Abstract
Background
This study was designed to determine the current status of diagnosis and treatment of valvular heart disease (VHD) in Korea.
Methods
A nationwide registry study was conducted in 45 hospitals in Korea involving adult patients with at least moderate VHD as determined by echocardiography carried out between September and October of 2019. Of a total of 4,094 patients with at least moderate VHD, 1,482 had severe VHD (age, 71.3 ± 13.5 years; 49.1% male). Echocardiographic data used for the diagnosis of each case of VHD were analyzed. Experts from each center determined the diagnosis and treatment strategy for VHD based on current guidelines and institutional policy. The clinical outcome was in-hospital mortality.
Results
Each valve underwent surgical or transcatheter intervention in 19.3% cases of severe mitral stenosis, 31.4% cases of severe primary mitral regurgitation (MR), 7.5% cases of severe secondary MR, 43.7% cases of severe aortic stenosis, 27.5% cases of severe aortic regurgitation, and 7.2% cases of severe tricuspid regurgitation. The overall in-hospital mortality rate for patients with severe VHD was 5.4%, and for secondary severe MR and severe tricuspid regurgitation, the rates were 9.0% and 7.5%, respectively, indicating a poor prognosis. In-hospital mortality occurred in 73 of the 1,244 patients (5.9%) who received conservative treatment and in 18 of the 455 patients (4.0%) who received a surgical or transcatheter intervention, which was significantly lower in the intervention group (P = 0.037).
Conclusions
This study provides important information about the current status of VHD diagnosis and treatment through a nationwide registry in Korea and helps to define future changes.
Keywords: Heart valve diseases, Diagnosis, Treatment, Outcome, Korea
Background
Valvular heart disease (VHD) accounts for a rapidly growing proportion of cardiovascular morbidity and mortality around the world [1]. Echocardiography has become an essential standard, not only for the diagnosis of VHD, but for determining treatment [2–5]. Although standard guidelines for diagnosing and treating VHD have been published in the United States and Europe, little is known about clinical situations in other countries.
The burden of VHD in Korea has changed significantly over the past 50 years due to the aging population, socioeconomic development, and advances in treatment [4, 5]. The burden of rheumatic valve disease has been dramatically reduced, and degenerative or secondary causes are now the leading cause of VHD [6–8]. Because the medical system in Korea is universally accessible, evaluating the effectiveness of echocardiography as an initial diagnostic tool for VHD is relatively straightforward, and VHD is often detected early.
The Korean Society of Echocardiography (KSE) implemented the Korean Valve Survey (KVS) registry as a major academic project, and the contemporary prevalence, etiology, and demographic profiles of VHD in Korea are reported in part 1 of the survey [8]. Part 2 focuses on patients with severe VHD who are eligible for consideration for surgical or percutaneous treatment. To examine diagnoses, we investigated the rates of key echocardiographic parameters and the implementation status of additional imaging for each case of severe VHD. In terms of treatment, we examined the rates and outcomes of surgery and transcatheter intervention in each case of severe VHD.
Methods
Study design and population
This nationwide, retrospective, multicenter, observational study of VHD was designed by the clinical practice guidelines committee of the KSE to investigate the position of VHD in Korea with the participation of 45 hospitals representing each region of the country. A list of participating sites and investigators is provided at the end.
Among patients aged 18 years and older who visited each participating hospital between September 1 and October 31, 2019, those with at least a moderate degree of VHD, as diagnosed by echocardiography, were included in this registry [8]. There was no limit on the number of enrolled patients at each center. Patients with severe native VHD were analyzed, and those previous surgical or transcatheter valve replacement or repair were excluded.
Data collection
Data were collected using a password-protected, web-accessible electronic case report form (eCRF; https://kmcecrf.kr/vhd) created a priori by consensus agreement of the Committee of Clinical Practice Guidelines of the KSE. Each eCRF included demographics, clinical information, echocardiographic findings, additional investigations, and therapeutic decisions. In-hospital mortality and cause of death were also investigated. The collected data were coded and stored, and access to them was strictly controlled. The attending physicians completed the eCRFs with assistance from clinical research coordinators. Data in the eCRFs were audited by the two study investigators (JWS and JBP).
Data analysis
A diagnosis of severe VHD was made based on echocardiograph results and an integrative approach following the VHD guidelines in effect at the time of enrollment. However, in severe mitral stenosis (MS), cutoffs of either 1.0 or 1.5 cm2 for the mitral valve area were according to each institution’s standards. We therefore reclassified patients with a mitral valve area of 1.5 cm2 or less as severe MS and included them in this study. A mitral valve area of 1.0 cm2 or less was separately classified as very severe MS. The reporting rates of echocardiographic parameters recommended in the guidelines for a diagnosis of severe VHD were investigated. The decision to perform further investigations, in the form of transesophageal echocardiography, stress echocardiography, cardiac computed tomography, coronary angiography, catheterization, or cardiac magnetic resonance, was made by the attending physicians at each center. Analysis was performed separately for each case of VHD. However, when severe dysfunction was observed in multiple valves in the same patient, the patient was included and analyzed each disease group.
Statistical analysis
Categorical variables were presented as frequencies and percentages. A chi-square or Fisher exact test was performed to test for differences in categorical variables between groups. Continuous variables were presented as mean ± standard deviations. Student t-tests were performed to measure the differences in continuous variables between the two groups. P-values of < 0.05 were considered statistically significant. Data were analyzed in Stata ver. 16.0 (Stata Corp).
Results
Baseline characteristics
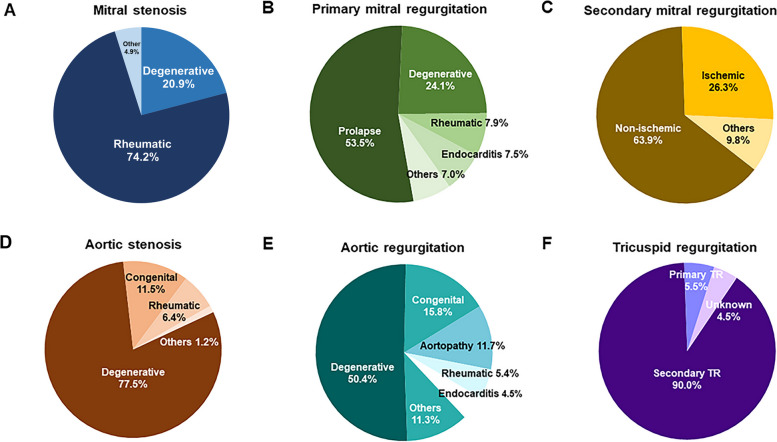
Table 1 shows the clinical and echocardiographic characteristics of patients with severe VHD in Korea. Among 1,482 patients, 244 (16.5%) had severe MS, 229 (15.5%) had severe primary mitral regurgitation (MR), 133 (8.9%) had severe secondary MR, 551 (37.2%) had severe aortic stenosis (AS), 222 (15.0%) had severe aortic regurgitation (AR), and 320 (21.6%) had severe tricuspid regurgitation (TR). Similar to the demographic characteristics of each case of VHD of at least a moderate degree shown in part 1 [8], patients with severe MS, severe primary MR, and severe AR were younger than those with severe secondary MR, severe AS, and severe TR. Most cases of severe MS and TR occurred in female patients, and most cases of severe AR occurred in males. Figure 1 shows the significant causes of each case of severe VHD and their proportions. Analysis of accompanying comorbidity showed characteristics according to the demographics of each VHD case, and the rate of atrial fibrillation was relatively high in cases of severe MS and severe TR.
Table 1.
Demographic, clinical, and echocardiographic characteristics of patients with severe valvular heart disease in Korea
| Characteristic | Severe MS (n = 244) |
Severe primary MR (n = 229) |
Severe secondary MR (n = 133) |
Severe AS (n = 551) |
Severe AR (n = 222) |
Severe TR (n = 320) |
|---|---|---|---|---|---|---|
| Clinical characteristic | ||||||
| Age (yr) | 65.8 ± 11.1 | 65.0 ± 15.7 | 71.6 ± 13.4 | 76.9 ± 10.2 | 65.8 ± 14.1 | 72.3 ± 12.9 |
| Male sex | 78 (32.0) | 108 (47.2) | 69 (51.9) | 284 (51.5) | 138 (62.7) | 143 (44.7) |
| Systolic blood pressure (mmHg) | 119.3 ± 18.4 | 122.8 ± 18.8 | 115.9 ± 20.1 | 126.7 ± 20.1 | 128.5 ± 18.1 | 118.4 ± 18.4 |
| Diastolic blood pressure (mmHg) | 70.5 ± 12.1 | 71.7 ± 13.2 | 68.4 ± 12.0 | 69.2 ± 12.6 | 65.2 ± 12.6 | 69.8 ± 12.8 |
| Body mass index (kg/m2) | 23.3 ± 3.5 | 23.5 ± 3.9 | 22.9 ± 3.4 | 23.6 ± 3.6 | 23.2 ± 3.7 | 23.4 ± 4.1 |
| History of smoking | 19 (8.0) | 17 (8.7) | 8 (6.6) | 24 (4.4) | 13 (7.5) | 13 (4.7) |
| NYHA Functional Classification | ||||||
| I | 91 (38.6) | 79 (64.6) | 18 (13.5) | 152 (28.4) | 91 (41.4) | 84 (26.2) |
| II | 119 (50.4) | 88 (38.6) | 56 (42.1) | 248 (46.3) | 81 (36.8) | 125 (39.1) |
| III | 20 (8.5) | 46 (20.2) | 33 (24.8) | 106 (19.8) | 37 (16.8) | 74 (23.1) |
| IV | 6 (2.5) | 15 (6.6) | 26 (19.6) | 30 (5.6) | 11 (5.0) | 37 (11.6) |
