Abstract
Background
The association between alterations in the oral microbiome and hand, foot, and mouth disease (HFMD) has been observed in previous studies. Our study, therefore, aimed to identify the structural changes in the oral microbiota and biomarkers in children with HFMD caused by enterovirus A 71 (EV-A71).
Methods
Children diagnosed with EV-A71 HFMD and healthy children recruited from April 2021 to September 2023 were included in the present study, and were categorized into EV-A71 and control groups, respectively. Oral swabs were collected and microbiota information was obtained using 16 S rRNA gene sequencing technology. Alpha-diversity and partial least squares discriminant analyses were conducted to compare microbial diversity, richness, and similarity between the two groups. Linear discriminant analysis effect size was employed to identify microbial taxa with significant differences, and determined the key genera among them.
Results
The study included a total of 80 children, with 50 assigned to the EV-A71 group and 30 to the control group. No significant differences were found between the two groups in terms of age (2.2 ± 1.2 vs. 2.7 ± 1.2 years; age range: 1–5 years; P = 0.114) or sex (56% vs. 60% boys, P = 0.726). The oral microbiota structure in the EV-A71 group differed from that in the control group. The EV-A71 group showed significant reductions in both the Shannon index (P = 0.037) and the abundance-based coverage estimator (ACE) index (P < 0.001). The key genus changes were marked by a significant decrease in the abundance of Capnocytophaga (P = 0.002) and Leptotrichia (P = 0.033) in the EV-A71 group.
Conclusion
In children with EV-A71 HFMD, the oral microbiota showed changes in composition, with a significant reduction in diversity and richness. The changes in key genera were a marked decrease in the abundance of Capnocytophaga and Leptotrichia.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-10233-2.
Keywords: Hand, foot, and mouth disease; Children; Oral microbiota; Short-chain fatty acids
Background
Hand, foot, and mouth disease (HFMD) is a rash-causing infectious disease caused by various non-polio enteroviruses, and is characterized by rashes or blisters on the hands, feet, buttocks and/or mouth, with an incidence rate ranging from 37–205/100,000 persons in China [1–3]. Children under 5 years of age are the main susceptible population [1, 3]. Enterovirus A 71 (EV-A71) and Coxsackievirus type A16 are the primary pathogens responsible for HFMD [4]; however, EV-A71, in particular, has a high affinity for the human nervous system and can lead to complications such as encephalitis, encephalomyelitis, cerebellar ataxia, neurogenic pulmonary edema, and pulmonary hemorrhage [5]. EV-A71, therefore, accounts for > 87% of fatal HFMD cases in China [6]. The pathogenesis of EV-A71 HFMD has not been fully elucidated, however, resulting in a lack of targeted antiviral drugs and specific treatment methods which can be utilized in clinical practice [7, 8].
The oral microbiota is an integral part of the human microbiome, and previous research has indicated that an imbalance or disruption in its composition is closely linked to HFMD [9, 10]. Compared to healthy controls, children with clinical symptoms of HFMD exhibit a notable reduction in the richness of their oral microbiota [9]. One study found that the level of enterovirus ribonucleic acid (RNA) in the saliva of children with HFMD was inversely related to the presence of Aggregatibacer segnis, but positively associated with Streptococcus gordonii and Trabulsiella farmeri [9]. These correlations suggest that alterations in the oral microbiota might play a role in the progression of HFMD. Moreover, a growing body of research has demonstrated that the oral microbiota is crucial in the progression of oral mucosal lesions (OMLs) [11–13]. It is well known that OMLs are a common pathological change in children with HFMD, and are the main contributors to oral pain and reluctance to eat during the course of the disease [1, 2]. Therefore, this study aimed to identify changes in the structure of the oral microbiota and characteristics in children with HFMD caused by EV-A71, through the use of 16 S rRNA gene sequencing technology. The results of our study will provide a theoretical basis for exploring the microbiological mechanisms of EV-A71-associated HFMD and the treatment of OMLs with probiotics.
Methods
Participants
Children diagnosed with HFMD, with EV-A71 infection confirmed at Suqian Hospital Affiliated to Xuzhou Medical University from April 2021 to September 2023, as well as healthy children undergoing physical examinations during the same period, were included in the present study, categorized into two groups, the EV-A71 and control groups, respectively. The criteria for including participants in the EV-A71 group were as follows: (1) Clinical diagnosis of HFMD was characterized by the presence of oral vesicular exanthema or ulcers, along with vesicular lesions on the hands, feet, and/or buttocks; (2) Real-time RT-PCR (reverse transcription-polymerase chain reaction) or nested RT-PCR of clinical samples (such as throat swabs, stool or rectal swabs, and blood) was positive for EV-A71; and (3) patients were in the acute phase of the disease (duration < 3 days). The following were the exclusion criteria: (1) age > 14 years; (2) use of antibiotics, probiotics, or related medications in the month prior to diagnosis; (3) history of genetic, metabolic, or oral diseases; (4) any concurrent infection; and (5) children not residing in Jiangsu Province. The exclusion criteria for the control group were identical to those for the EV-A71 group, and all control children were tested to confirm they were negative for EV-A71. For virus detection, real-time RT-PCR was performed using the SuperScript™ III Platinum™ One-Step qRT-PCR Kit (Thermo Fisher, Cergy-Pontoise, France), and nested RT-PCR was conducted with the SuperScript™ II RT Kit (Life Technologies, Ontario, Canada) and Platinum™ Taq DNA Polymerase Kit (Thermo Fisher, Barcelona, Spain). The protocol for the present study was approved by the ethics committee of Suqian Hospital Affiliated to Xuzhou Medical University (approval no. F201941). All parents or guardians were thoroughly informed about the study procedures and gave their written consent to participate.
