Abstract
Background
Myocardial ischemia/reperfusion (MI/R) injury is the main cause of death worldwide and poses a significant threat to cardiac health. Ginsenoside Rg1 has been shown to have inhibitory effects on inflammatory activation, oxidative stress, and cardiac injury, suggesting that Rg1 may have therapeutic effects on MI/R injury. However, the mechanism remains to be further studied.
Materials and methods
Left anterior descending coronary artery ligation was performed in Sprague-Dawley rats to construct an MI/R model in vivo. Organ index, electrocardiogram, infarct size, histopathological changes, and detection of cardiac injury and inflammatory factors in the rats were used to evaluate myocarditis, macrophage polarization, and fibrosis. We also used rat bone marrow-derived macrophages (BMDMs) to further investigate the effects of Rg1 on absent in melanoma 2 (AIM2) activation and macrophage polarization in vitro.
Results
Administration of Rg1 exhibited dose-dependent cardioprotective effects and effectively reduced MI/R injury. Rg1 significantly attenuated myocardial inflammation and inhibited M1 macrophage polarization during MI/R injury. Furthermore, Rg1 significantly reduced cardiac fibrosis in response to MI/R injury. This anti-fibrotic effect may contribute to the preservation of cardiac structure and function following an ischemic insult. Meanwhile, Rg1 effectively inhibited the activation of the AIM2 inflammasome in vitro, highlighting its potential as a key regulator of inflammatory pathways.
Conclusion
Our findings elucidate the multifaceted mechanisms underlying Rg1's cardioprotective effects, including its ability to mitigate inflammation, modulate macrophage polarization, and inhibit fibrosis.
Keywords: AIM2 inflammasome, Cardiac fibrosis, Ginsenoside Rg1, Myocardial ischemia-reperfusion injury (MIRI), Macrophage polarization
Graphical abstract
1. Introduction
Coronary artery disease (CAD) is an important global health issue with high morbidity and mortality rates. The main pathological manifestation is myocardial injury caused by ischemia-reperfusion [1]. Myocardial ischemia/reperfusion (MI/R) injury has attracted considerable attention because of its clinical significance and therapeutic challenges. MI/R injuries are prevalent after acute myocardial infarction [2]. Myocardial ischemia is characterized by the breakdown of myocardial cells in the aftermath of persistent ischemia, which progressively worsens cardiac pump performance. In most cases, reperfusion after ischemia can restore the normal structure of damaged myocardium and improve cardiac function. However, the abrupt resumption of blood flow accelerates the death of ischemic myocardial cells by triggering a series of intricate cellular processes, such as entry of inflammatory cells [3]. Therefore, inflammation is an important mechanism underlying cardiomyocyte injury.
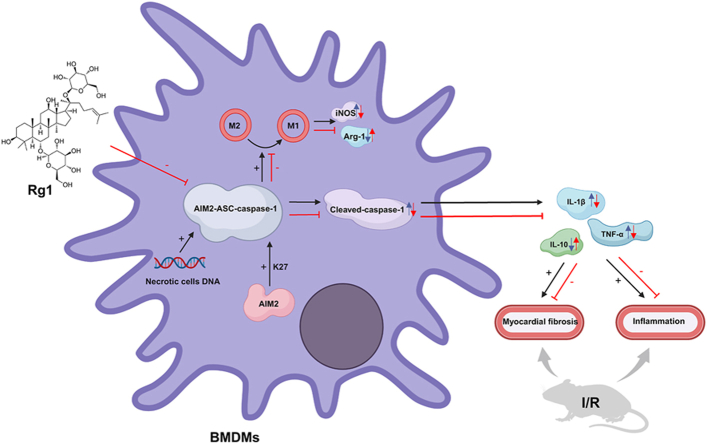
The relationship among MI/R, inflammatory corpuscle activation, and macrophage polarization is a popular research topic. Blocking the inflammatory reaction is one of the most crucial methods for preventing and treating MI/R injury because the myocardial inflammatory reaction is a prominent pathological hallmark of MI/R injury [4]. In the MI/R model, the AIM2-ASC-caspase 1 inflammasome activated by necrotic cell DNA increases the discharge of inflammatory mediators, elevates the degree of cardiac fibrosis, and exacerbates myocardial cells [5]. The protein ubiquitination system is one of the ways to regulate inflammasome activation, and K27-linked ubiquitination of absent in melanoma 2 (AIM2) promotes its activation of AIM2 inflammasome. Inflammasome assembly, ASC speck formation, and persistent caspase-1 activation have also been attributed to this process, which stabilizes AIM2 [6,7]. Macrophages also play a critical role in polarizing macrophages into pro-inflammatory M1 or anti-inflammatory M2 phenotypes. Inflammatory signals trigger inflammasome activation, which can cause macrophages to overproduce AIM2, boosting inflammasome activation and changing the phenotype from M2 to M1. AIM2 specifically regulates macrophage M1 polarization [8]. Therefore, inhibition of AIM2 inflammasome activation and M1 polarization of macrophages may attenuate MI/R-induced cardiac injury. However, few studies have been conducted on the treatment of MI/R injury via this pathway.
Ginsenoside Rg1 is a triterpenoid saponin isolated from the roots and rhizomes of Panax ginseng Meyer [9]. Rg1 has many pharmacological effects such as relieving depression, inhibiting acute kidney injury, and reducing cardiac dysfunction [[10], [11], [12]]. Another study [13] demonstrated that Rg1 inhibits the chemotherapy-induced phenotypic polarization of microglia from M2 to M1. A previous study showed that Rg1 alleviated myocardial cell apoptosis and inflammation through certain pathways [14]. However, whether Rg1 affects AIM2 inflammasome activation in cardiomyocytes and macrophage M1 polarization remains unknown. Thus, ginsenoside Rg1 is a promising therapeutic agent for MI/R injury and cardiac improvement. Our results revealed that Rg1 reduced cardiac inflammation and further explored the protective effect and potential mechanism of Rg1 in MI/R based on the AIM2 pathway.
2. Methods and materials
2.1. Drugs and reagents
Rg1 (purity≥98 %) was purchased from Shanghai Yuanye Bio-Technology CO., LTD (Shanghai, China). Diltiazem, the positive control drug, was purchased from Sigma-Aldrich.
2.2. Animals and MI/R model establishment
Male SD rats were purchased from Sibeifu Biotechnology Co., Ltd. (Beijing, China), weighing 250–300 g and 8–10 weeks old. The rats were kept together in a 12:12 h light-dark cycle-controlled facility with free access to water and standard rat chow. The MI/R model is based on methods reported in previous study [15]. The rats used in the sham surgery underwent the same process, but the ligature was left untied. Reperfusion was initiated by gently pulling the slipknot sutures in different directions after 40 min of ischemia and was performed for 7 days. All animal procedures were performed according to the regulations set out by the Animal Experimentation Ethics Committee of Nanjing University of Chinese Medicine.