| Unknown | 8 (3.2) | 1 (0.1) | 0 (0) | 15 (2.8) | 2 (0.1) | 0 (0) |
| NYHA class ≥ II | 145 (59.4) | 149 (65.4) | 115 (86.5) | 384 (69.8) | 129 (58.6) | 236 (73.8) |
| Hypertension | 90 (37.3) | 107 (46.9) | 72 (54.1) | 361 (65.6) | 135 (61.6) | 182 (57.1) |
| Diabetes | 43 (17.8) | 41 (18.0) | 42 (31.8) | 161 (29.3) | 26 (11.9) | 69 (21.6) |
| Dyslipidemia | 53 (22.1) | 35 (15.4) | 22 (16.7) | 180 (32.9) | 38 (17.4) | 76 (23.8) |
| Atrial fibrillation | 160 (66.4) | 76 (33.2) | 66 (49.6) | 80 (14.5) | 37 (16.8) | 222 (69.4) |
| Chronic dialysis | 7 (2.9) | 11 (4.8) | 12 (9.0) | 19 (3.5) | 7 (3.2) | 34 (10.6) |
| Chronic pulmonary disease | 14 (5.8) | 21 (9.3) | 16 (12.1) | 51 (9.3) | 13 (6.0) | 50 (15.7) |
| Previous myocardial infarction | 7 (2.9) | 8 (3.5) | 19 (14.3) | 39 (7.1) | 7 (3.2) | 27 (8.4) |
| Hemoglobin (g/dL) | 12.6 ± 2.2 | 12.3 ± 2.4 | 11.2 ± 2.1 | 11.8 ± 2.0 | 12.4 ± 2.3 | 11.2 ± 2.2 |
| Creatinine (mg/dL) | 1.1 ± 1.2 | 1.3 ± 1.7 | 1.7 ± 1.8 | 1.2 ± 1.1 | 1.2 ± 1.2 | 1.6 ± 1.6 |
| Creatinine clearance (mL/min) | 70.9 ± 23.4 | 70.2 ± 28.3 | 55.8 ± 33.0 | 66.7 ± 26.7 | 71.5 ± 27.1 | 55.2 ± 29.1 |
| NT-proBNP (pg/mL) | 3,790 ± 6,970 | 4,540 ± 9,668 | 10,763 ± 11,779 | 6,592 ± 10,766 | 7,714 ± 11,142 | 7,609 ± 13,123 |
| Echocardiographic characteristic | ||||||
| LVEDD (mm) | 48.6 ± 6.5 | 56.4 ± 7.7 | 61.9 ± 9.9 | 49.1 ± 7.3 | 61.5 ± 7.9 | 49.7 ± 9.2 |
| LVESD (mm) | 32.2 ± 6.3 | 36.8 ± 8.2 | 49.0 ± 11.9 | 32.5 ± 8.5 | 43.1 ± 8.5 | 35.3 ± 10.1 |
| LVEF (%) | 58.7 ± 8.9 | 63.9 ± 10.1 | 42.2 ± 16.9 | 62.1 ± 13.4 | 59.3 ± 10.9 | 55.7 ± 14.6 |
| IVS thickness (mm) | 9.2 ± 1.8 | 9.5 ± 1.9 | 9.3 ± 1.8 | 11.4 ± 2.2 | 10.3 ± 2.0 | 9.1 ± 1.7 |
| LV posterior wall thickness (mm) | 9.1 ± 1.7 | 9.5 ± 1.6 | 9.0 ± 1.6 | 11.0 ± 2.0 | 10.3 ± 1.9 | 9.1 ± 1.6 |
| LV mass index (g/m2) | 97.7 ± 28.8 | 128.7 ± 38.1 | 146.5 ± 42.6 | 131.4 ± 40.9 | 163.5 ± 46.4 | 102.3 ± 36.0 |
| TR Vmax (m/sec) | 2.9 ± 0.5 | 3.0 ± 0.7 | 3.3 ± 0.6 | 2.8 ± 0.5 | 2.6 ± 0.6 | 3.0 ± 0.8 |
| LA volume index (mL/m2) | 89.3 ± 44.2 | 87.5 ± 56.7 | 91.7 ± 52.9 | 54.3 ± 26.0 | 56.1 ± 30.2 | 82.9 ± 53.4 |
Values are presented as mean ± standard deviation or number (%)
MS mitral stenosis, MR mitral regurgitation, AS aortic stenosis, AR aortic regurgitation, TR tricuspid regurgitation, NYHA New York Heart Association, NT-proBNP N-terminal pro B-type natriuretic peptide, LVEDD left ventricular end-diastolic dimension, LVESD left ventricular end-systolic dimension, LVEF left ventricular ejection fraction, LV left ventricle, IVS interventricular septum, Vmax maximal velocity, LA left atrium
Fig. 1.
Etiology of each severe valvular heart disease in Korea. Pie charts showing the distribution of the etiology of (A) mitral stenosis, (B) primary mitral regurgitation, (C) secondary mitral regurgitation, (D) aortic stenosis, (E) aortic regurgitation, and (F) tricuspid regurgitation
Reporting rate of echocardiographic parameters recommended for diagnosis
Table 2 presents the reporting rates of echocardiographic parameters in patients with severe VHD. In cases of severe MS, the mitral valve area using two-dimensional (2D) planimetry was reported for 95.5% of patients (233 of 244), and the mean diastolic pressure gradient was reported for 98.8% of patients (241 of 244). The mitral valve area, as calculated using pressure half-time, was also reported in 218 patients (89.3%). This confirmed that most of the parameters used for diagnosing severe MS were faithfully reported. In cases of severe AS, calculation of aortic valve area using the continuity equation, as recommended by current guidelines, was reported for 96.7% of cases. Aortic valve (AV) peak velocity and transvalvular pressure gradients were also reported.
Table 2.
Reporting rate of echocardiographic parameters in patients with severe valvular heart disease
| Parameter | Severe MS (n = 244) |
Severe Primary MR (n = 229) |
Severe Secondary MR (n = 133) |
Severe AS (n = 551) |
Severe AR (n = 222) |
Severe TR (n = 320) |
|---|---|---|---|---|---|---|
| Valve area | ||||||
| By 2D planimetry | 233 (95.5) | - | - | 201 (36.5) | - | - |
| By PHT | 218 (89.3) | - | - | - | - | - |
| By continuity equation | - | - | - | 533 (96.7) | - | - |
| Transvalvular pressure gradient | 241 (98.8) | - | - | 545 (98.9) | - | - |
| AV peak velocity | - | - | - | 518 (94.0) | - | - |
| Velocity ratio | - | - | - | 247 (44.8) | - | - |
| Central large jet > 50% of LA area | - | 122 (53.3) | 101 (75.9) | - | - | - |
| Pulmonary vein systolic flow reversal | - | 68 (29.7) | 41 (30.8) | - | - | - |
| Vena contracta width | - | 26 (11.4) | 20 (15.0) | - | 101 (45.5) | 89 (27.8) |
| PISA radius at Nyquist 30–40 cm/sec | - | 190 (83.0) | 123 (92.5) | - | 37 (16.7) | 144 (45.0) |
| EROA | - | 149 (65.1) | 79 (59.4) | - | 39 (17.6) | 24 (7.5) |
| Regurgitant volume | - | 114 (49.8) | 53 (39.8) | - | 33 (14.9) | 12 (3.8) |
| Regurgitant fraction | - | 7 (3.1) | 7 (5.3) | - | 9 (4.1) | - |
| AR jet width to LVOT ratio (central jet) | - | - | - | - | 104 (46.8) | - |
| AR jet CSA/LVOT CSA (central jet) | - | - | - | - | 68 (30.6) | - |
| AR pressure half-time | - | - | - | - | 110 (49.5) | - |
| DTA diastolic flow reversal | - | - | - | - | 147 (66.2) | - |
| Central jet > 50% of RA | - | - | - | - | - | 226 (70.6) |
| TR jet area | - | - | - | - | - | 100 (31.3) |
| Systolic reversal of hepatic vein flow | - | - | - | - | - | 187 (58.4) |
| Tricuspid inflow E velocity (m/sec) | - | - | - | - | - | 21 (6.6) |
MS mitral stenosis, MR mitral regurgitation, AS aortic stenosis, AR aortic regurgitation, TR tricuspid regurgitation, D dimensional, PHT pressure half-time, PG pressure gradient, AV aortic valve, LA left atrium, PISA proximal isovelocity surface area, EROA effective regurgitant orifice area, LVOT left ventricular outflow tract, CSA cross-sectional area, DTA descending thoracic aorta, RA right atrium
However, the reporting rate for each parameters used to diagnose the regurgitation of each valve was relatively low, which can be interpreted as characteristic of valvular regurgitation, making integrated decisions about multiple parameters necessary. For a diagnosis of severe MS, proximal isovelocity surface area (PISA) radius, effective regurgitant orifice area (EROA), and regurgitant volume appeared to be used most often, and for the diagnosis of severe AR, the reporting rate of descending thoracic aorta diastolic flow reversal was high at 66.2%, and vena contracta width, AR jet width to left ventricular outflow tract ratio, and AR pressure half-time were reported in approximately half of the cases. In severe TR, the central jet > 50% of the right atrium was reported in 66.8% of cases. Systolic reversal of the hepatic vein flow was the next most reported at 56.3%, and the vena contracta width was reported in 89 patients, which was relatively low, at 26.8%. Although the reporting rate of PISA radius in cases of severe TR was 43.4%, cases of calculating EROA and regurgitant volume were remarkably low at 7.2% and 3.6%, respectively.
Additional imaging in diagnosis and treatment of severe VHD
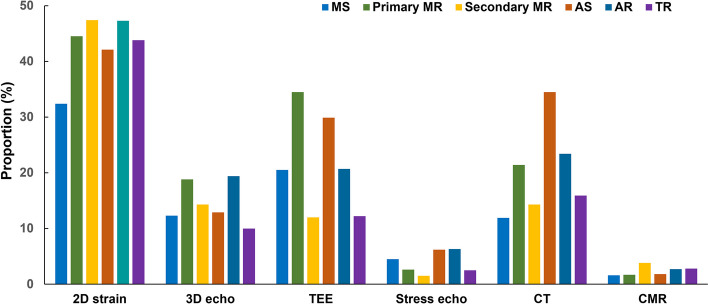
With respect to imaging modalities performed in addition to conventional transthoracic echocardiography, speckle tracking echocardiography was the most frequently performed advanced echocardiographic technique (43.3%), followed by transesophageal echocardiography at 30.0% and cardiac computed tomography at 17.8%. Figure 2 shows the proportion of additional imaging methods used for each disease. Transesophageal echocardiography was frequently used in cases of severe primary MR and severe AS, and cardiac computed tomography had a high utilization rate (34.5%) for severe AS.
Fig. 2.
Approaches based on the multimodal imaging for each valvular heart disease. MS, mitral stenosis; MR, mitral regurgitation; AS, aortic stenosis; AR, aortic regurgitation; TR, tricuspid regurgitation; D, dimensional; TEE, transesophageal echocardiography; CT, computed tomography; CMR, cardiac magnetic resonance
Treatment and outcomes of severe VHD
Of the 1,482 patients with severe VHD, intervention in the relevant valve was performed in 455 patients (30.7%). Surgical or transcatheter intervention was performed in 47 patients (19.3%) with severe MS, 73 (31.9%) with severe primary MR, 10 (7.5%) with severe secondary MR, 241 (43.7%) with severe AS, 61 (27.5%) with severe AR, and 23 (7.2%) with severe TR. The overall in-hospital mortality rate for patients with severe VHD was 5.3% (79 of 1,482 patients). Looking at each valve, in-hospital mortality was high at 9.0% and 7.5% for cases of secondary severe MR and severe TR, respectively, reflecting the poor prognosis of these valve diseases. In-hospital mortality occurred in 73 of the 1,244 patients (5.9%) who received conservative treatment and in 18 of the 455 patients (4.0%) who received the surgical or transcatheter intervention, which was significantly lower in the intervention group (P = 0.037).
