Abstract
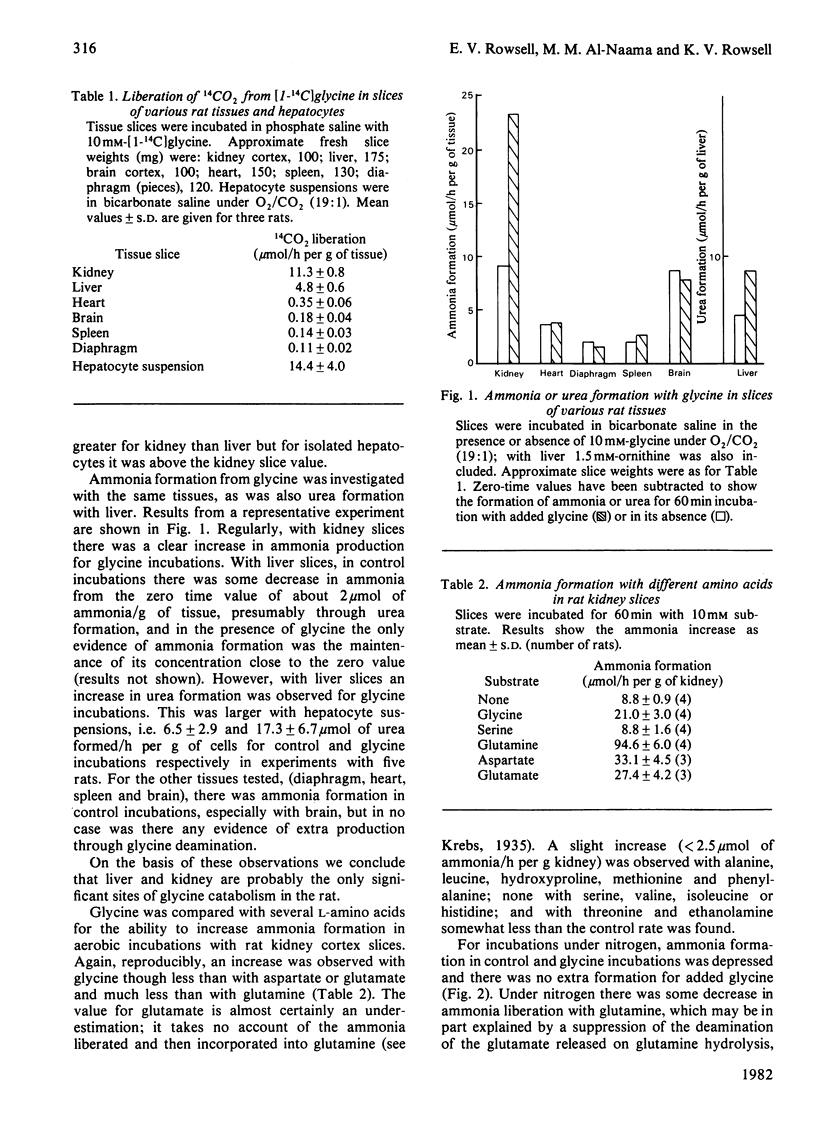
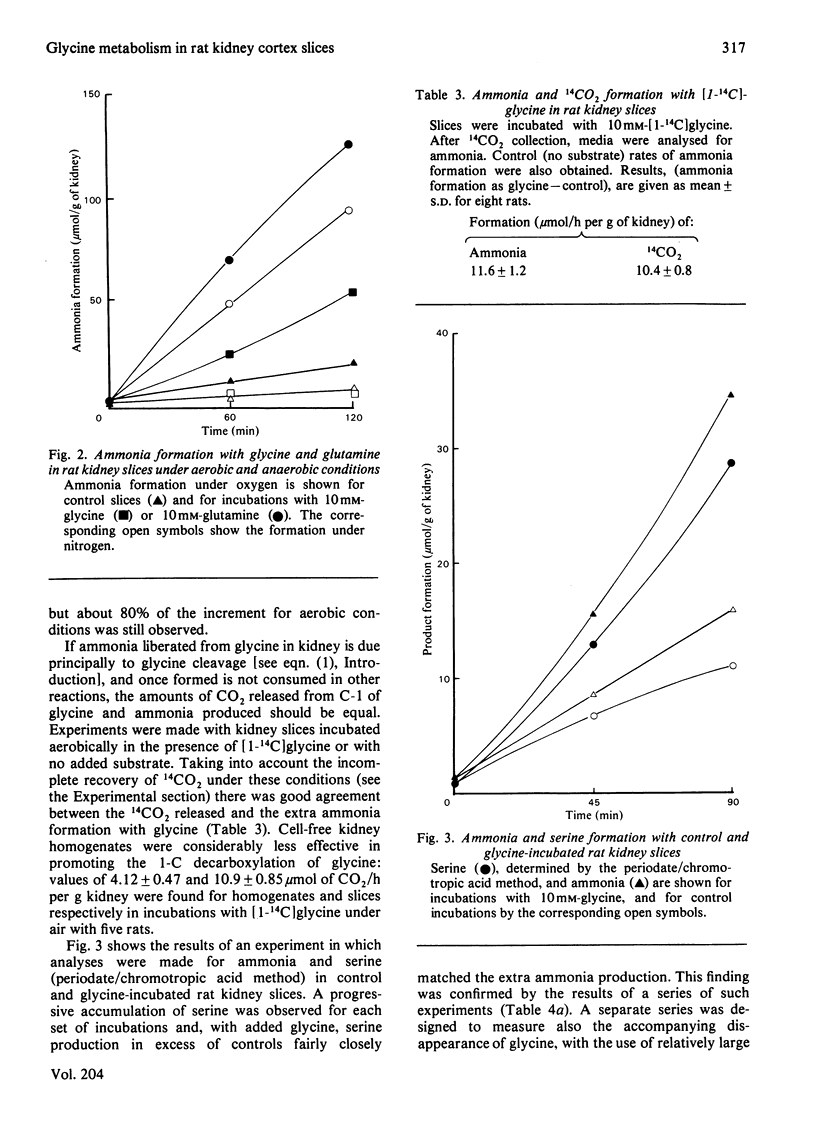
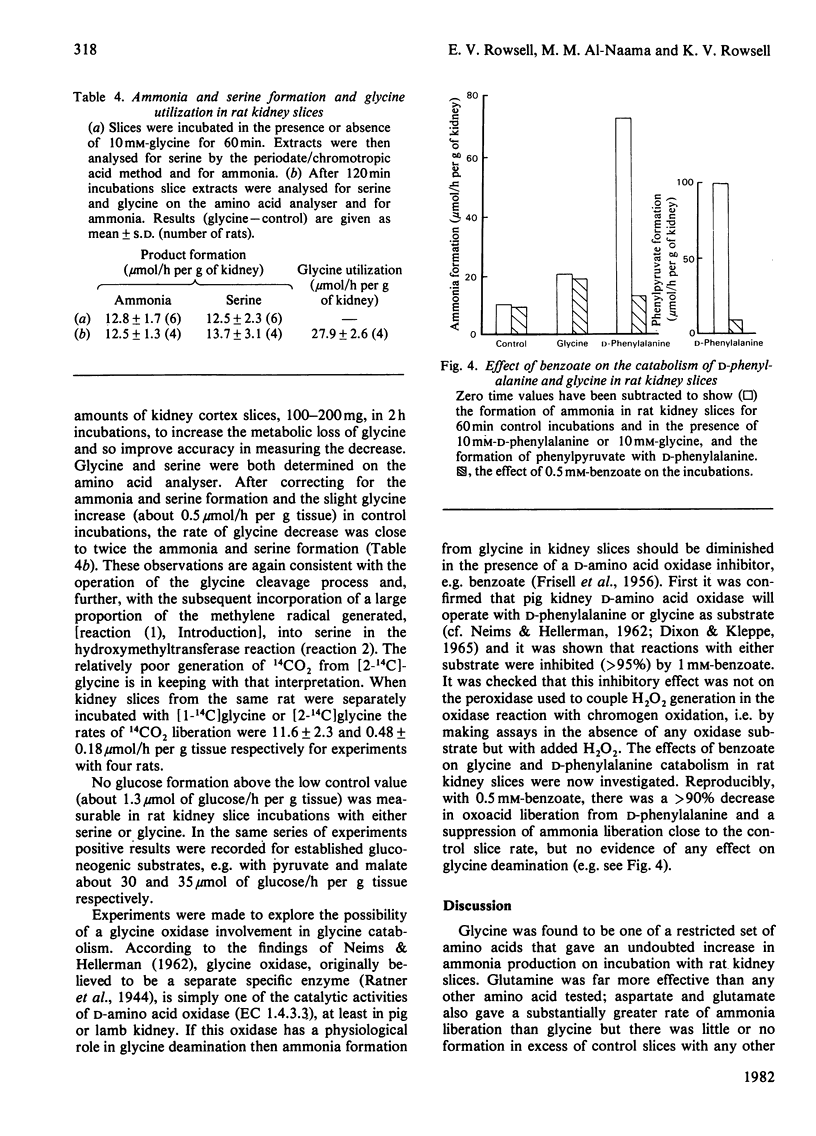
When rat kidney cortex slices were incubated with glycine or [1-14C]glycine, after correcting for metabolite changes with control slices, product formation and glycine utilization fitted the requirements of the equation: 2 Glycine leads to ammonia + CO2 + serine. Evidence is presented that degradation via glyoxylate, by oxidation or transamination, is unlikely to have any significant role in kidney glycine catabolism. It is concluded that glycine metabolism in rat kidney is largely via glycine cleavage closely coupled with serine formation. 1-C decarboxylation and urea formation with glycine in rat hepatocyte suspensions were somewhat greater than decarboxylation or ammonia formation in kidney slices, showing that in the rat, potentially, the liver is quantitatively the more important organ in glycine catabolism. There was no evidence of ammonia formation from glycine with rat brain cortex, heart, spleen or diaphragm and 1-C decarboxylation was very weak.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikawa T., Matsutaka H., Yamamoto H., Okuda T., Ishikawa E. Gluconeogenesis and amino acid metabolism. II. Inter-organal relations and roles of glutamine and alanine in the amino acid metabolism of fasted rats. J Biochem. 1973 Nov;74(5):1003–1017. [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttery P. J., Rowsell E. V. Enzymic assays for amoonia and L-glutamine in tissue extracts. Anal Biochem. 1971 Feb;39(2):297–310. doi: 10.1016/0003-2697(71)90418-0. [DOI] [PubMed] [Google Scholar]
- CANESSA-FISCHER M., SHALHOUB R., GLABMANS, DEHAAS J., PITTS R. F. Effects of infusions of ammonia, amides, and amino acids on excretion of ammonia. Am J Physiol. 1963 Feb;204:192–196. doi: 10.1152/ajplegacy.1963.204.2.192. [DOI] [PubMed] [Google Scholar]
