |
| Previous CRT-D implantation, n (%) | 48 (20.00) | 37 (33.33) | 12 (13.30) | 0.0066 | 0.162 | 0.0009 |
| Sinus rhythm, n (%) | 180 (75.00) | 89 (80.18) | 82 (91.10) | 0.287 | 0.0012 | 0.030 |
| Medications | ||||||
| Proton pump inhibitors, n (%) | 183 (76.25) | 92 (82.88) | 90 (100.00) | 0.161 | 0.0000002 | 0.00002 |
| Mineralcorticoid receptor antagonists, n (%) | 143 (59.58) | 57 (51.35) | 52 (57.80) | 0.148 | 0.767 | 0.365 |
| Furosemide, n (%) | 155 (64.58) | 100 (90.09) | 83 (92.20) | 0.0000004 | 0.0000004 | 0.600 |
| Furosemide, mg | 38.57 ± 47.45 | 61.18 ± 45.36 | 65.75 ± 82.26 | 0.006 | 0.002 | 0.844 |
| Beta blockers, n (%) | 201 (83.75) | 91 (81.98) | 85 (94.40) | 0.681 | 0.011 | 0.008 |
| ACE—inhibitors, n (%) | 26 (10.83) | 5 (4.50) | 76 (84.40) | 0.052 | 0 | 0 |
| Angiotensin receptor antagonists, n (%) | 13 (4.29) | – | 14 (15.60) | – | 0.002 | – |
| Angiotensin receptor neprilysin inhibitors, n (%) | 160 (66.67) | 90 (81.08) | – | 0.005 | – | – |
One-Way ANOVA except for *unpaired t test Dapagliflozin vs GL 2012 population and ** unpaired t test Dapagliflozin vs GL 2016 population. Bold values denote statistical significance at the p < 0.05 level
ACE, Angiotensin-Converting Enzyme; ARNI, Angiotensin Receptor-Neprilysin Inhibitor; BSA, Body Surface Area; COPD, Chronic Obstructive Pulmonary Disease; CRT-D, Cardiac Resynchronization Therapy Defibrillator; ICD, Implantable Cardioverter Defibrillator; ID, Insulin Dependent; NID, Non-insulin dependent
Table 2.
Clinical, biochemical and echocardiographic parameters of Dapagliflozin population (baseline vs. 6 months follow-up)
| Variables | Baseline | 6 M-follow-up | p value |
|---|---|---|---|
| Clinical data | |||
| Systolic blood pressure (mmHg) | 118.78 ± 16.78 | 117.50 ± 14.08 | 0.3659 |
| Diastolic blood pressure (mmHg) | 70.72 ± 10.49 | 70.05 ± 9.40 | 0.4336 |
| Heart rate (bpm) | 70.48 ± 13.35 | 68.52 ± 10.59 | 0.1184 |
| Quality of life (%) | 60.82 ± 12.06 | 77.29 ± 12.07 | < 0.0001 |
| Biochemical data | |||
| Creatinine (mg/dl) | 1.19 ± 0.40 | 1.18 ± 0.44 | 0.8072 |
| Glomerular filtrate (ml/min) | 68.96 ± 28.22 | 75.50 ± 27.23 | 0.1102 |
| Haemoglobin (g/dl) | 13.28 ± 1.81 | 13.27 ± 2.02 | 0.4271 |
| Echocardiographic data | |||
| LVEF (%) | 32.27 ± 8.34 | 35.92 ± 8.43 | < 0.0001 |
| LVEDV (ml) | 184.37 ± 66.05 | 171.92 ± 57.26 | 0.0281 |
| LVEDVi (ml/m2) | 96.25 ± 33.66 | 90.01 ± 30.05 | 0.0331 |
| LVESV (ml) | 128.62 ± 57.48 | 113.14 ± 47.90 | 0.0015 |
| LVESVi (ml/m2) | 66.55 ± 1.94 | 58.97 ± 1.64 | 0.0031 |
| SV (ml) | 56.59 ± 17.64 | 59.01 ± 16.10 | 0.1217 |
| SVi (ml/m2) | 29.69 ± 0.58 | 31.04 ± 0.53 | 0.1789 |
| LAVi (ml/m2) | 47.17 ± 17.85 | 42.42 ± 14.89 | 0.0013 |
| E/e′ ratio | 12.59 ± 5.47 | 10.99 ± 5.20 | 0.0012 |
