Abstract
Introduction
Post-COVID syndrome (PCS) is characterized by a polymorphism of symptoms with hypothetical pathophysiological mechanisms. Here, we aimed to analyze the profile of inflammatory cytokines in patients with PCS and to study the relationship between this profile, the clinical symptoms as well as the endothelial function in PCS.
Methods
Our analytical study involved all eligible patients (n = 66) with PCS included from April 2021 to December 2021. The serum concentration of cytokines IFN-γ, IL-1α, IL-1β, IL-6, IL-8, IL-10, IL-12p70, IL-27, IP-10, MCP-1 and TNF-α was quantified by flow cytometry. Endothelial function was explored by assessing microvascular flow and reactivity using thermal probes. A comparative study was carried out according to the presence of each PCS symptom.
Results
The average age of our patients was 55.9 ± 16.2 years. The sex ratio was 0.69. Forty-one patients (62%) presented with a severe form of acute infection. The most frequently reported symptoms were dyspnea (67%), fatigue (50%), and memory problems (32%). Fifty-seven patients (86%) had endothelial dysfunction. The majority of patients had increased levels of IP-10 (100%), IL-8 (95%), IFN-γ (95%), MCP-1 (80%), and TNF-α (70%). The serum concentration of IL-10 was below the threshold of quantification in 89% of subjects. The severe form of acute infection was associated with elevated IL-10, MCP-1, and IL-27. Increased IL-6 and IL-27 levels were associated with fatigue while IL-8 concentrations were higher in patients who reported dyspnea. Elevation of IL-8 level was more common in patients with profound impairment of endothelial function.
Conclusion
Our results further support the presence of endothelial dysfunction in PCS and show an elevation of pro-inflammatory cytokines with a downmodulation of the IL-10- anti-inflammatory response. In addition, immuno-clinical phenotypes emerge, such as an inflammatory profile mediated by IL-6 and IL-27 in fatigue and IL-8 in dyspnea. The identification of immuno-clinical phenotypes would allow a better understanding of the pathophysiology of PCS symptoms.
Keywords: Cytokines, Endothelial dysfunction, Flow cytometry, Post-COVID-19 syndrome, SARS-CoV-2
Introduction
While the COVID-19 pandemic is currently deemed under control, marked by the World Health Organization (WHO) lifting the public health emergency of international concern on May 5, 2023 [1], the enduring risks and the potential medium to long-term impact on human health remain incompletely understood. A new concern, known as Long COVID, Post-COVID Syndrome (PCS), or Post-COVID Conditions, has emerged. This condition is defined by the persistence, recurrence, or onset of new health issues that patients may experience beyond three months after being infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 virus), as outlined by the Center for Disease Control and Prevention (CDC) [2]. According to the High Authority for Health (HAS) in France, the definition hinges on three criteria: having been symptomatic of an infection linked to COVID-19, exhibiting at least one symptom persisting beyond four weeks from the onset of the acute episode, and presenting symptoms that cannot be explained by another diagnosis [3]. Despite the easing of the immediate crisis, the potential long-term health implications of COVID-19 are yet to be fully delineated.
In a comprehensive meta-analysis and systematic review encompassing 47,910 patients, researchers identified over 50 persistent or newly emerging symptoms following an acute episode of COVID-19, irrespective of the severity of the initial infection—whether severe or mild, pauci-symptomatic, or asymptomatic. Remarkably, more than 80% of patients reported experiencing at least one symptom associated with long COVID [4].
The symptoms associated with PCS can manifest in both somatic and psychological dimensions, exerting a significant impact on individuals’ overall quality of life. Recognizing the substantial repercussions of PCS, the Americans with Disabilities Act (ADA) took a decisive step in July 2021 by officially designating long COVID as a disability in the United States [5].
Given the current lack of objective tools for a precise diagnosis of this condition, it becomes imperative to delve into the pathophysiological mechanisms that underlie it. This exploration is crucial for steering research efforts toward the identification of diagnostic, therapeutic, and preventive solutions, ultimately enhancing the care provided to individuals suffering from this syndrome. In this context, numerous authors have focused their attention on PCS, examining diverse pathophysiological mechanisms such as the persistence of a viral reservoir in target organ tissues [6, 7], induced autoimmunity [8], and the lasting residual inflammatory response involving a continuous reaction to mitochondrial stress [9].
