Abstract
Background
Genetic polymorphisms in Toll-interacting protein (TOLLIP) have been documented in relation to clinical manifestations of interstitial lung disease (ILD). Nevertheless, the findings across studies present inconsistencies. The present meta-analysis endeavors to elucidate the nexus between genetic variations in TOLLIP and the onset and prognosis of interstitial lung disease (ILD), with the overarching aim of providing insight into the pathophysiological underpinnings of ILD.
Method
This systematic review was registered in PROSPERO. The OVID MEDLINE, OVID EMBASE, and Web of Science electronic databases were searched.
Results
Fourteen studies with a total of 4821 cases and 9765 controls were examined. The final TOLLIP variants to be included in this meta-analysis were rs5743890, rs111521887, and rs3750920. There were significantly fewer TOLLIP rs5743890 minor allele C carriers among individuals with interstitial lung disease (ILD) than among those without this condition (11.42% vs. 18.92%). Conversely, patients with ILD exhibited higher frequencies of rs111521887 minor allele G carriers (28.92% vs. 22.44%) and rs3750920 minor allele T carriers (40.06% vs. 34.00%). A potential association between rs5743890_C and a reduced incidence of ILD was plausible (p = 0.04, OR = 0.72, 95% CI = 0.53–0.99). Furthermore, a stratified analysis revealed that rs5743890_C was significantly associated with a decreased risk of IPF (p = 0.004, OR = 0.62, 95% CI = 0.44–0.86). There was a significant correlation between susceptibility to ILD and rs111521887 G (p < 0.00001, OR = 1.48, 95% CI = 1.33–1.65) and rs3750920 T (p < 0.00001, OR = 1.34, 95% CI = 1.26–1.44). The survival of IPF patients was correlated with the TOLLIP rs5743890 SNP, and patients with the rs5743890_C genotype had worse survival (p = 0.02, HR = 1.59, 95% CI = 1.07–2.36).
Conclusion
This study showed that rs5743890_C was associated with a lower incidence of ILD and a worse survival rate in patients with IPF. Rs111521887_G and rs3750920_T were found to be associated with an elevated risk of ILD incidence, while no significant association was observed with ILD prognosis. Furthermore, studies are warranted to validate our results and assess the effects of TOLLIP genetic variants on ILD.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13023-024-03410-8.
Keywords: TOLLIP, Genetic variants, Interstitial lung disease, Idiopathic pulmonary fibrosis, Meta-analyses
Introduction
Interstitial lung diseases (ILDs) are a heterogeneous group of pulmonary diseases characterized by fibrosis and inflammation in the pulmonary parenchyma [1]. Fibrotic ILDs, such as idiopathic pulmonary fibrosis (IPF), chronic hypersensitivity pneumonitis (CHP), and connective tissue disease-associated ILD (CTD-ILD), are usually associated with severe morbidity and early mortality.
Emerging evidence has revealed a genetic basis for the crucial role of the ILD incidence. The functions of identified susceptibility genes in ILD vary from host defence to cell‒cell adhesion to DNA repair [2]. These mutations account for only a small fraction of the risk of developing idiopathic interstitial pneumonia, and there has been no genetic variation associated with ILD results. In later studies, the Single nucleotide polymorphisms (SNPs) of some genes are reportedly associated with clinical features of lung fibrosis. The MUC5B rs35705950 SNP is a common and strong genetic risk factor for ILD in the general population and has been previously meta-analysed [3].
Toll-interacting protein (TOLLIP), which can inhibit the signaling of the TOLL-like receptor and the production of tumor necrosis factor-a (TNF-a) and IL-6, is involved in various diseases, such as Parkinson’s disease, Alzheimer’s disease, inflammatory bowel disease, myocardial hypertrophy, and idiopathic pulmonary fibrosis [4–9]. Note that TOLLIP can regulate the trajectory of lung disease in both positive and negative ways. Genetic variants of TOLLIP have been demonstrated to be associated with various chronic lung diseases, including idiopathic pulmonary fibrosis, asthma, primary graft dysfunction following lung transplantation, and pulmonary infections [9–12]. In 2013, a genome-wide association study led by Imre Noth et al., published in Lancet Respiratory Medicine, first identified variants in the TOLLIP gene as being associated with susceptibility to IPF and increased mortality, highlighting the gene’s significance in the disease’s pathogenesis [18]. Subsequent research in 2015 by Justin M. Oldham et al. revealed a significant interaction between TOLLIP SNPs and N-acetylcysteine (NAC) treatment, suggesting that further investigation into TOLLIP SNPs could offer new avenues for therapeutic strategies in IPF [8]. Located in proximity on chromosome 11p15.5, TOLLIP and MUC5B are crucial in the immune response, bronchial, and alveolar fibrosis. While both genes are involved in host defines, TOLLIP is particularly notable for its association with inflammatory signalling. However, studies on TOLLIP genetic variants and ILD have yielded conflicting results. To address this, we conducted a meta-analysis to examine the relationship between TOLLIP genetic variants and both the incidence and prognosis of ILD. Our aim was to clarify the role of these variants in the pathogenesis of ILD and to identify potential new avenues for therapeutic strategies.