Severe MS
Among 244 patients with severe MS, mitral valve replacement (MVR) was performed in 37 patients (15.2%) and percutaneous balloon mitral valvuloplasty (PMV) was performed in 10 patients (4.1%). In a comparison of treatment strategies, patients undergoing PMV were significantly younger than those in other groups (P = 0.005) (Table 3). The proportion of patients with New York Heart Association (NYHA) class II or greater symptoms and atrial fibrillation tended to be higher in the MVR and PMV groups compared with the conservative group, but no statistical significance was seen in differences among the three groups. Patients who underwent PMV tended to have a higher body mass index, lower creatinine levels, and higher creatinine clearance than did patients in other groups.
Table 3.
Demographic, clinical, and echocardiographic characteristics in patients with severe MS
| Characteristic | Severe MS | ||||
|---|---|---|---|---|---|
| Total (n = 244) | Conservative (n = 197) | MVR (n = 37) | PMV (n = 10) | P-valuea | |
| Clinical characteristic | |||||
| Age (yr) | 65.8 ± 11.1 | 66.6 ± 11.1 | 63.8 ± 9.8 | 55.4 ± 8.7 | 0.005* |
| Male sex | 78 (32.0) | 68 (34.5) | 8 (21.6) | 2 (20.0) | 0.229 |
| Body mass index (kg/m2) | 23.3 ± 3.5 | 23.2 ± 3.3 | 23.7 ± 4.2 | 25.6 ± 3.0 | 0.055 |
| NYHA class ≥ II | 145 (59.4) | 111 (56.3) | 27 (73.0) | 7 (70.0) | 0.140 |
| Hypertension | 90 (36.9) | 74 (37.6) | 15 (40.5) | 1 (10.0) | 0.195 |
| Diabetes | 43 (17.6) | 33 (16.8) | 10 (27.0) | 0 (0) | 0.124 |
| Dyslipidemia | 53 (21.7) | 44 (22.3) | 7 (18.9) | 2 (20.0) | 0.900 |
| Atrial fibrillation | 160 (65.6) | 126 (64.0) | 27 (73.0) | 7 (70.0) | 0.670 |
| Chronic dialysis | 7 (2.9) | 6 (3.1) | 1 (2.7) | 0 (0) | 0.999 |
| Chronic pulmonary disease | 14 (5.8) | 10 (5.2) | 3 (8.1) | 1 (10.0) | 0.613 |
| Previous MI | 7 (2.9) | 7 (3.6) | 0 (0) | 0 (0) | 0.763 |
| Hemoglobin (g/dL) | 12.6 ± 2.2 | 12.5 ± 2.3 | 13.0 ± 2.0 | 13.5 ± 1.8 | 0.310 |
| Creatinine (mg/dL) | 1.1 ± 1.2 | 1.1 ± 1.1 | 1.3 ± 1.6 | 0.8 ± 0.1 | 0.024* |
| Creatinine clearance (mL/min) | 70.9 ± 23.4 | 71.7 ± 24.8 | 63.8 ± 17.9 | 81.7 ± 12.3 | 0.032* |
| NT-proBNP (pg/mL) | 3,790 ± 6,970 | 3,523 ± 6,273 | 6,777 ± 11,661 | 1,277 ± 1,379 | 0.468 |
| Echocardiographic characteristic | |||||
| LVEDD (mm) | 48.6 ± 6.5 | 49.0 ± 6.6 | 47.3 ± 6.8 | 46.4 ± 3.1 | 0.216 |
| LVESD (mm) | 32.2 ± 6.3 | 32.4 ± 6.5 | 31.2 ± 6.1 | 32.0 ± 4.3 | 0.692 |
| LVEF (%) | 58.7 ± 8.9 | 59.1 ± 8.7 | 57.7 ± 10.6 | 55.1 ± 5.6 | 0.121 |
| TR Vmax (m/sec) | 2.9 ± 0.5 | 2.9 ± 0.5 | 3.1 ± 0.6 | 3.2 ± 0.5 | 0.104 |
| LA volume index (mL/m2) | 89.3 ± 44.2 | 87.7 ± 41.3 | 101.4 ± 57.8 | 71.0 ± 29.1 | 0.414 |
| MS etiology | |||||
| Rheumatic | 181 (74.2) | 140 (71.1) | 33 (89.2) | 8 (80.0) | 0.485 |
| Degenerative | 51 (20.9) | 45 (22.8) | 4 (10.8) | 2 (20.0) | |
| MVA by 2D planimetry (cm2) | 1.16 ± 0.24 | 1.18 ± 0.24 | 1.02 ± 0.19 | 1.11 ± 0.28 | < 0.001* |
| Very severe MS | 62 (25.4) | 41 (20.8) | 16 (43.2) | 5 (50.0) | 0.003* |
| MVA by PHT (cm2) | 1.23 ± 0.26 | 1.26 ± 0.26 | 1.09 ± 0.25 | 1.07 ± 0.19 | < 0.001* |
| Mean PG (mmHg) | 8.0 ± 3.7 | 7.6 ± 3.5 | 9.3 ± 3.3 | 12.6 ± 4.7 | < 0.001* |
| Treatment strategy | |||||
| Mechanical MVR | 25 (10.2) | 0 (0) | 25 (67.6) | 0 (0) | - |
| Bioprosthetic MVR | 12 (4.9) | 0 (0) | 12 (32.4) | 0 (0) | - |
| PMV | 10 (4.1) | 0 (0) | 0 (0) | 10 (100) | - |
| In-hospital mortality | 5 (2.0) | 3 (1.6) | 2 (5.4) | 0 (0) | - |
| Cardiac | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Noncardiac | 4 (1.6) | 2 (1.0) | 2 (5.4) | 0 (0) | |
| Unknown | 1 (0.4) | 1 (0.5) | 0 (0) | 0 (0) | |
Values are presented as mean ± standard deviation or number (%)
MS mitral stenosis, MVR mitral valve replacement, PMV percutaneous mitral valvuloplasty, NYHA New York Heart Association, MI myocardial infarction, NT-proBNP N-terminal pro B-type natriuretic peptide, LVEDD left ventricular end-diastolic dimension, LVESD left ventricular end-systolic dimension, LVEF left ventricular ejection fraction, TR tricuspid regurgitation, Vmax maximal velocity, LA left atrium, MVA mitral valve area, D dimensional, PHT pressure half-time, PG pressure gradient
aKruskal-Wallis rank test, Fisher exact test
*P < 0.05
In terms of echocardiographic characteristics, the transvalvular mean diastolic pressure gradient was significantly higher in the PMV group (12.6 ± 4.7 mmHg, P < 0.001) than in the conservative group (7.6 ± 3.5 mmHg) and MVR group (9.3 ± 3.3 mmHg). The mitral valve area as measured by 2D planimetry was smaller in patients who underwent MVR. However, the proportion of cases of very severe MS was greater in patients who underwent PMV. This result can be explained by the fact that PMV is performed in younger patients with an anatomically suitable condition and high transvalvular pressure gradient or symptomatic patients.
Among patients who required MVR, 25 (67.6%) underwent mechanical valve surgery, and 12 (32.4%) underwent bioprosthetic valve replacement. In-hospital mortality occurred in five patients (2.0%), three patients (1.6%) in the conservative group, and two patients (5.4%) in the MVR group. It did not occur in the PMV group, and no cases of cardiac mortality were reported among patients with severe MS.
Severe primary MR
Among 229 patients with severe primary MR, 73 (31.9%) underwent mitral valve intervention. Mitra l valve surgical repair was performed in 38 patients (16.6%), whereas surgical MVR was performed in 34 (14.8%). Primary MR patients who underwent mitral intervention were significantly younger, tended to be more symptomatic, and had fewer comorbidities compared with those who underwent conservative treatment (Table 4). In the intervention group, left ventricular end-diastolic dimension was significantly more significant than the conservative group, but there were no differences in left ventricular ejection fraction (LVEF), left atria volume index, and TR maximal velocity (Vmax). The most common etiology of severe primary MR was mitral valve prolapse (53.5%), and there was no significant difference between the two groups. Regarding MR quantification parameters, the PISA radius and PISA radius-driven EROA values were greater in the intervention group than in the conservative group. The in-hospital mortality rate was high in the conservative group, with nine patients (5.8%) in the conservative group and one patient (1.4%) in the intervention group, but not to a statistically significant level (P = 0.180).
Table 4.