Numerous authors have documented elevated levels of inflammatory cytokines in patients with PCS. Notably, these cytokine elevations have been observed in correlation with circulating endothelial cells (CEC), suggesting an influence on endothelial function [10]. The residual inflammation associated with sustained cytokine elevation and endothelial dysfunction (ED) could have potential systemic and organ-specific consequences, contributing to the manifestation of PCS symptoms [10–12]. Despite these intriguing connections, these mechanisms remain at the hypothetical stage. Thus, the primary aim of our study is to delineate the serum profile of inflammatory cytokines in PCS patients and explore its relationship with endothelial dysfunction. Furthermore, our secondary goal involves the establishment of immuno-clinical profiles through an examination of the interplay between inflammatory cytokines and various symptoms associated with PCS.
Materials and methods
Study population
This was a observational analytical study with prospective recruitment of patients, carried out at the Clinical Immunology Laboratory of the Pasteur Institute of Tunis in collaboration with the Department of Internal Medicine of the Mongi Slim Hospital, during the period from April 2021 to December 2021, having included patients in whom the diagnosis of SARS-CoV-2 infection was made between 4 and 24 weeks before the inclusion date and meeting the criteria definition of the PCS. Patients were recruited through face-to-face medical interviews, following the methodology outlined in the reference [13].
Were considered for this study patients aged > 18 years in whom the diagnosis of SARS-CoV-2 infection was established by Polymerase chain reaction (PCR) on nasopharyngeal swab and/or CT scan arguments at least 4 weeks before the date of inclusion and reporting at least one symptom meeting the PCS definition criteria and established by the TUN-EndCOV study [13]. We did not include, patients suffering from a chronic inflammatory disease or a neoplastic pathology, pregnant or breastfeeding patients and patients vaccinated against SARS-CoV-2. Finally, patients whose sample was not usable were excluded.
During the interview, carried out in the Internal Medicine department of Mongi Slim Hospital, we carried out for each patient:
The collection of epidemiological, clinical, and paraclinical data (cardiorespiratory history and risk factors, clinical form of the acute episode of SARS-CoV-2 infection, CT grades of lung involvement, and post-COVID symptoms).
The evaluation of endothelial function (EF).
the collection of a 5 ml blood sample by intravenous puncture, using a vacuum collection system on a dry tube. Samples were processed within 6 h of collection and sera were cryopreserved at -20 °C. The concentration of cytokines was subsequently quantified for all samples at a single time, at the Department Clinical of Immunology of the Pasteur Institute of Tunis.
Endothelial function exploration
Microvascular flow and reactivity were assessed with the “E4-Diagnose” Polymath Tunisia device [14]. This is a non-invasive device for measuring skin temperature at high resolution (0.002 °C). It thus uses the technique of digital thermo monitoring (DTM), independent of the operator, and is based on the principle of fully automated and standardized post-occlusion reactive hyperemia (PORH).
Thermal probes were attached to the index finger and thermal changes due to cuff occlusion were automatically recorded using Polymath software. Microvascular flow and reactivity were assessed in eligible patients after meeting the following conditions:
Be fasting for at least 4 h (including tobacco consumption).
Not having practiced intensive physical activity in the last 24 h.
Have a systolic blood pressure less than 160 mmHg.
Have a digital temperature above 27 °C.
Perform the test in a room whose temperature varied between 22 and 24 degrees.
As described by Charfeddine S. and colleagues [13], the thresholds accepted for judging endothelial function (Endothelial Quality Index (EQI) were as follows:
EQI ≥ 2: Good endothelial function (EF).
1.5 < EQI < 2: Moderate endothelial dysfunction.
1 < EQI ≤ 1.5: Severe endothelial dysfunction.
EQI ≤ 1: Very low endothelial function.
Serum cytokine measurement
The concentration of 11 cytokines (interferon (IFN)-γ, interleukin (IL)-1α, IL-1β, IL-6, IL-8, IL-10, IL-12p70, IL-27, Interferon gamma-induced protein 10 (IP-10), monocyte chemoattractant protein (MCP)-1 and Tumor Necrosis Factor (TNF)-α) was quantified in sera patients by the multiplex technique using the “Aimplex™ Human Inflammation” kit (Biosciences, Inc®) according to manufacturer procedures.