Method
Study registration
The systematic review was registered in PROSPERO (CRD42022308659). We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2009 statement.
Search strategy
To perform systematic retrieval, we searched the electronic databases OVID MEDLINE, OVID EMBASE, and Web of Science using the Medical Subject Headings term and a keyword on Jan 30, 2022. The search terms used were as follows: “interstitial lung diseases”, “ILD”, “genetic variation”, “variant”, “gene polymorphism”, “TOLLIP”, and “Toll-interacting protein”. The detailed search strategy is provided in the supplemental file. The duplication was removed. The references of retrieved publications were manually filtered for potentially relevant articles.
Inclusion criteria and exclusion criteria
The inclusion criteria were as follows: (a) contained original data and (b) provided adequate data to calculate odds ratios (ORs) and 95% confidence intervals (CIs). The exclusion criteria were as follows: (a) had overlapping data or (b) had family members studied because the analyses were based on linkage considerations. (c) English texts were not available. (d) Abstracts, reviews, comments and conference presentations.
Data extraction and quality assessment
The titles and abstracts of the retrieved studies were filtered based on our study selection criteria. The full texts of the remaining studies from the first screening were downloaded for further screening based on the study eligibility criteria. Two authors independently screened the studies, and any disagreements were resolved via discussion or adjudication by a third reviewer, if necessary.
Two authors independently collected data on the first author’s family name, year of publication, country, study design, ethnicity, number of participants, classification of ILD, allele frequency, hazard ratio (HR) and 95% confidence interval (CI) of survival associated with minor alleles. If a study contained several independent groups, the groups were listed.
The methodological quality of the included studies was assessed using the Newcastle–Ottawa quality assessment scale (NOS). A total of three domains—selection, comparability, and exposure—with eight numbered items yielded the highest total score of 9. For selection and exposure, each of seven numbered items was scored as 1 if the answer was yes, while for comparability, a maximum score of 2 was given for a numbered item. Studies with a score ≥ 6 were considered high-quality studies. Two authors performed the methodological quality assessment, and any disagreements were resolved via discussion or adjudication by a third reviewer, if necessary.
Statistical analysis
We performed the data analyses using RevMan software (version 5.4). Meta-analyses were performed to explore the associations between TOLLIP genotypes and susceptibility to and prognosis of ILD patients. Subgroup analysis was performed according to the classification of ILD. Time-to-event data were incorporated into the meta-analysis by the methods published by Jayne F Tierney et al. [13].
The effect size of the meta-analysis was estimated by incidence with 95% CIs and odds ratios (ORs). We assessed clinical diversity across studies through statistical heterogeneity using I2 and p values. I2 values of 25%, 50%, and 75% represented low, moderate, and high heterogeneity, respectively. Fixed-effect models (FEMs) were used for synthetic analyses. A random effects model was applied when heterogeneity was greater than 50% or P < 0.05. Sensitivity analysis was performed by excluding studies one by one to identify the potential source of heterogeneity. We assessed the risk of publication bias via funnel plots.
Results
Literature search and study characteristics
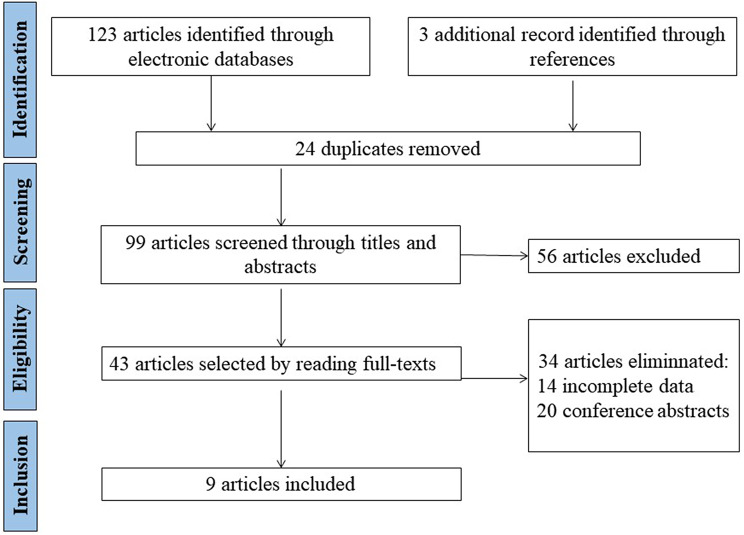
A total of 99 articles were retrieved via electronic and manual searching after removing duplications, with 43 selected articles for further evaluation based on title and abstract details. Of all the selected articles, 20 were meeting abstracts, and 14 lacked essential data; thus, these 34 articles were excluded. Among the 9 included articles, 3 studies contained complete data from two or three independent cohorts; thus, we listed them. A total of 14 eligible studies were ultimately analysed, involving 4821 patients and 9765 controls. The details are shown in Fig. 1. The characteristics of the eligible studies are shown in Tables 1 and 2.