Demographic, clinical, and echocardiographic characteristics in patients with severe MR
| Characteristic | Severe primary MR | Severe secondary MR | ||||||
|---|---|---|---|---|---|---|---|---|
| Total (n = 229) |
Conservative (n = 156) |
Intervention (n = 73) |
P-value | Total (n = 133) |
Conservative (n = 123) |
Intervention (n = 10) |
P-valuea | |
| Clinical characteristic | ||||||||
| Age (yr) | 65.0 ± 15.7 | 67.7 ± 15.0 | 59.4 ± 15.9 | < 0.001* | 71.6 ± 13.4 | 71.7 ± 13.6 | 69.9 ± 10.1 | 0.324 |
| Male sex | 108 (47.2) | 72 (46.1) | 36 (49.3) | 0.655 | 69 (51.9) | 59 (48.0) | 5 (50.0) | 0.999 |
| Body mass index (kg/m2) | 23.5 ± 3.9 | 23.4 ± 4.0 | 23.8 ± 3.9 | 0.407 | 22.9 ± 3.4 | 22.7 ± 3.4 | 24.3 ± 3.5 | 0.127 |
| NYHA class ≥ II | 149 (65.1) | 95 (60.9) | 54 (74.0) | 0.060 | 115 (86.5) | 106 (86.2) | 9 (90.0) | 0.999 |
| Hypertension | 107 (46.7) | 85 (54.5) | 22 (30.1) | < 0.001* | 72 (54.1) | 65 (52.8) | 7 (70.0) | 0.342 |
| Diabetes | 41 (17.9) | 32 (20.5) | 9 (12.3) | 0.127 | 42 (31.8) | 41 (33.3) | 1 (10.0) | 0.164 |
| Dyslipidemia | 35 (15.2) | 25 (16.0) | 10 (13.7) | 0.621 | 22 (16.7) | 21 (17.1) | 1 (10.0) | 0.999 |
| Atrial fibrillation | 76 (33.2) | 59 (37.8) | 17 (23.3) | 0.030* | 66 (49.6) | 61 (49.6) | 5 (50.0) | 0.999 |
| Chronic dialysis | 11 (4.8) | 8 (5.2) | 3 (4.1) | 0.999 | 12 (9.0) | 12 (9.8) | 0 (0) | - |
| Chronic pulmonary disease | 21 (9.3) | 19 (12.3) | 2 (2.8) | 0.025* | 16 (12.1) | 14 (11.4) | 2 (20.0) | 0.344 |
| Previous MI | 8 (3.5) | 4 (2.6) | 4 (5.5) | 0.276 | 19 (14.3) | 17 (13.8) | 2 (20.0) | 0.635 |
| Hemoglobin (g/dL) | 12.3 ± 2.4 | 12.2 ± 2.2 | 12.6 ± 2.6 | 0.441 | 11.2 ± 2.1 | 11.1 ± 2.1 | 11.9 ± 2.2 | 0.260 |
| Creatinine (mg/dL) | 1.3 ± 1.7 | 1.4 ± 1.9 | 1.2 ± 1.2 | 0.636 | 1.7 ± 1.8 | 1.8 ± 1.8 | 1.0 ± 0.3 | 0.040* |
| Creatinine clearance (mL/min) | 70.2 ± 28.3 | 66.0 ± 27.8 | 77.2 ± 28.1 | 0.013* | 55.8 ± 33.0 | 54.0 ± 33.5 | 73.2 ± 21.7 | 0.080 |
| NT-proBNP (pg/mL) | 4,540 ± 9,668 | 5,666 ± 11,243 | 1,912 ± 2,873 | 0.018* | 10,763 ± 11,780 | 10,975 ± 11,964 | 5,813 ± 4,275 | 0.868 |
| Echocardiographic characteristic | ||||||||
| LVEDD (mm) | 56.4 ± 7.7 | 55.4 ± 8.0 | 58.3 ± 6.8 | 0.004* | 61.9 ± 9.9 | 62.1 ± 10.2 | 58.2 ± 5.7 | 0.225 |
| LVESD (mm) | 36.8 ± 8.2 | 36.4 ± 8.7 | 37.6 ± 7.0 | 0.062 | 49.0 ± 11.9 | 49.4 ± 12.0 | 43.2 ± 8.2 | 0.111 |
| ≥ 40 | 53 (23.2) | 33 (21.3) | 20 (27.4) | 0.308 | - | - | - | - |
| > 70 | - | - | - | - | 10 (7.5) | 10 (8.1) | 0 (0) | - |
| LVEF (%) | 63.9 ± 10.1 | 63.5 ± 9.7 | 64.9 ± 11.4 | 0.174 | 42.2 ± 16.9 | 38.2 ± 15.1 | 49.5 ± 15.3 | 0.027* |
| ≤ 60 | 84 (35.6) | 61 (37.0) | 23 (32.4) | 0.600 | 119 (89.5) | 113 (91.9) | 6 (60.0) | 0.011* |
| LV mass index (g/m2) | 128.7 ± 38.1 | 128.8 ± 37.5 | 128.4 ± 39.5 | 0.929 | 146.5 ± 42.6 | 148.0 ± 42.4 | 127.8 ± 42.2 | 0.002* |
| TR Vmax (m/sec) | 3.0 ± 0.7 | 2.9 ± 0.6 | 3.1 ± 0.7 | 0.147 | 3.3 ± 0.6 | 3.3 ± 0.6 | 3.3 ± 0.6 | 0.863 |
| LA volume index (mL/m2) | 87.5 ± 56.7 | 88.8 ± 61.5 | 84.5 ± 44.6 | 0.552 | 91.7 ± 52.9 | 93.3 ± 54.4 | 77.0 ± 36.2 | 0.383 |
| MR etiology | 0.170 | 0.007* | ||||||
| Degenerative | 55 (24.0) | 41 (6.3) | 14 (19.4) | 0 (0) | 0 (0) | 0 (0) | ||
| Rheumatic | 20 (8.7) | 15 (9.6) | 5 (6.9) | 0 (0) | 0 (0) | 0 (0) | ||
| Mitral valve prolapse | 122 (53.2) | 81 (51.9) | 41 (56.9) | 0 (0) | 0 (0) | 0 (0) | ||
| Endocarditis | 17 (7.4) | 8 (5.1) | 9 (12.5) | 0 (0) | 0 (0) | 0 (0) | ||
| Ischemic | 0 (0) | 0 (0) | 0 (0) | 35 (26.3) | 32 (26.0) | 3 (30.0) | ||
| Nonischemic | 0 (0) | 0 (0) | 0 (0) | 85 (63.9) | 82 (66.7) | 3 (30.0) | ||
| Other | 15 (6.5) | 12 (7.7) | 3 (4.2) | 13 (9.8) | 9 (7.3) | 4 (40.0) | ||
| Central large jet > 50% of LA area | 87 (71.3) | 54 (67.5) | 33 (78.6) | 0.199 | 84 (83.2) | 77 (82.8) | 7 (87.5) | 0.744 |
| PV systolic flow reversal | 36 (52.9) | 25 (59.5) | 11 (42.3) | 0.167 | 23 (56.1) | 19 (52.8) | 4 (80.0) | 0.070 |
| Vena contracta width (cm) | 0.69 ± 0.15 | 0.71 ± 0.16 | 0.66 ± 0.14 | 0.428 | 0.72 ± 0.11 | 0.73 ± 0.11 | 0.68 ± 0.11 | 0.393 |
| PISA radius at Nyquist 30–40 cm/sec | 1.05 ± 0.26 | 1.00 ± 0.23 | 1.15 ± 0.28 | < 0.001* | 0.91 ± 0.19 | 0.91 ± 0.19 | 1.01 ± 0.21 | 0.087 |
| EROA (cm2) | 0.54 ± 0.25 | 0.49 ± 0.20 | 0.66 ± 0.31 | 0.003* | 0.43 ± 0.15 | 0.42 ± 0.15 | 0.50 ± 0.16 | 0.488 |
| Regurgitant volume (mL/beat) | 71.5 ± 24.3 | 68.7 ± 23.3 | 77.4 ± 25.6 | 0.085 | 64.1 ± 42.3 | 62.2 ± 42.2 | 87.4 ± 41.6 | 0.117 |
| Regurgitant fraction (%) | 56.5 ± 13.8 | 55.1 ± 16.6 | 60.1 ± 2.7 | 0.845 | 61.9 ± 0.0 | 59.1 ± 24.3 | 79.0 ± 0.0 | 0.574 |
| Treatment strategy | ||||||||
| Mechanical MVR | 17 (7.4) | 0 (0) | 17 (23.3) | - | 3 (2.2) | 0 (0) | 3 (27.3) | - |
| Bioprosthetic MVR | 17 (7.4) | 0 (0) | 17 (23.3) | - | 1 (0.7) | 0 (0) | 1 (9.1) | - |
| MV surgical repair | 38 (16.6) | 0 (0) | 38 (52.1) | - | 5 (3.8) | 0 (0) | 5 (45.4) | - |
| Other | 0 (0) | 0 (0) | 0 (0) | - | 1 (0.7) | 0 (0) | 1 (9.1) | - |
| In-hospital mortality | 10 (4.4) | 9 (5.8) | 1 (1.4) | 0.180 | 12 (9.0) | 11 (9.0) | 1 (9.1) | 0.999 |
| Cardiac | 3 (1.3) | 3 (1.9) | 0 (0) | 8 (6.0) | 8 (6.5) | 0 (0) | ||
| Noncardiac | 6 (2.6) | 6 (3.8) | 0 (0) | 4 (3.0) | 3 (2.5) | 1 (9.1) | ||
| Unknown | 1 (0.4) | 0 (0) | 1 (1.4) | 0 (0) | 0 (0) | 0 (0) | ||
Values are presented as mean ± standard deviation or number (%)
MR mitral regurgitation, NYHA New York Heart Association, MI myocardial infarction, NT-proBNP N-terminal pro B-type natriuretic peptide, LVEDD left ventricular end-diastolic dimension, LVESD left ventricular end-systolic dimension, LVEF left ventricular ejection fraction, LV left ventricle, TR tricuspid regurgitation, Vmax maximal velocity, LA left atrium, PV pulmonary vein, PISA proximal isovelocity surface area, EROA effective regurgitant orifice area; MVR, mitral valve replacement; MV, mitral valve
aWilcoxon rank sum test, Fisher exact test
*P < 0.05
Severe secondary MR
Among 133 patients with severe secondary MR, 10 (7.5%) underwent mitral valve intervention. Mitral valve surgical repair was performed in five patients (50.0%), surgical MVR was performed in four patients (40.0%), and one patient (10.0%) underwent transcatheter intervention. Secondary MR patients undergoing mitral intervention did not differ in demographic and clinical profiles, except for a lower serum creatinine level (1.8 ± 1.8 mg/dL vs. 1.0 ± 0.3 mg/dL, P = 0.040) compared with patients without intervention (Table 4). LVEF was higher in the intervention group (38.2% ± 15.1% vs. 49.5% ± 15.3%, P = 0.027). The most common etiology of severe secondary MR was nonischemic origin (63.9%), but the proportion of patients with nonischemic origin in the intervention group was only 30%. In-hospital mortality during the observation period occurred in 12 patients (9.0%), which was 11 patients (9.0%) in the conservative group and one patient (9.1%) in the mitral intervention group (P = 0.999).
Severe AS
Among 551 patients with severe AS, 241 (43.7%) underwent AV intervention. Surgical AV replacement (SAVR) was performed in 146 patients (26.5%), whereas transcatheter AV replacement (TAVR) was performed in 95 (17.2%). When comparing the three groups, patients with SAVR tended to be younger than those who received conservative care or TAVR (P = 0.073) (Table 5). The prevalence of patients with NYHA class II symptoms or more significant dyspnea was highest in the TAVR group, followed by the SAVR and conservative groups (P < 0.001).
Table 5.
