Abstract
As the current guidelines on myocardial revascularization recommend, transit-time flow measurement (TTFM) is increasingly used for intraoperative graft flow analysis during coronary artery bypass grafting (CABG) as a less invasive, more highly reproducible, and less time-consuming method. In addition to the morphological assessment using color Doppler, mean graft flow (Qm) > 15 ml/min, pulsatility index (PI) < 5.0, diastolic filling (DF) > 50%, and systolic reverse flow (SRF) < 4% have been reported to predict patent CABG grafts. However, it is difficult to determine the clear-cut cut-off value of these parameters, because they varies with the hemodynamic characters, including fractional flow reserve (FFR) of the target coronary artery. In addition to these parameters, we focused on fast Fourier transform (FFT) analysis, because the TTFM waveform morphology may be more important than Qm itself. FFT analysis is based on the principle that any periodic waveforms can be broken down into a series of pure sine waves or harmonics. Herein we review FFT analysis of the intraoperative TTFM waveforms for quality assessment of CABG grafts.
Keywords: Transit-time flow measurement, Fractional flow reserve, Coronary artery bypass grafting, Fast Fourier transform
Introduction
Transit-time flow measurement (TTFM) (Medi-Stim, Oslo, Norway), introduced in Japan in the late 1990s, is the most common method for intraoperative graft flow analysis during coronary artery bypass grafting (CABG), mainly due to its ease of less invasive, less time-consuming, and more highly reproducible use. The current European guidelines on myocardial revascularization recommend TTFM as Class IIa [1, 2]. A recent multicenter registry study also concluded that TTFM assessment should be routinely used in CABG procedures, even if interpretation depends on learning curves [3]. The TTFM is based on the principle to calculate blood flow volume from the difference in arrival time between ultrasonic transmission from the upstream and downstream transmission [4]. Many authors have attempted to validate TTFM seek the clear-cut values and algorithms of TTFM to predict CABG graft failure, using mean graft flow (Qm, mL/min); pulsatility index (PI) (= [maximal flow-minimal flow]/Qm); diastolic filling (DF, %) (= diastolic flow/systolic and diastolic flow); and systolic reverse flow (SRF, %) (= volume of backward flow/volume of forward flow) [4], as shown in Fig. 1.
Fig. 1.
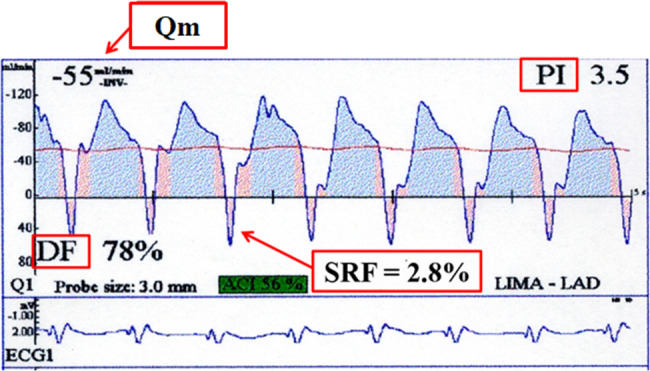
Parameters of intraoperative transit-time flow measurement (TTFM), mean graft flow (Qm); pulsatility index (PI); diastolic filling (DF); and systolic reverse flow (SRF)
In addition to the morphological evaluation by color Doppler, Qm > 15 ml/min, PI < 5.0, DF > 50%, SRF < 4%, and FFT ratio > 1.0 are the TTFM variables suggesting patent graft during CABG [4, 5]. A recent expert consensus suggests that Qm > 15 ml/min and PI < 5.0 are suggestive for patent grafts [6]. Another expert consensus advocates that Qm > 20 mL/min, PI < 3.0, and SFR < 3% for cut-off TTFM values to predict graft patency [7]. Di Giamarco et al. [8] and Tokuda et al. [9] reported that Qm, PI, and SFR predict one-year patency. Furthermore, it is reported that Qm, PI, and SFR predict mid-term (four and five- year) patency [10, 11]. A recent report also showed high PI (> 5.0) was an independent risk factor for major adverse cardiac and cerebrovascular events [12].