2.3. Grouping and treatment
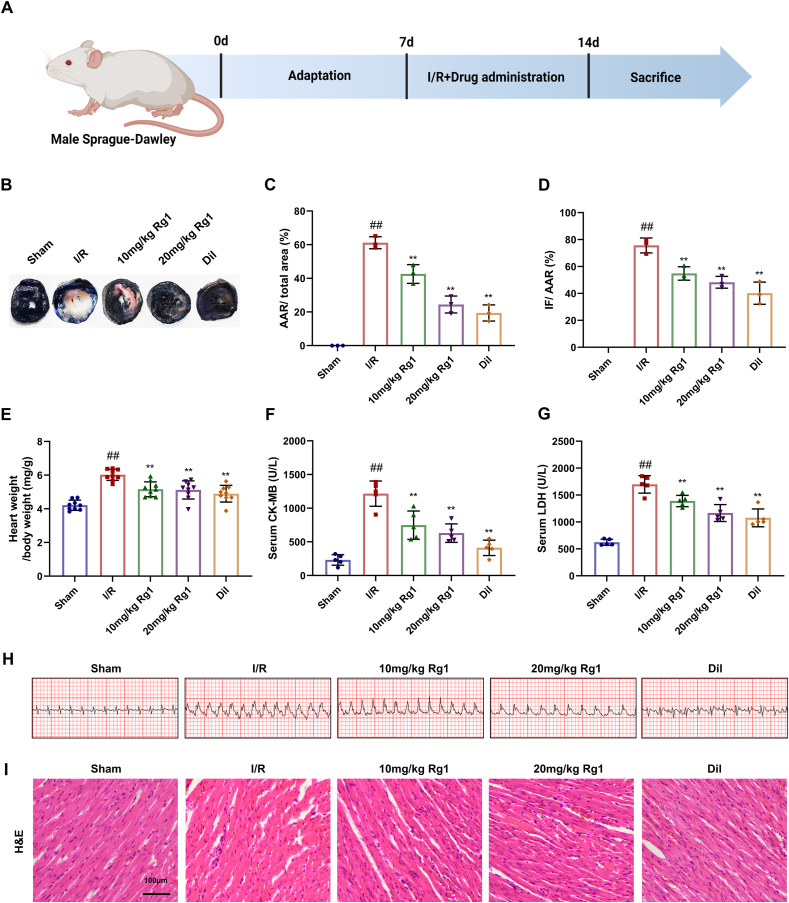
After one week of acclimatization, the rats underwent modeling and drug administration. The rats were randomly divided into five groups (n = 12): sham group (sham surgery), model group (MI/R), Rg1 group (10 and 20 mg/kg) [16] and diltiazem group (20 mg/kg) [17]. The dosage selection was based on the findings of previous studies. Distilled water (1 mL/100 g) was injected into the sham and MI/R groups. In the therapy group, Rg1 and Diltiazem were administered once daily for the first 2 h of ligation and once daily after ligation. The first batch (n = 3) of rats was taken after 3 days of modeling/administration for TTC staining, and the second batch (n = 9) of rats was taken after 7 days of modeling/administration for HE, biochemical index determination, Western blot, and tissue immunofluorescence staining, etc. [18]. A flowchart of the study design in vivo is shown in Fig. 1A.
Fig. 1.
Rg1 resists MI/R injury in a dose-dependent manner. (A) Flow chart of the study design in vivo; (B) TTC-Evans blue staining of cardiac tissues of rats; (C) AAR/total area; (D) IF/AAR; (E) Heart weight index; (F–G) The levels of LDH and CK-MB in serum; (H) Electrocardiography; (I) HE staining of cardiac tissues of rats (scale bar = 100 μm). Results are presented as means ± SD; ##P < 0.01 vs. Sham group, ∗∗P < 0.01 vs. I/R group.
2.4. Measurement of cardiac mass
A precision balance (Shimadzu, Japan) was used to measure the heart weight of the second batch (n = 9) of SD rats in each group, and the heart weight index (mg/g) was calculated.
2.5. Infarct size measurement of cardiac tissue
After MI/R damage, infarct size was assessed using TTC-Evans blue staining, as described in a previous study [19]. The myocardial infarction size (IF) is shown by white staining, the area at risk (AAR) by intense red staining, and the non-ischemic region by blue staining. Images were taken for statistical analysis using ImageJ software.
2.6. Electrocardiogram(ECG)detection
After seven days of modeling and administration, the rats in the different groups were anesthetized with 4 % pentobarbital sodium and analyzed for changes in their electrocardiogram.
2.7. Determination of biochemical parameters in serum
After being drawn from the ophthalmic vein, blood samples were stored at 4 °C for the night before being centrifuged for 15 min at 1000×g. Lactate dehydrogenase (LDH) assay kits (Beyotime, China) and creatine kinase isoenzyme (CK-MB) test kits were used to measure LDH and CK-MB activities.
2.8. Histopathological observation
HE and Masson's trichrome staining were performed according to methods reported in previous studies to detect pathological and morphological changes in myocardial tissue [20]. Formalin-fixed, paraffin-embedded sections of myocardial tissues were stained with hematoxylin for 5 min and eosin for 2 min or with a Masson trichrome staining kit (Solarbio, China).
2.9. Cell culture and treatment
Bone marrow-derived macrophages (BMDMs) were isolated from the femur and tibia of rats and cultured in RPMI 1640 medium (10 % fetal bovine serum and 10 % L929 cell-conditioned medium) for 7 days before conducting subsequent experiments [21]. Grouped as follows: Control, Model (necrotic cell DNA treatment), Rg1 groups (1,2,4 μM) [22]. In each group, RPMI 1640 media with 0.5 % fetal bovine serum and 1 g/mL LPS was utilized to activate rat BMDM cells for 4 h. The cells were treated with necrotic cell DNA labeled with Sytox Orange dye at a concentration of 800 ng DNA per 2 × 105 cells and incubated at 37 °C for 3 h. The DNA added to the control group was subjected to DNase I treatment, whereas the DNA added to the experimental and treatment groups remained untreated [23].
2.10. Immunofluorescence staining
After dewaxing and rehydration, paraffin slices of heart tissues were incubated overnight with primary antibodies against F4/80 (1:1,000, Thermo Fisher, Waltham, MA, USA) and AIM2 (1:1000; GeneTex, USA), and then with fluorescence-conjugated secondary antibodies, including Goat Anti-Rabbit, Anti-rat and Donkey Anti-Goat IgG H&L (1:5,000, Abcam, Cambridge, MA, USA). DAPI staining was performed, followed by sealing with a neutral resin. Each protein was quantified using ImageJ software.