Demographic, clinical, and echocardiographic characteristics of patients with severe AS
| Characteristic | Severe AS | ||||
|---|---|---|---|---|---|
| Total (n = 551) | Conservative (n = 310) | SAVR (n = 146) | TAVR (n = 95) | P-value | |
| Clinical characteristic | |||||
| Age (yr) | 76.9 ± 10.2 | 79.2 ± 9.9 | 69.4 ± 9.2 | 80.9 ± 5.5 | 0.073 |
| Male sex | 284 (51.5) | 166 (53.5) | 66 (45.2) | 52 (54.7) | 0.723 |
| Body mass index (kg/m2) | 23.6 ± 3.6 | 23.3 ± 3.9 | 24.0 ± 3.1 | 23.8 ± 3.4 | 0.144 |
| NYHA class ≥ II | 384 (69.7) | 188 (60.6) | 112 (76.7) | 84 (88.4) | < 0.001* |
| Hypertension | 361 (65.5) | 204 (66.0) | 84 (57.5) | 73 (76.8) | 0.374 |
| Diabetes | 161 (29.2) | 83 (26.8) | 44 (30.1) | 34 (35.8) | 0.315 |
| Dyslipidemia | 180 (32.7) | 95 (30.6) | 47 (32.2) | 38 (40.0) | 0.198 |
| Atrial fibrillation | 80 (14.5) | 47 (15.2) | 20 (13.7) | 13 (13.7) | 0.650 |
| Chronic dialysis | 19 (3.5) | 9 (2.9) | 4 (2.7) | 6 (6.3) | 0.459 |
| Chronic pulmonary disease | 51 (9.3) | 29 (9.4) | 15 (10.3) | 7 (7.4) | 0.070 |
| Previous MI | 39 (7.1) | 27 (8.7) | 8 (5.5) | 4 (4.2) | 0.518 |
| Hemoglobin (g/dL) | 11.8 ± 2.0 | 11.6 ± 2.1 | 12.5 ± 1.9 | 11.3 ± 1.7 | 0.864 |
| Creatinine (mg/dL) | 1.2 ± 1.1 | 1.2 ± 1.1 | 1.2 ± 1.1 | 1.3 ± 1.3 | 0.729 |
| Creatinine clearance (mL/min) | 66.7 ± 26.7 | 65.9 ± 29.3 | 72.6 ± 22.6 | 61.8 ± 23.1 | 0.709 |
| NT-proBNP (pg/mL) | 6,592 ± 10,766 | 7,537 ± 11,281 | 4,404 ± 7,503 | 6,023 ± 8,687 | 0.185 |
| Echocardiographic characteristic | |||||
| LVEDD (mm) | 49.1 ± 7.3 | 48.1 ± 7.0 | 50.9 ± 7.5 | 48.8 ± 7.2 | 0.061 |
| LVESD (mm) | 32.5 ± 8.5 | 31.8 ± 8.3 | 33.9 ± 8.6 | 31.9 ± 8.6 | 0.396 |
| LVEF (%) | 58.3 ± 12.7 | 58.5 ± 12.7 | 57.8 ± 13.2 | 58.4 ± 12.3 | 0.804 |
| ≤ 50 | 113 (20.5) | 59 (19.0) | 35 (24.0) | 19 (20.2) | 0.474 |
| LV mass index (g/m2) | 131.4 ± 40.9 | 127.3 ± 40.5 | 137.2 ± 40.1 | 135.3 ± 42.1 | 0.008* |
| TR Vmax (m/sec) | 2.8 ± 0.5 | 2.8 ± 0.5 | 2.7 ± 0.5 | 2.8 ± 0.5 | 0.513 |
| LA volume index (mL/m2) | 54.3 ± 26.0 | 53.5 ± 23.7 | 54.7 ± 30.7 | 56.5 ± 25.9 | 0.380 |
| AS etiology | < 0.001* | ||||
| Degenerative | 427 (77.5) | 245 (83.6) | 88 (60.7) | 94 (98.9) | |
| Rheumatic | 35 (6.4) | 23 (7.8) | 12 (8.3) | 0 (0) | |
| Congenital | 64 (11.6) | 21 (7.2) | 42 (29.0) | 1 (1.1) | |
| AV peak velocity (m/sec) | 4.6 ± 0.8 | 4.4 ± 0.9 | 4.8 ± 0.7 | 4.7 ± 0.8 | < 0.001* |
| AV mean PG (mmHg) | 50.9 ± 19.1 | 48.5 ± 19.9 | 54.2 ± 16.5 | 54.8 ± 18.5 | < 0.001* |
| AV area by 2D planimetry (cm2) | 0.79 ± 0.23 | 0.80 ± 0.21 | 0.84 ± 0.30 | 0.72 ± 0.22 | 0.107 |
| AV area by continuity equation (cm2) | 0.73 ± 0.22 | 0.77 ± 0.23 | 0.70 ± 0.19 | 0.68 ± 0.20 | < 0.001* |
| Velocity ratio | 0.23 ± 0.09 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.176 |
| Treatment strategy | - | ||||
| Mechanical AVR | 52 (9.4) | 0 (0) | 52 (35.6) | 0 (0) | |
| Bioprosthetic AVR | 94 (17.1) | 0 (0) | 94 (64.4) | 0 (0) | |
| TAVR | 95 (17.2) | 0 (0) | 0 (0) | 95 (100) | |
| In-hospital mortality | 32 (5.8) | 25 (8.1) | 6 (4.1) | 1 (1.1) | < 0.001* |
| Cardiac | 9 (1.6) | 9 (2.9) | 0 (0) | 0 (0) | |
| Noncardiac | 21 (3.8) | 16 (5.2) | 4 (2.7) | 1 (1.1) | |
| Unknown | 2 (0.4) | 0 (0) | 2 (1.4) | 0 (0) | |
Values are presented as mean ± standard deviation or number (%)
AS aortic stenosis, SAVR surgical aortic valve replacement, TAVR transcutaneous aortic valve replacement, NYHA New York Heart Association, MI myocardial infarction, NT-proBNP N-terminal pro B-type natriuretic peptide, LVEDD left ventricular end-diastolic dimension, LVESD left ventricular end-systolic dimension, LVEF left ventricular ejection fraction, LV left ventricle, TR tricuspid regurgitation, Vmax maximal velocity, LA left atrium, AV aortic valve, PG pressure gradient, D dimensional, AVR aortic valve replacement
*P < 0.05
The most common etiology of severe AS was degenerative valve disease (77.5%), and the second most common etiology was congenital heart disease, including bicuspid AV (11.6%). Compared with the conservative group, the LV mass index, AV peak velocity, and mean pressure gradient were higher, and the AV area estimated by the continuity equation was smaller in the SAVR or TAVR group. In the SAVR group, 52 patients (35.6%) underwent SAVR with a mechanical valve, and 94 (64.4%) underwent bioprosthetic atrial valve replacement. In-hospital mortality in the conservative group (8.1%) was significantly higher than those in the SAVR (4.1%) and TAVR groups (1.1%, P < 0.001).
Severe AR
Among 222 patients with severe AR, 61 (27.5%) underwent AV intervention. SAVR was performed in 55 patients, surgical AV repair was performed in three patients (4.9%), and TAVR was performed in three patients (4.9%) combined with significant AS. Demographics were similar between the conservative treatment and intervention groups, while more patients who underwent an AV intervention presented with NYHA functional class II or higher symptoms (Table 6). Patients in the intervention group had greater LV chamber size in both LV end-diastolic dimension (60.1 ± 7.4 vs. 65.4 ± 8.0, P < 0.001) and LV end-systolic volume (41.8 ± 7.9 vs. 46.7 ± 9.2, P < 0.001) compared with the conservative group. The intervention group had a statistically significant lower LVEF (55.8% ± 10.9% vs. 51.6% ± 12.5%, P = 0.014). As a result, the proportion of patients with LV end-systolic volume > 50 mm or LVEF ≤ 55% was 57.4% in the intervention group, greater than the 36.0% in the conservative group. LV mass index and TR Vmax were also higher in the intervention group compared with the conservative group. The most common etiology of severe AR was degenerative in both groups (55.9% vs. 36.1%). However, the intervention group had a relatively low rate of degenerative, rheumatic, or congenital etiologies, and a high rate of aorta pathology and endocarditis. Regarding AR measurement variables, AR pressure half-times tended to be shorter in the intervention group, and there were no differences in other variables. In-hospital mortality was not statistically different between groups but tended to be higher in the intervention group (1.9% vs. 8.2%, P = 0.063).
Table 6.
Demographic, clinical, and echocardiographic characteristics of patients with severe AR
| Characteristic | Severe AR | |||
|---|---|---|---|---|
| Total (n = 222) | Conservative (n = 161) | Intervention (n = 61) | P-value | |
| Clinical characteristic | ||||
| Age (yr) | 65.8 ± 14.1 | 65.8 ± 14.7 | 65.3 ± 12.9 | 0.812 |
| Male sex | 138 (62.7) | 61 (37.9) | 21 (34.4) | 0.748 |
| Body mass index (kg/m2) | 23.2 ± 3.7 | 23.0 ± 3.7 | 23.8 ± 3.4 | 0.170 |
| NYHA class ≥ 2 | 129 (58.1) | 83 (51.5) | 47 (77.0) | 0.001* |
| Hypertension | 135 (60.8) | 99 (61.5) | 37 (60.7) | 0.815 |
| Diabetes | 26 (11.7) | 18 (11.2) | 8 (13.1) | 0.768 |
| Dyslipidemia | 38 (17.1) | 33 (20.5) | 5 (8.2) | 0.059 |
| Atrial fibrillation | 37 (16.7) | 28 (17.4) | 10 (16.4) | 0.999 |
| Chronic dialysis | 7 (3.2) | 4 (2.5) | 3 (4.9) | 0.450 |
| Chronic pulmonary disease | 13 (5.9) | 9 (5.6) | 4 (6.6) | 0.661 |
| Previous MI | 7 (3.2) | 5 (3.1) | 2 (3.3) | 0.681 |
| Hemoglobin (g/dL) | 12.4 ± 2.3 | 12.5 ± 2.3 | 12.3 ± 2.2 | 0.649 |
| Creatinine (mg/dL) | 1.2 ± 1.2 | 1.2 ± 1.2 | 1.2 ± 1.0 | 0.966 |
| Creatinine clearance (mL/min) | 71.5 ± 27.1 | 71.3 ± 28.9 | 73.1 ± 22.9 | 0.709 |
| NT-proBNP (pg/mL) | 7,714.0 ± 11,142.0 | 8,993.9 ± 12,357.4 | 4,361.4 ± 6,076.6 | 0.033* |
| Echocardiographic characteristic | ||||
| LVEDD (mm) | 61.6 ± 7.9 | 60.1 ± 7.4 | 65.4 ± 8.0 | < 0.001* |
| LVESD (mm) | 43.2 ± 8.5 | 41.8 ± 7.9 | 46.7 ± 9.2 | < 0.001* |
| > 50 | 40 (18.0) | 21 (13.0) | 19 (31.1) | 0.003* |
| LVEF (%) | 54.7 ± 11.5 | 55.8 ± 10.9 | 51.6 ± 12.5 | 0.014* |
| ≤ 55 | 88 (39.6) | 55 (34.2) | 33 (54.1) | 0.011* |
| LVESD > 50 mm or LVEF ≤ 55% | 93 (41.9) | 58 (36.0) | 35 (57.4) | 0.006* |
| LV mass index (g/m2) | 163.5 ± 46.4 | 158.0 ± 47.0 | 178.2 ± 41.8 | 0.004* |
| TR Vmax (m/sec) | 2.6 ± 0.6 | 2.5 ± 0.5 | 2.8 ± 0.7 | 0.008* |