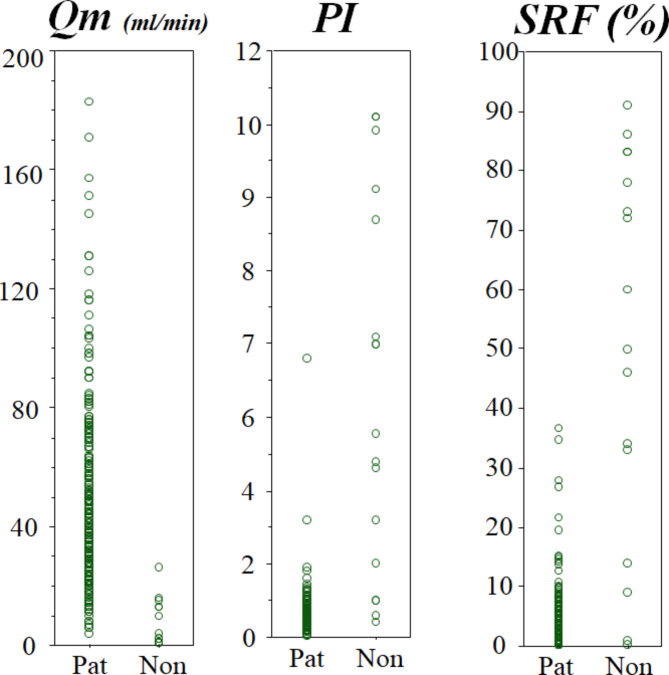
However, as shown in Fig. 2, it is difficult to determine the definitive cut-off values of Qm, PI, and SFR to detect non-patent grafts [11, 12]. These variables, especially Qm, vary with the dynamic characters, including systolic blood pressure and heart rate, coronary resistance, graft quality, and anastomotic quality [13]. Therefore, we have focused on fast Fourier transform (FFT) analysis, because the TTFM waveform morphology may be more important than Qm itself.
Fig. 2.
Distributions of the parameters of intraoperative transit-time flow measurement (TTFM), mean graft flow (Qm); pulsatility index (PI); and systolic reverse flow (SRF) [15]. Note that it is difficult to determine the definitive cut-off values of Qm, PI, and SFR to distinguish patent grafts (Pat) form non-patent grafts (Non)
FFT analysis
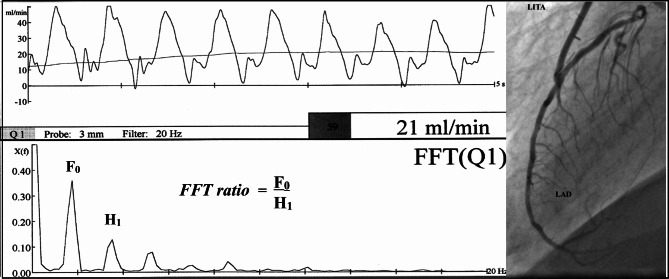
TTFM waveform morphology may be more important in relation to graft patency than Qm itself. However, the waveform morphology cannot reliably be assessed visually. To determine the clear-cut cut-off value of the waveform morphology to detect non-patent grafts, we focused on fast Fourier transform (FFT) analysis in 2001 [14, 15]. FFT analysis is based on the principle that any periodic waveforms can be broken down into a series of pure sine waves or harmonics. Harmonics exist at frequencies that are multiplies of the fundamental frequency and are described in terms of an amplitude and phase [16]. The greatest value of FFT is that it quantifies TTFM waveforms by transforming the waveforms into the frequency domain. When subjected to FFT analysis, a rectangular wave shows a clean decay waveform, while a disturbed waveform shows an amplified waveform rather than an attenuated waveform on FFT analysis. As a parameter representing gradual decrease in power of the harmonics of the fundamental frequency, the FFT ratio is calculated as F0/H1, where F0 is a power of the fundamental frequency and H1 is a power of the first harmonic on the FFT analysis of the blood flow curve, as shown in Fig. 3. A patent graft shows smooth attenuation of the FFT curve of graft flow, with F0/H1 ≥ 1.0, while a stenotic or occluded graft shows uneven attenuation with F0/H1 < 1.0.
Fig. 3.
Fast Fourier transform (FFT) analysis of intraoperative transit-time flow measurement (TTFM) waveform [15]. As a parameter representing gradual decrease in power of the harmonics of the fundamental frequency, the FFT ratio is calculated as F0/H1, where F0 is a power of the fundamental frequency and H1 is a power of the first harmonic
Four-quadrant matrix in relation between graft patency and TTFM (Fig. 4)
Fig. 4.
