Abstract
Background
Electrolyte imbalances are commonly observed in individuals diagnosed with myocardial infarction (MI). The levels of serum sodium have been linked to unfavorable outcomes in relation to MI. Additionally, there exists a correlation between serum sodium and serum chloride, although the combined influence of these electrolytes on the prognosis of MI patients has not been extensively studied. Consequently, our study aimed to examine whether an autonomous association exists between the sodium-to-chloride (Na/Cl) ratio and mortality rates during hospitalization among patients admitted to intensive care unit (ICU) with MI.
Methods
A retrospective cohort study analysis was conducted on the Na/Cl ratio within the ICU from 2008 to 2019. Patients diagnosed with MI were divided into two groups based on a predetermined cutoff value for the Na/Cl ratio. Various statistical models, including the Cox proportional hazard model, generalized additive model, and two-piecewise linear regression model, were employed to assess the relationship between the initial Na/Cl ratio upon admission and the likelihood of in-hospital mortality while accounting for other relevant covariates.
Results
After adjusting for all other factors, the study revealed that the Na/Cl ratio exhibited an independent association with in-hospital mortality (HR = 1.28; 95% CI: 1.11–1.47, P < 0.001). Further analysis indicated a nonlinear relationship between the Na/Cl ratio and in-hospital mortality among patients with MI, with a threshold at approximately 1.37. Specifically, if the Na/Cl ratio exceeded 1.37, there was a significant and progressively increasing likelihood of mortality during hospitalization (HR = 1.46; 95% CI: 1.20–1.77).
Conclusion
The in-hospital mortality of patients admitted to ICU with MI is predicted independently by the ratio of sodium to chloride (Na/Cl). A curvilinear correlation was observed between the Na/Cl ratio and in-hospital mortality, with a statistically significant threshold identified at 1.37.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-024-04351-7.
Keywords: Sodium-to-chloride ratio, Myocardial infarction, Mortality, MIMIC-IV
Introduction
Myocardial infarction (MI) is a prevalent and severe condition often observed in the intensive care unit (ICU), with a high mortality rate and significant financial burden [1]. Electrolyte imbalances are frequently encountered in patients with MI. Maintaining electrolyte stability is crucial for normal cellular metabolism, intracellular fluid osmolarity, acid‒base balance, and neuronal excitation. The presence of electrolyte imbalances in patients with MI can lead to complications such as arrhythmias, reduced myocardial contractility, and myocardial remodeling and ultimately impact the prognosis of these patients [2, 3]. Currently, serum electrolyte concentrations have been reported as indicators of prognosis in congestive heart failure (HF). Low serum sodium levels (hyponatremia) are recognized as adverse prognostic markers in patients with chronic HF [4, 5]. While chloride ions are the most important anions in plasma and tissue fluids, they have received less attention in cardiovascular diseases. To maintain charge balance and ensure normal cellular function, sodium and chloride, which are the primary cations and anions in the body, typically undergo simultaneous alterations. Studies indicate that there is a direct relationship between serum chloride and sodium levels in individuals diagnosed with HF [6]. Decreased serum chloride levels frequently align with reduced serum sodium concentrations, indicating more pronounced disruptions in electrolyte equilibrium [7]. Several studies have suggested a connection between hyponatremia and clinical results in univariate examination, although this correlation often vanishes when considering covariates, such as serum chloride levels [8–10]. Hence, taking into account both serum sodium and chloride levels may offer more comprehensive insight into the influence of electrolyte imbalances on disease prognosis.
Previous research has examined the ratio of sodium to chloride (Na/Cl) as a marker and observed its influence on the prognosis of patients with HF. It has been found that higher baseline Na/Cl ratios increase the risk of adverse outcomes [11]. However, no studies on the Na/Cl ratio have been conducted in patients with MI. Therefore, we conducted an analysis to evaluate the correlation between the Na/Cl ratio on admission and the prognosis of patients admitted to ICU with MI.
Materials and methods
Database
Data for this study were obtained from the MIMIC-IV database. Access to the MIMIC-IV database, containing real-time information on over 70,000 severely ill individuals who received treatment at Beth Israel Deaconess Medical Center from 2008 to 2019 [12], was granted by the Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center review committee after completing the collaborative institutional training initiative (CITI) course and passing the ethics examination (certification number 48693003). This access allows for downloading and utilization of the database. Data extraction was performed using PostgreSQL soft-ware(version13.3). In consideration of local legislation and institutional requirements, no ethical review or approval was mandated for this study. The study followed the moral guidelines outlined in the 1964 Helsinki Declaration and its subsequent revisions. Moreover, we carried out the investigation in accordance with the STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) guidelines [13].
Study cohort
This study included patients who were diagnosed with MI from 2008 to 2019. Inclusion criteria were determined based on the codes 41,000–41,100 of the International Classification of Diseases-9 (ICD-9) and codes I21-I23 of the International Classification of Diseases-10 (ICD-10). Several exclusion criteria were utilized, specifically, individuals under 18 years old, those with an ICU stay duration of fewer than 24 h, and patients lacking complete or accessible serum sodium or serum chloride information. For patients who had multiple admissions to the ICU, only information from their initial admission was considered for the analysis.
Exposure variable
The primary variable of interest in our study was the Na/Cl ratio. This ratio was calculated by dividing the serum sodium concentration by the serum chloride concentration using the blood samples obtained upon admission. The formula used for this calculation was serum sodium (mmol/L) divided by serum chloride (mmol/L).
Covariates
To extract the data, we utilized Structured Query Language and Navicat software (version 15). Within the initial 24 h of admission to the ICU, we collected data on various baseline characteristics of the patients. These included demographics (age and sex), classification of MI, vital signs (heart rate, mean blood pressure, respiratory rate, temperature, and oxygen saturation of blood), laboratory parameters (serum creatinine, white blood cell counts, hemoglobin, platelet counts, glucose, calcium, chloride, sodium, potassium, N-terminal pro-brain natriuretic peptide [NT-proBNP], and estimated glomerular filtration rate [eGFR]), comorbidities (HF, chronic pulmonary disease [COPD], liver disease, renal disease, and diabetes), scoring systems (Charlson comorbidity index [CCI] and Simplified Acute Physiology Score II [SAPSII]), and treatments and medications (percutaneous coronary intervention, mechanical ventilation, use of diuretics, and use of vasopressors). Hyponatremia and hypochloremia were delineated, whereby hyponatremia was characterized by serum sodium levels below 135mmol/L, and hypochloremia was characterized by serum chloride levels below 96mmol/L.