| TAPSE (mm) | 18.89 ± 3.90 | 19.92 ± 3.78 | 0.0036 |
| RVs’ (cm/sec) | 9.83 ± 2.10 | 10.70 ± 4.48 | 0.0138 |
| FAC (%) | 43.52 ± 9.73 | 46.84 ± 8.97 | 0.0044 |
| RVD1 (mm) | 36.74 ± 0.70 | 35.52 ± 0.60 | 0.1865 |
| ICV diameter (mm) | 18.19 ± 3.88 | 16.38 ± 4.09 | < 0.0001 |
| sPAP (mmHg) | 37.99 ± 12.97 | 32.98 ± 9.72 | < 0.0001 |
LVEF, Left ventricular ejection fraction; LVEDV, Left ventricle end-diastolic volume; LVEDVi, Left ventricle end-diastolic volume index; SV, Stroke volume; Svi, Stroke volume index; LAVi, Left atrium volume index; E, Early-wave transmitral diastolic velocity; e′, Early-diastolic velocity at tissue Doppler imaging; TAPSE, Tricuspid annular plane systolic excursion; RVs’, Tricuspid annular S′ velocity; FAC, Fractional area change; RVD, Right ventricle diameter; IVC, Inferior vena cava; sPAP, Pulmonary artery systolic pressure. Bold values denote statistical significance at the p < 0.05 level
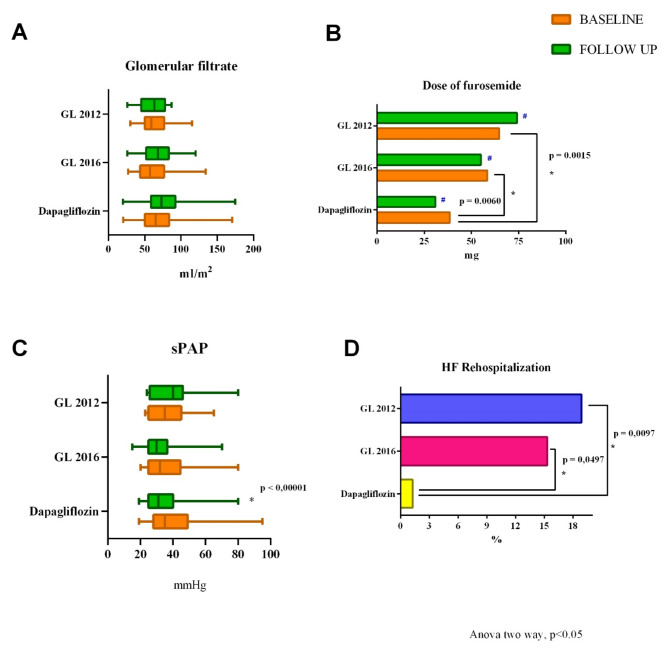
Fig. 1.
Changes from baseline to 6 months follow-up of clinical, biochemical, echocardiographic and pharmacological parameters of Dapagliflozin population. The graphs show that a significant improvement was observed in LVEF (A), LVEDV (B), E/e′ ratio (C), FAC (D), RVs’ (E), sPAP (F), and quality of life (G). In contrast, there were no significant changes in dose of furosemide (H) and glomerular filtration (I). LVEF, Left ventricular ejection fraction; LVEDV, Left ventricle end-diastolic volume; E, Early-wave transmitral diastolic velocity; e′, Early-diastolic velocity at tissue Doppler imaging; FAC, Fractional area change; RVs’, Tricuspid annular S′ velocity; sPAP, Pulmonary artery systolic pressure
Results based on hemodynamic profiles in the Dapagliflozin population
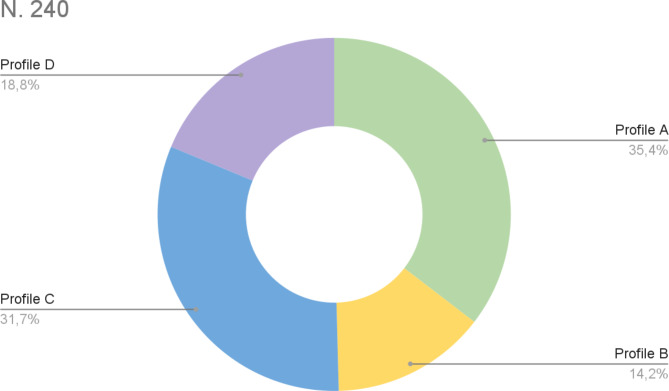
As reported in the Methods, considering E/e′ and SV, we divided patients into four hemodynamic profiles. Therefore, patients were classified as follows (Fig. 2):
85 patients as profile A (normal flow, normal pressure);
34 patients as profile B (normal flow, high pressure);
76 patients as profile C (low flow, normal pressure);
45 patients as profile D (low flow, high pressure).
Fig. 2.
Baseline hemodynamic profile of Dapagliflozin population: profile A (normal flow, normal pressure); profile B (normal flow, high pressure); profile C (low flow, normal pressure); profile D (low flow, high pressure)
No significant changes were recorded in the clinical and biochemical parameters even after they were divided into the four hemodynamic profiles (biochemical data not shown).
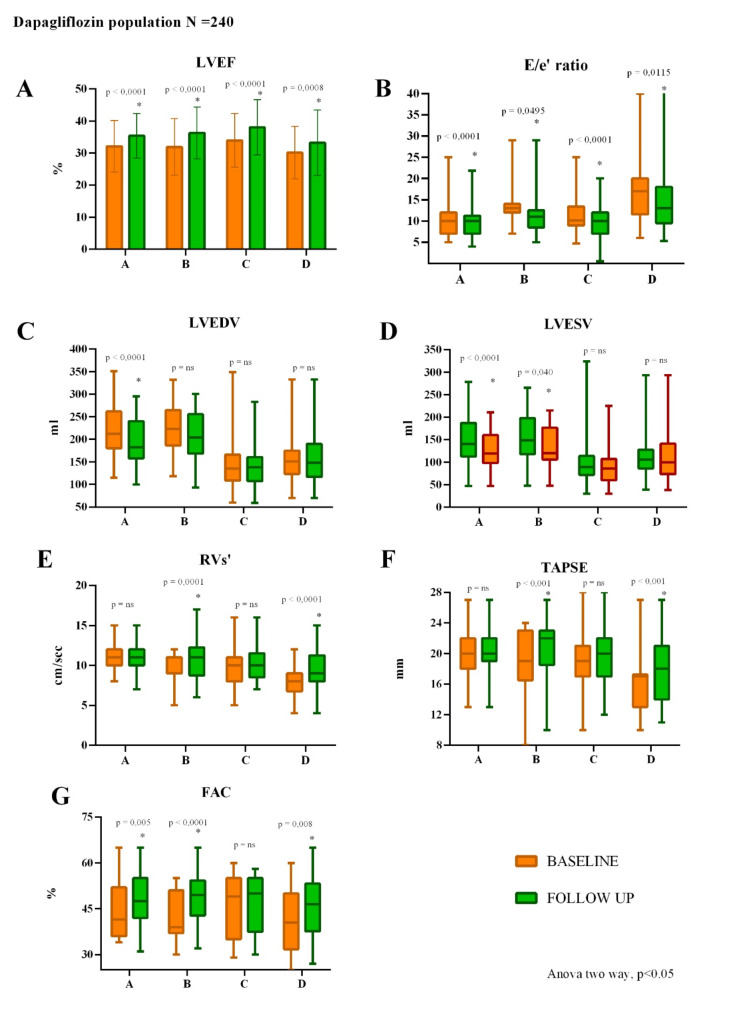
The main results of the echocardiographic parameters according to the four hemodynamic profiles are reported in Fig. 3.