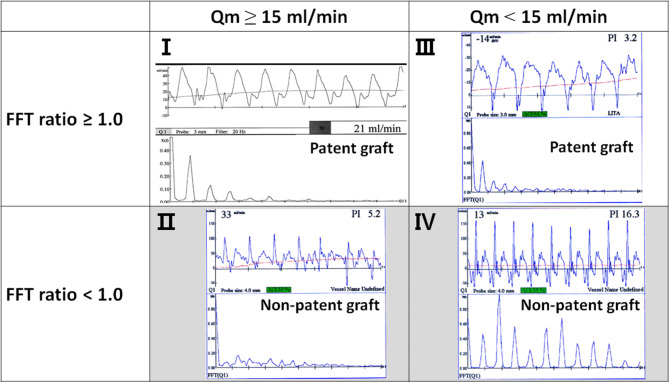
Four-quadrant matrix in relation between graft patency and the parameters of transit-time flow measurement (TTFM), mean graft flow (Qm) and fast Fourier transform (FFT) ratio
The first quadrant matrix (I) includes the graft with Qm ≥ 15 ml/min, whose FFT analysis shows smooth attenuation of the curve with F0/H1 ≥ 1.0. This quadrant matrix I suggests highly probable patency of the graft. The second quadrant matrix (II) includes the graft with Qm ≥ 15 ml/min, but whose FFT analysis shows rough attenuation of the curve with F0/H1 < 1.0. This quadrant matrix II suggests highly probable non-patency of the graft. The third quadrant matrix (III) includes the graft with Qm < 15 ml/min, but smooth attenuation of the curve with F0/H1 ≥ 1.0. This quadrant matrix III suggests highly probable patency of the graft. The fourth quadrant matrix (IV) includes the graft with Qm < 15 ml/min, whose FFT analysis shows rough attenuation of the curve with F0/H1 < 1.0. This quadrant matrix IV suggests highly probable non-patency of the graft.
In these ways, FFT is useful to predict the graft patency independently. When the TTFM indices are not good, such as Qm < 15 ml/min, PI > 5.0, or SFR > 4%, FFT analysis should be recommended. When the FFT ratio is 1.0 or higher, the graft anastomosis is adequate and the surgical indication for revascularization of the LAD is not adequate. When the FFT ratio is < 1.0, there may be a problem with the anastomosis, and therefore, graft revision should be considered [17].
Studies regarding FFT analysis of TTFM waveform
In 1999, Koenig et al. [18] reported in mongrel dogs during off-pump CABG that spectral analysis of graft flow waveforms was beneficial in detecting the anastomotic stenosis, compared to visual assessment of graft flow waveform morphology and/or Qm. They suggested that the first, second, and fourth harmonics of the magnitude component can be used to differentiate between patent and severely stenotic anastomoses.
In 2005, Kim et al. [19] assessed the validity of intraoperative TTFM, including FFT ratio, in 58 patients who underwent total arterial off-pump CABG. They concluded that TTFM is a reliable intraoperative tool to predict graft flow impairment, showing that the non-patent grafts demonstrated significantly lower Qm and FFT ratio and higher PI and SFR than patent grafts. In 2007, Hatada et al. [20] investigated the validity of Ha (= H5 + H6 + H7 + H8 + H9 + H10), where H5 as a power of the fifth harmonic, H6 as a power of the sixth harmonic, and sequentially as H7, H8, H9, and H10. They demonstrated that Ha in the group of patent grafts was significantly higher than that in the group of occluded grafts, suggesting harmonics of FFT analysis may become the parameter to express graft patency. In 2015, Uehara et al. [21] analysed the flow waveforms of the right gastroepiploic arterial (GEA) graft for right coronary artery (RCA), showing unique flow waveforms, by power spectral analysis based on the maximum entropy method in the frequency domain, and the non-linear least squares method in the time domain, which is more useful to investigate periodicities of time series of short data length such as graft flow data for 5 s than FFT. The patent grafts presented high power spectral at frequencies of 1, 2 and 3 Hz, while the non-patent grafts presented high power spectral in additional frequencies.