Fig. 1.
Study selection flowchart
Table 1.
Characteristics of individual studies on TOLLIP variants included in the meta-analysis of susceptibility to ILD
| References | Year | Country | Ethnicity | Disease | Numbers | Minor alleles (%) | Allele association | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | Case | Control | OR | 95% CI | p value | ||||||
| rs5743890 | ||||||||||||
| Patrícia Caetano Mota25 | 2022 | Portuguese | European | IPF | 64 | 74 | 14.80 | 18.20 | 0.78 | 0.41 | 1.48 | 0.45 |
| Javier Guzmán-Vargas14 | 2021 | Mexico | European | IPF | 93 | 174 | 0.00 | 41.52 | 0.01 | 0.00 | 0.10 | 0.00 |
| Jonsson, E. 15 | 2021 | Sweden | European | RA-ILD | 60 | 2350 | 20.00 | 19.42 | 1.04 | 0.63 | 1.70 | 0.01 |
| Francesco Bonella16 | 2021 | Germany | European | IPF | 62 | 50 | 11.29 | 11.00 | 1.03 | 0.45 | 2.38 | 0.95 |
| Brett Ley17 | 2017 | America | European | CHP/IPF | 442 | 503 | 15.27 | 14.20 | 1.09 | 0.84 | 1.4 | 0.51 |
| Imre Noth-118 | 2013 | America | European | IPF | 542 | 542 | 10.98 | 15.04 | 0.70 | 0.54 | 0.90 | 0.01 |
| Imre Noth-218 | 2013 | America | European | IPF | 544 | 687 | 9.00 | 14.99 | 0.56 | 0.43 | 0.72 | 0.00 |
| Imre Noth-3 18 | 2013 | America | European | IPF | 324 | 702 | 10.03 | 17.02 | 0.54 | 0.41 | 0.73 | 0.00 |
| In all | 2131 | 5082 | 11.42 | 18.92 | ||||||||
| rs111521887 | ||||||||||||
| Patrícia Caetano Mota25 | 2022 | Portuguese | European | IPF | 64 | 74 | 19.50 | 12.80 | 1.65 | 0.86 | 3.16 | 0.13 |
| Javier Guzmán-Vargas14 | 2021 | Mexico | European | IPF | 93 | 174 | 43.75 | 44.23 | 0.98 | 0.47 | 2.04 | 0.96 |
| Jonsson, E.15 | 2021 | Sweden | European | RA-ILD | 60 | 2350 | 33.33 | 19.55 | 2.06 | 1.4 | 3.03 | 0.00 |
| Imre Noth-118 | 2013 | America | European | IPF | 542 | 542 | 28.97 | 21.03 | 1.53 | 1.26 | 1.86 | 0.00 |
| Imre Noth-218 | 2013 | America | European | IPF | 544 | 687 | 25.00 | 17.98 | 1.52 | 1.25 | 1.85 | 0.00 |
| Imre Noth-318 | 2013 | America | European | IPF | 324 | 702 | 22.99 | 19.02 | 1.27 | 1.01 | 1.59 | 0.04 |
| In all | 1627 | 4529 | 28.92 | 22.44 | ||||||||
| rs3750920 | ||||||||||||
| Patrícia Caetano Mota25 | 2022 | Portuguese | European | IPF | 64 | 74 | 50.00 | 41.20 | 1.43 | 0.89 | 2.3 | 0.14 |
| Francesco Bonella16 | 2021 | Germany | European | IPF | 62 | 50 | 45.97 | 46.00 | 1.00 | 0.59 | 1.69 | 1.00 |
| Fingerlin, T. E.19 | 2013 | America | European | IIP | 1615 | 4683 | 50.19 | 43.80 | 1.29 | 1.19 | 1.40 | 0.00 |
| Imre Noth-118 | 2013 | America | European | IPF | 542 | 542 | 29.15 | 21.03 | 1.54 | 1.27 | 1.88 | 0.00 |
| Imre Noth-218 | 2013 | America | European | IPF | 544 | 687 | 25.00 | 17.98 | 1.52 | 1.25 | 1.85 | 0.00 |
| In all | 2827 | 6036 | 40.06 | 34.00 | ||||||||
OR: odds ratio; CI: confidence interval; IPF: idiopathic pulmonary fibrosis; RA: rheumatoid arthritis; ILD: interstitial lung diseases; IIP: idiopathic interstitial pneumonia; CTD-ILD: connective tissue disease associated interstitial lung diseases; IPAF: Interstitial pneumonitis with autoimmune features; CHP: chronic hypersensitivity pneumonitis
Table 2.