Sample collection, DNA extraction and quality control
All oral swab samples were collected from the hospital as follows: patients did not eat or drink anything within 30 min of providing the sample; the staff member obtaining the sample thoroughly washed and disinfected their hands prior to the procedure; a sterile swab was inserted into the patient’s oral cavity and thoroughly rubbed against the buccal mucosa, moving upward and downward while rotating the swab for approximately 30 s, and a tongue depressor was used to keep the mouth open if necessary; a second swab was collected from the buccal mucosa on the contralateral side using the same method. After sampling, both swabs were placed together in the same cryovial and stored at -80 °C. After collecting all the samples, genomic DNA was extracted following the manufacturer’s instructions using the BeaverBeads™ Saliva DNA Kit (Beaver Corp., Suzhou, China). The extracted DNA’s purity, concentration, and integrity were tested using a NanoDrop 2000 spectrophotometer (Thermo Fisher, Tuas, Singapore), Qubit 3.0 fluorescence quantifier (Thermo Fisher, Tuas, Singapore), and 1% agarose gel electrophoresis, respectively [14]. Samples were considered of adequate quality for sequencing. if the A260/280 ratio was between 1.8 and 2.0, the concentration was ≥ 100 ng/µL, and there was a clear main band present [14].
16 S rRNA gene sequencing
The fourth hypervariable (V4) region was chosen as the target for 16 S rRNA amplification. The primers, 515 F (5′-GTGCGCAGCMGCCGGTAA-3′) and 806R (5′-GACTACHVGGGTWTCTAAT-3′), were designed and synthesized by BGI Genomics Co., Ltd (Shenzhen, China). First, a 50 ng DNA sample with good quality and its corresponding fusion primers were prepared. Next, the polymerase chain reaction (PCR) system and parameters for amplification were set up, and the products were purified using DNA magnetic beads (BGI, Shenzhen, China) to finalize the library construction with 2×Phanta Max Master Mix (VAZYME, Nanjing, China) polymerase. Fragment size and concentration of the library samples were assessed using an Agilent 2100 Expert (Asiagene Technology, Shanghai, China). After passing the quality check, sequencing was performed using the Illumina HiSeq X Ten platform.
Bioinformatics analysis
The raw sequencing data was processed, and clean data obtained as follows: (1) reads that matched the primers were processed using Cutadapt version 3.0 (https://cutadapt.readthedocs.io/en/stable/) to remove any primer and adapter contamination, resulting in isolated fragments of the target region; (2) a 25 bp sliding window was applied, and if the quality score within the window dropped below 20, the sequence was trimmed from that point onwards, but if the trimmed read was shorter than 50 bp, it was discarded entirely; (3) reads with undetermined bases (N) were eliminated; and (4) low-complexity reads, which contain simple repetitive patterns or repeated nucleotide sequences, were discarded. Forward and reverse reads from each DNA fragment were merged to form complete sequences (tags) using FLASH version 1.2.11. The merging conditions were as follows: (1) a minimum overlap length of 10 bp, and (2) a mismatch rate in the overlap region ≤ 0.2.
We used USEARCH version 7.0.1090 (www.drive5.com/usearch/) to cluster the merged tags into operational taxonomic units (OTUs). The main process was as follows: (1) tags were clustered at 97% similarity using UPARSE to obtain the representative OTU sequences, after which (2) chimeric sequences produced by PCR were eliminated from the representative OTU sequences using UCHIME version 4.2.40, with the gold database version 20,110,519 as the reference. Using USEARCH, we assigned the original reads to predefined OTUs and calculated the abundance of each OTU across all samples. Once the representative OTU sequences were obtained, the RDP Classifier (version 2.14, https://sourceforge.net/projects/rdp-classifier/) was employed to compare these sequences against the database for species annotation, using a confidence threshold of 0.6. The filtering of annotation results was conducted as follows: (1) OTUs without annotation results were removed from the analysis, and (2) OTUs with annotation results not relevant to the species under investigation were excluded. The leftover OTUs were employed in later analysis. Using QIIME software (https://qiime2.org) for alpha (α)-diversity analysis, the Shannon and abundance-based coverage estimator (ACE) indices were selected to compare microbial diversity and richness differences between the two groups [15]. For beta (β)-diversity analysis, partial least squares discriminant analysis (PLS-DA) was used to assess microbial community differences between the two groups. The analysis was conducted using the mixOmics package in R (v3.2.1) to visualize group separation based on microbial composition. Linear discriminant analysis effect size (LEfSe) differential analysis uses linear discriminant analysis (LDA) to estimate the effect size of each taxon’s abundance on differences, and to identify significantly different species [16], with a default LDA score > 2.0. The analyses for LDA and LEfSe were carried out using the LEfSe toolkit (http://huttenhower.sph.harvard.edu/galaxy).