Mao et al. [22, 23] demonstrated that the FFT analysis can be applied to evaluate CABG graft flow using multi-scale models of a 3D LITA-LAD graft of different stenosis in LAD to get different magnitude of competitive flows. Their in vitro results showed that the FFT ratio > 1.0 is preserved for the patent LITA-LAD graft with any degree of stenosis. Alternatively, Jia et al. [16] reported that main wave (H0), the amplitude of the first harmonic (H1), and the frequency of the first harmonic are predictive parameters for graft failure, although they concluded that FFT of TTFM waveforms can be used to evaluate graft quality.
All these studies suggest very important roles of FFT analysis of TTFM waveforms to predict the graft patency. Although we have advocated FFT ratio as an index, several other indexes also have been proposed. We need further investigation to reveal which parameter of FFT is the best to predict unsuccessful grafts.
FFT ratio as an independent parameter
As a determinant of lesion-specific myocardial ischemia from coronary artery disease, fractional flow reserve (FFR) has been well validated, which a ratio of distal coronary pressure to proximal pressure measured under conditions of maximal hyperemia [24]. An FFR cutoff of 0.75 to 0.80 distinguishes between nonfunctionally from functionally significant coronary artery stenosis, as current guidelines recommend the use of FFR to complement coronary angiography [25]. CABG strategy is also shifting to FFR-guided, although its advantage over angiography-guided CABG is not clarified.
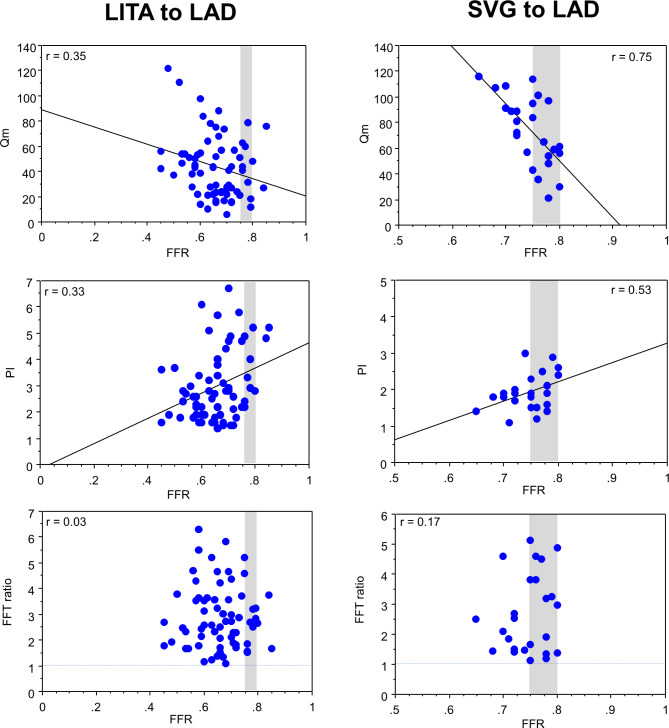
We retrospectively collected the data of 74 in-situ LITA grafts and 27 saphenous vein grafts (SVGs) to LAD, which fulfilled the following requirements: (1) preoperatively, the FFR of the target LAD (FFRLAD) with moderate stenosis on coronary angiography was measured to determine the surgical indication, (2) intraoperatively, TTFM was applied for graft analysis, (3) postoperatively, the patency of the LITA-LAD graft was confirmed by multi-slice computed tomography angiography, and (4) no ischemia in the LAD area was also identified on postoperative stress myocardial perfusion scintigraphy [26]. The LITA to LAD showed statistically significant negative correlations of the FFRLAD with Qm (r = -0.35; p = 0.039), while significant positive correlations were observed with PI (r = 0.33; p = 0.008) and SRF (r = 0.37; p = 0.002). No correlations of FFRLAD with FFT ratio of the LITA grafts were observed. The SVG to LAD showed statistically significant negative correlations of the FFRLAD with Qmax (r = -0.65, p = 0.004), Qmin (r = -0.43, p = 0.044), and Qm (r = -0.75, p = 0.001), while significant positive correlations were observed with PI (r = 0.53; p = 0.033) and SRF (r = 0.61; p = 0.009). The SVG to LAD did not show any correlations of FFRLAD with DF and FFT ratio. In summary, both LITAs and SVGs to LAD show negative correlations of FFRLAD with Qm, while showed positive correlations of FFRLAD with PI and SFR, as shown in Fig. 5. It is noted that the FFT ratio is not influenced by FFR.
Fig. 5.