The serum concentration of each cytokine of interest was evaluated by comparing the intensity of the fluorescent signals obtained to that of a range of standards prepared by serial dilution of a known concentration of the cytokine studied. The sera as well as the standards were placed in a 96-well plate provided by the manufacturer and incubated for 1 h at room temperature. After removing the solution from the wells by a vacuum pump, the wells were washed 3 times. The cytokines were then detected by using the biotinylated detection antibody directed against the desired cytokine after 30 min-incubation. After 3 washes, PE-labeled Streptavidin was added to each well. Fluorescence signals of the beads were acquired by a flow cytometer (FACS Canto II, Becton Dickinson). The used threshold values align with the quantification values provided by the “Aimplex TM Human Inflammation” kit from Biosciences, Inc®.
Statistical analysis
Data were processed using SPSS version 25.0 software. Qualitative variables were described by absolute and relative frequencies. Quantitative variables were described by means and standard deviations. We compared the frequencies of positivity (above the threshold) and the average concentrations of cytokines as well as the frequencies of ED in the groups according to the main parameters studied.
According to the conditions of application, the comparison of percentages required the use of the Pearson chi-square test or the Fisher exact test. The comparison of means/medians between the two groups was carried out using the Student’s t-test or the non-parametric Mann-Whitney test as appropriate. ANOVA or Kruskal-Wallis tests were used to compare means/medians between the three groups. Furthermore, a correlation between quantitative variables (cytokine levels, age, EF) was assessed using the Spearman correlation test. For all tests, the significance level was set at a p-value < 0.05. Multiple logistic regression models were built in order to assess the impact of the clinical and biological features on PCS symptoms. Subsequently, the Logit formula was obtained from logistic regression adjustments together with receiver operating curve (ROC) analyses in order to assess the performances (sensitivity and specificity) of the fitting models.
Results
Study population description
Among the 75 patients initially recruited, nine subjects were secondarily excluded because the samples were not usable. The average time between diagnosis of SARS-CoV-2 infection and the date of inclusion was 82.8 ± 53.4 days. The mean age of our patients was 55.9 ± 16.2 years with extremes ranging from 22 years to 81 years and 67% of subjects were over 50 years old. They were 39 women (59%) and 27 men (41%) with a sex ratio of 0.69.
Among our patients, nine (14%) presented with pre-existing heart disease: coronary insufficiency (n = 3), heart rhythm disorders (n = 3), heart failure (n = 2), and valvular disease (n = 1). We noted chronic respiratory pathology in 12% (n = 8) of the study population: asthma in 7 patients and COPD in one patient.
Regarding the acute episode of SARS-CoV-2 infection, 41 patients (62%) presented a severe form of infection while 25 subjects (38%) had a mild to moderate form. A chest computed tomography (CT) scan was performed during the acute phase of SARS-CoV-2 infection in 36 patients (55%). Among these patients, severe damage to the lung parenchyma was noted in 17 subjects (47%). The characteristics of enrolled patients are summarized in Table 1.
Table 1.
Baseline characteristics of enrolled patients
| Patients | N = 66 |
|---|---|
| Agea (years) | 55.9 ± 16.2 |
| Age categories | |
| < 40 years | 13 (20%) |
| 40–59 years | 22 (33%) |
| ≥ 60 years | 31 (47%) |
| Gender ratio (Male/Female) | 0.69 (27/39) |
| Time from COVID-19 episode (days) | 82.8 ± 53.4 |
| BMIa, b | 27 ± 3.4 |
| Obesity (BMI ≥ 30) | 10 (15%) |
| High blood pressure | 24 (36%) |
| Diabetes | 16 (24%) |
| Dyslipidemia | 11 (17%) |
| COVID-19 form | |
| Mild | 24 (36%) |
| Moderate | 1 (2%) |
| Severe | 41 (62%) |
| Chest CT scan damage extent c | |
| < 10% | 3 (8%) |
| 10–25% | 6 (17%) |
| 25–50% | 10 (28%) |
| 50–75% | 17 (47%) |
| > 75% | 0 (0%) |
amean ± SD; bBMI: Body Mass Index; c Performed in only 36 patients, CT: Computed Tomography
Among the symptoms of PCS identified in our patients (Table 2), dyspnea (n = 44; 67%) and fatigue (n = 33; 50%) were the most frequently reported symptoms.
Table 2.