Fig. 3.
Changes from baseline to 6 months follow-up of echocardiographic parameters of the four hemodynamic profiles. A LVEF significantly improved in all profiles. B E/e′ significantly improved in all profile. C LVEDV significantly improved only in profile A. D LVESV significantly improved only in profile A and B. E RVs’ significantly improved in congestive profiles (B and D). F TAPSE significantly improved in congestive profiles (B and D). G FAC significantly improved in profile A, B and D. LVEF, Left ventricular ejection fraction; E, Early-wave transmitral diastolic velocity; e′, Early-diastolic velocity at tissue Doppler imaging; LVEDV, Left ventricle end-diastolic volume; LVESV, Left ventricle end-systolic volume; RVs’, Tricuspid annular S′ velocity; TAPSE, Tricuspid annulus plane systolic excursion; FAC, Fractional area change
Specifically, LVEF and E/e′ ratio significantly improved in all profiles, while LVEDV had a remarkable improvement only in profile A (p < 0.001), and LVESV improved significantly in profile A (p < 0.001) and B (p 0.040). Moreover, TAPSE significantly improved only for the congestive profiles B and D (p < 0.001); accordingly, RVs’ had a significant improvement in the same profiles (profile B: p 0.001; profile D p < 0.001).
Furthermore, FAC had a remarkable reduction for profile A (p 0.005), B (p < 0.001), and D (p 0.008), while no significant changes were recorded for profile C.
RVD1 improves only in profile B (p 0.037) and D (p 0.002). Finally, LAVi had a significant improvement only in profile A (p 0.001) and B (p 0.029).
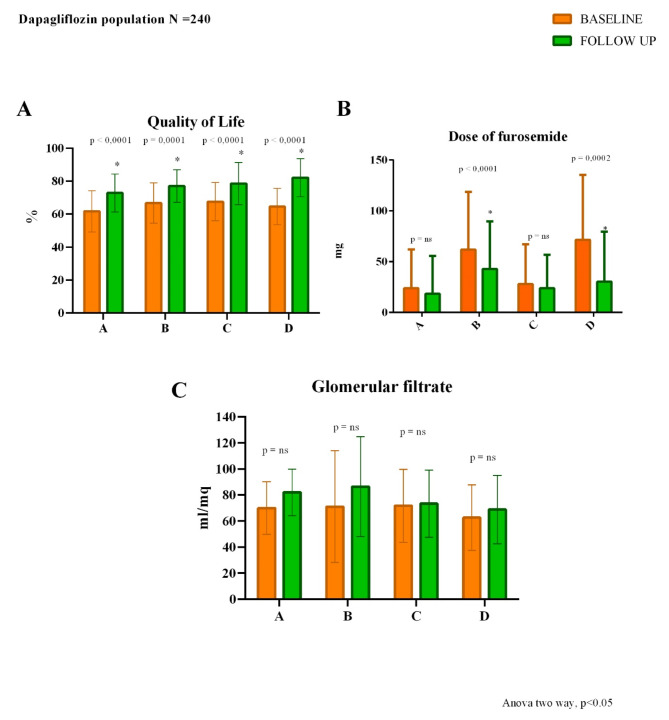
Moreover, patients of all four hemodynamic profiles significantly improved in QoL (profile A, profile B, and D p < 0.001 and profile C p 0.004) (Fig. 4) and in NYHA class (Fig. 5).
Fig. 4.
Changes from baseline to 6 months follow-up of quality of life (A), dose of furosemide (B) and glomerular filtrate (C)
Fig. 5.