Correlations of the preoperative fractional flow reserve (FFR) of the left anterior descending artery (LAD) and the parameters of intraoperative transit-time flow measurement (TTFM) of the in-situ left internal thoracic artery (LITA) graft and saphenous vein graft (SVG) to the LAD, including mean flow (Qm), pulsatility index (PI), and fast Fourier transform (FFT) ratio [26]. Note that the FFT ratio is not influenced by FFR
Honda et al. also compared the TTFM indices of the LITA-LAD graft between three patient groups divided according to preoperative FFR [27]. In the group with higher FFR ≧ 0.75, Qm was significantly lower and PI was significantly higher than the groups with FFR <0.70 and 0.70 ≦ FFR <0.75. Also, it was reported that intraoperative TTFM parameters (Qm and PI) of the LITA graft to the LAD are strongly affected by preoperative quantitative flow ratio (QFR) values, derived from the novel method for evaluating the functional significance of coronary stenosis by computation of FFR in the vessel based on 3-dimensional angiographic reconstruction and fluid dynamics algorithms [28, 29].
Recently, we reported a case with interesting flow characteristics of an in-situ LITA graft with angiographically competitive flow to the LAD, during the first CABG with aortic valve replacement (AVR) and during re-AVR seven years later [30]. Although intraoperative TTFM showed lower Qm and higher PI, suggesting inadequate anastomosis, FFT analysis of TTFM presented gradual waning of the amplitude, as shown in patent grafts.
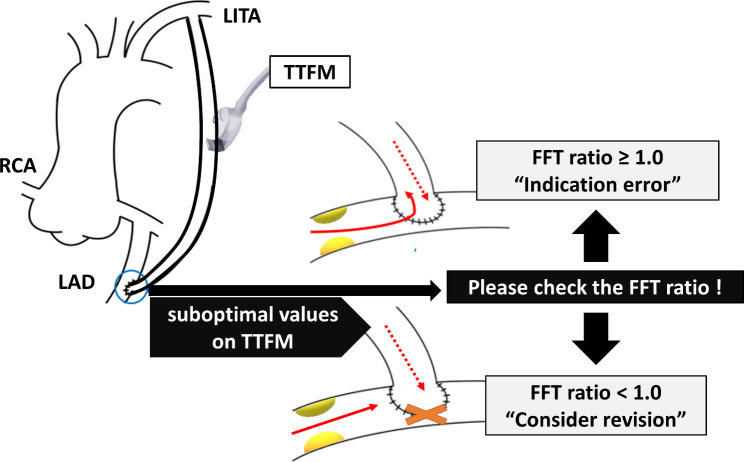
Clinical application of FFT analysis (Fig. 6)
Fig. 6.
Clinical application of fast Fourier transform (FFT) analysis. When the FFT ratio is ≥ 1.0, even if other transit-time flow measurement (TTFM) variables are suboptimal, the graft anastomosis can be considered satisfactory, and the surgical indication for revascularization is not adequate. Conversely, when the FFT ratio is < 1.0, there may be a potential issue with the anastomosis, and therefore graft revision should be considered. LITA: left internal thoracic artery, RCA: right coronary artery, LAD: left anterior descending artery
Since the FFT ratio is not influenced by FFR, FFT analysis of the TTFM waveforms may be recommend, especially for the graft to the LAD with gray-zone FFF (FFRLAD >0.75). When the FFT ratio is ≥ 1.0, even if other TTFM variables are suboptimal, the graft anastomosis can be considered satisfactory, and the surgical indication for revascularization is not adequate. Conversely, when the FFT ratio is < 1.0, there may be a potential issue with the anastomosis, and therefore graft revision should be considered.
Limitations
This review has several limitations. First, there is limited data of how FFT analysis correlates with long-term clinical outcomes. We need further investigation of the predictive value of FFT analysis for graft patency at one year, five years, or beyond. Second, there is the risk of overfitting when applying FFT to a relatively small sample size in our previous studies [14, 15, 17, 26]. Third, the lack of correlation of FFR with the FFT ratio could indicate that the FFT ratio might miss certain aspects of graft functionality that FFR captures. As is obvious, the FFT ratio should be used in combination with other TTFM parameters, Qm, PI, and SFR.
Conclusion
As shown in experimental and clinical studies, FFT analysis of the TTFM waveforms is useful to judge the patency of the CABG grafts, even with competitive flow, as an independent index upon FFR of the target coronary artery.