Statistical analysis
In the present study, normally distributed quantitative data are presented as mean ± standard deviation and non-normally distributed continuous variables are presented as median (1st quartile, 3rd quartile). The sex composition ratios between the two groups were analyzed using the chi-squared test for independence with a 2 × 2 contingency table to determine statistical differences. Levene’s test assessed the homogeneity of variances, whereas the Kolmogorov-Smirnov test determined the normality of the quantitative data distribution between the two groups. If both assumptions were met, a t-test was performed; otherwise, the rank-sum test was used. A P-value of less than 0.05 was considered statistically significant, with all analyses performed using SPSS version 26.0 (IBM Corp., Armonk, NY, USA).
Results
Participants
A total of 80 children participated in this study – 50 in the EV-A71 group (28 males, 22 females) and 30 in the control group (18 boys, 12 girls) – ranging in age from 1 to 5 years. There was no significant difference in age (2.2 ± 1.2 vs. 2.7 ± 1.2 years; P = 0.114), sex distribution (56% vs. 60% boys; P = 0.726), or number of teeth (16.8 ± 4.3 vs. 18.3 ± 2.7; P = 0.065) between the EV-A71 and control groups, respectively. All clinical samples (such as throat swabs, stool or rectal swabs, and blood) from the children tested negative for Coxsackievirus, Echovirus, Herpesvirus, and mpox by real-time RT-PCR or nested RT-PCR.
Diversity analysis and dimensionality reduction analysis
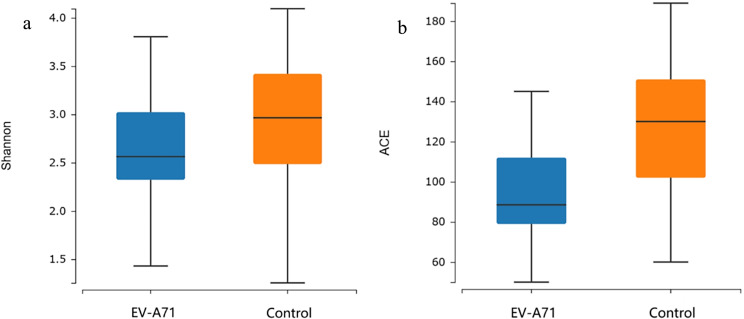
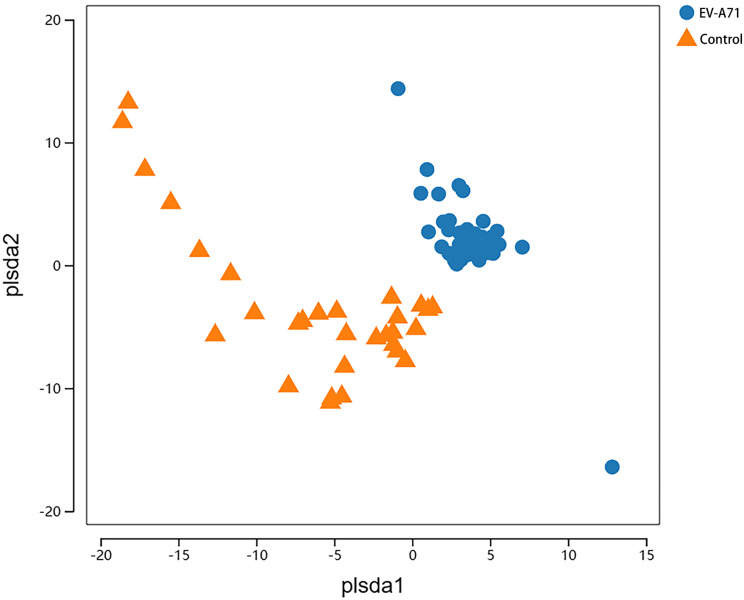
A total of 1,611 OTUs were obtained from the two groups – 837 in the EV-A71 group and 774 in the control group (Figs. 1) – 462 of which were common between both groups. The Shannon diversity indices (Fig. 2a) were 2.563 (2.324, 3.023) and 2.965 (2.486, 3.420) in the EV-A71 and control groups, respectively, while the ACE indices (Fig. 2b) were 88.5 (79.0, 112.0) and 130.0 (102.0, 151.0), respectively. Compared with the control group, the Shannon (P = 0.037) and ACE (P < 0.001) indices of the EV-A71 group were significantly reduced. The PLS-DA score chart shows a clear distinction between the EV-A71 and control groups (Fig. 3).
Fig. 1.