Outcomes
The primary result was mortality in the hospital due to any cause.
Statistical analysis
The patients were divided into two groups, namely, the low Na/Cl ratio group and the high Na/Cl ratio group, using the optimal cutoff value of the Na/Cl ratio determined by X-tile software (Version 3.6.1, Yale University School of Medicine) [14]. Mean and standard deviation were reported for continuous variables, whereas categorical variables were presented as percentages. For parameters that had a skewed distribution, median values and interquartile ranges were computed. Pearson’s chi-square test was employed to compare categorical variables, while unpaired Student’s t test and Mann‒Whitney U test were utilized to compare continuous variables with normal distribution and those with skewed distribution, respectively. The Kaplan-Meier (KM) method was used to plot unadjusted survival curves, and the log-rank test was used to compare differences between the two Na/Cl ratio groups. The hazard ratios (HRs) and their corresponding 95% inter-confidence intervals (CIs) of in-hospital mortality were estimated using multivariable Cox regression analysis. Multiple variables were adjusted for to evaluate the independent role of the Na/Cl ratio. The selection of covariates was based on existing literature and clinical experience [15, 16].Four adjustment models were employed: Model I, which did not account for any variables; Model II, which accounted for age and sex; Model III, which accounted for Model II as well as HF, diabetes, renal disease, liver disease, COPD, SAPSII, and CCI; and Model IV, which accounted for Model III in addition to heart rate, mean blood pressure, glucose, eGFR, white blood cell count, hemoglobin, and NT-proBNP. By utilizing a generalized additive model (GAM) [17, 18], we investigated the nonlinear correlation between the two variables and identified the threshold through a two-step linear regression model. To conduct sensitivity analysis, the Na/Cl ratio was transformed into a categorical variable. Median values were then calculated for each group based on the Na/Cl ratio, and these values were used as continuous variables to determine linear trends [19]. In addition, we performed stratification and interaction investigations. R version 3.6.3 (R Foundation, Vienna, Austria) and Free Statistics software (version 1.8) were utilized for conducting statistical analyses. The determination of statistical significance was based on two-sided P values of 0.05.
Results
Participant selection
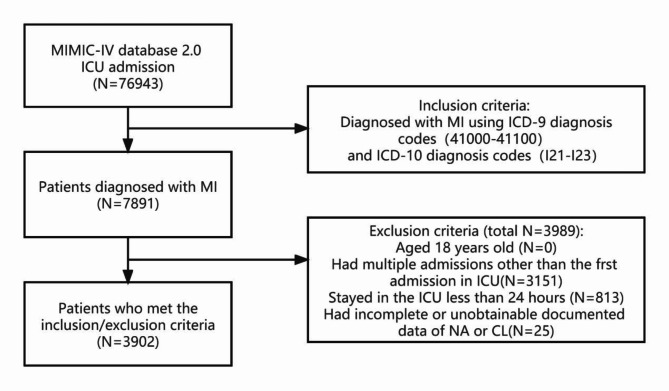
From a pool of 76,943 MIMIC-IV ICU admissions, a grand total of 7891 individuals diagnosed with MI were found. Figure 1 depicts the flowchart that illustrates the process of research. A total of 3,902 patients were included in the analysis after excluding individuals below the age of 18, patients with an ICU stay of less than 24 h, and those with incomplete data.
Fig. 1.
Flowchart of patient selection. ICU, intensive care unit; MI, Myocardial Infarction; ICD, International Classification of Diseases; NA, Sodium; CL, Chloride
Baseline characteristics
The study population’s baseline characteristics are presented in Table 1. Two groups were formed with the enrolled patients: the low Na/Cl ratio group (Na/Cl ratio < 1.37) and the high Na/Cl ratio group (Na/Cl ratio ≥ 1.37). The cut-off value of 1.37 was determined by X-tile software and as the threshold based on the subsequent use of a two-piecewise linear regression model to explore the non-linear relationship between the Na/Cl ratio and in-Hospital Mortality. The average ratio of Na/Cl for the entire group was 1.4 ± 0.1, with 2,631 patients (67.4%) categorized in the high Na/Cl ratio category. Of the study participants, 37.3% were women, with an average age of 70.2 ± 13.4 years, totaling 3,647 individuals. Significant disparities were noted between the two groups regarding gender (P = 0.038). The group with a greater Na/Cl ratio exhibited a higher incidence of specific coexisting conditions, including HF, liver disease, renal disease, diabetes, classification of MI type 2, and the use of diuretics and vasopressors. Furthermore, individuals belonging to the high Na/Cl ratio category exhibited elevated age, heart rate, respiratory rate, and temperature, along with increased scores in all the assessment systems. In addition, they showed increased platelet counts, glucose, serum creatinine, calcium, potassium, and NT-proBNP, as well as extended durations of hospital stay and ICU stay. Conversely, their chloride, sodium, and eGFR levels were lower.
Table 1.