Author contributions
YT: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Visualization; Writing—original draft. AM: Supervision; Data curation; Project administration; Writing—review & editing. KY: Data curation; Project administration; Methodology; Validation; Writing—review & editing. KA: Data curation; Project administration. KA: Data curation; Project administration. KM: Data curation; Project administration. WN: Data curation; Project administration. YT; Supervision: Data curation; Project administration; Writing—review & editing.
Funding
No funding was received for the preparation of this manuscript.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
None.
Ethical approval
Not applicable.
Support/Grant information
None.
Institutional Research Review Board Approval
HM19-323, October 15, 2019.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kolh P, Windecker S, Alfonso F, Collet JP, Cremer J, Falk V, et al. 2014 ESC/EACTS guidelines on myocardial revascularization: the task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio- thoracic surgery (EACTS). Eur J Cardiothorac Surg. 2014;46:517–92. [DOI] [PubMed] [Google Scholar]
- 2.Evidence-based recommendations on MiraQ for assessing graft flow during coronary artery bypass graft surgery. NICE medical technologies guidance. Published November 2011. Last updated February 2018. https://www.nice.org.uk/Search?q.thetVeriQtsystem. Accessed April 29, 2024.
- 3.Laali M, Bouchot O, Fouquet O, Maureira P, Verhoye JP, Corbi P, et al. Analysis of a multicenter registry on evaluation of transit-time flow in coronary artery disease surgery. JTCVS Open. 2023;16:401–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takami Y, Takagi Y. Roles of Transit-Time Flow Measurement for coronary artery bypass surgery. Thorac Cardiovasc Surg. 2018;66:426–33. [DOI] [PubMed] [Google Scholar]
- 5.Gaudino M, Antoniades C, Benedetto U, Deb S, Di Franco A, Di Giammarco G, et al. ATLANTIC (Arterial Grafting International Consortium) Alliance. Mechanisms, consequences, and prevention of coronary graft failure. Circulation. 2017;136:1749–64. [DOI] [PubMed] [Google Scholar]
- 6.Gaudino M, Sandner S, Di Giammarco G, Di Franco A, Arai H, Asai T, et al. The Use of Intraoperative Transit Time Flow Measurement for coronary artery bypass surgery: systematic review of the evidence and Expert Opinion statements. Circulation. 2021;144:1160–71. [DOI] [PubMed] [Google Scholar]
- 7.Akhrass R, Bakaeen FG. Intraoperative graft patency validation: friend or foe? JTCVS Tech. 2021;7:131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Giammarco G, Pano M, Cirmeni S, Pelini P, Vitolla G, Di Mauro M. Predictive value of intraoperative transit-time flow measurement for short-term graft patency in coronary surgery. J Thorac Cardiovasc Surg. 2006;132:468–74. [DOI] [PubMed] [Google Scholar]
- 9.Tokuda Y, Song MH, Ueda Y, Usui A, Akita T. Predicting early coronary artery bypass graft failure by intraoperative transit time flow measurement. Ann Thorac Surg. 2007;84:1928–33. [DOI] [PubMed] [Google Scholar]
- 10.Tokuda Y, Song MH, Oshima H, Usui A, Ueda Y. Predicting midterm coronary artery bypass graft failure by intraoperative transit time flow measurement. Ann Thorac Surg. 2008;86:532–6. [DOI] [PubMed] [Google Scholar]
- 11.Zeng C, Li X, Dai Y, Zhou Y, Li C, Liu N, Wang J. Transit time flow measurement predicts graft patency in off-pump coronary artery bypass grafting upon 5-year angiographic follow-up. J Cardiothorac Surg. 2021;16:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim HH, Kim JH, Lee SH, Yoo KJ, Youn YN. Transit-time flow measurement and outcomes in coronary artery bypass graft patients. Semin Thorac Cardiovasc Surg. 2023;35:217–27. [DOI] [PubMed] [Google Scholar]
- 13.Jaber SF, Koenig SC, BhaskerRao B, VanHimbergen DJ, Cerrito PB, Ewert DJ, et al. Role of graft flow measurement technique in anastomotic quality assessment in minimally invasive CABG. Ann Thorac Surg. 1998;66:1087–92. [DOI] [PubMed] [Google Scholar]