Frequencies of Post-COVID symptoms
| Symptoms | N = 66 |
|---|---|
| Dyspnea | 44 (67%) |
| Fatigue | 33 (50%) |
| Memory disorders | 21 (32%) |
| Myalgia | 19 (29%) |
| Chest pain | 18 (27%) |
| Headache | 13 (20%) |
| Palpitation | 13 (20%) |
| Cough | 13 (20%) |
| Sleep disorders | 10 (15%) |
| Anosmia | 9 (14%) |
| Ageusia | 7 (11%) |
| Abdominal pain | 4 (6%) |
Endothelial function exploration
In the present study, the endothelial function examination revealed ED in 57 patients (86%), including 39 subjects (59%) who had a severe ED or very low EF.
The frequency of ED was then compared according to the clinical form of acute SARS-CoV-2 infection as well as the symptoms of PCS. ED was more common in the group of patients who had a severe form of acute SARS-CoV-2 infection, with a p-value close to statistical significance (p = 0.072). Regarding the symptoms, ED was significantly associated with dyspnea occurrence (p = 0.031) (Fig. 1).
Fig. 1.
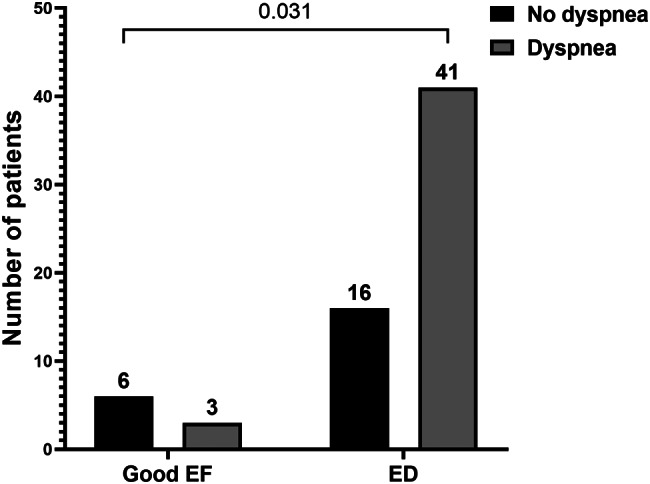
Significant association between ED and dyspnea. ED: Endothelial dysfunction, EF: Endothelial function
Cytokine measurement
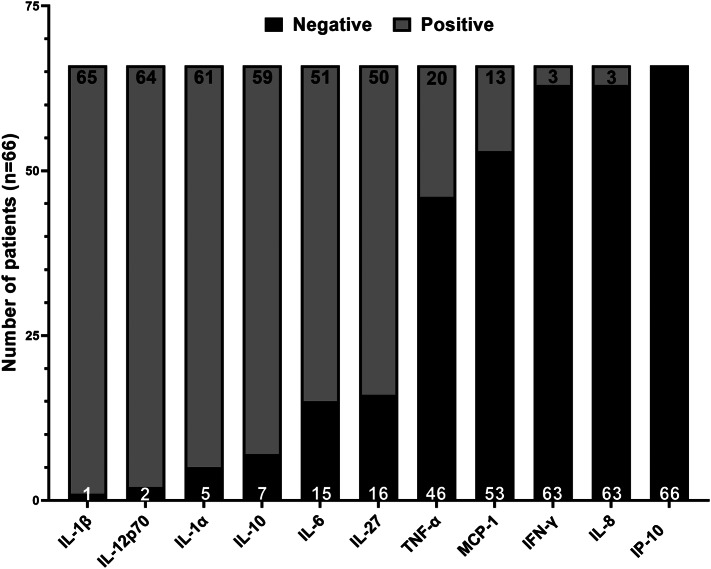
In all patients, an increase in the serum concentration of at least two proinflammatory cytokines among the assessed parameters was observed. As shown in Fig. 2, serum levels of IP-10, IL-8, IFN-γ, MCP-1, and TNF-α were elevated in the majority of our patients. However, it is noteworthy that the blood concentration of IL-10 was below the normal threshold (5pg/ml) in 89% of the study population (n = 59) including 38 patients (58%) who had non-detectable levels of this cytokine. Furthermore, the serum levels of IL-1α, IL-1β, and IL-12p70 were found to be below the quantification thresholds in the majority of patients.
Fig. 2.