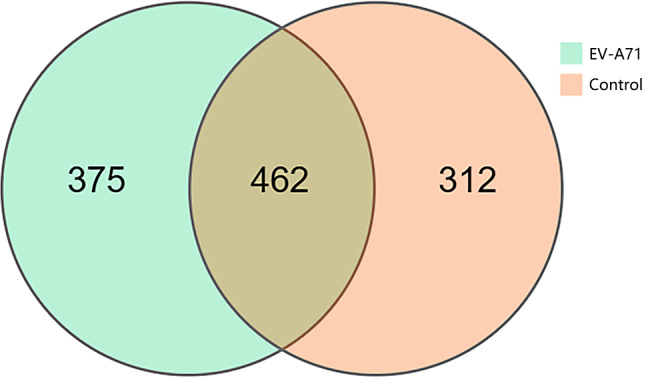
Comparison of the number of OTUs obtained from the EV-A71 and control groups, with the number in the overlapping section representing the OTUs common to both groups. OTU, operational taxonomic unit; EV-A71, enterovirus A 71
Fig. 2.
Alpha diversity analysis of the oral microbiota in the EV-A71 and control groups, with the five lines from bottom to top representing the minimum value, first quartile, median, third quartile, and maximum value, respectively. (a) Shannon index; (b) ACE index. ACE, abundance-based coverage estimator
Fig. 3.
PLS-DA analysis of the oral microbiota at the OTUs level for the EV-A71 and control groups, with the horizontal and vertical axes representing relative distances. PLS-DA, partial least squares discriminant analysis
Structure and abundance ratio of oral microbiota
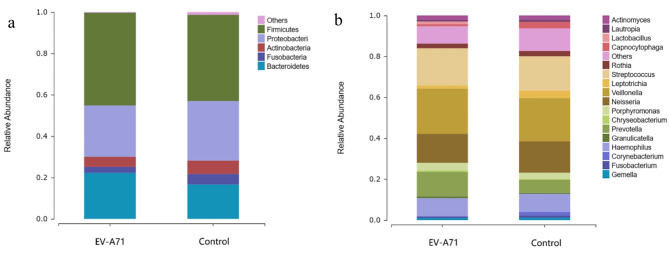
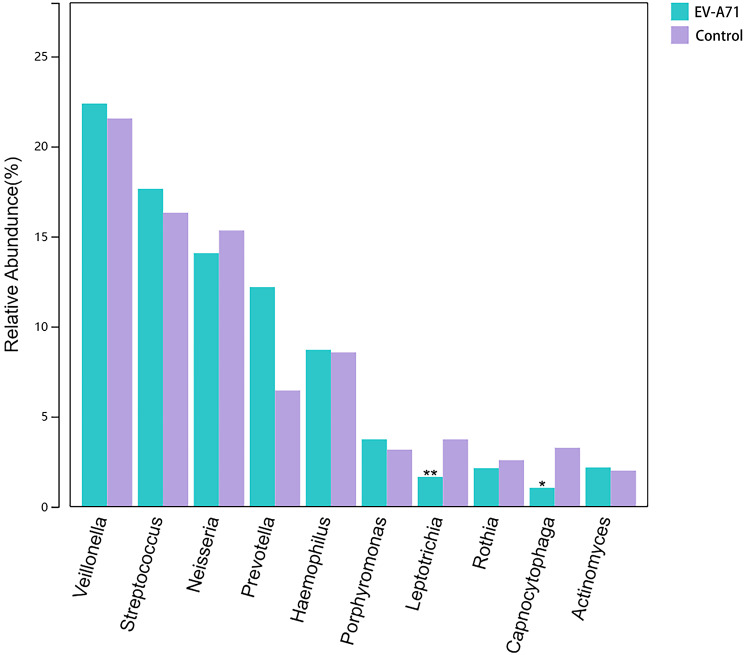
At the phylum level (Fig. 4a), the main components of both the EV-A71 and control groups were Firmicutes, Proteobacteria, Bacteroidetes, Actinobacteria, and Fusobacteria. At the genus level (Fig. 4b), the top ten abundant bacteria in the control group were Veillonella (21.147%), Streptococcus (16.769%), Neisseria (15.321%), Haemophilus (8.834%), Prevotella (6.325%), Leptotrichia (3.678%), Porphyromonas (3.185%), Capnocytophaga (3.166%), Rothia (2.588%), and Actinomyces (2.011%), while those in the EV-A71 group were Veillonella (22.082%), Streptococcus (18.179%), Neisseria (14.074%), Prevotella (11.928%), Haemophilus (8.724%), Porphyromonas (3.737%), Rothia (2.205%), Actinomyces (2.135%), Leptotrichia (1.646%), and Lactobacillus (1.253%).
Fig. 4.