2.11. Western blot assay
The left ventricle and cell samples were homogenized in ice-cold RIPA lysis solution that was ice-cold. The Bradford BCA assay was used to calculate protein concentration (Beyotime, China). The total protein (30 mg) of each sample was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to 0.2 μm polyvinylidene fluoride membrane (Millipore, USA) to be immunoblot-analyzed. Then sealed the membrane in the closed buffer for 1 h and further treated overnight with primary antibodies at 4 °C and then the second antibody was incubated at 25 °C for 1 h. After washing with TBST, the protein bands were detected using a Tanon gel imaging system (Bio-Rad, China), and the grayscale values of the protein bands were analyzed using ImageJ software.
2.12. Preparation of necrotic cell DNA
Rat ventricular myocytes were incubated at 37 °C with 500 mM hydrogen peroxide for 3 h to induce cell necrosis. Subsequently, Sytox Orange dye was added for staining, followed by washing and centrifugation at 20,000×g for 5 min. Necrotic cells were lysed using a high-throughput tissue homogenizer (Scientz, China). DNase inactivation reagent was added to the cell lysates to obtain necrotic DNA.
2.13. Sytox orange dye-phagocytosis experiment
During the Sytox orange dye phagocytosis experiment, macrophages were exposed to white light, and necrotic cell DNA labeled with Sytox orange dye is shown in red. Cellular immunofluorescence was stained with ASC, where the formation of the ASC speck indicated inflammasome activation, and necrotic cell DNA used in this fraction was not labeled with SYTOX Orange dye. After 24 h of DNA/drug treatment with or without necrotic cell DNA/drugs, the cells were stained with ASC primary and secondary antibodies (Abcam, Cambridge, MA, USA). DAPI staining was performed and after applying an anti-fluorescence quenching agent, the samples were mounted for fluorescence microscopy.
2.14. Flow cytometry
M1 type (CD11b+CD16/32+) and M2 type (CD11b+CD206+) macrophages were detected using flow cytometry. The necrotic cell DNA was not labeled with SYTOX Orange dye. After 24 h of treatment with or without necrotic cell DNA/drugs, cells were subjected to staining with FITC-CD11b monoclonal antibody (BioLegend, San Diego, CA, USA) and CD16/32 primary antibody (Thermo Fisher, Waltham, MA, USA) followed by APC-conjugated secondary antibody (Thermo Fisher, Waltham, MA, USA), or CD11b + primary antibody (BioLegend, San Diego, CA, USA) and CD206 primary antibody (Thermo Fisher, Waltham, MA, USA) followed by PE-conjugated secondary antibody (Thermo Fisher, Waltham, MA, USA). Subsequently, the corresponding cell proportions were analyzed using flow cytometry (BD FACSCanto II, USA).
2.15. Quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR)
Total RNA was extracted by Trizol Reagent ® (Sigma, USA) and using qPCR SYBR Green Master Mix (High Rox Plus) quantitative PCR Kit (Yeasen, China) were subjected to reverse transcription. The expression of genes encoding interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), interleukin-10 (IL-10), inducible nitric oxide synthase (iNOS), and arginase-1 (Arg-1) mRNA was quantified by real-time PCR. The primer sequences are listed in Table 1 below.
Table 1.
Primes sequences.
| Primer | Forward (5′-3′) | Reverse(5′-3′) |
|---|---|---|
| TNF-α | GGCTTTCGGAACTCACTGGA | CCCGTAGGGCGATTACAGTC |
| IL-1β | TTGAGTCTGCACAGTTCCCC | GTCCTGGGGAAGGCATTAGG |
| IL-10 | CATTCCATCCGGGGTGACAA | GTAGATGCCGGGTGGTTCAA |
| iNOS | TGGAGCGAGTTGTGGATTGTC | TATCCGTCTCGTCCGTGGCA |
| Arg-1 | CCAGAAGAATGGAAGAGTCAGTGT | GCAGATATGCAGGGAGTCACC |
| GAPDH | GCATCTTCTTGTGCAGTGCC | GATGGTGATGGGTTTCCCGT |
2.16. Statistical analysis
All data was expressed as means ± SD. Statistical differences were analyzed using GraphPad Prism 8. One-way analysis of variance (ANOVA) with Dunnett's multiple comparison test was used to compare multiple groups. Statistical significance was set at P < 0.05.
3. Results
3.1. Rg1 resists MI/R injury in a dose-dependent manner
We investigated the effects of Rg1 on MI/R injury in rats. TTC and Evans staining revealed that the MI/R group had significantly increased AAR and IF areas in the myocardium. However, these levels decreased following administration. The High-dose Rg1 group had a lower degree of infarction than the low-dose group, suggesting that Rg1 could reduce infarct size in a dose-dependent manner (Fig. 1B–D). This treatment also reduced the heart weight index after MI/R injury (Fig. 1E). Furthermore, two sensitivity index analyses of cardiac injury markers in the serum showed that the activities of LDH and CK-MB significantly increased in the MI/R group (Fig. 1F and G). Dramatic ST segment elevation was observed in the MI/R group (Fig. 1H). HE staining revealed intact morphology, closely arranged cells, and a regular fiber structure in the sham group. However, the MI/R group showed ruptured necrotic myocardial cells with irregular organization and leukocyte infiltration (Fig. 1I). In contrast, after treatment with Rg1 and Diltiazem, these pathological changes were reversed, and the effects of Rg1 (20 mg/kg) were better than those of Rg1 (10 mg/kg).
3.2. Rg1 reduces myocardial inflammation induced by MI/R injury
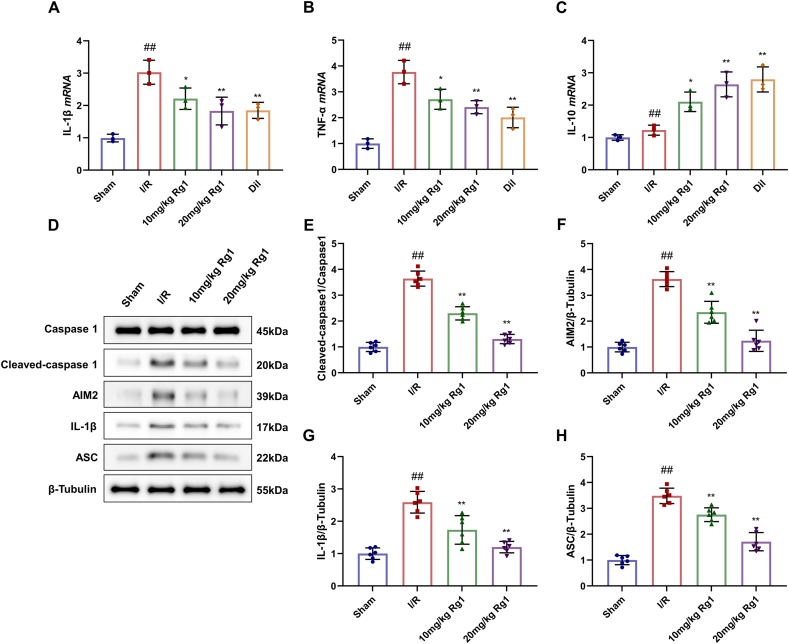
To further investigate whether the reduction of MI/R injury by Rg1 is related to the inhibition of inflammation, we measured the mRNA levels of three inflammatory markers in cardiac tissues. In the MI/R group, the levels of IL-1β and TNF-α mRNA were greatly raised, but the changes of IL-10 were not significant. However, their levels decreased in the Rg1 and diltiazem groups, except for IL-10 mRNA (Fig. 2A–C).