Frequency of cytokine elevation in our patients. The frequency of patients with an elevated concentration of cytokines (above the threshold) is shown in the 66 included patients
We conducted a comparative analysis of cytokine positivity frequencies (above the threshold) and average concentrations among different groups, focusing on key parameters. Specifically, we compared these metrics across the three main age groups, exploring the correlation between age and cytokine levels.
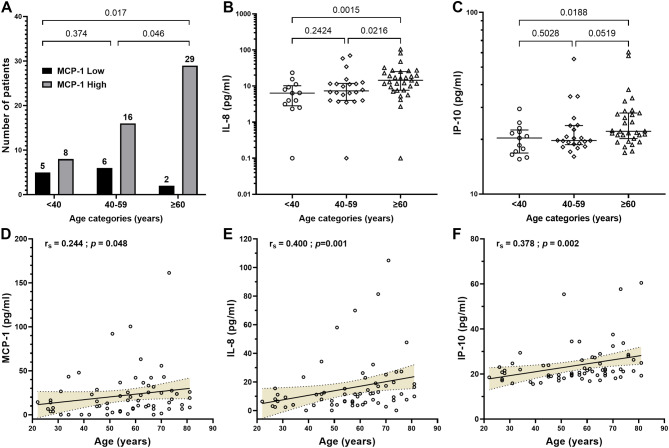
Significantly elevated MCP-1 levels were observed more frequently in patients aged 60 and above (p = 0.017) as depicted in Fig. 3A. Additionally, the median levels of IL-8 and IP-10 were notably higher in the 60 years and older age group compared to the other groups (Fig. 3B and C). Moreover, a positive correlation was identified between age and concentrations of MCP-1, IL-8, and IP-10 (r = 0.244, p = 0.048; r = 0.400, p = 0.001; r = 0.378, p = 0.002, respectively), further illustrated in Fig. 3D and E, and 3F.
Fig. 3.
MCP-1, IL-8 and IP-10 levels according to age. Patients with PCS were stratified in three age-groups. Frequencies of increased levels of MCP-1 (A) as well as median levels (B) of IL-8 (B) and IP-10 (C) are compared between groups. (D, E, F) Correlation between MCP1, IL-8 and IL-10 levels and age are shown
In terms of gender, our data revealed higher MCP-1 levels in men, approaching statistical significance (p = 0.071). However, the comparative analyses did not unveil statistical differences in cytokine levels between groups based on other epidemiological parameters (data not shown).
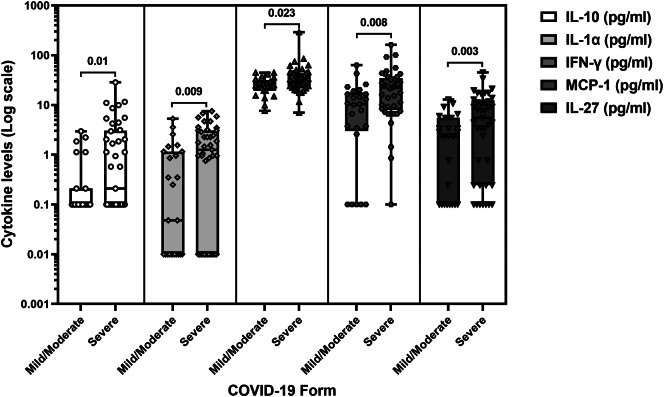
A noteworthy observation emerged in the context of the severity of acute SARS-CoV-2 infection. The incidence of elevated IL-10, MCP-1, or IL-27 levels was more frequent in patients who experienced a severe form of the infection (p = 0.039, p = 0.023, and p = 0.019, respectively) (Table 3). Moreover, individuals with a severe COVID-19 presentation exhibited significantly higher levels of IL-10 (p = 0.010), IL-1-α (p = 0.009), IFN-γ (p = 0.023), MCP-1 (p = 0.008), and IL-27 (p = 0.003), as illustrated in Fig. 4.
Table 3.
Cytokine level elevation frequencies according to the clinical form of SARS-CoV-2 infection
| Cytokine | COVID-19 form | p-value | |
|---|---|---|---|
| Severe (n = 41) | Mild/Moderate (n = 25) | ||
| IL-1β | 2% | 0% | 1 |
| IL-10 | 17% | 0% | 0.039 |
| IL-6 | 24% | 20% | 0.769 |
| IL-1α | 10% | 4% | 0.642 |
| IFN-γ | 98% | 92% | 0.552 |
| IL-12p70 | 5% | 0% | 0.522 |
| IL-8 | 98% | 92% | 0.552 |
| TNF-α | 66% | 76% | 0.423 |
| IP-10 | 100% | 100% | 1 |
| MCP-1 | 90% | 64% | 0.023 |
| IL-27 | 34% | 8% | 0.019 |
Fig. 4.