Composition and proportions of the main taxonomic groups in the oral microbiota for the EV-A71 and control groups at (a) phylum and (b) genus levels
Screening key bacterial markers
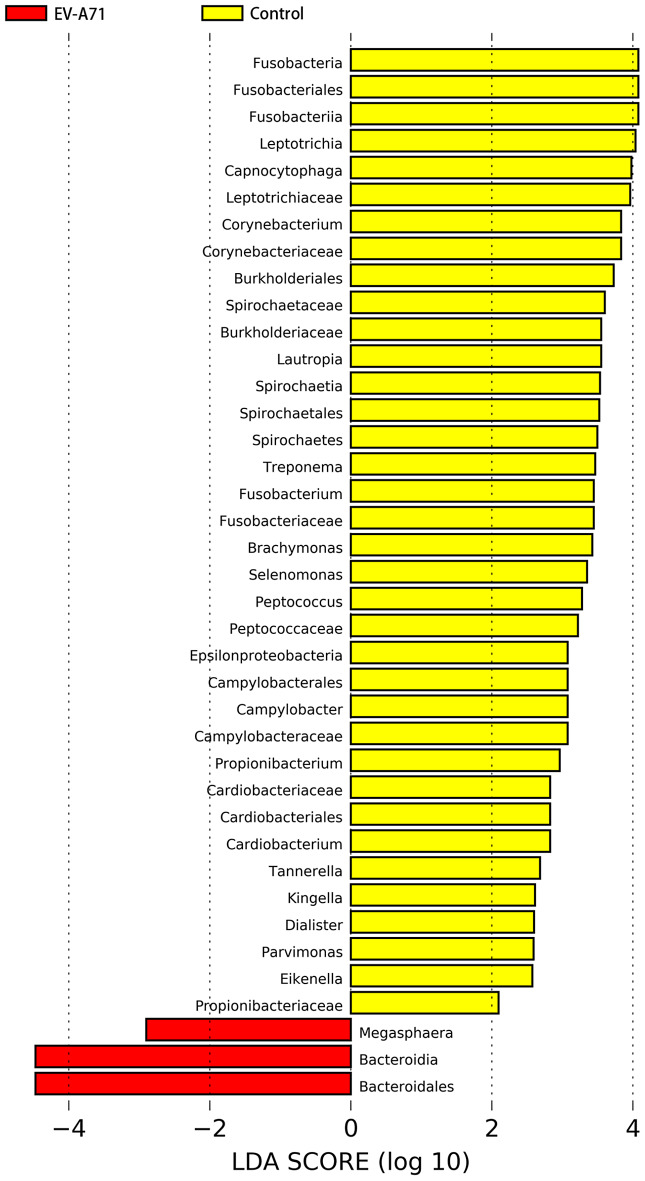
The preset LDA value was 2.0, and an absolute LDA score greater than this value indicated a statistically significant difference. The oral microbiota of the EV-A71 group showed changes at various taxonomic levels compared to the control group (Fig. 5), including two bacteria at the phylum level, four at the class level, six at the order level, nine at the family level, and 18 at the genus level. Among them, 18 genera with significant differences are Leptotrichia, Capnocytophaga, Corynebacterium, Lautropia, Treponema, Fusobacterium, Brachymonas, Selenomonas, Peptococcus, Campylobacter, Propionibacterium, Megasphaera, Cardiobacterium, Tannerella, Kingella, Dialister, Parvimonas, and Eikenella. The genera with the top 10 highest abundances in the two groups were identified as key genera, with the average abundance and significance of the difference test presented for each group. As shown in Fig. 6, the abundances of Capnocytophaga (P = 0.035) and Leptotrichia (P = 0.002) were significantly reduced in the EV-A71 group.
Fig. 5.
Significantly different taxa with LDA scores greater than the preset value, representing statistically significant biomarkers. The default preset value was 2.0 (only taxa with absolute LDA scores > 2 are displayed in the figure). LDA, linear discriminant analysis
Fig. 6.
The average relative abundance and significance of the difference test of each of the top 10 most abundant species. “**” indicates a P-value ≤ 0.01, “*” indicates a P-value between 0.01 and 0.05, and no symbol is shown for P-values > 0.05
Discussion
In this study, 16 S rRNA gene sequencing was employed to analyze the oral microbiota of children affected by EV-A71 HFMD. The results indicated that children with EV-A71 HFMD exhibited decreased diversity and richness in their oral microbiota, with a notable decrease in the abundance of key genera, namely Capnocytophaga and Leptotrichia.
This study found the diversity and richness of the oral microbiota both decreased significantly, suggesting that the structure of the oral microbiota had changed. The oral cavity is well known to be the second largest reservoir of microorganisms in the human body, and its balance is crucial for maintaining the function and physiological activities of the oral mucosa [17, 18]. There is mounting evidence to indicate a close correlation between changes in the oral microbiota and infections with various viruses in the human body, such as human immunodeficiency virus, novel coronavirus, and human papillomavirus [19–24]. After infection with these viruses, the oral microbiota generally exhibit decreased diversity and richness, which corresponds with the results obtained in this study. This decrease in diversity and richness may lead to ‘crosstalk’ among the oral microbiota, mucosal epithelium, and immune cells [25, 26], and a reduction in the type and number of symbiotic bacteria can cause an imbalance in the ratio of regulatory to helper T-cells, resulting in mucosal inflammatory damage [27, 28]. Additionally, changes in the metabolic products associated with dysbiosis can compromise the integrity and barrier function of the mucosal epithelium [25, 28]. Therefore, dysbiosis of the oral microbiota may, to some extent, promote the progression of EV-A71 HFMD, especially in terms of damage to the oral mucosa. Recent studies have demonstrated that regulating the microbiota balance can improve the outcomes of oral infections, ulcers, and even OMLs caused by radiotherapy and chemotherapy administered for head and neck tumors [29–32]. Therefore, supplementation with probiotics to increase the richness and diversity of symbiotic oral bacteria may help alleviate oral pain and appetite loss in children with EV-A71 HFMD.