Analysis of the association between the TOLLIP variants and susceptibility to ILD
| Variants | Population | No. of studies | No. of alleles | Test of association | Test of heterogeneity | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | OR | 95%CI | p value | Model | p value | I2 | |||
| rs5743890, C | Overall | 8 | 4170 | 10,147 | 0.72 | 0.53-0.99 | 0.04 | RE | <0.0000 | 80% |
| IPF | 6 | 3186 | 4440 | 0.62 | 0.44-0.86 | 0.004 | RE | 0.004 | 71% | |
| rs111521887, G | Overall | 6 | 3100 | 9022 | 1.48 | 1.33-1.65 | <0.0000 | FE | 0.3 | 17% |
| rs3750920, T | Overall | 5 | 5654 | 12,072 | 1.34 | 1.26-1.44 | <0.0000 | FE | 0.23 | 29% |
OR: odds ratio; CI: confidence interval; RE: R random effects model; FE: F fixed effects model
Methodological quality
Of the 9 articles, 8 had an NOS score ≥ 6, indicating high study quality, while one was rated as being of low quality (NOS score < 6). The NOS scores are shown in supplemental Table S1.
All the selected studies were thoroughly analysed. The three TOLLIP variants rs5743890, rs111521887 and rs3750920 were ultimately included in this meta-analysis.
Allele frequency of the TOLLIP variants
The frequency of Rs5743890_C was lower in patients with ILD than in controls (11.42% vs. 18.92%). In contrast, the frequencies of rs111521887_G (28.92% vs. 22.44%) and rs3750920_T (40.06% vs. 34.00%) were greater in patients with ILD. The details are shown in Table 1.
Meta-analysis of the TOLLIP minor alleles and susceptibility to ILD
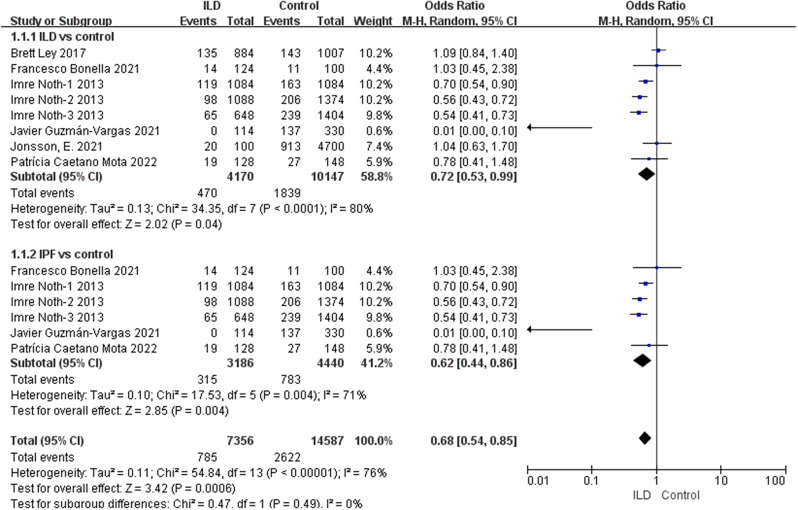
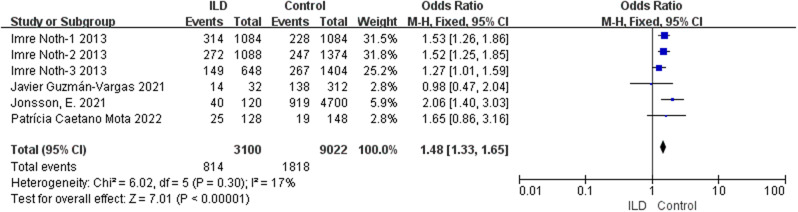
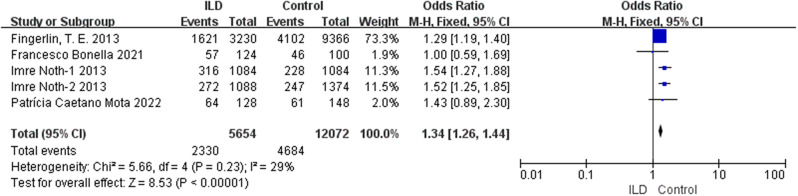
According to our comprehensive meta-analysis encompassing various types of ILD, rs5743890_C exhibited a potential association with a reduced risk of ILD (p = 0.04, OR = 0.72, 95% CI = 0.53–0.99) and a significant association with a decreased risk of idiopathic pulmonary fibrosis (IPF) (p = 0.004, OR = 0.62, 95% CI = 0.44–0.86) (Fig. 2). Notably, the analysis revealed a substantial association between susceptibility to ILD and rs111521887_G (p < 0.00001, OR 1.48, 95% CI = 1.33–1.65) (Fig. 3), as well as the rs3750920_T allele (p < 0.00001, OR 1.34, 95% CI = 1.26–1.44) (Fig. 4). The details are shown in Table 3.
Fig. 2.
Analysis of the association between rs5743890_C and susceptibility to ILD
Fig. 3.
Analysis of the association between rs111521887_G and susceptibility to ILD
Fig. 4.
Analysis of the association between rs3750920_T and susceptibility to ILD
Table 3.