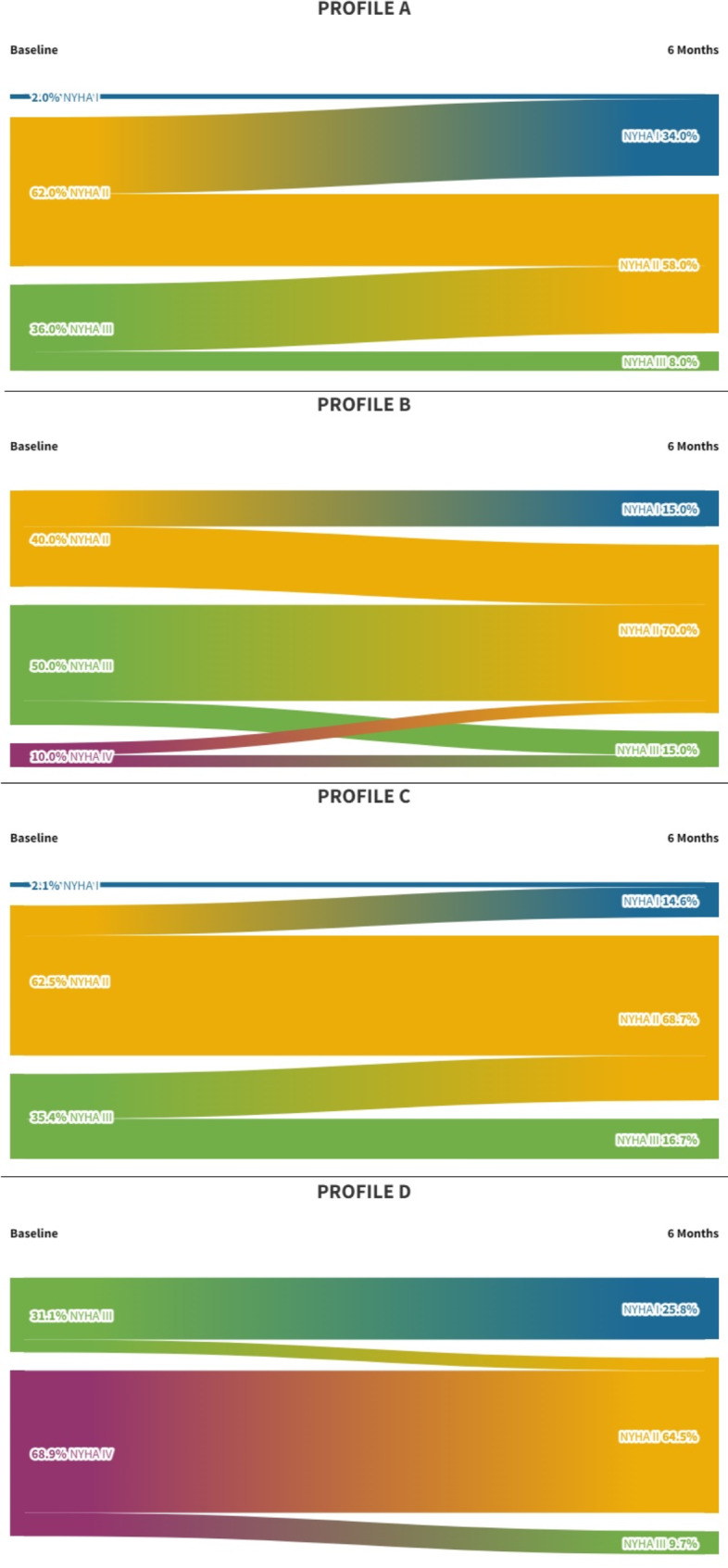
Changes from baseline to 6 months follow-up of NYHA class according to the four hemodynamic profiles. The Sankey diagrams show that patients of all four hemodynamic profiles significantly improved in the NYHA class. NYHA, New York Heart Association
Contrary to the results obtained from the analysis of the total population, the dose of furosemide had no significant changes for profiles A and C but had a significant decrease in profiles B (p < 0.001) and D (p 0.001) (Fig. 4).
Comparison of Dapagliflozin population with GL 2012 and GL 2016
As reported in Methods, our population was compared with two retrospective HF cohorts defined as GL 2012 and GL 2016 groups. The demographic and anamnestic data of GL 2012 and GL 2016 groups are reported in Table 1. All the groups were comparable for demographic parameters.
The analysis of echocardiographic parameters, from baseline to 6 months follow-up, highlighted a significant decrease of sPAP in the Dapagliflozin population (p < 0.00001), while no changes were recorded in GL 2012 and GL 2016 groups (Fig. 6). It must be emphasized that the basal sPAP of the three groups was similar.
Fig. 6.
Comparison of GL 2012, GL 2016 and Dapagliflozin population in terms of glomerular filtrate (A), dose of loop diuretic (B), sPAP (C) and HF rehospitalizations (D). GL, Guideline; HF, Heart Failure; sPAP, Pulmonary artery systolic pressure; #, p (baseline vs. follow up) > 0.05
Moreover, at the baseline evaluation, GL 2012 group needed a significantly higher dose of loop diuretic compared to the baseline dose of furosemide of the Dapagliflozin group, while there were no differences between the basal loop diuretic dose comparing GL 2012 group and GL 2016 group.
Finally, DAPA patients had significantly fewer rehospitalizations (1.25%) compared with the other two groups (GL 2012 18.89%, p 0.0097; GL 2016 15.32%, p 0.0497) (Fig. 6).
Discussion
Our real-world, multicenter, and observational study demonstrates how Dapagliflozin can produce beneficial effects in HFrEF patients in all hemodynamic conditions from clinical, instrumental, and QoL points of view. The favorable effects are particularly evident in congestive patients, typically those with more advanced HF.
Several clinical trials on SGLT2i focused on the prognostic effects of SGLT2i in rehospitalization and mortality rates; conversely, a few real-world data provide an idea of the hemodynamic and pathophysiological mechanisms involved [26, 27].