Baseline characteristics of the study participants
| Variables | Total | Quartile of Na/Cl ratio | P | |
|---|---|---|---|---|
| < 1.37 | ≥ 1.37 | |||
| N | n = 3902 | n = 2631 | n = 1271 | |
| Demographics | ||||
| Sex, n (%) | 0.038 | |||
| Male | 2448(62.7) | 1680(63.9) | 768(60.4) | |
| Female | 1454(37.3) | 951(36.1) | 503(39.6) | |
| Age, n (%) | 70.2 ± 13.4 | 69.9 ± 13.3 | 70.8 ± 13.7 | 0.049 |
| Classification of MI, n (%) | ||||
| MI type 1 | 3066 (78.6) | 2155 (81.9) | 911 (71.7) | < 0.001 |
| MI type 2 | 829(21.2) | 469(17.8) | 360(28.3) | < 0.001 |
| Acute MI | 938(24.0) | 746(28.4) | 192(15.1) | < 0.001 |
| MI in anterior wall | 373(9.6) | 260(9.9) | 113(8.9) | 0.324 |
| NSTEMI | 1693(43.4) | 1128(42.9) | 565(44.5) | 0.351 |
| Comorbidities, n (%) | ||||
| Heart failure | 1931(49.5) | 1120(42.6) | 811(63.8) | < 0.001 |
| COPD | 100(2.6) | 67(2.5) | 33(2.6) | 0.926 |
| Liver disease | 1226(31.4) | 690(26.2) | 536(42.2) | < 0.001 |
| Renal disease | 1173(30.1) | 750(28.5) | 423(33.3) | 0.002 |
| Diabetes | 554(14.2) | 344(13.1) | 210(16.5) | 0.004 |
| Scoring system | ||||
| CCI | 7.0 ± 2.7 | 6.6 ± 2.6 | 7.7 ± 2.7 | < 0.001 |
| SAPSII | 38.6 ± 13.8 | 38.0 ± 13.6 | 39.8 ± 14.1 | < 0.001 |
| Vital signs | ||||
| Heart rate(beats/minute) | 82.6 ± 14.9 | 81.8 ± 14.3 | 84.4 ± 15.8 | < 0.001 |
| MBP(mmHg) | 77.9 ± 10.2 | 77.4 ± 9.6 | 78.9 ± 11.1 | < 0.001 |
| RR(times/minute) | 19.4 ± 3.5 | 19.1 ± 3.4 | 20.1 ± 3.7 | < 0.001 |
| Temperature(℃) | 36.8 ± 0.5 | 36.8 ± 0.6 | 36.8 ± 0.5 | 0.016 |
| SaO2(%) | 96.9 ± 2.0 | 97.1 ± 2.0 | 96.5 ± 2.2 | < 0.001 |
| Laboratory parameters | ||||
| White cell count(10^9/L) | 9.9(7.4,13.0) | 9.9(7.4,13.0) | 9.9(7.4,13.1) | 0.733 |
| Hemoglobin(g/dL) | 11.6 ± 2.2 | 11.6 ± 2.2 | 11.6 ± 2.3 | 0.954 |
| Platelets (K/µL) | 225.4 ± 102.0 | 219.8 ± 99.6 | 236.9 ± 105.9 | < 0.001 |
| Glucose(mg/dl) | 135.6(118.5,170.5) | 132.8(118.0,161.0) | 145.7(119.6,192.6) | < 0.001 |
| Serum creatinine(mg/dl) | 1.0(0.8,1.6) | 1.0(0.7,1.4) | 1.3(0.8,2.3) | < 0.001 |
| eGFR(ml/min/1.73m2) | 71.5 ± 32.3 | 76.8 ± 29.6 | 60.5 ± 34.8 | < 0.001 |
| Calcium(mmol/L) | 8.7 ± 0.8 | 8.6 ± 0.8 | 8.9 ± 0.9 | < 0.001 |
| Chloride(mmol/L) | 105.1 ± 6.1 | 107.3 ± 4.9 | 100.6 ± 5.8 | < 0.001 |
| Sodium(mmol/L) | 139.5 ± 4.7 | 139.8 ± 4.4 | 139.0 ± 5.3 | < 0.001 |
| Hypochlorhydria | 578 (14.8) | 64 (2.4) | 514 (40.4) | < 0.001 |
| Hyponatremia | 1047 (26.8) | 604 (23) | 443 (34.9) | < 0.001 |
| Potassium(mmol/L) | 4.7 ± 0.8 | 4.7 ± 0.8 | 4.8 ± 0.9 | 0.001 |
| NT-proBNP(pg/mL) | 3678(1012,10156) | 3364(903,9340) | 4668(1431,11865) | < 0.001 |
| Na/Cl ratio | 1.4 ± 0.1 | 1.3 ± 0.0 | 1.4 ± 0.1 | < 0.001 |
| Treatments and medications | ||||
| PCI | 658(16.9) | 509(19.3) | 149(11.7) | < 0.001 |
| MV | 1050(26.9) | 720(27.4) | 330(26) | 0.355 |
| Use of diuretic | 2486(63.7) | 1604(61) | 882(69.4) | < 0.001 |
| Use of vasopressor | 387(9.9) | 237(9) | 150(11.8) | 0.006 |
| Use of antibiotic | 3339 (85.6) | 2229 (84.7) | 1110 (87.3) | 0.03 |
| Outcomes | ||||
| In-hospital mortality, n (%) | 455(11.7) | 250(9.5) | 205(16.1) | < 0.001 |
| Los hospital(days) | 10.8 ± 10.6 | 10.3 ± 9.7 | 12.0 ± 12.2 | < 0.001 |
| Los ICU(days) | 4.1 ± 5.1 | 3.9 ± 4.8 | 4.5 ± 5.8 | < 0.001 |
Na/Cl ratio, sodium-to-chloride ratio; MI, Myocardial Infarction; NSTEMI, Non-ST-Segment Elevation Myocardial Infarction; COPD, Chronic obstructive pulmonary disease; CCI, Charlson comorbidity index; SAPSII, Simplified Acute Physiology Score II; MBP, Mean blood pressure; RR, respiratory rate; SaO2,oxygen saturation of blood; eGFR, Estimated Glomerular Filtration Rate; NT-proBNP, N-terminal pro-brain natriuretic peptide; PCI, Percutaneous Coronary Intervention; MV, mechanical ventilation; Los hospital, Length of stay in hospital; Los ICU, length of intensive care unit stay time
Outcomes
During the initial hospital stay, a total of 455 individuals (11.7%) lost their lives, and it was observed that those with an elevated Na/Cl ratio had a higher probability of mortality while being hospitalized (16.1% compared to 9.5%; P < 0.001).