Interestingly, we found that although both the Shannon and ACE indices were significantly lower in the EV-A71 group compared to the control group, there was substantial overlap in the range of values between the two groups, with the overlap being more pronounced for the Shannon index. As is well known, the Shannon index reflects both the richness and evenness of the microbial community, whereas the ACE index primarily estimates species richness. Therefore, the observed overlap between these indices may suggest that while microbial richness (captured by the ACE index) was altered, the evenness of species distribution (captured by the Shannon index) was less affected. This implies that EV-A71 infection may lead to a reduction in specific microbial populations, thereby impacting richness, but does not significantly disrupt the balance of the remaining microbial community.
Leptotrichia belongs to the phylum Fusobacteriota, and Capnocytophaga belongs to the phylum Bacteroidetes, both of which are Gram-negative anaerobic bacteria. The final products of their carbohydrate fermentation include lactic acid, acetic acid, and succinate. These short-chain fatty acids (SCFAs) have been shown in previous studies to play important roles in the regulation of inflammatory responses, mucosal barrier stability, and tissue repair during viral infections. Specifically, it has been reported that: (1) SCFAs can bind to G protein-coupled receptors (GPR41 and GPR43), activating the adenosine monophosphate-activated protein kinase (AMPK) pathway, therby reducing the release of inflammatory cytokines such as interleukin (IL)-2, -6, and-8, and tumor necrosis factor (TNF)-α [33, 34]; (2) SCFAs may reduce oxidative stress response and promote epithelial cell proliferation, accelerating the repair and regeneration of damaged mucosal tissues [35]; (3) SCFAs are also known to promote the expression of tight junction proteins, which can enhance the physical barrier function of the oral mucosa and limit pathogen invasion [36, 37]; and (4) SCFAs provide a mildly acidic environment that supports local beneficial microbiota, indirectly inhibiting the colonization of opportunistic pathogens [38, 39]. Therefore, given the known functions of SCFAs, a decrease in the abundance of Capnocytophaga and Leptotrichia, as observed in our study, could potentially impair oral mucosal integrity and self-repair in children with EV-A71-associated HFMD. Further studies are needed to directly examine these mechanisms.
Previous studies on the oral microbiota of patients with HFMD have grouped subjects based on the presence or absence of symptoms or the severity of the disease, but none have categorized them according to the pathogenic agents [9, 10]. For instance, Ho et al. [9] used 16 S sequencing technology to compare and analyze the oral microbiota of symptomatic (17 children), asymptomatic (10 children), and healthy (11 children) cohorts, and found that compared to healthy children, the diversity of the oral microbiota among symptomatic patients decreased, while the richness of the microbiota among asymptomatic cohorts increased. They identified Streptococcus as the key genus in the symptomatic group, and its abundance increased significantly, and was positively correlated with the level of enterovirus RNA in the saliva. Similarly, Zhang et al. [10] used 16 S sequencing to analyze the oral microbiota in children with severe and mild HFMD, and found that compared to mild cases (4 children), patients with severe cases (16 children) did not exhibit significant changes in the diversity and richness of the oral microbiota. They did, however, observe an increase in the abundance of Megasphaera and Alloprevotella and a decrease in the abundance of Streptococcus and Gemella. The results of the two aforementioned studies differ from those of the present study, which may be due to differences in the sample size, 16 S primer sequences, geographic location, and types of viruses causing HFMD among the studies, but suggests that changes in the oral microbiota caused by EV-A71-associated HFMD may have unique characteristics. This possibility should, therefore, be considered in the future development of oral probiotics for the treatment of HFMD.
The present study did have some limitations. First, study participants were limited to children; therefore, these results may not translate to adults, although the incidence of HFMD in adults is very low. Second, the present study did not investigate whether there was a correlation between the oral microbiota and the clinical symptoms of EV-A71-associated HFMD. Finally, the present study had a relatively small sample size, which may have increased the influence of confounding factors on the results.
Conclusions
In conclusion, the structure of the oral microbiota in children with HFMD caused by EV-A71 showed alterations, with a significant reduction in the overall diversity and richness. Notably, a significant decrease in the abundance of Capnocytophaga and Leptotrichia was identified as a key change at the genus level. The results of the present study provide a theoretical basis for future research on the oral microecology of EV-A71-associated HFMD and offer directions for the development of probiotics for adjuvant therapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Youqing Shen and Jing Zhang for their assistance during the writing process.
Abbreviations
- HFMD
Hand, foot, and mouth disease
- EV-A71
Enterovirus A 71
- RNA
Ribonucleic acid
- OMLs
Oral mucosal lesions
- rDNA
Ribosomal deoxyribonucleic acid
- PCR
Polymerase chain reaction
- OTU
Operational taxonomic unit
- ACE
Abundance-based coverage estimator
- LEfSe
Linear discriminant analysis effect size
- LDA
Linear discriminant analysis
- PLS-DA
Partial least squares discriminant analysis
- SCFAs
Short-chain fatty acids
- AMPK
Adenosine monophosphate-activated protein kinase
- IL
Interleukin
- TNF
Tumor necrosis factor
Author contributions
NS, RW, and TL: conceptualization and performance of the experiments, methodology, investigation, data curation, writing of the original draft, and finalization of the manuscript; XL: performance of the experiments and formal analyses; SL, YJ and TN: performance of the experiments, validation, and supervision; SZ, and JQ: conceptualization, supervision, funding acquisition, project administration, and writing – review, and editing. All authors read and approved the final manuscript.