Levels of IL-10, IL-1α, IFN-γ, MCP-1 and IL-27 in patients according to the severity of the SARS-CoV-2 infection
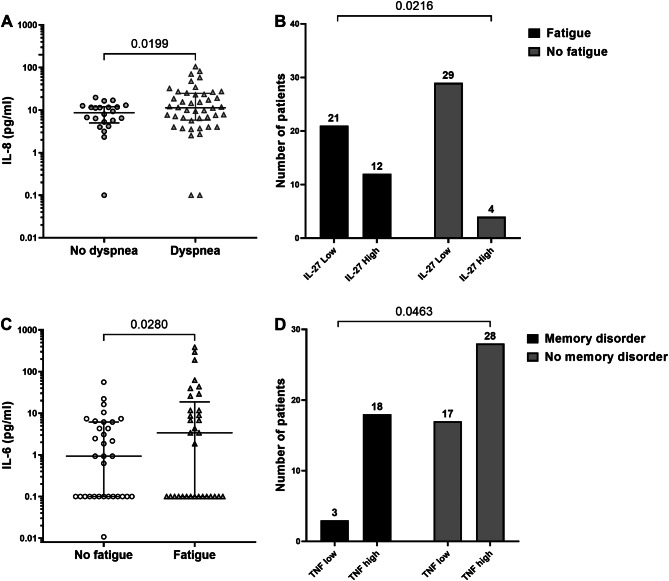
We then compared the frequencies of positivity and the average levels of cytokines in the groups based on reported symptoms within PCS. As illustrated in Fig. 5A, the IL-8 level exhibited a significant increase in the group of patients reporting dyspnea (p = 0.0199). Notably, patients with a reported cough displayed significantly lower IL-1β levels (p = 0.014) (data not shown). Additionally, elevated IL-27 levels were significantly associated with fatigue (p = 0.021) (Fig. 5B). Moreover, patients experiencing fatigue demonstrated had significant higher levels of IL-6 (p = 0.028) (Fig. 5C) Lastly, the elevation increase of TNF-α levels was significantly more prevalent in the group patients reporting memory problems (p = 0.046) (Fig. 5D).
Fig. 5.
Association between cytokine elevation and PCS symptoms
Multiple logistic regression revealed that dyslipidemia, IL-10, MCP-1 and IL-27 levels were significantly associated with fatigue risk (Table 4). Hence, for each increase of MCP-1 and IL-27 by 1 pg/ml, the risk of fatigue increases by respectively 7.3% and 26.9%.
Table 4.
Factors associated with fatigue risk
| Item | Fatigue risk | |
|---|---|---|
| OR [95% CI] | p-value | |
| IL-10 | 0.552 [0.315–0.965] | 0.037 |
| MCP-1 | 1.073 [1.004–1.147] | 0.035 |
| IL-27 | 1.269 [1.012–1.592] | 0.019 |
Model summary: Nagelkerke R2 = 0.654; Omnibus p-value = 0.002
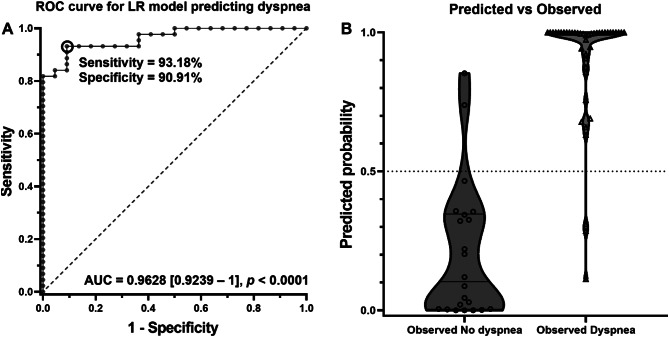
The most parsimonious model for symptom PCS prediction was this for dyspnea (Fig. 6). The multiple logistic regression carried out by integrating age, gender, BMI, history of high blood pressure (HBP) and diabetes, smoking habit, severe COVID-19, an EQI < 1.5 and the following cytokine ratios: IL-1α/IL-10, IL-1β/IL-10, IL-6/IL-10 and TNF/IL-10 predicted dyspnea occurrence with a 92.4% precision, Nagelkerke R2 = 0.795, p = 1.4 E-7. The area under the ROC curve (AUC) for the dyspnea prediction was 0.9628 [0.9239–1] (p < 0.0001) with a 93.18% sensitivity and a 90.91% specificity (Fig. 6). The following formula characterizes the fitting model: Logit dyspnea = -6.286 + (0.017*Age) + (2.783*gender†) + (0.092*BMI) + (0.881*HBP) + (3.983*diabetes) + (5.495*smoking habit) + (1.469*severe COVID-19) + (0.12*IL-1α/IL-10) + (0.005*IL-1β/IL-10) + (0.007*IL-6/IL-10) + (0.017*TNF/IL-10) + (5.221*EQI < 1.5). (†Gender: Male = 1, Female = 0). Subsequently, dyspnea risk can be estimated by exponentiating the Logit result.