Na/Cl ratio and in-hospital mortality
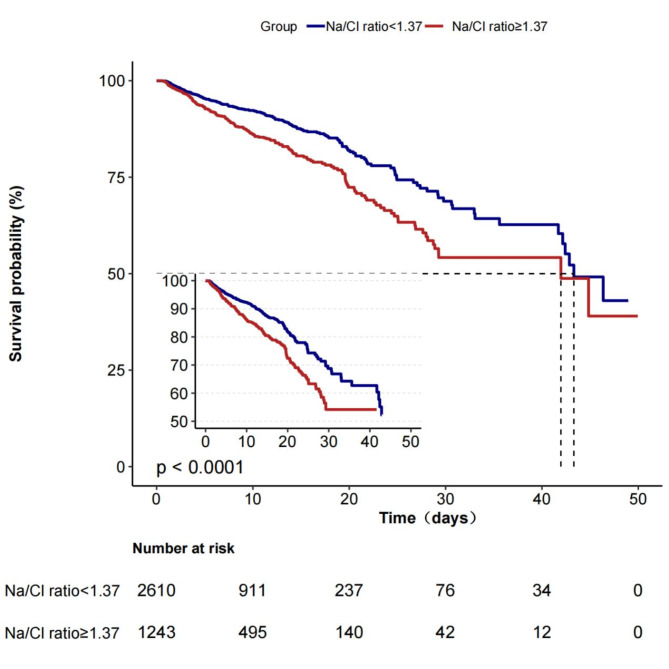
During hospitalization, the high Na/Cl ratio group exhibited a notably reduced survival rate in comparison to the low Na/Cl ratio group, as indicated by Kaplan‒Meier analysis (P < 0.001) (Fig. 2). The dependent variable chosen for investigation in the univariate analysis was in-hospital mortality, which served as the outcome indicator of interest, to examine its correlation with different independent variables. The examination revealed numerous elements that were strongly linked to mortality during hospitalization, such as age, liver disease, CCI, SAPSII, heart rate, mean blood pressure, respiratory rate, temperature, oxygen saturation of blood serum creatinine, white blood cell count, calcium, chloride, NT-proBNP, eGFR, Na/Cl ratio, use of diuretics, use of vasopressors, MV, MI type 2, acute MI, MI in anterior wall, and NSTEMI (Supplementary Table 1). In the unadjusted Cox hazard regression model, when the Na/Cl ratio was considered a continuous variable, a notable correlation was found between a higher Na/Cl ratio and a heightened likelihood of in-hospital death. In particular, for every 0.1 increase in the Na/Cl ratio, there was a 25% increase in the rate of mortality during hospitalization (HR = 1.25, 95% CI: 1.1–1.42). After accounting for all possible confounding factors, the connections were slightly strengthened and continued to be statistically significant, with an HR of 1.28 (95% CI: 1.11–1.47) (Table 2). In the fully adjusted model, when the Na/Cl ratio was considered as a categorized variable, individuals in the high Na/Cl ratio category experienced a 46% higher chance of in-hospital death compared to those in the low Na/Cl ratio category (Table 2). However, isolated serum sodium or serum chloride levels are not significantly associated with in-hospital mortality (Supplementary Table 2).
Fig. 2.
Kaplan–Meier survival curves for in-hospital mortality of patients with myocardial infarction depending on the Na/Cl ratio. The numbers listed under the chart indicate the number of patients at risk at each time point. Na/Cl ratio, sodium-to- chloride ratio
Table 2.
Association between Na/Cl ratio and in-hospital mortality in multiple regression model
| Model I | Model II | Model III | Model IV | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||
| Na/Cl ratio as Continuous variables per 0.1 increase | |||||||||
| In-hospital mortality | 1.25 (1.1 ~ 1.42) | 0.001 | 1.26 (1.11 ~ 1.43) | < 0.001 | 1.3 (1.14 ~ 1.48) | < 0.001 | 1.28 (1.11 ~ 1.47) | 0.001 | |
| Na/Cl ratio as categorical variables | |||||||||
| In-hospital mortality | Na/Cl ratio < 1.37 | 1(Ref) | 1(Ref) | 1(Ref) | 1(Ref) | ||||
| Na/Cl ratio ≥ 1.37 | 1.46 (1.21 ~ 1.75) | < 0.001 | 1.46(1.22 ~ 1.76) | < 0.001 | 1.53 (1.27 ~ 1.86) | < 0.001 | 1.46 (1.20 ~ 1.78) | < 0.001 | |
Model I: didn’t adjusted for confounders. Model II: adjusted for age and sex
Model III: Model II + heart failure, diabetes, renal disease, liver disease, chronic obstructive pulmonary disease, Charlson comorbidity index, SAPSII
Model IV: Model III + heart rate, mean blood pressure, glucose, eGFR, white cell counts, hemoglobin, NT-proBNP, use of antibiotic
Na/Cl ratio, sodium-to-chloride ratio; HR, hazard Ratio; CI, Confidence interval; SAPSII, Simplified Acute Physiology Score II;
eGFR, Estimated Glomerular Filtration Rate; NT-proBNP, N-terminal pro-brain natriuretic peptide
Nonlinear relationship between Na/Cl ratio and in-hospital mortality
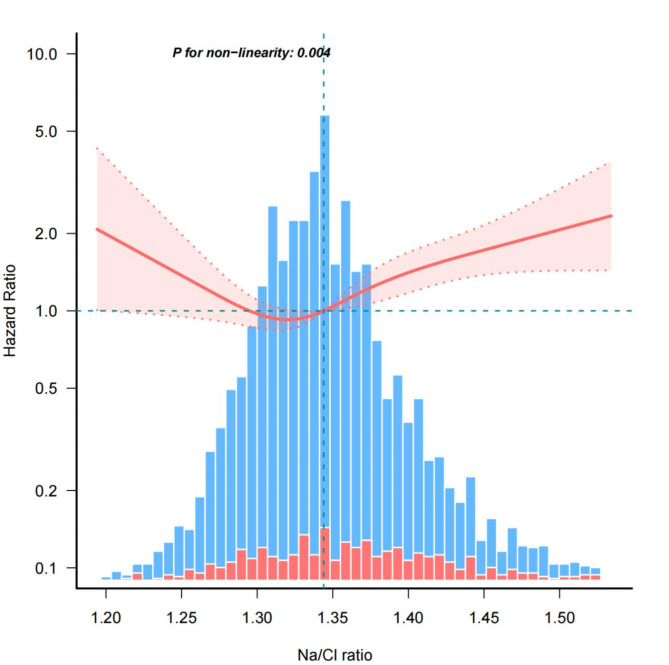
Figure 3 reveals the observation of a nonlinear correlation between in-hospital mortality and the Na/Cl ratio after accounting for various covariates. Table 3 determined a threshold of 1.37 for the Na/Cl ratio using a two-piecewise linear regression model. After surpassing the threshold, there was a significant increase in the risk of mortality during hospitalization (HR = 1.3; 95% CI: 1.01–1.69; P = 0.046). Nevertheless, there was no notable correlation between dosage and response below the limit (HR = 0.78; 95% CI: 0.53–1.12; P = 0.18).
Fig. 3.
Nonlinear dose-response relationship between Na/Cl ratio and in-hospital mortality. The red line and salmon pink area represent the estimated values and their corresponding 95% confidence intervals, respectively. The blue bars represent the cases where in-hospital mortality did not occur and red bars represent the cases where in-hospital mortality did occur. HRs are adjusted for age, sex, failure, diabetes, renal disease, liver disease, chronic obstructive pulmonary disease, Charlson comorbidity index, SAPSII, heart rate, mean blood pressure, glucose, eGFR, white cell counts, hemoglobin, NT-proBNP, use of antibiotic. SAPSII, Simplified Acute Physiology Score II; eGFR, Estimated Glomerular Filtration Rate; NT-proBNP, N-terminal pro-brain natriuretic peptide
Table 3.