- Cossins E. A., Sinha S. K. The interconversion of glycine and serine by plant tissue extracts. Biochem J. 1966 Nov;101(2):542–549. doi: 10.1042/bj1010542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIES B. M. A., YUDKIN J. Studies in biochemical adaptation; the origin or urinary ammonia as indicated by the effect of chronic acidosis and alkalosis on some renal enzymes in the rat. Biochem J. 1952 Nov;52(3):407–412. doi: 10.1042/bj0520407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON M., KLEPPE K. D-AMINO ACID OXIDASE. 3. EFFECT OF PH. Biochim Biophys Acta. 1965 Mar 22;96:383–389. doi: 10.1016/0005-2787(65)90558-7. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Park C. R. Control of gluconeogenesis in liver. I. General features of gluconeogenesis in the perfused livers of rats. J Biol Chem. 1967 Jun 10;242(11):2622–2636. [PubMed] [Google Scholar]
- FRISELL W. R., HELLERMAN L., LOWE H. J. Flavoenzyme catalysis: substrate-competitive inhibition of D-amino acid oxidase. J Biol Chem. 1956 Nov;223(1):75–83. [PubMed] [Google Scholar]
- FRISELL W. R., MEECH L. A., MACKENZIE C. G. A simplified photometric analysis for serine and formaldehyde. J Biol Chem. 1954 Apr;207(2):709–716. [PubMed] [Google Scholar]
- Kikuchi G. The glycine cleavage system: composition, reaction mechanism, and physiological significance. Mol Cell Biochem. 1973 Jun 27;1(2):169–187. doi: 10.1007/BF01659328. [DOI] [PubMed] [Google Scholar]
- Krebs H. A., De Gasquet P. Inhibition of gluconeogenesis by alpha-oxo acids. Biochem J. 1964 Jan;90(1):149–154. doi: 10.1042/bj0900149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., Dierks C., Gascoyne T. Carbohydrate synthesis from lactate in pigeon-liver homogenate. Biochem J. 1964 Oct;93(1):112–121. doi: 10.1042/bj0930112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A. Metabolism