Therefore, our study aimed to highlight the huge diversity of HF patients. Per this, we introduce a hemodynamic subclassification of our population, categorized by congestion and perfusion balance. Current literature offers numerous suggestions for thought in this regard. Dini et al. [28] used a subdivision into hemodynamic profiles very similar to ours to evaluate prognostically patients affected by HFrEF who introduced ARNIs into therapy.
Firstly, we did not observe significant changes in blood pressure and heart rate values, confirming excellent hemodynamic tolerability of Dapagliflozin. Indeed, 32% of our Dapagliflozin population was not treated with ARNI, mainly due to intolerance (symptomatic hypotension and renal impairment).
On the other hand, in our clinics only approximately 10% of HFrEF patients do not take SGLT2i, mostly due to recurrent genitourinary tract infections. This data is in line with real world studies and data [29].
Echocardiographycally, our data highlighted an increased global contractile function of the LV (LVEF and SVi), simultaneously reducing the LVEDV and LVEDVi. Regarding the changes in LV remodelling, a positive effect of SGLT2i on LV remodelling has been found in patients with type 2 diabetes and/or HFrEF [30–32]. Accordingly, DAPA-MODA study [33] recorded an improvement in left ventricular geometry. Moreover, as already shown in the literature [34, 35], SGLT2i affects body weight, and consequently body surface area (BSA); accordingly, in our patients, there was a reduction of BSA at follow-up (data not shown) with a simultaneous improvement of SVi but not of SV. Nonetheless, by dividing the population into haemodynamic profiles, LVEDV had a remarkable improvement only in profile A (p < 0.0001), while LVEF significantly improved in all profiles. Precisely, the improvement in global cardiac contractility is not only due to a remodeling of the cardiac chambers but also to RV function because RV and LV interaction contributes to cardiac dyssynchrony [36]. RV dysfunction may impair LV function by reducing LV preload and unfavorably affecting the systolic and diastolic interaction via the intra-ventricular septum and the pericardium (ventricular interdependence) [36]. In HFrEF patients, changes in RV performance may be a sensitive indicator of variations in LV function [36, 37].
To date, the literature has little evidence of the effects of SGLT2i on RV contractility and on improving the degree of hemodynamic compensation. Only a small and monocentric study by Mustapic et al. [38] highlighted how SGLT2i substantially improved RV contractility after three months. Accordingly, our multicentric study extends this observation after six months of follow-up in a larger population, especially in the congestive profiles.
Improving the RV function opens another scenario: a reduced RV contractility negatively affects the application of OMT. In particular, some real-world studies identified how a right dysfunction was associated with a more frequent interruption of ARNI [39]. Moreover, baseline RV dysfunction hampers ARNI up‐titration, as demonstrated in our recent study [39].
Consequently, the use of this class of drugs could potentially increase the patients' tolerability to ARNI due to the improvement in the RV's performance.
Likewise, we observed an improvement in the hemodynamic profile of the enrolled population, possibly due to the improved performance of the RV. Moreover, our data highlighted how Dapagliflozin is effective in all hemodynamic profiles of HFrEF without affecting renal function and hemodynamic parameters, strongly supporting the concept of SGLT2i as the first pillar in the management of HFrEF [40].
The analysis of the biochemical data highlights how, at follow-up, there were no substantial changes in the creatinine and glomerular filtration data. Indeed, DAPA-CKD [41] and DECLARE-TIMI [8] demonstrate a nephroprotective effect of Dapagliflozin. Nephroprotection is an essential element of SGLT2i effects [42]; indeed, one of the major difficulties of physicians using HF medications is reaching a compromise between the optimization of medical therapy and the protection of renal function.
It must be emphasized that HFrEF represents a therapeutic challenge with multiple objectives. Indeed, it is needed to maintain an adequate hemodynamic balance and guarantee an adequate QoL [43]. Our results show how Dapagliflozin constitutes a fair compromise in this sense, providing clinical benefit while positively affecting subjectively perceived QoL. Accordingly, in DAPA-HF patients who received Dapagliflozin were more likely to have a clinically relevant improvement in their QoL after only eight months of treatment, as assessed by the Kansas City Cardiomyopathy Questionnaire (KCCQ) [10]. The beneficial effects of SGLT2i appeared even earlier in the DEFINE-HF, in which Dapagliflozin increased the proportion of patients achieving a combined endpoint of improved functional status (as measured by the KCCQ), or ≥ 20% reduction in NT-proBNP [44]. Our real-world observations perfectly fit with the results of RCT, supporting the use of Dapagliflozin as early as possible for managing HF patients.
Intriguingly, a previous investigation showed a good impact on physical function measured with a 5 m gait speed [45]. In this regard, it is essential to note that although the dose of loop diuretic remains constant in follow-up, there is a substantial difference in dosage when comparing the Dapagliflozin population to GL 2012 and GL 2016 groups. The mechanism is certainly due, at least in part, to the glycosuric effect of SGLT2i [46].
Finally, we reported fewer HF hospitalizations in Dapagliflozin population, compared to GL 2012 and GL 2016 groups. These data are in line with the literature [10], and the rate reduction of HF rehospitalization is a fundamental objective due to the evident prognostic impact.
In conclusion, the classification of HF based on LVEF gives a static image of this disease. SGLT2i and their beneficial effect independent of this echocardiographic parameter open the doors to a new definition of HF. Indeed, the HF patient is dynamic, and therapy should be personalized based on the hemodynamic characteristics. From this perspective, the LVEF, although constituting an essential cornerstone, can no longer represent the only prognostic parameter. Still, it should be accompanied by new parameters that can capture the complexity of this disease.