Threshold effect analysis of Na/Cl ratio on in-hospital mortality
| Threshold of Na/Cl ratio | HR | 95% CI | P value |
|---|---|---|---|
| < 1.37 | 0.78 | 0.53,1.12 | 0.18 |
| ≥ 1.37 | 1.3 | 1.01,1.69 | < 0.046 |
| Log-likelihood ratio test | 0.018 |
Adjuested for age, sex, failure, diabetes, renal disease, liver disease, chronic obstructive pulmonary disease, SAPSII, Charlson comorbidity index, heart rate, mean blood pressure, glucose, eGFR, white cell counts, hemoglobin, NT-proBNP, use of antibiotic. SAPSII, Simplified Acute Physiology Score II; eGFR, Estimated Glomerular Filtration Rate; NT-proBNP, N-terminal pro-brain natriuretic peptide
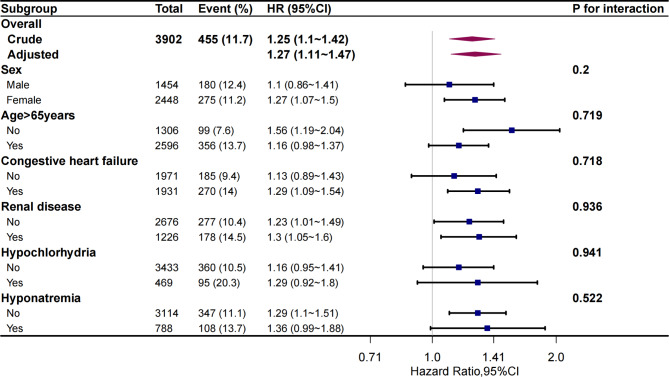
Subgroup analyses
The subgroup analysis, which categorized participants by age (< 65 and ≥ 65 years), sex (male and female), HF, renal disease, hypochlorhydria, and hyponatremia, revealed that the Na/Cl ratio consistently correlated with in-hospital mortality. No notable interactions were witnessed (Fig. 4).
Fig. 4.
Risk of the primary outcome for Na/Cl ratio in different subgroups of patients. Hazard ratio (HR) was adjusted for age, sex, failure, diabetes, renal disease, liver disease, chronic obstructive pulmonary disease, Charlson comorbidity index, SAPSII, heart rate, mean blood pressure, glucose, eGFR, white cell counts, hemoglobin, NT-proBNP, use of antibiotic. SAPSII, Simplified Acute Physiology Score II; eGFR, Estimated Glomerular Filtration Rate; NT-proBNP, N-terminal pro-brain natriuretic peptide
Discussion
In this retrospective study, we established a significant association between the Na/Cl ratio and the mortality rate of patients with MI who were admitted to ICU during their hospital stay. As the Na/Cl ratio increased, we observed a corresponding increase in the rate of mortality among patients during their hospital stay. Significantly, this association remained meaningful even after accounting for potential confounding variables. Additionally, our examination uncovered a nonlinear correlation and a point of impact between the Na/Cl ratio and the rate of mortality during hospitalization. We noticed a positive relationship between the two variables when the ratio of Na/Cl surpassed 1.37. In contrast, if the Na/Cl ratio was less than 1.37, the association was not statistically significant. To summarize, our results emphasize the separate factors that increase the risk of in-hospital death in patients admitted to ICU with MI, which include the Na/Cl ratio and an Na/Cl ratio of at least 1.37. The significance of monitoring and controlling electrolyte imbalances in these patients is emphasized by these findings.
Hyponatremia, a common electrolyte imbalance, is frequently observed in patients with MI. In previous research, it has been documented that the occurrence of hyponatremia in these individuals varies between 12.5% and 23.2% [20]. In our study, we found a slightly higher incidence of 26.8%, which can be attributed to our focus on patients admitted to ICU with MI cases. These patients often have underlying diseases or more severe conditions, increasing their susceptibility to developing hyponatremia. In contrast to serum sodium, the reporting of serum chloride levels in patients with MI is limited. Only one study by João Pedro Ferreira and colleagues reported that 35.1% of patients with MI had serum chloride levels below 100 mmol/L [16]. In our study, we observed a similar proportion of 33.9% of patients with serum chloride levels below 100 mmol/L, indicating a comparable occurrence. The development of hyponatremia in MI is multifactorial. First, ischemia and hypoxia lead to neuroendocrine changes, resulting in increased cell membrane permeability and accelerated excretion of sodium ions, potassium ions, and chloride ions [21]. Additionally, the activation of the renin-angiotensin system promotes renal vascular constriction, reducing endogenous creatinine clearance. This, in turn, leads to decreased water excretion in the diluting segment of the renal unit [22]. Moreover, MI triggers an elevation in arginine vasopressin levels, causing the insertion of aquaporin-2 water channels into the cell membrane of the renal collecting duct [23, 24]. As a result, there is an increase in the re-absorption of unbound water, which ultimately leads to a reduction in the levels of sodium and chloride in the blood serum due to the expansion of blood volume. In summary, low sodium levels are commonly seen in individuals who have experienced an MI, and the presence of this condition is affected by multiple factors. Having knowledge of the fundamental processes can assist in the control and therapy of this imbalance in bodily fluids.
Researchers have investigated the correlation between serum sodium levels and the outlook of individuals diagnosed with MI. Several research studies have discovered a correlation between low sodium levels (hyponatremia) and higher rates of both short-term and long-term death in individuals who have experienced an MI [15, 25, 26]. However, contrasting findings have been reported in other studies, with one study indicating that hyponatremia might not autonomously forecast mortality in these patients with MI [27]. On the other hand, the significance of serum chloride levels in MI prognosis is not yet clear, as no specific studies have been conducted in this area. However, recent studies have found that low levels of chloride in the body at the beginning are linked to an increased chance of death in the short and long term for individuals with acute or chronic HF [6, 28]. Furthermore, a study discovered that in individuals with MI and compromised heart function, decreased levels of chloride in the blood were linked to death only when combined with reduced levels of sodium, whereas there was no connection when sodium levels in the blood were within the normal range or increased [16]. It is crucial to take into account the correlation between serum sodium and chloride based on these discoveries. Hence, our research aimed to examine the correlation between the ratio of Na/Cl and the mortality rate during hospitalization among individuals diagnosed with MI. The results of our study indicated that a greater ratio of Na/Cl was linked to a heightened likelihood of mortality. Moreover, the high Na/Cl ratio group exhibited a greater prevalence of hyponatremia (34.9% vs. 23%) and a significantly elevated occurrence of hypochloremia (40.4% vs. 2.4%) in comparison to the low Na/Cl ratio group. This suggests that individuals with an elevated Na/Cl ratio experience a greater incidence of reduced levels of both sodium and chloride in their blood, which has a detrimental effect on the prognosis of the patient. Hence, an elevated Na/Cl ratio could potentially indicate a more advanced condition in patients with MI.