- 14.Takami Y, Ina H. A simple method to determine anastomotic quality of coronary artery bypass grafting in the operating room. Cardiovasc Surg. 2001;9:499–503. [DOI] [PubMed] [Google Scholar]
- 15.Takami Y, Ina H. Relation of intraoperative flow measurement with postoperative quantitative angiographic assessment of coronary artery bypass grafting. Ann Thorac Surg. 2001;72:1270–4. [DOI] [PubMed] [Google Scholar]
- 16.Jia Y, Xu H, Su P, Gao J, Gu S, Liu Y, An X, Yan J, Zhang X. Predictive value of graft patency and major adverse cardiac and cerebrovascular events (MACCEs) in coronary artery bypass grafting (CABG) based on Fourier transform (FFT). J Thorac Dis. 2021;13(5):2705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noda M, Takami Y, Amano K, Sakurai Y, Akita K, Maekawa A, et al. Relation of fractional flow reserve with transit time coronary artery bypass graft flow measurement. Ann Thorac Surg. 2021;111:134–40. [DOI] [PubMed] [Google Scholar]
- 18.Koenig SC, VanHimbergen DJ, Jaber SF, Ewert DL, Cerrito P, Spence PA. Spectral analysis of graft flow for anastomotic error detection in off-pump CABG. Eur J Cardiothorac Surg. 1999;16(Suppl 1):S83–7. [DOI] [PubMed] [Google Scholar]
- 19.Kim KB, Kang CH, Lim C. Prediction of graft flow impairment by intraoperative transit time flow measurement in off-pump coronary artery bypass using arterial grafts. Ann Thorac Surg. 2005;80:594–8. [DOI] [PubMed] [Google Scholar]
- 20.Hatada A, Yoshimasu T, Kaneko M, Kawago M, Yuzaki M, Honda K. Relation of waveform of transit-time flow measurement and graft patency in coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2007;134:789–91. [DOI] [PubMed] [Google Scholar]
- 21.Uehara M, Takagi N, Muraki S, Yanase Y, Tabuchi M, Tachibana K, et al. New parameter of the right gastroepiploic arterial graft using the power spectral analysis device named MemCalc soft. Eur J Cardiothorac Surg. 2015;48:887–92. [DOI] [PubMed] [Google Scholar]
- 22.Mao B, Wang W, Zhao Z, Zhao X, Li L, Zhang H, Liu Y. On the relationship between competitive flow and FFT analysis of the flow waves in the left internal mammary artery graft in the process of CABG. Biomed Eng Online. 2016;15(Suppl 2):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mao B, Feng Y, Duan M, Dong Y, Li G, Li B, et al. A novel method to determine the cause of left internal mammary artery instant non-patency based on transit time flow measurement. Front Physiol. 2022;13:901280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spaan JA, Piek JJ, Hoffman JI, Siebes M. Physiological basis of clinically used coronary hemodynamic indices. Circulation. 2006;113:446–55. [DOI] [PubMed] [Google Scholar]
- 25.Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery revascularization: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice guidelines. Circulation. 2022;145:e4–17. [DOI] [PubMed] [Google Scholar]
- 26.Takami Y, Maekawa A, Yamana K, Akita K, Amano K, Sakurai Y, et al. Effects of Fractional Flow Reserve on Coronary Artery Bypass Graft Flow to Left Anterior descending artery. Circ J. 2023;87:1672–9. [DOI] [PubMed] [Google Scholar]
- 27.Honda K, Okamura Y, Nishimura Y, Uchita S, Yuzaki M, Kaneko M, et al. Graft flow assessment using a transit time flow meter in fractional flow reserve-guided coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2015;149:1622–8. [DOI] [PubMed] [Google Scholar]
- 28.Chen C, Zhao Y, Li W, Zhang K, Dang H, Liu T, et al. Relation of quantitative flow ratio with transit time coronary artery bypass graft flow measurement. Front Cardiovasc Med. 2022;9:975759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang P, Wang C, Hu Z, Hou Z, Song L, Dong Y, et al. Preoperative quantitative flow ratio, intraoperative transit time flow measurement parameters, and their predictive value for short-term graft failure after coronary artery bypass grafting. Circ J. 2024 Jun;5. 10.1253/circj.CJ-24-0078. Online ahead of print. [DOI] [PubMed]
- 30.Takami Y, Maekawa A, Yamana K, Akita K, Amano K, Sakurai Y, et al. Flow characteristics of in-situ internal thoracic artery graft with competitive flow. J Cardiol Cases. 2023;28:242–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.