Fig. 2.
Effects of Rg1 on myocardial inflammation induced by MI/R injury. (A–C) Levels of IL-1β, TNF-α, and IL-10 mRNA in serum; (D)Western Blot of rat cardiac tissues; (E–H) Protein levels of Cleaved-caspase1, AIM2, IL-1β, and ASC. Results were presented as means ± SD; ##P < 0.01 vs. Sham group, ∗P < 0.05, ∗∗P < 0.01 vs. I/R group.
Western blot assay demonstrated the expression of Cleaved-caspase-1, AIM2, IL-1β, and ASC critically ran up in myocardial cells of MI/R rats and the expression of them in the Rg1 groups declined (Fig. 2D–H). Thus, pretreatment with Rg1 attenuated MI/R-induced myocardial inflammation.
3.3. Rg1 inhibits M1 polarization of macrophages in MI/R injury
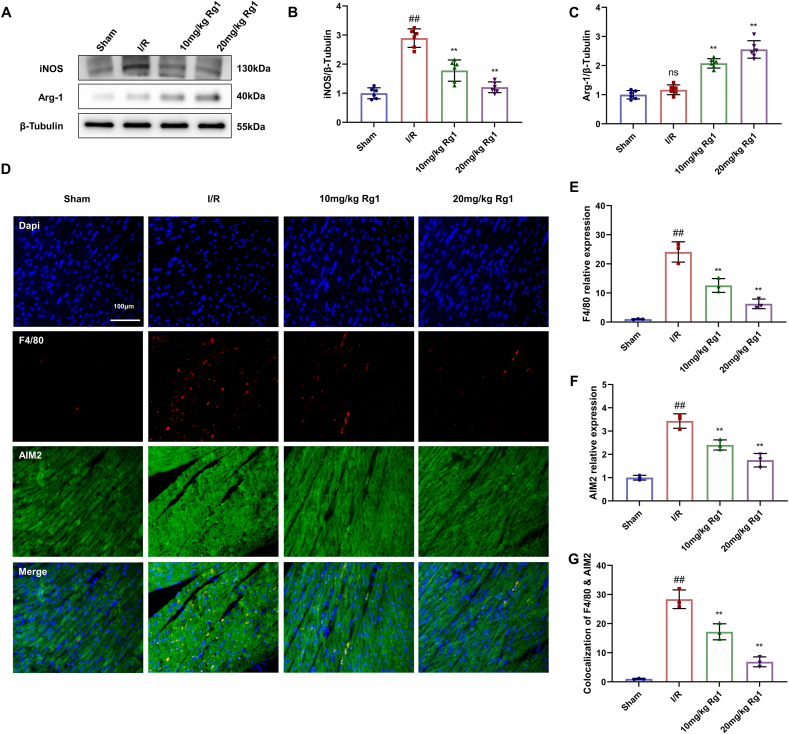
INOS and Arg-1 are functional markers of M1 and M2 macrophages, respectively. Further research found that iNOS was significantly increased in the MI/R group, but was noticeably reduced, and Arg-1 was increased in the Rg1 groups compared to the MI/R group (Fig. 3A–C). DNA from necrotic cardiomyocytes can activate the inflammasome AIM2 to drive macrophage M1 polarization. Immunohistochemical staining was used to analyze the heart tissue of rats; the nuclei and myocardial fibers in the sham group were arranged neatly, and the inflammasome AIM2 was evenly distributed. In the MI/R group, inflammasome AIM2 was overactivated, and the co-localization of F4/80 and AIM2 was significantly increased. However, the Rg1 group showed the opposite results (Fig. 3D–G). These results demonstrate that macrophage M1 polarization and AIM2 activation were inhibited by Rg1 treatment.
Fig. 3.
Rg1 inhibits macrophage M1 polarization in MI/R injury. (A) Western Blot in heart tissues; (B–C) The levels of iNOS and Arg-1; (D) Immunohistochemical staining of cardiac tissue (scale bar = 100 μm); (E–F) The relative expression of Macrophage marker F4/80 and inflammasome indicator AIM2 in heart tissues; (G) Immunofluorescence co-localization results of F4/80 and AIM2. Results were presented as means ± SD; ##P < 0.01 vs. Sham group, ∗∗P < 0.01 vs. I/R group.
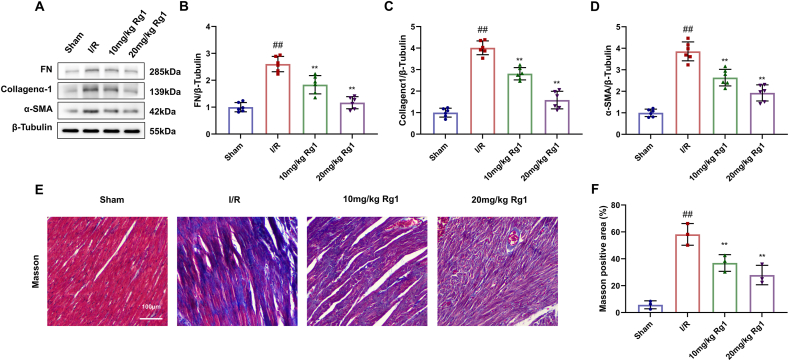
3.4. Rg1 reduces cardiac fibrosis in MI/R injury
The protein expression levels of myocardial fibrosis markers FN, Collagenα-1, and α-SMA were determined by Western blot. Their levels in the MI/R group were highly increased, but significantly decreased in the Rg1 group (Fig. 4A–D). Masson's trichrome staining was performed on the heart tissue. The nuclei and collagen fibers of cardiomyocytes in the sham group are blue, and the cytoplasm, muscle, and red blood cells are red. Collagen fibers in the MI/R group increased significantly. However, Rg1-treated rats had significantly reduced cardiomyocytes, indicating that Rg1 significantly reduced myocardial fibrosis caused by MI/R injury (Fig. 4E–F).
Fig. 4.