Study limitation
Our results cannot be extended to patients with HF with mildly reduced ejection fraction (HFmrEF) or preserved ejection fraction (HFpEF). Furthermore, our real-world patients were enrolled in the maximum OMT tolerated for several years at enrolment, and the follow-up was relatively short. The three groups are comparable in terms of age, gender and BSA, but there are differences in comorbidities. Moreover, patients with HFrEF are hemodynamically dynamic, so these data must be verified over a more extended period. Our results also require further validation on larger sample sizes.
Conclusions
HFrEF represents an ongoing therapeutic challenge, and the number of patients in this category constantly increases. To date, cardiologists are faced with increasingly complex therapeutic choices and increasingly personalized patient management. Precisely, in our real world study, we enrolled different etiologies of HFrEF patients, showing the effectiveness of this drug in different contexts, as well as in different hemodynamic profiles, and the benefits are evident already at 6 months. Furthermore, the patients who are worse off (profile D) respond best to the drug, suggesting a beneficial effect on the hemodynamic balance.
Additionally, although this data needs to be validated on a larger population, Dapagliflozin improves the performance of the RV, suggesting greater tolerance of the other therapeutic pillars for the treatment of HF. Furthermore, Dapagliflozin is well tolerated by the patient and improves the subjective perception of QoL.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- ARNI
Angiotensin receptor-neprilysin inhibitor
- ASE
American Society of Echocardiography
- CKD
Chronic kidney disease
- EACVI
European Association of Cardiovascular Imaging
- EAE
European Association of Echocardiography
- ESC
European Society of Cardiology
- FAC
Fractional area change
- FU
Follow-up
- HF
Heart failure
- HFmrEF
Heart failure with mildly reduced ejection fraction
- HFpEF
Heart failure with preserved ejection fraction
- HFrEF
Heart Failure with reduced ejection fraction
- HRQoL
Health-related quality of life
- IVC
Inferior vena cava
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- LA
Left atrium
- LVEDV
Left ventricular end-diastolic volume
- LVEF
Left ventricular ejection fraction
- LVESV
Left ventricular end-systolic volume
- LVOT
Left ventricular outflow tract
- OMT
Optimal medical therapy
- RV
Right ventricle
- RVD1
Basal RV diameter
- RVs’
Tricuspid annular S′ velocity
- SGLT2i
Sodium-Glucose Cotransporter-2 inhibitors
- sPAP
Pulmonary artery systolic pressure
- SV
Stroke volume
- TAPSE
Tricuspid annulus plane systolic excursion
- VTI
Time-velocity integral
Author contributions
Conceptualization: CV, MC, VV, Methodology: FL, PM, AR, RDF, MC, VV, Formal analysis: FL, AR, MC, VV, Data Curation: FL, PM, AR, RDF, DM, CM, MCo, AV, MG, PM, VM, FF, EDS, CI, AC, NV, ES, SB, GD, CGT, GS, MC, VV, Echocardiography: FL, AR, AV, MG, DM, FF, EDS, PM, ES, SB, GD. Investigation: FL, PM, AR, RDF, DM, CM, MCo, AV, MG, PM, VM, FF, EDS, NV, ES, SB, GD, CGT, GS, MC, VV, Writing—original draft preparation: FL, PM, AR, RDF, MC, VV, Writing—review and editing: FL, PM, AR, GS, LF, CV, MC, VV, Supervision: CV, MC, VV. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ciccarelli M, Giallauria F, Carrizzo A, Visco V, Silverio A, Cesaro A, Calabro P, De Luca N, Mancusi C, Masarone D, et al. Artificial intelligence in cardiovascular prevention: new ways will open new doors. J Cardiovasc Med (Hagerstown). 2023;24(Suppl 2):e106–15. [DOI] [PubMed] [Google Scholar]
- 2.Visco V, Esposito C, Rispoli A, Di Pietro P, Izzo C, Loria F, Di Napoli D, Virtuoso N, Bramanti A, Manzo M et al: The favourable alliance between CardioMEMS and levosimendan in patients with advanced heart failure. ESC Heart Fail 2024. [DOI] [PMC free article] [PubMed]
- 3.Visco V, Esposito C, Manzo M, Fiorentino A, Galasso G, Vecchione C, Ciccarelli M. A Multistep Approach to Deal With Advanced Heart Failure: A Case Report on the Positive Effect of Cardiac Contractility Modulation Therapy on Pulmonary Pressure Measured by CardioMEMS. Front Cardiovasc Med. 2022;9: 874433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campanile A, Visco V, De Carlo S, Ferruzzi GJ, Mancusi C, Izzo C, Mongiello F, Di Pietro P, Virtuoso N, Ravera A et al: Sacubitril/Valsartan vs. Standard Medical Therapy on Exercise Capacity in HFrEF Patients. Life (Basel) 2023, 13(5). [DOI] [PMC free article] [PubMed]
- 5.Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e876–94. [DOI] [PubMed] [Google Scholar]
- 6.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H, Butler J, Celutkiene J, Chioncel O, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–726. [DOI] [PubMed] [Google Scholar]
- 7.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H, Butler J, Celutkiene J, Chioncel O, et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2023;44(37):3627–39. [DOI] [PubMed] [Google Scholar]
- 8.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019;380(4):347–57. [DOI] [PubMed] [Google Scholar]