of amino-acids: The synthesis of glutamine from glutamic acid and ammonia, and the enzymic hydrolysis of glutamine in animal tissues. Biochem J. 1935 Aug;29(8):1951–1969. doi: 10.1042/bj0291951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., Notton B. M., Hems R. Gluconeogenesis in mouse-liver slices. Biochem J. 1966 Dec;101(3):607–617. doi: 10.1042/bj1010607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKADA H. I. GLUTAMIC-GLYCINE TRANSAMINASE FROM RAT LIVER. J Biol Chem. 1964 Feb;239:468–471. [PubMed] [Google Scholar]
- NEIMS A. H., HELLERMAN L. Specificity of the D-amino acid oxidase in relation to glycine oxidase activity. J Biol Chem. 1962 Mar;237:PC976–PC978. [PubMed] [Google Scholar]
- OWEN E. E., ROBINSON R. R. Amino acid extraction and ammonia metabolism by the human kidney during the prolonged administration of ammonium chloride. J Clin Invest. 1963 Feb;42:263–276. doi: 10.1172/JCI104713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITTS R. F., DEHAAS J., KLEIN J. Relation of renal amino and amide nitrogen extraction to ammonia production. Am J Physiol. 1963 Feb;204:187–191. doi: 10.1152/ajplegacy.1963.204.2.187. [DOI] [PubMed] [Google Scholar]
- Pitts R. F., Damian A. C., MacLeod M. B. Synthesis of serine by rat kidney in vivo and in vitro. Am J Physiol. 1970 Sep;219(3):584–589. doi: 10.1152/ajplegacy.1970.219.3.584. [DOI] [PubMed] [Google Scholar]
- Pitts R. F., MacLeod M. B. Synthesis of serine by the dog kidney in vivo. Am J Physiol. 1972 Feb;222(2):394–398. doi: 10.1152/ajplegacy.1972.222.2.394. [DOI] [PubMed] [Google Scholar]
- Pitts R. F. Metabolism of amino acids by the perfused rat kidney. Am J Physiol. 1971 Apr;220(4):862–867. doi: 10.1152/ajplegacy.1971.220.4.862. [DOI] [PubMed] [Google Scholar]
- RICHERT D. A., AMBERG R., WILSON M. Metabolism of glycine by avian liver. J Biol Chem. 1962 Jan;237:99–103. [PubMed] [Google Scholar]