Characteristics of individual studies on TOLLIP variants included in the meta-analysis of survival
| References | Year | Country | Ethnicity | Disease | n | HR | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| rs5743890 | ||||||||
| Associations with transplant-free survival | ||||||||
| Francesco Bonella16 | 2021 | Germany | European | IPF | 37 | 2.22 | 1.15 | 4.29 |
| Chad A. Newton-120 | 2019 | America | European | IPF | 499 | 1.41 | 1.10 | 1.81 |
| Chad A. Newton-220 | 2019 | America | European | IPAF | 233 | 0.65 | 0.37 | 1.13 |
| Chad A. Newton-320 | 2019 | America | European | CTD-ILD | 241 | 0.90 | 0.45 | 1.83 |
| Brett Ley-117 | 2017 | America | European | CHP/IPF | 142 | 0.94 | 0.42 | 2.10 |
| Brett Ley-217 | 2017 | America | European | CHP/IPF | 72 | 0.85 | 0.40 | 1.82 |
| Associations with survival | ||||||||
| Chad A. Newton-120 | 2019 | America | European | IPF | 499 | 1.46 | 1.08 | 1.98 |
| Chad A. Newton-220 | 2019 | America | European | IPAF | 233 | 0.64 | 0.34 | 1.23 |
| Chad A. Newton-320 | 2019 | America | European | CTD-ILD | 241 | 0.80 | 0.36 | 1.77 |
| Brett Ley-117 | 2017 | America | European | CHP/IPF | 142 | 0.91 | 0.39 | 2.14 |
| Brett Ley-217 | 2017 | America | European | CHP/IPF | 72 | 0.78 | 0.27 | 2.26 |
| rs3750920 | ||||||||
| Associations with survival | ||||||||
| Francesco Bonella16 | 2021 | Germany | European | IPF | 37 | 0.77 | 0.33 | 1.84 |
| Takuma Isshiki9 | 2021 | Japan | Asian | FILD | 102 | 0.95 | 0.61 | 1.49 |
| rs111521887 | ||||||||
| Associations with survival | ||||||||
| Patrícia Caetano Mota-125 | 2022 | Portuguese | European | IPF | 21 | 1.65 | 0.83 | 3.26 |
| Patrícia Caetano Mota-225 | 2022 | Portuguese | European | IPF | 2 | 5.99 | 0.74 | 48.20 |
CTD-ILD, connective tissue disease associated interstitial lung diseases; IPAF: Interstitial pneumonitis with autoimmune features; IPF: idiopathic pulmonary fibrosis; CHP: chronic hypersensitivity pneumonitis; FILD: fibrosing ILD
Meta-analysis of the TOLLIP genotype and survival of patients with ILD
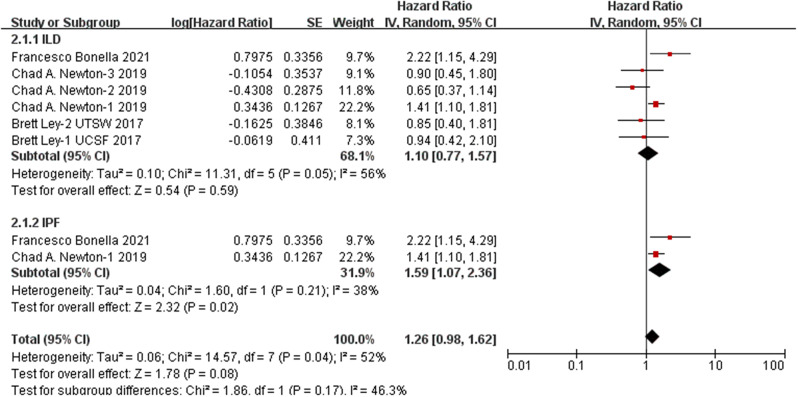
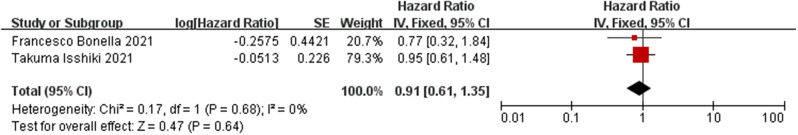
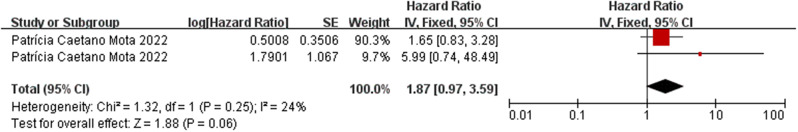
The presence of the TOLLIP rs5743890 SNP was not found to be associated with either overall survival or transplant-free survival among patients with ILD (survival: p = 0.59, HR: 1.10, 95% CI: 0.77–1.57; transplant-free survival: p = 0.27, HR: 1.14, 95% CI: 0.90–1.46). However, a stratified analysis revealed that rs5743890_C was significantly associated with worse survival in patients diagnosed with IPF (p = 0.02, hazard ratio (HR) 1.59, 95% CI = 1.07–2.36) (Fig. 5). No significant association was detected between rs3750920 and the survival of patients with ILD (p = 0.64, HR = 0.91, 95% CI = 0.61–1.35) (Fig. 6). Similarly, there was no significant association between rs111521887 and the survival of ILD patients (p = 0.06, HR 1.87, 95% CI = 0.97–3.59) (Fig. 7). The details are shown in shown in Table 4.