Funding
The present study was supported by the Jiangsu Province Maternal and Child Health Research Project (F202153 and F201941) and the Suqian Science and Technology Plan Project (Z2019154).
Data availability
All raw reads were deposited in the National Center for Biotechnology Information Sequence Read Archive database (accession no. PRJNA1121660; https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA1121660).
Declarations
Ethics committee approval and consent to participate
The parents or guardians of the children included in the present study provided informed consent, and the study design was approved by the ethics committee of Suqian Hospital Affiliated to Xuzhou Medical University (June 14, 2020). All experiments were performed in accordance with guidelines of the Declaration of Helsinki (2013).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nan Shen, Rang Wu and Tiantian Lu contributed equally to this work.
Contributor Information
Suyue Zhu, Email: zsyzsy7878@163.com.
Jibing Qiao, Email: 826457@qq.com.
References
- 1.Li X-W, Ni X, Qian S-Y, Wang Q, Jiang R-M, Xu W-B, et al. Chinese guidelines for the diagnosis and treatment of hand, foot and mouth disease (2018 edition). World J Pediatr. 2018;14:437–47. [DOI] [PubMed] [Google Scholar]
- 2.Yang Q, Liu F, Chang L, Lai S, Teng J, Duan J, et al. Molecular epidemiology and clinical characteristics of enteroviruses associated HFMD in Chengdu, China, 2013–2022. Virol J. 2023;20:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu P, Ji W, Li D, Li Z, Chen Y, Dai B, et al. Current status of hand-foot-and-mouth disease. J Biomed Sci. 2023;30:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi Y, Chen P, Bai Y, Xu X, Liu Y. Seroprevalence of coxsackievirus A6 and enterovirus A71 infection in humans: a systematic review and meta-analysis. Arch Virol. 2023;168:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee KY. Enterovirus 71 infection and neurological complications. Korean J Pediatr. 2016;59:395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu S-L, Pan H, Liu P, Amer S, Chan T-C, Zhan J, et al. Comparative epidemiology and virology of fatal and nonfatal cases of hand, foot and mouth disease in mainland China from 2008 to 2014. Rev Med Virol. 2015;25:115–28. [DOI] [PubMed] [Google Scholar]
- 7.Swain SK, Panda S, Sahu BP, Sarangi R. Activation of host cellular signaling and mechanism of enterovirus 71 viral proteins associated with hand, foot and mouth disease. Viruses. 2022;14:2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bello AM, Roshorm YM. Recent progress and advances towards developing enterovirus 71 vaccines for effective protection against human hand, foot and mouth disease (HFMD). Biologicals. 2022;79:1–9. [DOI] [PubMed] [Google Scholar]
- 9.Ho SX, Min N, Wong EPY, Chong CY, Chu JJH. Characterization of oral virome and microbiome revealed distinctive microbiome disruptions in paediatric patients with hand, foot and mouth disease. Npj Biofilms Microbiomes. 2021;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang N, Mou D, Li T, Chen Z, Ma C, Liang L, et al. Integrated analysis reveals important differences in the gut and oropharyngeal microbiota between children with mild and severe hand, foot, and mouth disease. Emerg Microbes Infect. 2023;12:2192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Min Z, Yang L, Hu Y, Huang R. Oral microbiota dysbiosis accelerates the development and onset of mucositis and oral ulcers. Front Microbiol. 2023;14:1061032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Liu K, Sun X, Shi X, Zhao G, Yang Z. Microbiome landscape of lesions and adjacent normal mucosal areas in oral lichen planus patient. Front Microbiol. 2022;13:992065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim YJ, Choi YS, Baek KJ, Yoon SH, Park HK, Choi Y. Mucosal and salivary microbiota associated with recurrent aphthous stomatitis. BMC Microbiol 2016;16;Suppl 1:57. [DOI] [PMC free article] [PubMed]
- 14.Wu R, Jiang Y, Yan J, Shen N, Liu S, Yin H, et al. Beneficial changes in gut microbiota after phototherapy for neonatal hyperbilirubinemia. Biomed Rep. 2024;20:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barger K, Langsetmo L, Orwoll ES, Lustgarten MS. Investigation of the diet-gut-muscle axis in the osteoporotic fractures in men study. J Nutr Health Aging. 2020;24:445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin DJ, Yang LS, Wang Z. Research updates: the role of interaction between oral microbiota, immune cells, and epithelial barrier in oral mucosal homeostasis and pathogenesis. Sichuan Da Xue Bao Yi Xue Bao. 2022;53:188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwiertz A. Microbiota of the human body: implications in health and disease. Adv Exp Med Biol. 2016;902:v. [PubMed] [Google Scholar]