In the high Na/Cl ratio group, we observed a higher proportion of elevated NT-proBNP levels and lower oxygen saturation, indicating more severe congestion in the body. Additionally, in the high Na/Cl ratio group, eGFR was lower, possibly due to impaired cardiac function after MI, leading to compromised renal blood flow and poorer re-absorption function in the kidneys. These pathological factors contribute to low serum sodium and low serum chloride levels. The exact reasons for the poorer prognosis in hyponatremia are not fully understood, but potential mechanisms include significantly suppressed calcium channel currents resulting from decreased extracellular sodium concentration, which can significantly impair myocardial contractility [29]. Hyponatremia can also cause arrhythmias, conduction blockages [30], and even trigger apical ballooning syndrome [31]. Additionally, activation of neurohormones caused by hyponatremia can lead to fibroblast proliferation in the myocardium, resulting in irreversible structural changes [32], ultimately leading to HF. Similarly, low serum chloride is also associated with neurohormonal activation, leading to various adverse outcomes and an increased risk of death [33]. Recent research has explored the relationship between the sodium-chloride difference (SCD) and 30-day mortality risk in patients with AMI undergoing PCI, revealing that low SCD is associated with an increased risk of mortality during the 30-day follow-up [34]. While both studies investigated the impact of electrolyte imbalance on outcomes in this patient population, our study took a more comprehensive approach by quantitatively assessing the imbalance between serum sodium and serum chloride, as represented by the sodium-to-chloride ratio (Na/Cl ratio). This allowed us to explore the specific effects of this electrolyte disparity on organ function, particularly cardiac and renal function, which have important implications for clinical outcomes among patients admitted to ICU diagnosed with MI.
In cases of pathological conditions, the rise in the ratio of Na/Cl is primarily caused by the decrease in levels of both sodium and chloride in the bloodstream, with a more significant decline in chloride levels. Why is lower serum chloride associated with higher mortality risk? Perhaps chloride levels can provide more prognostic information than sodium levels. Chloride, a vital electrolyte, plays a pivotal role in maintaining optimal fluid balance and is intricately linked to the heart, renal, and neurohormonal systems, widely acknowledged as the primary drivers of HF’s development and progression. Despite the historical emphasis on sodium’s role in HF by clinicians and guidelines, recent evidence has underscored the more substantial contribution of chloride to the pathophysiology and prognosis of HF. Hypochloremia, independently predicting adverse outcomes in both acute and chronic HF, further emphasizes the critical role of chloride in managing HF [35] .A survival analysis was performed, examining the occurrence of hypochloremia and hyponatremia upon admission. The findings revealed that serum chloride exhibited a more significant predictive role in determining mortality among patients with acute decompensated HF compared to serum sodium. Furthermore, the predictive effect of serum chloride on mortality in HF patients remained unaffected by serum sodium levels [36]. A separate study conducted in China also confirmed that lower levels of serum chloride upon admission were independently linked to short-term all-cause mortality and readmission, with no observed interaction with serum sodium levels [37]. This implies that a decrease in serum chloride levels may offer supplementary predictive data for individuals with HF. In our study, we found that after adjusting for covariates, the Na/Cl ratio was associated with in-hospital mortality in patients admitted to ICU diagnosed with MI, while individual sodium or chloride levels did not show significant correlation. Thus, it can be concluded that the sodium/chloride ratio has better predictive capacity than sodium or chloride levels alone. Evaluating patient prognosis by combining chloride and sodium ion concentrations improves the predictive ability compared to using only sodium ion concentration, but the specific mechanisms behind this require further research.
This research presents the initial findings regarding the inverse correlation between the ratio of Na/Cl and the mortality rate during hospitalization among patients admitted to ICU with MI. By employing restricted cubic spline analysis, we achieved a heightened level of accuracy in depicting the L-shaped curve correlation between the Na/Cl ratio and the likelihood of in-hospital fatality among these individuals. Our calculations yielded a threshold value of approximately 1.37, below which and above which the effects were markedly different. On the other hand, a distinct study investigated the correlation between the ratio of Na/Cl and the rate of mortality after six months in individuals with HF [11]. The study revealed that the mortality rate showed an upward trend when the Na/Cl ratio dropped below 1.3 or surpassed 1.4. These findings differ slightly from our own, possibly due to variations in the study population. Our study primarily examined patients admitted to ICU with MI in the United States, whereas their research primarily concentrated on patients with HF from China.
For this research, we evaluated the Na/Cl ratio both as a continuous factor and by dividing it into groups for multivariate adjusted Cox regression and K‒M survival analysis. Furthermore, we conducted stratified analyses considering the occurrence of hyponatremia and hypochloremia and performed sensitivity analyses, all of which consistently reinforced strong findings. Assessing the Na/Cl ratio to evaluate the likelihood of in-hospital mortality in patients admitted to ICU with MI can aid doctors in improving their predictive skills for unfavorable outcomes and developing suitable treatment strategies. According to our research, it is typically observed that patients admitted to ICU with MI who have a Na/Cl ratio higher than 1.37 often experience congestion and impaired kidney function, which can result in electrolyte reabsorption disorders. In light of this, it is important to prioritize fluid control, enhance urine production, and promptly provide electrolyte supplements for managing these situations. Additionally, our study emphasizes a possible correlation between sodium and chloride levels, where low chloride levels may have a significant impact on the outcome of individuals with MI. In a clinical setting, it is essential to recognize and take proactive measures to handle hypochloremia. Therefore, it becomes essential to evaluate patient prognosis by considering both serum sodium and chloride levels together.