- 9.Sarafidis PA, Tsapas A. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2016;374(11):1092. [DOI] [PubMed] [Google Scholar]
- 10.McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Belohlavek J, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019;381(21):1995–2008. [DOI] [PubMed] [Google Scholar]
- 11.Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N Engl J Med. 2022;387(12):1089–98. [DOI] [PubMed] [Google Scholar]
- 12.Packer M, Anker SD, Butler J, Filippatos G, Ferreira JP, Pocock SJ, Carson P, Anand I, Doehner W, Haass M, et al. Effect of Empagliflozin on the Clinical Stability of Patients With Heart Failure and a Reduced Ejection Fraction: The EMPEROR-Reduced Trial. Circulation. 2021;143(4):326–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Packer M, Butler J, Zannad F, Filippatos G, Ferreira JP, Pocock SJ, Carson P, Anand I, Doehner W, Haass M, et al. Effect of Empagliflozin on Worsening Heart Failure Events in Patients With Heart Failure and Preserved Ejection Fraction: EMPEROR-Preserved Trial. Circulation. 2021;144(16):1284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mone P, Ciccarelli M, Jankauskas SS, Guerra G, Vecchione C, Visco V, Santulli G. SGLT2 inhibitors and GLP-1 receptor agonists: which is the best anti-frailty drug? Lancet Healthy Longev. 2024;5(9): 100632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santulli G, Varzideh F, Forzano I, Wilson S, Salemme L, de Donato A, Lombardi A, Rainone A, Nunziata L, Jankauskas SS, et al. Functional and Clinical Importance of SGLT2-inhibitors in Frailty: From the Kidney to the Heart. Hypertension. 2023;80(9):1800–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mone P, Guerra G, Lombardi A, Illario M, Pansini A, Marro A, Frullone S, Taurino A, Sorriento D, Verri V, et al. Effects of SGLT2 inhibition via empagliflozin on cognitive and physical impairment in frail diabetic elders with chronic kidney disease. Pharmacol Res. 2024;200: 107055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forrester JS, Diamond G, Chatterjee K, Swan HJ. Medical therapy of acute myocardial infarction by application of hemodynamic subsets (first of two parts). N Engl J Med. 1976;295(24):1356–62. [DOI] [PubMed] [Google Scholar]
- 18.Forrester JS, Diamond GA, Swan HJ. Correlative classification of clinical and hemodynamic function after acute myocardial infarction. Am J Cardiol. 1977;39(2):137–45. [DOI] [PubMed] [Google Scholar]
- 19.Abbas AE, Khoury Abdulla R, Aggrawal A, Crile J, Lester SJ, Boura J. A novel echocardiographic hemodynamic classification of heart failure based on stroke volume index and left atrial pressure. Echocardiography. 2017;34(10):1417–25. [DOI] [PubMed] [Google Scholar]
- 20.Mele D, Pestelli G, Dini FL, Dal Molin D, Smarrazzo V, Trevisan F, Luisi GA, Ferrari R. Novel Echocardiographic Approach to Hemodynamic Phenotypes Predicts Outcome of Patients Hospitalized With Heart Failure. Circ Cardiovasc Imaging. 2020;13(4): e009939. [DOI] [PubMed] [Google Scholar]
- 21.Mele D, Andrade A, Bettencourt P, Moura B, Pestelli G, Ferrari R. From left ventricular ejection fraction to cardiac hemodynamics: role of echocardiography in evaluating patients with heart failure. Heart Fail Rev. 2020;25(2):217–30. [DOI] [PubMed] [Google Scholar]
- 22.Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, Brueckmann M, Ofstad AP, Pfarr E, Jamal W, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396(10254):819–29. [DOI] [PubMed] [Google Scholar]
- 23.Polito MV, Silverio A, Rispoli A, Vitulano G, Auria F, De Angelis E, Loria F, Gigantino A, Bonadies D, Citro R, et al. Clinical and echocardiographic benefit of Sacubitril/Valsartan in a real-world population with HF with reduced ejection fraction. Sci Rep. 2020;10(1):6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Kardiol Pol. 2016;74(10):1037–147. [DOI] [PubMed] [Google Scholar]
- 25.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA et al: ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012, 14(8):803–869. [DOI] [PubMed]
- 26.Severino P, Maestrini V, Mariani MV, Birtolo LI, Scarpati R, Mancone M, Fedele F. Structural and myocardial dysfunction in heart failure beyond ejection fraction. Heart Fail Rev. 2020;25(1):9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butler J, Packer M, Filippatos G, Ferreira JP, Zeller C, Schnee J, Brueckmann M, Pocock SJ, Zannad F, Anker SD. Effect of empagliflozin in patients with heart failure across the spectrum of left ventricular ejection fraction. Eur Heart J. 2022;43(5):416–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dini FL, Carluccio E, Bitto R, Ciccarelli M, Correale M, D’Agostino A, Dattilo G, Ferretti M, Grelli A, Guida S, et al. Echocardiographically defined haemodynamic categorization predicts prognosis in ambulatory heart failure patients treated with sacubitril/valsartan. ESC Heart Fail. 2022;9(2):1107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim H, Lee SH, Cho JH, Lee H, Yim HW, Yoon KH, Kim HS. Discontinuation rate and reason for discontinuation after sodium-glucose cotransporter 2 inhibitor prescription in real clinical practice. J Clin Pharm Ther. 2020;45(6):1271–7. [DOI] [PubMed] [Google Scholar]