| LA volume index (mL/m2) | 56.1 ± 30.2 | 52.5 ± 25.8 | 67.1 ± 39.1 | 0.030* |
| AR etiology | < 0.001* | |||
| Degenerative | 112 (50.4) | 90 (55.9) | 22 (36.1) | |
| Rheumatic | 12 (5.4) | 10 (6.2) | 2 (3.3) | |
| Congenital | 35 (15.8) | 28 (17.4) | 7 (11.5) | |
| Aorta pathology | 26 (11.7) | 14 (8.7) | 12 (19.7) | |
| Endocarditis | 10 (4.5) | 3 (1.9) | 7 (11.5) | |
| Other | 25 (11.3) | 14 (8.7) | 11 (18.0) | |
| AR jet width to LVOT ratio (central jet) | 0.827 | |||
| Mild (< 25) | 2 (1.5) | 1 (1.1) | 1 (2.4) | |
| Moderate (25–64) | 47 (34.6) | 33 (34.7) | 14 (34.1) | |
| Severe (≥ 65) | 87 (64.0) | 61 (64.2) | 26 (63.4) | |
| AR jet CSA/LVOT CSA (central jet) | 0.502 | |||
| Mild (5–20) | 6 (6.6) | 5 (7.2) | 1 (4.5) | |
| Moderate (21–59) | 24 (26.4) | 20 (29.0) | 4 (18.2) | |
| Severe (≥ 60) | 61 (67.0) | 44 (63.8) | 17 (77.3) | |
| AR PHT (msec) | 366.9 ± 132.2 | 380.6 ± 122.9 | 324.8 ± 152.2 | 0.056 |
| AR vena contracta width (cm) | 0.64 ± 0.16 | 0.64 ± 0.15 | 0.65 ± 0.19 | 0.673 |
| Regurgitant volume (mL/beat) | 65.8 ± 27.2 | 66.2 ± 30.0 | 64.7 ± 17.5 | 0.897 |
| Regurgitant fraction (%) | 47.3 ± 12.0 | 44.7 ± 12.2 | 56.5 ± 6.4 | 0.244 |
| EROA (cm2) | 0.36 ± 0.15 | 0.35 ± 0.16 | 0.41 ± 0.14 | 0.393 |
| DTA diastolic flow reversal | 140 (95.2) | 95 (94.1) | 45 (97.8) | 0.564 |
| Treatment strategy | - | |||
| Mechanical AVR | 27 (12.2) | 0 (0) | 27 (44.3) | |
| Bioprosthetic AVR | 28 (12.6) | 0 (0) | 28 (45.9) | |
| AV surgical repair | 3 (1.3) | 0 (0) | 3 (4.9) | |
| TAVR | 3 (1.3)a | 0 (0) | 3 (4.9) | |
| In-hospital mortality | 8 (3.6) | 3 (1.9) | 5 (8.2) | 0.063 |
| Cardiac | 2 (0.9) | 2 (1.2) | 0 (0) | |
| Noncardiac | 3 (1.4) | 1 (0.6) | 2 (3.2) | |
| Unknown | 3 (1.4) | 0 (0) | 3 (4.9) | |
Values are presented as mean ± standard deviation or number (%)
AR aortic regurgitation, NYHA New York Heart Association, MI myocardial infarction, NT-proBNP N-terminal pro B-type natriuretic peptide, LVEDD left ventricular end-diastolic dimension, LVESD left ventricular end-systolic dimension, LVEF left ventricular ejection fraction, LV left ventricle, TR tricuspid regurgitation, Vmax maximal velocity, LA left atrium, LVOT left ventricular outflow tract, CSA cross-sectional area, PHT pressure half-time, EROA effective regurgitant orifice area, DTA descending thoracic aorta, AVR aortic valve replacement, TAVR transcutaneous aortic valve replacement
aAR cases combined with aortic stenosis underwent TAVR
*P < 0.05
Severe TR
Among 320 patients with severe TR, 23 (7.2%) underwent tricuspid valve (TV) intervention. Surgical TV replacement was performed in six patients, and 17 underwent tricuspid annuloplasty or valvuloplasty. Patients who underwent TV surgery were significantly younger and had fewer comorbidities with preserved renal function compared with patients who received conservative care (Table 7). The TR vena contracta width in patients who underwent TV surgery was significantly larger than in the conservative group (0.98 ± 0.22 cm vs. 0.82 ± 0.29 cm, P = 0.036). Additionally, the intervention group exhibited a higher incidence of hepatic vein systolic reversal and TR jets > 50% of the right atrium area. In-hospital mortality occurred in 22 patients (7.4%) in the conservative group and two patients (8.7%) in the intervention group. This result was not statistically significant (P = 0.827).
Table 7.
Demographic, clinical, and echocardiographic characteristics of patients with severe TR
| Characteristic | Severe TR | |||
|---|---|---|---|---|
| Total (n = 320) | Conservative (n = 297) | Intervention (n = 23) | P-valuea | |
| Clinical characteristic | ||||
| Age (yr) | 72.3 ± 12.9 | 72.9 ± 13.0 | 65.1 ± 7.5 | < 0.001* |
| Male sex | 143 (44.7) | 137 (46.1) | 6 (26.1) | 0.059 |
| Body mass index (kg/m2) | 23.4 ± 4.1 | 23.4 ± 4.2 | 23.9 ± 3.5 | 0.310 |
| NYHA class ≥ II | 235 (73.4) | 217 (73.1) | 18 (78.3) | 0.556 |
| Hypertension | 182 (56.9) | 176 (59.3) | 6 (26.1) | 0.002* |
| Diabetes | 69 (21.6) | 63 (21.2) | 6 (26.1) | 0.627 |
| Dyslipidemia | 75 (23.4) | 66 (22.2) | 9 (39.1) | 0.069 |
| Atrial fibrillation | 222 (69.4) | 205 (69.0) | 17 (73.9) | 0.642 |
| Chronic dialysis | 33 (10.3) | 32 (10.8) | 1 (4.3) | 0.329 |
| Chronic pulmonary disease | 50 (15.6) | 47 (15.8) | 3 (13.0) | 0.822 |
| Previous MI | 27 (8.4) | 26 (8.8) | 1 (4.3) | 0.464 |
| Hemoglobin (g/dL) | 11.2 ± 2.2 | 11.1 ± 2.2 | 11.8 ± 2.2 | 0.160 |
| Creatinine (mg/dL) | 1.6 ± 1.6 | 1.6 ± 1.6 | 1.0 ± 0.3 | 0.084 |
| Creatinine clearance (mL/min) | 55.2 ± 29.1 | 54.5 ± 29.7 | 66.0 ± 17.3 | 0.038* |
| NT-proBNP (pg/mL) | 7,609 ± 13,123 | 7,280.5 ± 12,165.4 | 5,422.5 ± 9,996.4 | 0.494 |
| Echocardiographic characteristic | ||||
| LVEDD (mm) | 49.6 ± 9.2 | 49.7 ± 9.3 | 48.7 ± 7.9 | 0.633 |
| LVESD (mm) | 35.2 ± 10.1 | 35.3 ± 10.2 | 34.3 ± 8.3 | 0.806 |
| LVEF (%) | 53.1 ± 14.5 | 52.9 ± 14.6 | 55.8 ± 13.0 | 0.310 |
| LV mass index (g/m2) | 102.1 ± 36.0 | 102.8 ± 36.4 | 93.7 ± 29.4 | 0.210 |
| TR Vmax (m/sec) | 3.0 ± 0.8 | 3.1 ± 0.8 | 2.8 ± 0.5 | 0.207 |
| TAPSE (mm) | 1.7 ± 0.5 | 1.6 ± 0.5 | 1.8 ± 0.6 | 0.654 |
| TV S’ (cm/sec) | 10.3 ± 3.9 | 10.2 ± 3.9 | 10.7 ± 2.9 | 0.552 |
| LA volume index (mL/m2) | 82.9 ± 53.4 | 81.3 ± 50.8 | 112.7 ± 86.7 | 0.369 |
| TR etiology | ||||
| Functional | 287 (90.0) | 268 (90.2) | 19 (82.6) | 0.298 |
| Primary | 18 (5.6) | 16 (5.4) | 2 (8.7) | |
| Central jet > 50% of RA area | 184 (57.5) | 167 (56.2) | 17 (73.9) | 0.048* |
| PISA radius at Nyquist 30–40 cm/sec (cm) | 0.93 ± 0.22 | 0.91 ± 0.21 | 1.03 ± 0.29 | 0.100 |
| Jet area (cm2) | 20.6 ± 8.0 | 20.9 ± 8.2 | 18.4 ± 5.9 | 0.385 |
| Vena contracta width (cm) | 0.83 ± 0.28 | 0.82 ± 0.29 | 0.98 ± 0.22 | 0.036* |
| Systolic reversal of hepatic vein flow | 150 (46.9) | 135 (45.5) | 15 (65.2) | 0.045* |
| Tricuspid inflow E velocity (m/sec) | 1.1 ± 0.9 | 1.2 ± 1.0 | 0.9 | - |
| EROA (cm2) | 0.51 ± 0.19 | 0.5 ± 0.2 | 0.5 ± 0.0 | 0.673 |
| Regurgitant volume (2D PISA) (mL/beat) | 58.7 ± 26.5 | 59.1 ± 27.7 | 54.1 | - |
| Treatment strategy | - | |||
| TV replacement | 6 (1.9) | 0 (0) | 6 (26.1) | |
| Tricuspid annuloplasty | 7 (2.2) | 0 (0) | 7 (30.4) | |
| Tricuspid valvuloplasty | 10 (3.1) | 0 (0) | 10 (43.5) | |
| In-hospital mortality | 24 (7.5) | 22 (7.4) | 2 (8.7) | 0.827 |
| Cardiac | 5 (1.6) | 5 (1.6) | 0 (0) | |
| Noncardiac | 19 (5.9) | 17 (5.7) | 2 (7.7) | |
Values are presented as mean ± standard deviation or number (%)
TR tricuspid regurgitation, NYHA New York Heart Association, MI myocardial infarction, NT-proBNP N-terminal pro B-type natriuretic peptide, LVEDD left ventricular end-diastolic dimension, LVESD left ventricular end-systolic dimension, LVEF left ventricular ejection fraction, LV left ventricle, Vmax maximal velocity, TAPSE tricuspid annular plane systolic excursion, TV tricuspid valve, RA right atrium, PISA proximal isovelocity surface area, EROA effective regurgitant orifice area, D dimensional
aWilcoxon rank sum test, Fisher exact test
*P < 0.05
Discussion
The principal findings of this study are as follows: (1) in stenotic valve diseases such as severe MS and severe AS, the most accurate diagnoses were based on key parameters, but in regurgitant valve diseases such as severe MR, AR, and TR, the reporting rate of quantitative parameters was not sufficient, as expected; (2) surgical or transcatheter intervention was performed in 19.3% of cases of severe MS, 31.4% of cases of severe primary MR, 7.5% of cases of severe secondary MR, 43.7% of cases of severe AS, 27.5% of cases of severe AR, and 7.2% of cases of severe TR; and (3) the overall in-hospital mortality rate for patients with severe VHD was 5.4%. In-hospital mortality occurred in 73 of the 1,244 patients (5.9%) who received conservative treatment and 18 of the 455 patients (4.0%) who received surgical or transcatheter intervention, and it was significantly lower in the intervention group. This study is provides valuable statistical information on contemporary diagnosis, treatment, and in-hospital outcomes for severe VHD in Korea.