This study also has several limitations that should be acknowledged. First, we only assessed serum sodium and chloride levels at admission, without considering their temporal changes. Unfortunately, our study did not report the hormone levels that are known to be significant contributors to the development of hyponatremia and hypochloremia following MI. Additionally, it is crucial to mention that this research is of a retrospective nature, which brings about the potential for unmeasured confounding variables. In conclusion, to improve the dependability and applicability of our results, it would be advantageous to have a larger number of participants.
Conclusion
The results of our study indicate that a high ratio of Na/Cl is strongly linked to a higher likelihood of mortality during hospitalization in patients admitted to ICU diagnosed with MI. Furthermore, there seems to be a possible correlation between serum sodium and chloride concentrations, underscoring the significance of assessing both factors simultaneously when assessing patient outlook and directing treatment approaches in a medical environment.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors want to express their sincere gratitude to the creators and maintainers of this database.
Abbreviations
- ICU
Intensive care unit
- NSTEMI
Non-ST-Segment Elevation Myocardial Infarction
- COPD
Chronic obstructive pulmonary disease
- CCI
Charlson comorbidity index
- SAPSII
Simplified Acute Physiology Score
- MBP
Mean blood pressure
- RR
Respiratory rate
- SaO2
Oxygen saturation of blood
- PCI
Percutaneous Coronary Intervention
- MV
Mechanical ventilation
- NT-proBNP
N-terminal pro-brain natriuretic peptide
- WBC
White blood cell count
- eGFR
Estimated Glomerular Filtration Rate
- SAPSII
Simplified acute physiology score II
- Los hospital
Length of stay in hospital
- Los ICU
Length of intensive care unit stay time
- HR
Hazard Ratio
- CI
Confidence interval
Author contributions
All authors have participated in the work and take public responsibility for appropriate portions of the content, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. WD is responsible for the design of this work. CR contributed to the acquisition and analysis of the data. DX contributed to the interpretation of the data. CS is responsible for data statistics. WD and MY are involved in drafting and critically revising the manuscript. The final manuscript was read and endorsed by all authors.
Data availability
The clinical data used to support the findings of this study were supplied by Monitoring in Intensive Care Database IV version 2.0 (MIMIC-IV v.2.0). Although the database is publicly and freely available, researchers must complete the National Institutes of Health’s web-based course known as Protecting Human Research Participants to apply for permission to access the database. Data are available to researchers on request for purposes of reproducing the results or replicating the procedure by directly contacting the corresponding author.
Declarations
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by the Institutional Review Boards of Massachusetts Institute of Technology (Cambridge, MA, USA) and Beth Israel Deaconess Medical Center (Boston, MA, USA). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Valley TS, Iwashyna TJ, Cooke CR, Sinha SS, Ryan AM, Yeh RW, Nallamothu BK. Intensive care use and mortality among patients with ST elevation myocardial infarction: retrospective cohort study. BMJ. 2019;365:l1927. 10.1136/bmj.l1927. [DOI] [PMC free article] [PubMed]
- 2.Harrington DH, Stueben F, Lenahan CM. ST-Elevation myocardial infarction and Non-ST-Elevation myocardial infarction: Medical and Surgical interventions. Crit Care Nurs Clin North Am. 2019;31:49–64. 10.1016/j.cnc.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Zijlstra F, Suryapranata H, de Boer M-J. ST-segment elevation myocardial infarction: historical perspective and new horizons. Neth Heart J. 2020;28:93–8. 10.1007/s12471-020-01443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balling L, Schou M, Videbæk L, Hildebrandt P, Wiggers H, Gustafsson F. Prevalence and prognostic significance of hyponatraemia in outpatients with chronic heart failure. Eur J Heart Fail. 2011;13:968–73. 10.1093/eurjhf/hfr086. [DOI] [PubMed] [Google Scholar]
- 5.Rusinaru D, Tribouilloy C, Berry C, Richards AM, Whalley GA, Earle N, et al. Relationship of serum sodium concentration to mortality in a wide spectrum of heart failure patients with preserved and with reduced ejection fraction: an individual patient data meta-analysis(†): Meta-Analysis Global Group in chronic heart failure (MAGGIC). Eur J Heart Fail. 2012;14:1139–46. 10.1093/eurjhf/hfs099. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Peng R, Li X, Yu J, Chen X, Zhou Z. Serum chloride as a novel marker for adding prognostic information of mortality in chronic heart failure. Clin Chim Acta. 2018;483:112–8. 10.1016/j.cca.2018.04.028. [DOI] [PubMed] [Google Scholar]
- 7.Lorenz JN, Kotchen TA, Ott CE. Effect of na and cl infusion on loop function and plasma renin activity in rats. Am J Physiol. 1990;258:F1328–35. 10.1152/ajprenal.1990.258.5.F1328. [DOI] [PubMed] [Google Scholar]
- 8.Amornmarn R, Prempree T, Viravathana T, Donavanik V, Wizenberg MJ. A therapeutic approach to early vocal cord carcinoma. Acta Radiol Oncol. 1985;24:321–5. 10.3109/02841868509136059. [DOI] [PubMed] [Google Scholar]
- 9.Grodin JL, Simon J, Hachamovitch R, Wu Y, Jackson G, Halkar M, et al. Prognostic role of serum chloride levels in Acute Decompensated Heart failure. J Am Coll Cardiol. 2015;66:659–66. 10.1016/j.jacc.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Testani JM, Hanberg JS, Arroyo JP, Brisco MA, Maaten JM ter, Wilson FP et al. Hypochloraemia is strongly and independently associated with mortality in patients with chronic heart failure. Eur J Heart Fail. 2016;18:660–8. 10.1002/ejhf.477 [DOI] [PMC free article] [PubMed]
- 11.Fu Z, Zhang X, Zhao X, Wang Q. U-Shaped relationship of Sodium-to-chloride ratio on admission and mortality in Elderly patients with heart failure: a retrospective cohort study. Curr Probl Cardiol. 2023;48:101419. 10.1016/j.cpcardiol.2022.101419. [DOI] [PubMed] [Google Scholar]
- 12.Johnson A, Bulgarelli L, Pollard T, Horng S, Celi LA, Mark R. MIMIC-IV: PhysioNet; 2023.