Fig. 5.
Analysis of the association between rs5743890_C and the survival of patients with ILD
Fig. 6.
Analysis of the association between rs3750920_T and the survival of patients with ILD
Fig. 7.
Analysis of the association between 111521887_G and the survival of patients with ILD
Table 4.
Analysis of the association between the TOLLIP polymorphisms and survival of patients with ILD
| Variants | Population | No. of studies | Test of association | Test of heterogeneity | ||||
|---|---|---|---|---|---|---|---|---|
| HR | 95%CI | p value | Model | p value | I2 | |||
| Associations with survival | ||||||||
| rs5743890 | Overall | 6 | 1.10 | 0.77-1.57 | 0.59 | RE | 0.04 | 56% |
| IPF | 2 | 1.59 | 1.07-2.36 | 0.02 | FE | 0.21 | 38% | |
| rs3750920 | Overall | 2 | 0.91 | 0.61-1.36 | 0.64 | FE | 0.67 | 0% |
| rs111521887 | IPF | 1 | 1.87 | 0.97-3.59 | 0.06 | FE | 0.25 | 24% |
| Associations with transplant-free survival | ||||||||
| rs5743890 | Overall | 5 | 1.14 | 0.90-1.46 | 0.27 | FE | 0.13 | 44% |
HR, hazard ratio; CI, confidence interval; RE, R random effects model; FE, F fixed effects model
Publication bias
Publication biases were evaluated with funnel plots. The funnel plots of any comparisons were symmetrical (Supplemental Figures S1-3). Therefore, publication bias was considered unlikely.
Heterogeneity source and sensitivity analysis
Heterogeneity was observed, especially in the meta-analysis of rs5743890_C and susceptibility to ILD. Sensitivity analysis performed by excluding studies one by one did not reveal any apparent possible heterogeneity (data not shown).
Discussion
To the best of our knowledge, this is the first inaugural meta-analysis conducted on the relationship between TOLLIP variants and interstitial lung disease (ILD). Our findings indicate that the frequency of rs5743890_C is reduced among ILD patients, whereas the frequencies of rs111521887_G and rs3750920_T are elevated. Specifically, our analysis revealed that rs5743890_C is associated with a decreased risk of ILD, while rs5743890_C is also linked to a decreased survival rate in individuals with IPF. On the other hand, rs111521887_G and rs3750920_T are associated with an increased incidence of ILD, although they are not significantly associated with ILD incidence or prognosis.
According to a prior genome-wide association study (GWAS) conducted in three stages, carriers of the minor allele of rs5743890 were found to have a reduced risk of developing IPF but a heightened risk of mortality [18]. Our meta-analysis examined the impact of rs5743890 on the prognosis of patients with ILD and included a study indicating that the minor allele of rs5743890 was associated with worse survival and disease progression in patients with IPF [16]. It is worth noting that, to incorporate all available data, the results included in our meta-analysis were not adjusted for other variables. We observed that, in another study included in our analysis, the association between the minor allele of rs5743890 and the survival of IPF patients was not statistically significant after we adjusted for variables such as age, sex, non-Hispanic white ethnicity, baseline forced vital capacity percent predicted, and baseline diffusion capacity of the lung for carbon monoxide percent predicted [20].
In this study, we identified the association between the minor alleles rs111521887 and rs3750920 and an increased incidence of ILD. However, these alleles were not found to be associated with the prognosis of ILD. Among the studies included in our analysis, only one small sample study did not reach the conclusion that the presence of rs111521887_G increased the incidence of ILD [14]. Moreover, the results of two genome-wide association studies (GWASs) supported that rs3750920_T was a risk factor for ILD [18, 19]. In a study involving a Japanese population, the frequency of rs3750920_T was lower in patients with fibrotic ILD, particularly among non-IPF patients [9]. Interestingly, although our meta-analysis revealed that rs3750920_T was not associated with the prognosis of ILD, previous studies have revealed a significant correlation between the response to N-acetylcysteine (NAC) therapy and rs3750920. These findings suggest that NAC may be an effective therapy for IPF patients with the rs3750920 TT genotype but may be associated with a trend toward harm in those with the CC genotype. Additionally, in a retrospective study involving patients with IPF and interstitial pneumonia with autoimmune features (IPAF), rs3750920 TT was more common in the positive antinuclear antibody (ANA) group, in which NAC exposure appeared to be beneficial for transplant-free survival [21].
Although these data could not be incorporated into the present study, it is worth noting that several other TOLLIP genetic variants have also been reported to be associated with the clinical phenotype of ILD. According to the previously mentioned three-stage genome-wide association study (GWAS), rs5743894_G significantly increased the risk of IPF. Furthermore, rs3829223_C, rs908225_T, rs5743944_A, and rs5743900_G also exhibited associations with susceptibility to IPF in both the primary GWAS and replication [18]. In a retrospective and prospective study, the presence of the rs5743899 GG genotype was associated with a rapid deterioration in forced vital capacity over time among patients diagnosed with chronic hypersensitivity pneumonitis (CHP) [22].
