Abstract
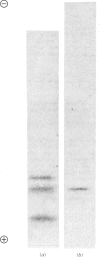
A method was devised to purify branched-chain oxo acid dehydrogenase (BCOAD) from rat kidney which retains endogenous kinase activity. Incorporation of 32P into purified enzyme parallels the time course of enzyme inhibition by ATP. Phosphorylation occurs on a serine residue(s) of the 46000-mol.wt. subunit of the enzyme complex. Endogenous phosphatase activity is not present after purification, and added pyruvate dehydrogenase phosphate phosphatase does not re-activate BCOAD or liberate 32P from previously labelled enzyme. These results demonstrate that BCOAD can be regulated by an endogenous protein kinase and that the phosphorylation-cycle enzymes regulating BCOAD appear to be distinct from those associated with pyruvate dehydrogenase complex.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Danner D. J., Lemmon S. K., Besharse J. C., Elsas L. J., 2nd Purification and characterization of branched chain alpha-ketoacid dehydrogenase from bovine liver mitochondria. J Biol Chem. 1979 Jun 25;254(12):5522–5526. [PubMed] [Google Scholar]
- Dawson A. G., Hird F. J., Morton D. J. Oxidation of leucine by rat liver and kidney. Arch Biochem Biophys. 1967 Nov;122(2):426–433. doi: 10.1016/0003-9861(67)90216-0. [DOI] [PubMed] [Google Scholar]
- DePaoli-Roach A. A., Roach P. J., Larner J. Multiple phosphorylation of rabbit skeletal muscle glycogen synthase. Comparison of the actions of different protein kinases capable of catalyzing phosphorylation in vitro. J Biol Chem. 1979 Dec 10;254(23):12062–12068. [PubMed] [Google Scholar]
- Goodman H. M. Site of action of insulin in promoting leucine utilization in adipose tissue. Am J Physiol. 1977 Aug;233(2):E97–103. doi: 10.1152/ajpendo.1977.233.2.E97. [DOI] [PubMed] [Google Scholar]
- Huang K. P., Robinson J. C. A rapid and sensitive assay method for protein kinase. Anal Biochem. 1976 May 7;72:593–599. doi: 10.1016/0003-2697(76)90571-6. [DOI] [PubMed] [Google Scholar]
- Hughes W. A., Halestrap A. P. The regulation of branched-chain 2-oxo acid dehydrogenase of liver, kidney and heart by phosphorylation. Biochem J. 1981 May 15;196(2):459–469. doi: 10.1042/bj1960459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence J. C., Jr, Larner J. Activation of glycogen synthase in rat adipocytes by insulin and glucose involves increased glucose transport and phosphorylation. J Biol Chem. 1978 Apr 10;253(7):2104–2113. [PubMed] [Google Scholar]
- Odessey R. Direct evidence for the inactivation of branched-chain oxo-acid dehydrogenase by enzyme phosphorylation. FEBS Lett. 1980 Dec 1;121(2):306–308. doi: 10.1016/0014-5793(80)80369-3. [DOI] [PubMed] [Google Scholar]
- Odessey R., Goldberg A. L. Leucine degradation in cell-free extracts of skeletal muscle. Biochem J. 1979 Feb 15;178(2):475–489. doi: 10.1042/bj1780475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odessey R. Reversible ATP-induced inactivation of branched-chain 2-oxo acid dehydrogenase. Biochem J. 1980 Oct 15;192(1):155–163. doi: 10.1042/bj1920155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker P. J., Randle P. J. Active and inactive forms of branched-chain 2-oxoacid dehydrogenase complex in rat heart and skeletal muscle. FEBS Lett. 1980 Apr 7;112(2):186–190. doi: 10.1016/0014-5793(80)80176-1. [DOI] [PubMed] [Google Scholar]
- Parker P. J., Randle P. J. Inactivation of rat heart branched-chain 2-oxoacid dehydrogenase complex by adenosine triphosphate. FEBS Lett. 1978 Nov 1;95(1):153–156. doi: 10.1016/0014-5793(78)80072-6. [DOI] [PubMed] [Google Scholar]
- Pettit F. H., Yeaman S. J., Reed L. J. Purification and characterization of branched chain alpha-keto acid dehydrogenase complex of bovine kidney. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4881–4885. doi: 10.1073/pnas.75.10.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]