Effect of Rg1 on cardiac fibrosis in MI/R injury. (A) Western Blot in heart tissues; (B–D) Protein expression levels of FN, Collagena-1, and α-SMA; (E) Masson's trichrom staining of myocardial fibrosis (scale bar = 100 μm); (F) Masson positive area. Results are presented as means ± SD; ##P < 0.01 vs. Sham group, ∗∗P < 0.01 vs. I/R group.
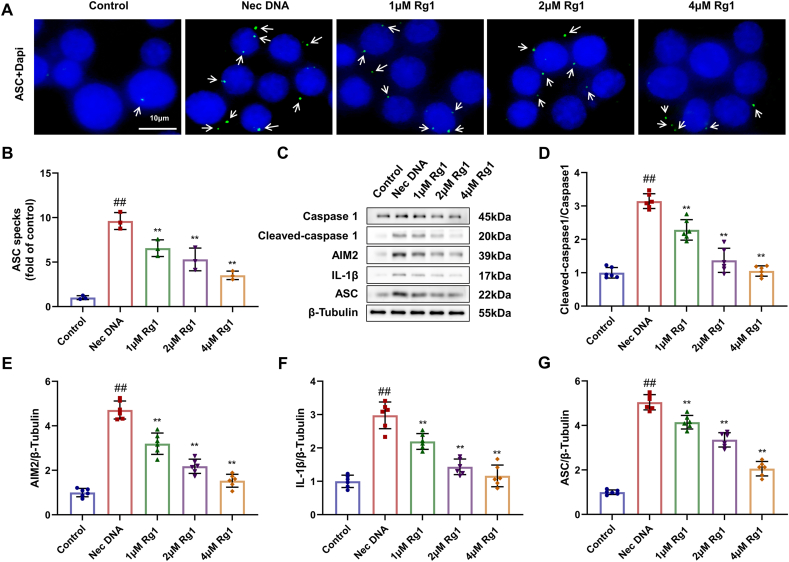
3.5. Rg1 inhibits AIM2 inflammasome activation in vitro
To further investigate whether ginsenoside Rg1 also exhibits an inhibitory effect on AIM2 inflammasome activation in vitro, ASC spots, a marker of inflammasome activation, and inflammatory markers AIM2, IL-β were monitored. Immunofluorescence results showed that the number of ASC specks in the BMDMs of the model group treated with necrotic cell DNA significantly increased. The number of cells treated with Rg1 was dramatically lower than that in the model group (Fig. 5A and B). Western blot showed that the protein levels of Cleaved-caspase 1, AIM2, IL-1β, and ASC in the model group treated with DNA from necrotic cells were considerably higher than those in the control group. However, the administration of Rg1 greatly downregulated the expression of these proteins, indicating that AIM2 inflammasome activation was inhibited (Fig. 5C–G).
Fig. 5.
Inhibitory effect of Rg1 on AIM2 inflammasome activation in vitro. (A) Results of different doses of Rg1's treatment on BMDMs were assayed by Cell immunohistochemical staining (scale bar = 10 μm); (B) Results of ASC specks (fold of control); (C–G) Protein expression levels of Cleaved-caspase 1, AIM2, IL-1β and ASC. Results are presented as means ± SD; ##P < 0.01 vs. Control group, ∗∗P < 0.01 vs. Nec DNA group.
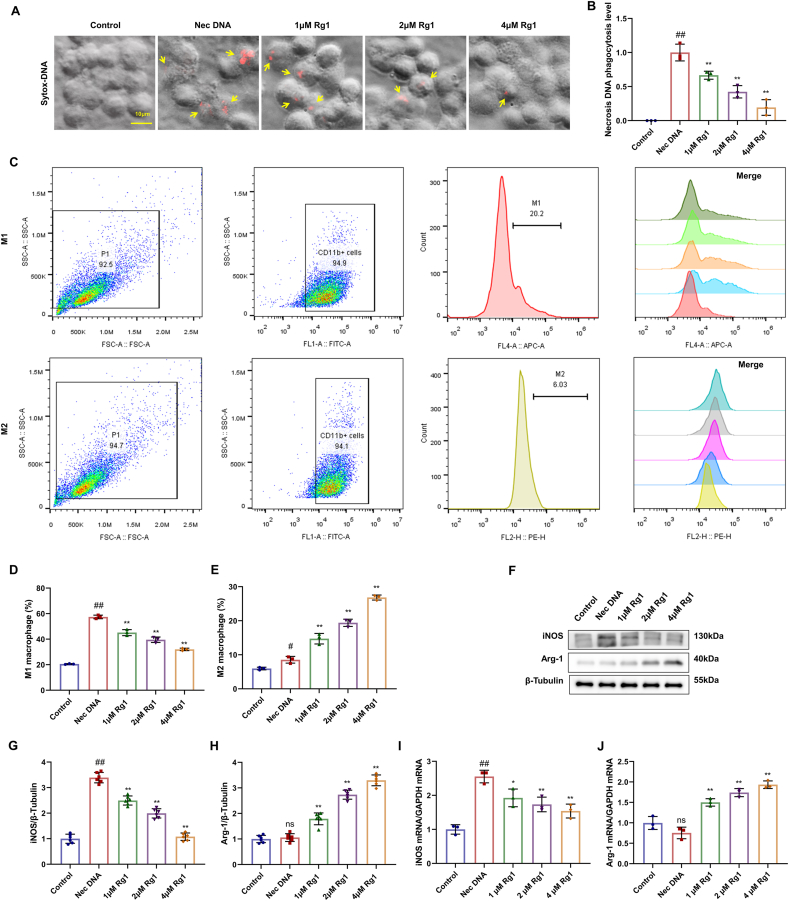
3.6. Rg1 inhibits macrophage M1 polarization in vitro
BMDMs were stained with SYTOX orange dye, and the DNA in the necrotic macrophages was stained red. No marker was detected in the control group. The necrotic cell DNA treatment group exhibited an obvious increase in macrophage markers and DNA phagocytosis levels, whereas the Rg1 treatment group showed a decrease with increasing concentration (Fig. 6A–B). These results indicate that Rg1 treatment downregulates DNA phagocytosis in macrophages.
Fig. 6.
Inhibitory effect of Rg1 on M1 polarization of macrophages during MI/R injury in vitro. (A) Sytox orange dye staining of rat BMDM cells (scale bar = 10 μm); (B) Phagocytosis DNA levels in macrophages; (C–E) The M1/M2 phenotype of macrophages and the corresponding cell proportion were detected by phagocytosis flow cytometry; (F) Western blot; (G–H) The protein expression levels of iNOS and Arg-1. (I–J) The mRNA expression of iNOS and Arg-1. Results are presented as means ± SD; ##P < 0.01 vs. Control group, ∗∗P < 0.01 vs. Nec DNA group.