- 13.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of Observational studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–7. 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 14.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–9. 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg A, Hammerman H, Petcherski S, Nassar M, Zdorovyak A, Yalonetsky S, et al. Hyponatremia and long-term mortality in survivors of acute ST-elevation myocardial infarction. Arch Intern Med. 2006;166:781–6. 10.1001/archinte.166.7.781. [DOI] [PubMed] [Google Scholar]
- 16.Ferreira JP, Girerd N, Duarte K, Coiro S, McMurray JJV, Dargie HJ, et al. Serum chloride and sodium interplay in patients with Acute myocardial infarction and heart failure with reduced ejection fraction: an analysis from the high-risk myocardial infarction database Initiative. Circ Heart Fail. 2017. 10.1161/CIRCHEARTFAILURE.116.003500. [DOI] [PubMed] [Google Scholar]
- 17.Kong X, Huang X, Zhao M, Xu B, Xu R, Song Y, et al. Platelet Count affects efficacy of folic acid in preventing First Stroke. J Am Coll Cardiol. 2018;71:2136–46. 10.1016/j.jacc.2018.02.072. [DOI] [PubMed] [Google Scholar]
- 18.Yu X, Chen J, Li Y, Liu H, Hou C, Zeng Q, et al. Threshold effects of moderately excessive fluoride exposure on children’s health: a potential association between dental fluorosis and loss of excellent intelligence. Environ Int. 2018;118:116–24. 10.1016/j.envint.2018.05.042. [DOI] [PubMed] [Google Scholar]
- 19.Park S-Y, Freedman ND, Haiman CA, Le Marchand L, Wilkens LR, Setiawan VW. Association of Coffee Consumption with Total and cause-specific mortality among nonwhite populations. Ann Intern Med. 2017;167:228–35. 10.7326/M16-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madan VD, Novak E, Rich MW. Impact of change in serum sodium concentration on mortality in patients hospitalized with heart failure and hyponatremia. Circ Heart Fail. 2011;4:637–43. 10.1161/CIRCHEARTFAILURE.111.961011. [DOI] [PubMed] [Google Scholar]
- 21.WaliM V, Yatiraj S. Study of serum sodium and potassium in acute myocardial infarction. J Clin Diagn Res. 2014;8:CC07–9. 10.7860/JCDR/2014/10417.5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar S, Berl T, Sodium. Lancet. 1998;352:220–8. 10.1016/S0140-6736(97)12169-9. [DOI] [PubMed] [Google Scholar]
- 23.Deen PM, Knoers NV. Vasopressin type-2 receptor and aquaporin-2 water channel mutants in nephrogenic diabetes insipidus. Am J Med Sci. 1998;316:300–9. 10.1097/00000441-199811000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Szczepanska-Sadowska E. The heart as a target of Vasopressin and other Cardiovascular peptides in Health and Cardiovascular diseases. Int J Mol Sci. 2022. 10.3390/ijms232214414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Havránek Š, Bělohlávek J, Škulec R, Kovárník T, Dytrych V, Linhart A. Long-term prognostic impact of hyponatremia in the ST-elevation myocardial infarction. Scand J Clin Lab Invest. 2011;71:38–44. 10.3109/00365513.2010.535012. [DOI] [PubMed] [Google Scholar]
- 26.Singla I, Zahid M, Good CB, Macioce A, Sonel AF. Effect of hyponatremia (< 135 mEq/L) on outcome in patients with non-ST-elevation acute coronary syndrome. Am J Cardiol. 2007;100:406–8. 10.1016/j.amjcard.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 27.Lazzeri C, Valente S, Chiostri M, Attanà P, Picariello C, Gensini GF. Usefulness of hyponatremia in the acute phase of ST-elevation myocardial infarction as a marker of severity. Am J Cardiol. 2012;110:1419–24. 10.1016/j.amjcard.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Grodin JL, Verbrugge FH, Ellis SG, Mullens W, Testani JM, Tang WHW. Importance of abnormal chloride homeostasis in stable chronic heart failure. Circ Heart Fail. 2016;9:e002453. 10.1161/CIRCHEARTFAILURE.115.002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Movafagh S, Cleemann L, Morad M. Regulation of cardiac ca(2+) channel by extracellular na(+). Cell Calcium. 2011;49:162–73. 10.1016/j.ceca.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mouallem M, Friedman E, Shemesh Y, Mayan H, Pauzner R, Farfel Z. Cardiac conduction defects associated with hyponatremia. Clin Cardiol. 1991;14:165–8. 10.1002/clc.4960140214. [DOI] [PubMed] [Google Scholar]
- 31.AbouEzzeddine O, Prasad A. Apical ballooning syndrome precipitated by hyponatremia. Int J Cardiol. 2010;145:e26–9. 10.1016/j.ijcard.2008.12.195. [DOI] [PubMed] [Google Scholar]
- 32.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–8. 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 33.Article R, Kataoka H, Cardiology. Open Access Plasma Renin Activity after Diuretic Treatment in Patients with Stable Heart Failure: With Special Reference to its Association with Electrolyte Chloride. COA 2021. 10.33140/COA.06.02.01
- 34.Terlecki M, Kocowska-Trytko M, Kurzyca A, Pavlinec C, Zając M, Rusinek J, et al. The sodium-chloride difference: a marker of prognosis in patients with acute myocardial infarction. Eur J Clin Invest. 2024;54:e14157. 10.1111/eci.14157. [DOI] [PubMed] [Google Scholar]
- 35.Zandijk AJL, van Norel MR, Julius FEC, Sepehrvand N, Pannu N, McAlister FA, et al. Chloride in Heart failure: the neglected Electrolyte. JACC Heart Fail. 2021;9:904–15. 10.1016/j.jchf.2021.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Grodin JL, Testani JM, Tang WHW. Reply: Hypochloremia in Acute Decompensated Heart failure. J Am Coll Cardiol. 2015;66:2683–4. 10.1016/j.jacc.2015.09.081. [DOI] [PubMed] [Google Scholar]
- 37.Fu Z, An L, Lu X, Sheng L, Liu H. Serum chloride is inversely Associated with 3 months outcomes in Chinese patients with heart failure, a Retrospective Cohort Study. Front Cardiovasc Med. 2022;9:855053. 10.3389/fcvm.2022.855053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The clinical data used to support the findings of this study were supplied by Monitoring in Intensive Care Database IV version 2.0 (MIMIC-IV v.2.0). Although the database is publicly and freely available, researchers must complete the National Institutes of Health’s web-based course known as Protecting Human Research Participants to apply for permission to access the database. Data are available to researchers on request for purposes of reproducing the results or replicating the procedure by directly contacting the corresponding author.