- Ross B. D., Hems R., Krebs H. A. The rate of gluconeogenesis from various precursors in the perfused rat liver. Biochem J. 1967 Mar;102(3):942–951. doi: 10.1042/bj1020942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowsell E. V., Snell K., Carnie J. A., Al-Tai A. H. Liver-L-alanine-glyoxylate and L-serine-pyruvate aminotransferase activities: an apparent association with gluconeogenesis. Biochem J. 1969 Dec;115(5):1071–1073. doi: 10.1042/bj1151071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowsell K. V., Ali T. A. A difference in the incorporation of 14C into hippurate glycine from DL-(2-14C)-glutamate and DL-(5-14C)glutamate in guinea pigs. Biochem J. 1975 Nov;152(2):357–363. doi: 10.1042/bj1520357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAGERS R. D., GUNSALUS I. C. Intermediatry metabolism of Diplococcus glycinophilus. I. Glycine cleavage and one-carbon interconversions. J Bacteriol. 1961 Apr;81:541–549. doi: 10.1128/jb.81.4.541-549.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Kochi H., Sato N., Kikuchi G. Glycine metabolism by rat liver mitochondria. 3. The glycine cleavage and the exchange of carboxyl carbon of glycine with bicarbonate. J Biochem. 1969 Jan;65(1):77–83. [PubMed] [Google Scholar]
- Squires E. J., Hall D. E., Brosnan J. T. Arteriovenous differences for amino acids and lactate across kidneys of normal and acidotic rats. Biochem J. 1976 Oct 15;160(1):125–128. doi: 10.1042/bj1600125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. S., Richardson K. E. Isolation and characterization of an L-alanine: glyoxylate aminotransferase from human liver. J Biol Chem. 1967 Aug 25;242(16):3614–3619. [PubMed] [Google Scholar]
- WEINHOUSE S., FRIEDMANN B. Metabolism of labeled 2-carbon acids in the intact rat. J Biol Chem. 1951 Aug;191(2):707–717. [PubMed] [Google Scholar]