- 30.Santos-Gallego CG, Vargas-Delgado AP, Requena-Ibanez JA, Garcia-Ropero A, Mancini D, Pinney S, Macaluso F, Sartori S, Roque M, Sabatel-Perez F, et al. Randomized Trial of Empagliflozin in Nondiabetic Patients With Heart Failure and Reduced Ejection Fraction. J Am Coll Cardiol. 2021;77(3):243–55. [DOI] [PubMed] [Google Scholar]
- 31.Soga F, Tanaka H, Tatsumi K, Mochizuki Y, Sano H, Toki H, Matsumoto K, Shite J, Takaoka H, Doi T, et al. Impact of dapagliflozin on left ventricular diastolic function of patients with type 2 diabetic mellitus with chronic heart failure. Cardiovasc Diabetol. 2018;17(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee MMY, Brooksbank KJM, Wetherall K, Mangion K, Roditi G, Campbell RT, Berry C, Chong V, Coyle L, Docherty KF, et al. Effect of Empagliflozin on Left Ventricular Volumes in Patients With Type 2 Diabetes, or Prediabetes, and Heart Failure With Reduced Ejection Fraction (SUGAR-DM-HF). Circulation. 2021;143(6):516–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pascual-Figal DA, Zamorano JL, Domingo M, Morillas H, Nunez J, Cobo Marcos M, Riquelme-Perez A, Teis A, Santas E, Caro-Martinez C, et al. Impact of dapagliflozin on cardiac remodelling in patients with chronic heart failure: The DAPA-MODA study. Eur J Heart Fail. 2023;25(8):1352–60. [DOI] [PubMed] [Google Scholar]
- 34.Bolinder J, Ljunggren O, Kullberg J, Johansson L, Wilding J, Langkilde AM, Sugg J, Parikh S. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97(3):1020–31. [DOI] [PubMed] [Google Scholar]
- 35.Han Y, Li YF, Ye CW, Gu YY, Chen X, Gu Q, Xu QQ, Wang XM, He SM, Wang DD. Effects of dapagliflozin on body weight in patients with type 2 diabetes mellitus: Evidence-based practice. Exp Ther Med. 2024;27(4):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta S, Khan F, Shapiro M, Weeks SG, Litwin SE, Michaels AD. The associations between tricuspid annular plane systolic excursion (TAPSE), ventricular dyssynchrony, and ventricular interaction in heart failure patients. Eur J Echocardiogr. 2008;9(6):766–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Popescu BA, Antonini-Canterin F, Temporelli PL, Giannuzzi P, Bosimini E, Gentile F, Maggioni AP, Tavazzi L, Piazza R, Ascione L et al: Right ventricular functional recovery after acute myocardial infarction: relation with left ventricular function and interventricular septum motion. GISSI-3 echo substudy. Heart 2005, 91(4):484–488. [DOI] [PMC free article] [PubMed]
- 38.Mustapic I, Bakovic D, Susilovic Grabovac Z, Borovac JA: Impact of SGLT2 Inhibitor Therapy on Right Ventricular Function in Patients with Heart Failure and Reduced Ejection Fraction. J Clin Med 2022, 12(1). [DOI] [PMC free article] [PubMed]
- 39.Visco V, Radano I, Campanile A, Ravera A, Silverio A, Masarone D, Pacileo G, Correale M, Mazzeo P, Dattilo G, et al. Predictors of sacubitril/valsartan high dose tolerability in a real world population with HFrEF. ESC Heart Fail. 2022;9(5):2909–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen L, Jhund PS, Docherty KF, Vaduganathan M, Petrie MC, Desai AS, Kober L, Schou M, Packer M, Solomon SD, et al. Accelerated and personalized therapy for heart failure with reduced ejection fraction. Eur Heart J. 2022;43(27):2573–87. [DOI] [PubMed] [Google Scholar]
- 41.Heerspink HJL, Stefansson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, et al. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020;383(15):1436–46. [DOI] [PubMed] [Google Scholar]
- 42.Chertow GM, Vart P, Jongs N, Toto RD, Gorriz JL, Hou FF, McMurray JJV, Correa-Rotter R, Rossing P, Sjostrom CD, et al. Effects of Dapagliflozin in Stage 4 Chronic Kidney Disease. J Am Soc Nephrol. 2021;32(9):2352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Visco V, Esposito C, Vitillo P, Vecchione C, Ciccarelli M. It is easy to see, but it is better to foresee: a case report on the favourable alliance between CardioMEMS and levosimendan. Eur Heart J Case Rep. 2020;4(4):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nassif ME, Windsor SL, Tang F, Khariton Y, Husain M, Inzucchi SE, McGuire DK, Pitt B, Scirica BM, Austin B, et al. Dapagliflozin Effects on Biomarkers, Symptoms, and Functional Status in Patients With Heart Failure With Reduced Ejection Fraction: The DEFINE-HF Trial. Circulation. 2019;140(18):1463–76. [DOI] [PubMed] [Google Scholar]
- 45.Mone P, Varzideh F, Jankauskas SS, Pansini A, Lombardi A, Frullone S, Santulli G. SGLT2 Inhibition via Empagliflozin Improves Endothelial Function and Reduces Mitochondrial Oxidative Stress: Insights From Frail Hypertensive and Diabetic Patients. Hypertension. 2022;79(8):1633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fathi A, Vickneson K, Singh JS. SGLT2-inhibitors; more than just glycosuria and diuresis. Heart Fail Rev. 2021;26(3):623–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.