For cellular immunofluorescence staining and flow cytometry, the model group treated with necrotic cell DNA showed no significant changes compared with the control group. However, the proportion of M1-polarized macrophages decreased and the proportion of M2-polarized macrophages treated with Rg1 increased with increasing concentrations (Fig. 6C–E). The iNOS protein expression level was significantly higher in the model group than in the control group. However, iNOS protein expression decreased significantly, whereas Arg-1 protein expression increased significantly after treatment with Rg1 (Fig. 6F–H). The qRT-PCR results were consistent with these conclusions (Fig. 6I–J). These findings were consistent with the in vivo results, indicating that Rg1 treatment effectively downregulated M1 macrophage polarization.
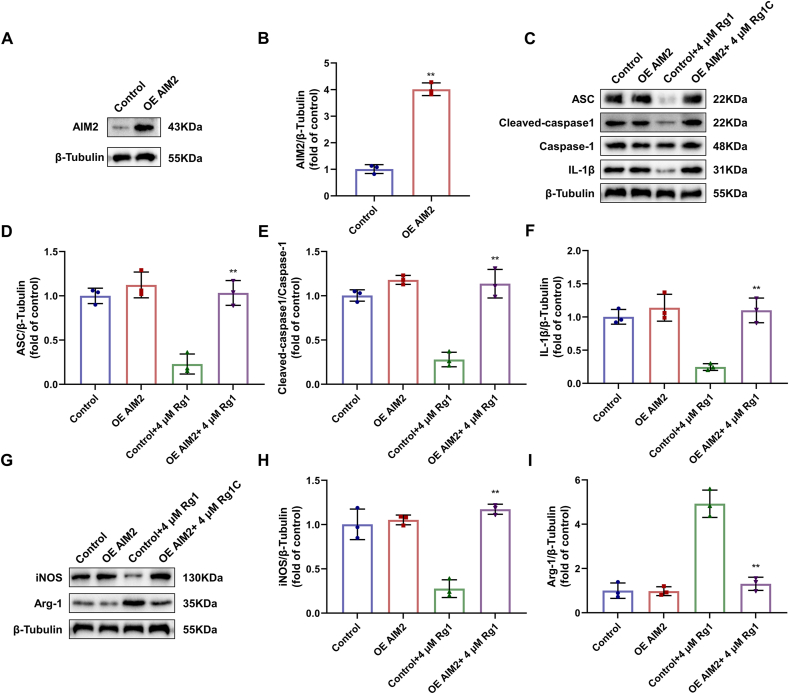
3.7. Rg1 inhibits macrophage M1 polarization by inhibiting AIM2 inflammasome activation
To further verify the mechanism of action of Rg1, we used an AIM2 overexpression plasmid. Western blotting confirmed successful transfection (Fig. 7A–B). When AIM2 was overexpressed, ASC, cleaved-caspase 1, and caspase-1 levels were increased in the OE AIM2+ 4 μM Rg1 group compared with the Control+4 μM Rg1 group, indicating that the inhibitory effect of Rg1 on the activation of AIM2 inflammasome was reversed. INOS and Arg-1, which are markers of macrophage polarization, were detected, and it was found that iNOS increased and Arg-1 decreased. These results indicated that the polarization of M1 macrophages was enhanced when AIM2 inflammasome activation increased. Therefore, the inhibitory effect of Rg1 on the M1 polarization of macrophages was also reversed. These results proved that Rg1 inhibited the M1 polarization of macrophages by inhibiting the activation of the AIM2-ASC-caspase-1 inflammasome.
Fig. 7.
Reversal of inhibitory effect of Rg1 during AIM2 overexpression in vitro. (A–B) The levels of AIM2 of rat BMDM cells; (C) Western blot of rat BMDM cells; (D–F) The protein expression levels of ASC, cleaved-caspase 1 and IL-1β; (G) Western blot of rat BMDM cells; (H–I) The protein expression levels of iNOS and Arg-1. Results are presented as means ± SD; ∗∗P < 0.01 vs. OE AIM2 group.
4. Discussion
Our study demonstrated that Rg1 reduced cardiac inflammation and fibrosis in myocardial I/R injury by inhibiting macrophage polarization. Inflammatory response plays a crucial role in the occurrence and development of injury. MI/R induces the release of various proinflammatory factors and infiltration of inflammatory cells, leading to myocardial dysfunction and necrosis [24]. It is a key factor that induces adverse left ventricular remodeling in patients with myocardial infarction. Therefore, inhibition of the inflammatory response has become a significant measure for reducing MI/R injury and improving the prognosis of patients with myocardial infarction. Our findings highlight the multifaceted cardioprotective effects of Rg1, suggesting its potential as a therapeutic candidate for mitigating the detrimental consequences of MI/R injury, including suppression of inflammation, macrophage modulation, and reduction of fibrosis.
MI/R injuries remain a pivotal area of investigation owing to their substantial impact on global health. The role of inflammasomes in MI/R injury is an emerging area of interest. Inflammasomes are multiprotein complexes that respond to cellular stress and activate caspase-1, leading to the release of pro-inflammatory cytokines IL-1β and IL-18. Various inflammasomes, including NLRP3 and AIM2, are implicated in the exacerbation of myocardial injury during reperfusion [25]. As the initial sensor of danger signals in myocardial ischemic injury, AIM2 inflammasome is widely activated after myocardial ischemic infarction and participates in the regulation of myocardial fibrosis after injury [26,27]. Targeting these inflammasomes holds promise for attenuating inflammatory responses and ameliorating myocardial damage.
Macrophages have emerged as key players in the MI/R process, and their polarization phenotype plays a critical role in determining cardiac outcomes. M2 macrophages have anti-inflammatory properties and inhibit myocardial inflammation by secreting IL-10 [28]. The balance between the M1 and M2 macrophages influences tissue damage and repair during MI/R. Strategies aimed at modulating macrophage polarization may have therapeutic potential for promoting tissue repair and limiting inflammation-induced injury. The complex interplay between inflammasomes, macrophage polarization, and cardiac injury has been increasingly elucidated in recent studies on MI/R [29]. Many pattern recognition receptors expressed on macrophages induce inflammatory response [5]. Macrophages also play a key role in coordinating inflammatory responses [30,31]. Understanding these intricate mechanisms at the molecular level opens new avenues for targeted therapeutic interventions that could revolutionize the management of MI/R injuries and improve patient outcomes. However, further studies are needed to uncover the precise signaling pathways involved and validate the efficacy and safety of potential interventions in clinical settings.