Epidemiology and characteristics of severe VHD in Korea
The clinical characteristics of severe VHD in Korea did not differ significantly from those of significant VHD, as shown in part 1 [8]. Patients diagnosed with severe AS were older and had more comorbidities, such as hypertension and diabetes, compared with other cases of severe VHD patients. Patients with severe secondary MR often had symptoms of NYHA class II or higher, the highest levels of N-terminal prohormone of brain natriuretic peptide, and relatively high creatinine levels. In contrast, patients diagnosed with severe MS were younger, nearly 70% were female, and most had good systemic conditions with fewer underlying diseases.
Adverse cardiac remodeling is the primary determinant of prognosis in patients with VHD [9]. These myocardial and cardiac chamber changes are caused by volume/pressure factors and concomitant disease affected by the specific form of VHD [9–15]. In our registry, severe secondary MR, and severe AR presented with an enlarged left ventricle dimension. Decreases in ejection fraction were more pronounced in cases of severe secondary MR. Patients with severe AS showed increased thickness of the left ventricle wall, and atrial volume was greater in severe mitral valve disease and severe TR. Except for TR, TR Vmax was highest in cases of severe AR, followed by severe secondary MR.
The etiology of each severe VHD is no different than that described in part 1, which reported the etiology of significant VHD. In cases of MS, practical survey results showed that many institutions adhere to a definition of severe MS as being no larger than 1.0 cm2. The definition was therefore revised to 1.5 cm2 or less and applied uniformly, resulting in an increase in the number of severe patients within the registry [5]. Still, in each case of severe VHD, the main etiology of MS was rheumatic, that of primary MR was mitral valve prolapse, secondary MR was nonischemic cause, AS and AR were degenerative, and TR was functional. This main etiology is not expected to change in the near future. However, because the degenerative portion is likely to increase in all types of severe VHD, it can serve as a point of comparison for future changes in the epidemiology of VHD in Korea. As the number of newly occurring cases of rheumatic MS rapidly decreases, interest in degenerative MS related to mitral annular calcification and risk stratification is growing [5, 16–18].
Diagnostic approaches for severe VHD in Korea
Echocardiography is an essential test for diagnosing VHD and for determining the prognosis and timing of intervention in patients with severe VHD because it evaluates the etiology, severity, cardiac remodeling, and hemodynamic consequences. The increased use of multimodality imaging has resulted in a significant improvement in our understanding of the complicated aspects of VHD in recent years [13, 19–21]. Although 2D echocardiography remains the most popular imaging modality, evaluation of patients with VHD requires multimodality imaging for in-depth investigation of the underlying mechanism of valve dysfunction, precise quantification of disease severity, and consideration of any extravalvular issues. Advances in both surgical and transcatheter procedures have resulted in an increased demand for precise multimodality imaging tools to aid in patient and procedure selection [22, 23]. However, no statistical data on how much it is actually used in nationwide practice is available. This study did not target patients for whom specific treatment was planned, and the results should be interpreted with the understanding that additional imaging rates were investigated in patients diagnosed with severe VHD at 45 hospitals nationwide. Transesophageal echocardiography was performed as additional imaging in 30% of cases, coronary angiography in 18.9%, and cardiac computed tomography in 17.8%, which is not considered to be a low rate. Speckle tracking echocardiography is applied in 43.3% of cases. This reflects a great need for speckle tracking echocardiography to be used clinically, as it is easy to perform alongside conventional echocardiography and has several proven prognostic implications for patients with severe VHD [15, 24–27].
In addition, current guidelines emphasize an integrated diagnostic approach that comprehensively applies various parameters related to each case of VHD [2, 3, 28, 29]. However, it is challenging to measure and report multiple parameters. The reporting rate data for each major echocardiographic parameter shown through the KVS is significant because it reflects the current practice of echocardiographic assessment in VHD. Because the KVS systemically collected and analyzed echocardiographic data from 45 major university hospitals or hospitals over a specific period, and all participating institutions have echocardiologists certified in echocardiography by the KSE, any interpretation should assume the data are reliable.
Treatment approaches for severe VHD in Korea
The main treatment strategies for patients with severe VHD are conservative or interventional treatment, which includes surgical treatment and transcatheter intervention [2–5]. As both surgical and transcatheter intervention showed advances in choices of treatment strategy, favorable data for early intervention for severe VHD has recently accumulated [30–32], and the role of imaging for successful intervention is being emphasized [22, 23]. Representative transcatheter interventions for severe VHD currently available in Korea include PMV, mitral transcatheter edge-to-edge repair (TEER), and transcatheter AV implantation. In addition, it is expected that various interventions, including tricuspid TEER for severe TR, transcatheter MVR for severe MS, or mixed mitral valve disease, will be possible soon. In addition, the use of mechanical valves is expected to decrease, even in surgical valve replacement, as valve-in-valve procedures become possible via a transcatheter approach in cases of structural degeneration of bioprosthetic valves. In other words, surgical MVR or PMV is currently applied as an intervention method in severe MS, but transcatheter MVR is expected to be used more often in the future. In severe TR, the intervention group underwent all surgical interventions, but the transcatheter approach will also be applied to the disease. In this study, the overall in-hospital mortality of the intervention group was lower than that of conservative treatment, which can be interpreted in various ways. In most comparative trials of intervention and conservative treatment for severe VHD, the early outcome (i.e., 30-day mortality) in the intervention group tends to be worse, but the long-term prognosis improved. Because this study is retrospective in design and may be subject to various confounding factors associated with the intervention, a simple comparison cannot be made. However, the significant improvement in in-hospital mortality in the intervention group, particularly in patients with severe AS, can be accepted as meaningful.
Limitations
This study had several limitations. First, because it was a cross-sectional study, we were unable to determine any temporal patterns in prevalence and incidence. Second, despite our best efforts to identify the causes of VHD, it is difficult to assign a definitive etiology to any VHD cases due to the limited number of patients for whom surgical specimens were available. Third, referral bias may have affected our findings; because most participant institutions were universities or referral hospitals, the data may include more severe cases that required hospitalization or intervention. Fourth, although information was collected nationwide from 45 centers, differences at each institution may exist, and caution is needed when interpreting the results. Fifth, our data lacked specific information regarding the indications and symptoms of VHD patients, and other parameters were missing for some patients. Last, the clinical outcome in this study was in-hospital mortality, which provides no information on other meaningful outcomes, such as long-term survival or quality of life.
Conclusions
This Korean national hospital-based registry study supplied up-to-date statistics on clinical and echocardiographic characteristics of severe VHD. This study provides important information on the current status of diagnosis and treatment of severe VHD in Korea and helps to define future changes.
Acknowledgements
The authors are grateful to the Korean Society of Echocardiography for the generous support of the Korean Valve Survey.
List of the participating centers and principal investigators of the Korean Valve Survey:
KHK, MD, PhD, Chonnam National University Hospital; Wook-Jin Chung, MD, PhD, Gachon University Gil Medical Center; Chan Seok Park, MD, PhD, The Catholic University of Korea, Bucheon St. Mary’s Hospital; Hyo-Suk Ahn, MD, PhD; The Catholic University of Korea, Uijeongbu St. Mary’s Hospital; WBC, MD, PhD, The Catholic University of Korea, Seoul St. Mary’s Hospital; Eun Joo Cho, MD, PhD, The Catholic University of Korea, Yeouido St. Mary’s Hospital; JSC, MD, PhD, The Catholic University of Korea, Daejeon St. Mary’s Hospital; Dong Ryeol Ryu, MD, PhD, Kangwon National University School of Medicine and Kangwon National University Hospital; Dong Heon Yang, MD, PhD, Kyungpook National University Hospital; Jeong Rang Park, MD, PhD, Gyeongsang National University School of Medicine and Gyeongsang National University Hospital; Woo-Shik Kim, MD, PhD, Kyung Hee University Healthcare System; Il Suk Sohn, MD, PhD, Kyung Hee University Hospital at Gangdong; ICK, MD, PhD, HK, MD, PhD, Keimyung University Dongsan Medical Center; Jin Oh Na, MD, PhD, Korean University Guro Hospital; SMP, MD, PhD, Korea University Anam Hospital; Hwang Sun Ho, MD, Gwangju Veterans Hospital; Choi Ji-Yong, MD, PhD, Daegu Catholic University Medical Center; Tae-Ho Park, MD, PhD, Dong-A University Medical Center; Yong Hyun Park, MD, PhD, Pusan National University Yangsan Hospital; Jung Hyun Choi, MD, Pusan National University Hospital; Hack-Lyoung Kim, MD, PhD, Seoul Metropolitan Government-Seoul National University Boramae Medical Center; JBP, MD, PhD, Seoul National University Hospital; EKK, MD, PhD, Samsung Medical Center; Hye Sun Seo, MD, PhD, Soonchunhyang University Bucheon Hospital; JSP, MD, PhD, Ajou University School of Medicine; JWS, MD, Wonju Severance Christian Hospital, Yonsei University College of Medicine; CYS, MD, PhD, Severance Cardiovascular Hospital, Yonsei University College of Medicine; Eui-Young Choi, MD, PhD, Gangnam Severance Hospital, Yonsei University College of Medicine; Jang-Won Son, MD, PhD, Yeungnam University Hospital; Shin-Jae Kim, MD, PhD, Ulsan University Hospital; SL, MD, PhD, Asan Medical Center; Sang Jae Rhee, MD, PhD, Wonkwang University Hospital; In-Jeong Cho, MD, PhD, Ewha Womans University Seoul Hospital; Young Sup Byun, MD, Inje University Sanggye Paik Hospital; JSS, MD, PhD, Inje University Busan Paik Hospital; Sung-Hee Shin, MD, PhD, Inha University Hospital; Se-Jung Yoon, MD, PhD, National Health Insurance Service Ilsan Hospital; SHL, MD, PhD, Jeonbuk National University Hospital; Jong Wook Beom, MD, Jeju National University Hospital; BJS, MD, PhD; Chungnam National University Hospital and Chungnam National University School of Medicine; Ju-Hee Lee, MD, PhD, Dae-Hwan Bae, MD, Chungbuk National University Hospital, Chungbuk National University College of Medicine; Sung-Ai Kim, MD, Hallym Sacred Heart Hospital and Hallym University College of Medicine; Dae Gyun Park, MD, PhD, Hallym University Kangdong Sacred Heart Hospital; Min-Kyung Kang, MD, PhD, Hallym University Kangnam Sacred Heart Hospital; Kyung-Soon Hong, MD, PhD, Hallym University Chuncheon Sacred Heart Hospital; Ran Heo, MD, PhD, Hanyang University Medical Center, Hanyang University College of Medicine.