The precise roles of TOLLIP genetic variants in ILD remain enigmatic. TOLLIP, acting as an inhibitor, plays a pivotal role in downregulating the production of proinflammatory cytokines, promoting autophagy, and facilitating intracellular trafficking [23, 24]. A previous study revealed differences in TOLLIP expression among IPF patients bearing various TOLLIP minor alleles. Notably, TOLLIP expression was reduced in the lung tissue of minor allele carriers, decreasing by 20% in patients with rs5743890_C, 40% in those with rs111521887_G, and 50% in those with rs5743894_G [18]. The decreased TOLLIP expression observed in minor allele carriers suggested that TOLLIP deficiency may be involved in the pathogenesis of IPF.
This study is subject to several limitations. Significant heterogeneity was observed, particularly in the meta-analysis examining the relationship between rs5743890_C and susceptibility to interstitial lung disease (ILD). Although subgroup analyses were performed based on ILD classification, they did not entirely mitigate the observed heterogeneity, which could be attributed to the diverse study designs. It is important to recognize that multiple factors, such as age, sex, and smoking status, can influence the clinical phenotype of ILD. Consequently, the inherent heterogeneity of the study populations across the included studies might have contributed to the overall heterogeneity in our analysis. Additionally, certain subgroup analyses were limited by the limited number of included studies, and genotype distribution data were unavailable.
Conclusion
This study represents the first meta-analysis investigating the relationship between TOLLIP genetic variants and clinical features of interstitial lung disease (ILD). The analysis revealed several key findings: the frequency of rs5743890_C was notably lower in ILD patients, while the frequencies of rs111521887_G and rs3750920_T were greater. Specifically, rs5743890_C was associated with a decreased risk of ILD incidence and emerged as a significant factor in the survival of patients with IPF. Conversely, the presence of 111521887_G and rs3750920_T was associated with an increased risk of ILD incidence, but no significant associations were identified regarding ILD prognosis. Further research is warranted to validate our findings and to comprehensively assess the impacts of TOLLIP genetic variants on ILD.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
None declared.
Abbreviations
- Acronym
Full Form
- TOLLIP
Toll-Interacting Protein
- ILD
Interstitial Lung Disease
- IPF
Idiopathic Pulmonary Fibrosis
- CHP
Chronic Hypersensitivity Pneumonitis
- CTD-ILD
Connective Tissue Disease-Associated ILD
- SNP
Single Nucleotide Polymorphism
- OR
Odds Ratio
- CI
Confidence Interval
- HR
Hazard Ratio
- NOS
Newcastle–Ottawa Quality Assessment Scale
- PROSPERO
International Prospective Register of Systematic Reviews
- IPAF
Interstitial Pneumonitis with Autoimmune Features
Author contributions
Jiang and Li conceived the study, Li and Cui designed the study forms, and Jiang guided this study. Li searched the literature; Li and Cui screened the studies for inclusion and extracted data; Li and Cui assessed methodological quality; and Li organized the data. All the authors drafted and revised the manuscript.
Funding
The work is supported by the 1.3.5 Project for Disciplines of Excellence-Clinical Research Incubation Project, West China Hospital, Sichuan University (No. 2019HXFH002).
Data availability
The datasets supporting the conclusions of this article are included within the article (and its additional files).
Declarations
Ethics approval and consent to participate
This study does not involve ethics, as it is a systematic review and meta-analysis.
Consent for publication
All the authors have agreed to be listed and have read and approved the manuscript for publication.