Ginsenoside Rg1, which is known for its protective effects against cerebral ischemic injury, has gained significant attention in cardiovascular research [[32], [33], [34]]. Ginsenosides exert their protective effects on the heart through various mechanisms, and our study provides further insights into the mechanism of action of Rg1. Notably, ginsenosides have shown promising results in inhibiting the activation of inflammasomes such as AIM2, leading to a reduction in pro-inflammatory cytokine release. As mentioned in a previous study [35], ginsenosides Rg1 and Rh3, the active ingredients of RGE, substantially inhibit macrophage pyroptosis by inhibiting AIM2 inflammasome activation. This anti-inflammatory effect has significant potential for attenuating the inflammatory response induced by myocardial ischemia and subsequent tissue damage, thereby promoting myocardial repair.
This study provides additional evidence that the protective effects of Rg1 against MI/R damage are mediated by an intrinsic mechanism. In contrast to the protective mechanism of other drugs reported in recent years [[36], [37], [38]], this study confirmed that Rg1 can alleviate MI/R injury by inhibiting the activation of the AIM2 inflammasome, thereby inhibiting the polarization of macrophages, which provides some ideas for the field of cardiovascular disease, from animal experiments to clinical treatment. This study also provides a reference for exploring the mechanism of action of ginsenosides in the treatment of inflammatory injury [23]. However, this study used in vitro cells or animal models, and whether it is feasible for clinical application as a single drug remains unknown. Although Rg1 showed a strong inhibitory effect on AIM2 inflammasome activation, its selective inhibitory effect on the activation of other inflammasomes such as NLRP3 remains unknown.
5. Conclusions
In conclusion, our study demonstrated that Rg1 has dose-dependent cardioprotective effects against MI/R injury, reducing inflammation, inhibiting AIM2 inflammasome activation, and M1 macrophage polarization, while preserving the cardiac structure, indicating its potential as a therapeutic candidate for MI/R injury.
CRediT authorship contribution statement
Xiaojin Xu: Methodology, Validation, and. Qing Wu: wrote the original manuscript. Ke Pei: Conceptualization, and. Meng Zhang: Visualization. Chenhan Mao: Methodology. Xinxin Zhong: Methodology. Yunfan Huang: Software. Yang Dai: Investigation, and. Rui Yin: Writing – review & editing. Zhaoyang Chen: Writing – review & editing. Xindong Wang: Methodology, Investigation, Formal analysis.
Declaration of Competing interest
The authors declare no potential conflicts of interest concerning the research, authorship, or publication of this article.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81973766, 82274469, and 82204995), Natural Science Foundation of Jiangsu Province (BK20211387), and Natural Science Foundation of Nanjing University of Chinese Medicine (No. XZR2021024, XZR2023052), Youth Science Foundation of Jiangsu Province Academy of Traditional Chinese Medicine (QNKXYJ202102, QNKXYJ202104), and National Administration of Traditional Chinese Medicine through the Construction Project of Inheritance Studio of National Famous Traditional Chinese Medicine Experts ([2022] No. 75), and Traditional Chinese Medicine Science and Technology Plan Project of Jiangsu Province (No. MS2023030).
References
- 1.Mokhtari-Zaer A., Marefati N., Atkin S.L., et al. The protective role of curcumin in myocardial ischemia-reperfusion injury. J Cell Physiol. 2018;234(1):214–222. doi: 10.1002/jcp.26848. [DOI] [PubMed] [Google Scholar]
- 2.Kato R., Foëx P. Myocardial protection by anesthetic agents against ischemia-reperfusion injury: an update for anesthesiologists. Can J Anaesth. 2002;49(8):777–791. doi: 10.1007/BF03017409. [DOI] [PubMed] [Google Scholar]
- 3.Koeppen M., Lee J.W., Seo S.W., et al. Hypoxia-inducible factor 2-alpha-dependent induction of amphiregulin dampens myocardial ischemia-reperfusion injury. Nat Commun. 2018;9(1):816. doi: 10.1038/s41467-018-03105-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silvis M.J.M., Kaffka Genaamd Dengler S.E., Odille C.A., et al. Damage-Associated molecular patterns in myocardial infarction and heart Transplantation: the Road to Translational success. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.599511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamidzadeh K., Christensen S.M., Dalby E., et al. Macrophages and the Recovery from acute and Chronic inflammation. Annu Rev Physiol. 2017;79:567–592. doi: 10.1146/annurev-physiol-022516-034348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo Y., Li L., Xu T., et al. HUWE1 mediates inflammasome activation and promotes host defense against bacterial infection. J Clin Invest. 2020;130(12):6301–6316. doi: 10.1172/JCI138234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Q., Zhao X., Tang H., et al. USP22 suppresses the NLRP3 inflammasome by degrading NLRP3 via ATG5-dependent autophagy. Autophagy. 2023;19(3):873–885. doi: 10.1080/15548627.2022.2107314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chai D., Zhang Z., Shi S.Y., et al. Absent in melanoma 2-mediating M1 macrophages facilitate tumor rejection in renal carcinoma. Transl Oncol. 2021;14(4) doi: 10.1016/j.tranon.2021.101018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang Y.Y., Guo W.X., Lu Z.F., et al. Ginsenoside Rg1 promotes the Migration of Olfactory Ensheathing cells via the PI3K/Akt pathway to repair rat Spinal Cord injury. Biol Pharm Bull. 2017;40(10):1630–1637. doi: 10.1248/bpb.b16-00896. [DOI] [PubMed] [Google Scholar]
- 10.Liu Z., Pan H., Zhang Y., et al. Ginsenoside-Rg1 attenuates sepsis-induced cardiac dysfunction by modulating mitochondrial damage via the P2X7 receptor-mediated Akt/GSK-3β signaling pathway. J Biochem Mol Toxicol. 2022;36(1) doi: 10.1002/jbt.22885. [DOI] [PubMed] [Google Scholar]
- 11.Guo J., Wang R., Min F. Ginsenoside Rg1 ameliorates sepsis-induced acute kidney injury by inhibiting ferroptosis in renal tubular epithelial cells. J Leukoc Biol. 2022;112(5):1065–1077. doi: 10.1002/JLB.1A0422-211R. [DOI] [PubMed] [Google Scholar]
- 12.Li J., Gao W., Zhao Z., et al. Ginsenoside Rg1 reduced microglial activation and mitochondrial dysfunction to alleviate depression-Like Behaviour via the GAS5/EZH2/SOCS3/NRF2 Axis. Mol Neurobiol. 2022;59(5):2855–2873. doi: 10.1007/s12035-022-02740-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi D.D., Huang Y.H., Lai C.S.W., et al. Ginsenoside Rg1 Prevents chemotherapy-induced Cognitive Impairment: Associations with microglia-mediated cytokines, Neuroinflammation, and Neuroplasticity. Mol Neurobiol. 2019;56(8):5626–5642. doi: 10.1007/s12035-019-1474-9. [DOI] [PubMed] [Google Scholar]