Abbreviations
- AR
Aortic regurgitation
- AS
Aortic stenosis
- AV
Aortic valve
- D
Dimensional
- eCRF
Electronic case report form
- EROA
Effective regurgitant orifice area
- KSE
Korean Society of Echocardiography
- KVS
Korean Valve Survey
- LA
Left atria
- LV
Left ventricular
- LVEF
Left ventricular ejection fraction
- MR
Mitral regurgitation
- MS
Mitral stenosis
- MVR
Mitral valve replacement
- NYHA
New York Heart Association
- PISA
Proximal isovelocity surface area
- PMV
Percutaneous balloon mitral valvuloplasty
- SAVR
Surgical aortic valve replacement
- TAVR
Transcatheter aortic valve replacement
- TEER
Transcatheter edge-to-edge repair
- TR
Tricuspid regurgitation
- TV
Tricuspid valve
- VHD
Valvular heart disease
- Vmax
Maximal velocity
Authors’ contributions
Conceptualization: HYK, CYS, KHK, JWH; Data curation: HYK, ICK, JWS, JBP, SL, EKK, SMP, WBC, JSC, JSP, JSS, SHL, BJS, HK, DHK, JWH; Formal analysis: HYK, HJL; Investigation: HYK, ICK, JWS, JBP, SL, EKK, SMP, WBC, JSC, JSP, JSS, SHL, BJS, HK, DHK; Methodology: CYS; Project administration: KHK, JWH; Resources: KHK; Supervision: HYK, CYS, KHK, DHK; Validation: HK; Visualization: HJL, CYS; Writing–original draft: HJL, HYK, ICK, HK, CYS, KHK; Writing–review & editing: all authors. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the Institutional Review Board of Chonnam National University Hospital (No. CNUH-2021–203) and the participating centers, each of which waived the requirement for written informed consent because of the study’s retrospective nature and the anonymized approach to data analysis.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hyung Yoon Kim and Hee Jeong Lee contributed equally to this work as co-first authors.
Contributor Information
Chi Young Shim, Email: cysprs@yuhs.ac.
Kye Hun Kim, Email: christiankyehun@hanmail.net.
on behalf of the Korean Valve Survey Registry Investigators:
Wook-Jin Chung, Chan Seok Park, Hyo-Suk Ahn, Eun Joo Cho, Dong Ryeol Ryu, Dong Heon Yang, Jeong Rang Park, Woo-Shik Kim, Il Suk Sohn, Jin Oh Na, Hwang Sun Ho, Choi Ji-Yong, Tae-Ho Park, Yong Hyun Park, Jung Hyun Choi, Hack-Lyoung Kim, Hye Sun Seo, Eui-Young Choi, Jang-Won Son, Shin-Jae Kim, Sang Jae Rhee, In-Jeong Cho, Young Sup Byun, Sung-Hee Shin, Se-Jung Yoon, Jong Wook Beom, Ju-Hee Lee, Dae-Hwan Bae, Sung-Ai Kim, Dae Gyun Park, Min-Kyung Kang, Kyung-Soon Hong, and Ran Heo
References
- 1.Coffey S, Roberts-Thomson R, Brown A, Carapetis J, Chen M, Enriquez-Sarano M, et al. Global epidemiology of valvular heart disease. Nat Rev Cardiol. 2021;18:853–64. [DOI] [PubMed] [Google Scholar]
- 2.Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e72-227. [DOI] [PubMed] [Google Scholar]
- 3.Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561–632. [DOI] [PubMed] [Google Scholar]
- 4.Lee SH, Yoon SJ, Sun BJ, Kim HM, Kim HY, Lee S, et al. 2023 Korean Society of Echocardiography position paper for the diagnosis and management of valvular heart disease, part I: aortic valve disease. J Cardiovasc Imaging. 2024;32:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shim CY, Kim EK, Cho DH, Park JB, Seo JS, Son JW, et al. 2023 Korean Society of Echocardiography position paper for the diagnosis and management of valvular heart disease, part II: mitral and tricuspid valve disease. J Cardiovasc Imaging. 2024;32:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watkins DA, Johnson CO, Colquhoun SM, Karthikeyan G, Beaton A, Bukhman G, et al. Global, regional, and national burden of rheumatic heart disease, 1990–2015. N Engl J Med. 2017;377:713–22. [DOI] [PubMed] [Google Scholar]
- 7.Jang SY, Ju EY, Seo SR, Choi JY, Park SJ, Kim DK, et al. Changes in the etiology of valvular heart disease in the rapidly aging Korean population. Int J Cardiol. 2014;174:355–9. [DOI] [PubMed] [Google Scholar]
- 8.Choi YJ, Son JW, Kim EK, Kim IC, Kim HY, Seo JS, et al. Epidemiologic profile of patients with valvular heart disease in Korea: a nationwide hospital-based registry study. J Cardiovasc Imaging. 2023;31:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ajmone Marsan N, Delgado V, Shah DJ, Pellikka P, Bax JJ, Treibel T, et al. Valvular heart disease: shifting the focus to the myocardium. Eur Heart J. 2023;44:28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudiktyo E, Soesanto AM, Cramer MJ, Yonas E, Teske AJ, Siswanto BB, et al. Global left ventricular myocardial work efficiency in patients with severe rheumatic mitral stenosis and preserved left ventricular ejection fraction. J Cardiovasc Imaging. 2023;31:191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park JB. Prognostic value of left atrial volume in patients with progressive mitral stenosis: a possible analogy with left ventricular mass in the setting of pressure overload. J Cardiovasc Imaging. 2019;27:134–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Güvenç RÇ, Aruğaslan E, Güvenç TS, Karadeniz FÖ, Kaşıkçıoğlu H, Çam N. An analysis of myocardial efficiency in patients with severe asymptomatic mitral regurgitation. J Cardiovasc Imaging. 2020;28:267–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park SJ, Dweck MR. Multimodality imaging for the assessment of severe aortic stenosis. J Cardiovasc Imaging. 2019;27:235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akintoye E, Saijo Y, Braghieri L, Badwan O, Patel H, Dabbagh MM, et al. Impact of age and sex on left ventricular remodeling in patients with aortic regurgitation. J Am Coll Cardiol. 2023;81:1474–87. [DOI] [PubMed] [Google Scholar]
- 15.Kim DY, Seo J, Cho I, Lee SH, Lee S, Hong GR, et al. Prognostic implications of biventricular global longitudinal strain in patients with severe isolated tricuspid regurgitation. Front Cardiovasc Med. 2022;9:908062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seo J, Jeong H, Cho I, Hong GR, Ha JW, Shim CY. Sex differences in mitral annular calcification and the clinical implications. Front Cardiovasc Med. 2021;8:736040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee HJ, Seo J, Gwak SY, Kim K, Cho I, Hong GR, et al. Risk factors and outcomes with progressive mitral annular calcification. J Am Heart Assoc. 2023;12:e030620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasmussen JH, Fredgart MH, Lindholt JS, Johansen JB, Sandgaard N, Yousef AH, et al. Mitral annulus calcification and cardiac conduction disturbances: a DANCAVAS sub-study. J Cardiovasc Imaging. 2022;30:62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim K, Lee SJ, Seo J, Suh YJ, Cho I, Hong GR, et al. Assessment of aortic valve area on cardiac computed tomography in symptomatic bicuspid aortic stenosis: utility and differences from Doppler echocardiography. Front Cardiovasc Med. 2022;9:1035244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim IC, Chang S, Hong GR, Lee SH, Lee S, Ha JW, et al. Comparison of cardiac computed tomography with transesophageal echocardiography for identifying vegetation and intracardiac complications in patients with infective endocarditis in the era of 3-dimensional images. Circ Cardiovasc Imaging. 2018;11:e006986. [DOI] [PubMed] [Google Scholar]
- 21.Seo J, Song S, Suh YJ, Kim YJ, Hong GR, Ha JW, et al. Contemporary multimodality imaging for cardiovascular Behçet disease. JACC Cardiovasc Imaging. 2020;13:2435–44. [DOI] [PubMed] [Google Scholar]
- 22.Reid A, Blanke P, Bax JJ, Leipsic J. Multimodality imaging in valvular heart disease: how to use state-of-the-art technology in daily practice. Eur Heart J. 2021;42:1912–25. [DOI] [PubMed] [Google Scholar]
- 23.Agricola E, Ancona F, Bartel T, Brochet E, Dweck M, Faletra F, et al. Multimodality imaging for patient selection, procedural guidance, and follow-up of transcatheter interventions for structural heart disease: a consensus document of the EACVI Task Force on Interventional Cardiovascular Imaging: part 1: access routes, transcatheter aortic valve implantation, and transcatheter mitral valve interventions. Eur Heart J Cardiovasc Imaging. 2023;24:e209–68. [DOI] [PubMed] [Google Scholar]
- 24.Lee SH, Lhagvasuren P, Seo J, Cho I, Kim DY, Hong GR, et al. Prognostic implications of left ventricular global longitudinal strain in patients with surgically treated mitral valve disease and preserved ejection fraction. Front Cardiovasc Med. 2022;8:775533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Namazi F, van der Bijl P, Hirasawa K, Kamperidis V, van Wijngaarden SE, Mertens B, et al. Prognostic value of left ventricular global longitudinal strain in patients with secondary mitral regurgitation. J Am Coll Cardiol. 2020;75:750–8. [DOI] [PubMed] [Google Scholar]
- 26.Meucci MC, Butcher SC, Galloo X, van der Velde ET, Marsan NA, Bax JJ, et al. Noninvasive left ventricular myocardial work in patients with chronic aortic regurgitation and preserved left ventricular ejection fraction. J Am Soc Echocardiogr. 2022;35:703–11. [DOI] [PubMed] [Google Scholar]
- 27.Vollema EM, Sugimoto T, Shen M, Tastet L, Ng AC, Abou R, et al. Association of left ventricular global longitudinal strain with asymptomatic severe aortic stenosis: natural course and prognostic value. JAMA Cardiol. 2018;3:839–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30:303–71. [DOI] [PubMed] [Google Scholar]
- 29.Baumgartner H, Hung J, Bermejo J, Chambers JB, Edvardsen T, Goldstein S, et al. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2017;30:372–92. [DOI] [PubMed] [Google Scholar]
- 30.Kang DH, Park SJ, Lee SA, Lee S, Kim DH, Kim HK, et al. Early surgery or conservative care for asymptomatic aortic stenosis. N Engl J Med. 2020;382:111–9. [DOI] [PubMed] [Google Scholar]
- 31.Kim K, Cho I, Ko KY, Lee SH, Lee S, Hong GR, et al. Early aortic valve replacement in symptomatic normal-flow, low-gradient severe aortic stenosis: a propensity score-matched retrospective cohort study. Korean Circ J. 2023;53:744–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang DH, Park SJ, Sun BJ, Cho EJ, Kim DH, Yun SC, et al. Early surgery versus conventional treatment for asymptomatic severe mitral regurgitation: a propensity analysis. J Am Coll Cardiol. 2014;63:2398–407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.