- 19.Zhang Y, D’Souza G, Fakhry C, Bigelow EO, Usyk M, Burk RD, et al. Oral human papillomavirus associated with differences in oral microbiota beta diversity and microbiota abundance. J Infect Dis. 2022;226:1098–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramos Peña DE, Pillet S, Grupioni Lourenço A, Pozzetto B, Bourlet T, Motta ACF. Human immunodeficiency virus and oral microbiota: mutual influence on the establishment of a viral gingival reservoir in individuals under antiretroviral therapy. Front Cell Infect Microbiol. 2024;14:1364002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie Y, Sun J, Hu C, Ruan B, Zhu B. Oral microbiota is associated with immune recovery in human immunodeficiency virus-infected individuals. Front Microbiol. 2021;12:794746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li K, Methé BA, Fitch A, Gentry H, Kessinger C, Patel A, et al. Gut and oral microbiota associations with viral mitigation behaviors during the COVID-19 pandemic. Front Cell Infect Microbiol. 2022;12:966361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iebba V, Zanotta N, Campisciano G, Zerbato V, Di Bella S, Cason C, et al. Profiling of oral microbiota and cytokines in COVID-19 patients. Front Microbiol. 2021;12:671813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dahlstrom KR, Sikora AG, Liu Y, Chang C-C, Wei P, Sturgis EM, et al. Characterization of the oral microbiota among middle-aged men with and without human papillomavirus infection. Oral Oncol. 2023;142:106401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Şenel S. An overview of physical, microbiological and immune barriers of oral mucosa. Int J Mol Sci. 2021;22:7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown JL, Johnston W, Delaney C, Rajendran R, Butcher J, Khan S, et al. Biofilm-stimulated epithelium modulates the inflammatory responses in co-cultured immune cells. Sci Rep. 2019;9:15779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dutzan N, Kajikawa T, Abusleme L, Greenwell-Wild T, Zuazo CE, Ikeuchi T, et al. A dysbiotic microbiome triggers T(H)17 cells to mediate oral mucosal immunopathology in mice and humans. Sci Transl Med. 2018;10:463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen B, Sun L, Zhang X. Integration of microbiome and epigenome to decipher the pathogenesis of autoimmune diseases. J Autoimmun. 2017;83:31–42. [DOI] [PubMed] [Google Scholar]
- 29.Kamal Y, Kandil M, Eissa M, Yousef R, Elsaadany B. Probiotics as a prophylaxis to prevent oral candidiasis in patients with Sjogren’s syndrome: a double-blinded, placebo-controlled, randomized trial. Rheumatol Int. 2020;40:873–9. [DOI] [PubMed] [Google Scholar]
- 30.Cappello F, Rappa F, Canepa F, Carini F, Mazzola M, Tomasello G, et al. Probiotics can cure oral aphthous-like ulcers in inflammatory bowel disease patients: a review of the literature and a working hypothesis. Int J Mol Sci. 2019;20:5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dou X, Li G, Wang S, Shao D, Wang D, Deng X, et al. Probiotic-loaded calcium alginate/fucoidan hydrogels for promoting oral ulcer healing. Int J Biol Macromol. 2023;244:125273. [DOI] [PubMed] [Google Scholar]
- 32.Mirza MA, Aruna D, Irukulla M. Efficacy of Bacillus clausii UBBC – 07 spores in the amelioration of oral mucositis in head and neck cancer patients undergoing radiation therapy. Cancer Treat Res Commun. 2022;31:100523. [DOI] [PubMed] [Google Scholar]
- 33.Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91–119. [DOI] [PubMed] [Google Scholar]
- 34.Lee AR, Wilson KR, Clarke M, Engel S, Tscharke DC, Gebhardt T, et al. A: GPR41 and GPR43 regulate CD8(+) T cell priming during herpes simplex virus type 1 infection. Front Immunol. 2024;15:1332588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin WY, Lin JH, Kuo YW, Chiang PR, Ho HH. Probiotics and their metabolites reduce oxidative stress in middle-aged mice. Curr Microbiol. 2022;79:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yue X, Wen S, Long-Kun D, Man Y, Chang S, Min Z, et al. Three important short-chain fatty acids (SCFAs) attenuate the inflammatory response induced by 5-FU and maintain the integrity of intestinal mucosal tight junction. BMC Immunol. 2022;23:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ornelas A, Dowdell AS, Lee JS, Colgan SP. Microbial metabolite regulation of epithelial cell-cell interactions and barrier function. Cells. 2022;11:944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhan Z, Tang H, Zhang Y, Huang X, Xu M. Potential of gut-derived short-chain fatty acids to control enteric pathogens. Front Microbiol. 2022;13:976406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Kuo S, Shu M, Yu J, Huang S, Dai A, et al. Staphylococcus epidermidis in the human skin microbiome mediates fermentation to inhibit the growth of Propionibacterium acnes: implications of probiotics in acne vulgaris. Appl Microbiol Biotechnol. 2014;98:411–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw reads were deposited in the National Center for Biotechnology Information Sequence Read Archive database (accession no. PRJNA1121660; https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA1121660).