- 14.Luo M., Yan D., Sun Q., et al. Ginsenoside Rg1 attenuates cardiomyocyte apoptosis and inflammation via the TLR4/NF-kB/NLRP3 pathway. J Cell Biochem. 2020;121(4):2994–3004. doi: 10.1002/jcb.29556. [DOI] [PubMed] [Google Scholar]
- 15.Cai W., Liu L., Shi X., et al. Alox15/15-HpETE Aggravates myocardial ischemia-reperfusion injury by promoting cardiomyocyte ferroptosis. Circulation. 2023;147(19):1444–1460. doi: 10.1161/CIRCULATIONAHA.122.060257. [DOI] [PubMed] [Google Scholar]
- 16.Jin C., Wang Z.Z., Zhou H., et al. Ginsenoside Rg1-induced antidepressant effects involve the protection of astrocyte gap junctions within the prefrontal cortex. Prog Neuro-Psychopharmacol Biol Psychiatry. 2017;75:183–191. doi: 10.1016/j.pnpbp.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Shan X., Lv Z.Y., Yin M.J., et al. The protective effect of Cyanidin-3-Glucoside on myocardial ischemia-reperfusion injury through ferroptosis. Oxid Med Cell Longev. 2021;2021 doi: 10.1155/2021/8880141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao J.X., Li X.L., Hu J.X., et al. Mesenchymal stromal cell-derived exosomes attenuatemyocardial ischaemia-reperfusion injury throughmiR-182-regulated macrophage polarization. Cardiovasc Res. 2019;115(7):1205–1216. doi: 10.1093/cvr/cvz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao M., Wang Z., Jiang L., et al. Oxytocin ameliorates high glucose- and ischemia/reperfusion-induced myocardial injury by suppressing pyroptosis via AMPK signaling pathway. Biomed Pharmacother. 2022;153 doi: 10.1016/j.biopha.2022.113498. [DOI] [PubMed] [Google Scholar]
- 20.Liu W., Chen C., Gu X., et al. AM1241 alleviates myocardial ischemia-reperfusion injury in rats by enhancing Pink1/Parkin-mediated autophagy. Life Sci. 2021;272 doi: 10.1016/j.lfs.2021.119228. [DOI] [PubMed] [Google Scholar]
- 21.Lu B., Wu C., Azami N.L.B., et al. Babao Dan improves neurocognitive function by inhibiting inflammation in clinical minimal hepatic encephalopathy. Biomed Pharmacother. 2021;135 doi: 10.1016/j.biopha.2020.111084. [DOI] [PubMed] [Google Scholar]
- 22.Chu S.F., Zhang Z., Zhou X., et al. Ginsenoside Rg1 protects against ischemic/reperfusion-induced neuronal injury through miR-144/Nrf2/ARE pathway. Acta Pharmacol Sin. 2019;40(1):13–25. doi: 10.1038/s41401-018-0154-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Komada T., Chung H., Lau A., et al. Macrophage Uptake of necrotic cell DNA activates the AIM2 inflammasome to regulate a proinflammatory phenotype in CKD. J Am Soc Nephrol. 2018;29(4):1165–1181. doi: 10.1681/ASN.2017080863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ong S.B., Hernandez-Resendiz S., Crespo-Avilan G.E., et al. Inflammation following acute myocardial infarction: multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol Ther. 2018;186:73–87. doi: 10.1016/j.pharmthera.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strowig T., Henao-Mejia J., Elinav E., et al. Inflammasomes in health and disease. Nature. 2012;481(7381):278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 26.Díaz-Araya G., Vivar R., Humeres C., et al. Cardiac fibroblasts as sentinel cells in cardiac tissue: receptors, signaling pathways and cellular functions. Pharmacol Res. 2015;101:30–40. doi: 10.1016/j.phrs.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Kawaguchi M., Takahashi M., Hata T., et al. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011;123(6):594–604. doi: 10.1161/CIRCULATIONAHA.110.982777. [DOI] [PubMed] [Google Scholar]
- 28.de Couto G. Macrophages in cardiac repair: Environmental cues and therapeutic strategies. Exp Mol Med. 2019;51(12):1–10. doi: 10.1038/s12276-019-0269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long J., Liu X.K., Kang Z.P., et al. Ginsenoside Rg1 ameliorated experimental colitis by regulating the balance of M1/M2 macrophage polarization and the homeostasis of intestinal flora. Eur J Pharmacol. 2022;917 doi: 10.1016/j.ejphar.2022.174742. [DOI] [PubMed] [Google Scholar]
- 30.Varol C., Mildner A., Jung S. Macrophages: development and tissue specialization. Annu Rev Immunol. 2015;33:643–675. doi: 10.1146/annurev-immunol-032414-112220. [DOI] [PubMed] [Google Scholar]
- 31.Murray P.J., Wynn T.A. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11(11):723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han Y., Li X., Yang L., et al. Ginsenoside Rg1 attenuates cerebral ischemia-reperfusion injury due to inhibition of NOX2-mediated calcium homeostasis dysregulation in mice. J Ginseng Res. 2022;46(4):515–525. doi: 10.1016/j.jgr.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin M., Sun W., Gong W., et al. Ginsenoside Rg1 protects against transient focal cerebral ischemic injury and suppresses its systemic metabolic changes in cerabral injury rats. Acta Pharm Sin B. 2015;5(3):277–284. doi: 10.1016/j.apsb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie W., Zhou P., Sun Y., et al. Protective effects and Target Network analysis of ginsenoside Rg1 in cerebral ischemia and reperfusion injury: a Comprehensive Overview of experimental studies. Cells. 2018;7(12) doi: 10.3390/cells7120270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim T.H., Lee S.M. The effects of ginseng total saponin, panaxadiol and panaxatriol on ischemia/reperfusion injury in isolated rat heart. Food Chem Toxicol. 2010;48(6):1516–1520. doi: 10.1016/j.fct.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 36.Xu L.J., Chen R.C., Ma X.Y., et al. Scutellarin protects against myocardial ischemia-reperfusion injury by suppressing NLRP3 inflammasome activation. Phytomedicine. 2020;68 doi: 10.1016/j.phymed.2020.153169. [DOI] [PubMed] [Google Scholar]
- 37.Wang R., Wang M., Zhou J., et al. Calenduloside E suppresses calcium overload by promoting the interaction between L-type calcium channels and Bcl2-associated athanogene 3 to alleviate myocardial ischemia/reperfusion injury. J Adv Res. 2021;34:173–186. doi: 10.1016/j.jare.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang L., Yin X., Chen Y.H., et al. Proteomic analysis reveals ginsenoside Rb1 attenuates myocardial ischemia/reperfusion injury through inhibiting ROS production from mitochondrial complex I. Theranostics. 2021;11(4):1703–1720. doi: 10.7150/thno.43895. [DOI] [PMC free article] [PubMed] [Google Scholar]