Competing interests
The authors have declared no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Johannson KA, Chaudhuri N, Adegunsoye A, Wolters PJ. Treatment of fibrotic interstitial lung disease: current approaches and future directions. Lancet. 2021;398(10309):1–11. [DOI] [PubMed] [Google Scholar]
- 2.Adegunsoye A, Vij R, Noth I. Integrating Genomics into Management of Fibrotic interstitial lung disease. Chest. 2019;155(5):1026–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei R, Li C, Zhang M, Jones-Hall YL, Myers JL, Noth I, et al. Association between MUC5B and TERT polymorphisms and different interstitial lung disease phenotypes. Transl Res. 2014;163(5):494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryan TA, Phillips EO, Collier CL, Jb Robinson A, Routledge D, Wood RE, et al. Tollip coordinates parkin-dependent trafficking of mitochondrial-derived vesicles. EMBO J. 2020;39(11):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makioka K, Yamazaki T, Takatama M, Ikeda M, Murayama S, Okamoto K, et al. Immunolocalization of Tom1 in relation to protein degradation systems in Alzheimer’s disease. J Neurol Sci. 2016;365:101–7. [DOI] [PubMed] [Google Scholar]
- 6.Fernandes P, MacSharry J, Darby T, Fanning A, Shanahan F, Houston A, et al. Differential expression of key regulators of toll-like receptors in ulcerative colitis and Crohn’s disease: a role for Tollip and peroxisome proliferator-activated receptor gamma? Clin Exp Immunol. 2016;183(3):358–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Jiang XL, Liu Y, Jiang DS, Zhang Y, Zhang R, et al. Toll-interacting protein (Tollip) negatively regulates pressure overload-induced ventricular hypertrophy in mice. Cardiovasc Res. 2014;101(1):87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oldham JM, Ma SF, Martinez FJ, Anstrom KJ, Raghu G, Schwartz DA et al. TOLLIP, MUC5B, and the Response to N-Acetylcysteine among Individuals with Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med. 2015;192(12):1475–1482. [DOI] [PMC free article] [PubMed]
- 9.Isshiki T, Koyama K, Homma S, Sakamoto S, Yamasaki A, Shimizu H, et al. Association of rs3750920 polymorphism in TOLLIP with clinical characteristics of fibrosing interstitial lung diseases in Japanese. Sci Rep. 2021;11(1):16250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu S, Liu X, Chen L, Wang Y, Zhang M, Wang M, et al. Polymorphisms of TLR2, TLR4 and TOLLIP and tuberculosis in two independent studies. Biosci Rep. 2020;28(8):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah JA, Vary JC, Chau TT, Bang ND, Yen NT, et al. Human TOLLIP regulates TLR2 and TLR4 signalling, and its polymorphisms are associated with susceptibility to tuberculosis. J Immunol. 2012;15(4):1737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang C, Jiang D, Francisco D, Berman R, Wu Q, et al. Tollip SNP rs5743899 modulates human airway epithelial responses to rhinovirus infection. Clin Exp Allergy. 2016;46(12):1549–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guzmán-Vargas J, Ambrocio-Ortiz E, Pérez-Rubio G, Ponce-Gallegos MA, Hernández-Zenteno RJ, Mejía M, et al. TERT, DS Differential genomic Profile in, and between COPD patients with Emphysema, IPF, and CPFE Syndrome. Front Med (Lausanne). 2021;8:7251449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jönsson E, Ljung L, Norrman E, Freyhult E, Ärlestig L, Dahlqvist J, et al. Pulmonary fibrosis in relation to genetic loci in an inception cohort of patients with early rheumatoid arthritis from northern Sweden. Rheumatology (Oxford). 2021;16:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonella F, Campo I, Zorzetto M, Boerner E, Ohshimo S, Theegarten D, et al. Potential clinical utility of MUC5B und TOLLIP single nucleotide polymorphisms (SNPs) in the management of patients with IPF. Orphanet J Rare Dis. 2021;16(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ley B, Newton CA, Arnould I, Elicker BM, Henry TS, Vittinghoff E, et al. The MUC5B promoter polymorphism and telomere length in patients with chronic hypersensitivity pneumonitis: an observational cohort-control study. Lancet Respir Med. 2017;08(8):30216–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noth I, Zhang Y, Ma SF, Flores C, Barber M, Huang Y, et al. Broderick SM, genetic variants associated with idiopathic pulmonary fibrosis susceptibility and mortality: a genome-wide association study. Lancet Respir Med. 2013;1(4):309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peljto AL, Selman M, Kim DS, Murphy E, Tucker L, Pardo A, et al. The MUC5B promoter polymorphism is associated with idiopathic pulmonary fibrosis in a Mexican cohort but is rare among Asian ancestries. Chest. 2015;147(2):460–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newton CA, Oldham JM, Ley B, Anand V, Adegunsoye A, Liu G, et al. Telomere length and genetic variant associations with interstitial lung disease progression and survival. Eur Respir J. 2019;53(4):1801641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oldham JM, Witt LJ, Adegunsoye A, Chung JH, Lee C, Hsu S, et al. N-acetylcysteine exposure is associated with improved survival in anti-nuclear antibody seropositive patients with usual interstitial pneumonia. BMC Pulm Med. 2018;18(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katayanagi S, Setoguchi Y, Kitagawa S, Okamoto T, Miyazaki Y. Alternative gene expression by TOLLIP variant is Associated with lung function in chronic hypersensitivity pneumonitis. Chest. 2022;02(2):1–19. [DOI] [PubMed] [Google Scholar]
- 23.Lee HJ, Chung KC. PINK1 positively regulates IL-1β-mediated signaling through Tollip and IRAK1 modulation. J Neuroinflammation. 2012;9:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azurmendi HF, Mitra S, Ayala I, Li L, Finkielstein CV, Capelluto DG. Backbone (1)H, (15)N, and (13)C resonance assignments and secondary structure of the Tollip CUE domain. Mol Cells. 2010;30(6):581–5. [DOI] [PubMed] [Google Scholar]
- 25.Soares 25MPC, Vasconcelos ML, Ferreira CD, Lima AC, Manduchi BA, Moore E, Melo JH, Novais-Bastos N, Pereira H, Guimarães JM, Moura S, Marques CS, Morais JA. Predictive value of common genetic variants in idiopathic pulmonary fibrosis survival. J Mol Med (Berl). 2022;100(9):1341–53. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article (and its additional files).