Fig. 6.
A: Multiple logistic regression for dyspnea prediction. ROC curve for the dyspnea prediction model. B: Predicted versus observed plot: if probability is ≥ 0.5 the model predicts dyspnea
Endothelial function and cytokines
In our study, we noted an association approaching statistical significance between the elevation in IL-8 levels and an EQI ≤ 1.5 (severe ED or very low EF) (p = 0.064) (Fig. 7).
Fig. 7.
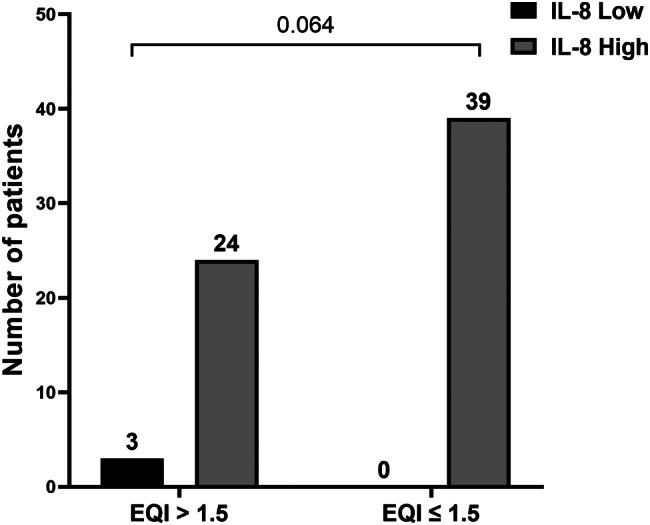
IL-8 elevation according to the Endothelial Quality Index (EQI)
The correlation study between EQI values and cytokine levels revealed no significant correlation (Table 5).
Table 5.
Correlation between cytokine levels and the endothelial Quality Index (EQI)
| Spearman’s rank correlation coefficient (rs) | p | |
|---|---|---|
| IL-1β and EQI | 0.067 | 0.593 |
| IL-10 and EQI | -0.191 | 0.124 |
| IL-6 and EQI | -0.031 | 0.802 |
| IL-1α and EQI | -0.223 | 0.072 |
| IFN-γ and EQI | -0.220 | 0.076 |
| IL-12p70 and EQI | 0.158 | 0.205 |
| IL-8 and EQI | -0.016 | 0.897 |
| TNF-α and EQI | 0.033 | 0.791 |
| IP-10 and EQI | -0.130 | 0.299 |
| MCP-1 and EQI | -0.087 | 0.487 |
| IL-27 and EQI | -0.098 | 0.435 |
Multivariate analysis by multiple logistic regression showed that patients’ age was significantly associated with an EQI < 1.5, OR [95% CI] = 1.124 [1.02–1.238], p = 0.018. Moreover, IL-1α level was significantly associated with increased risk of an EQI < 1, OR [95% CI] = 2.617 [1.17–5.855], p = 0.019. This finding indicates that each 1 pg/ml increase in IL-1α, increases risk of EQI < 1 by 161.7%.
Discussion
Post-COVID Syndrome corresponds to a spectrum of clinical conditions that emerge following the initial recovery from SARS-CoV-2 infection [2]. It represents a public health problem and a source of disability [5, 15]. According to a comprehensive meta-analysis and systematic review, over 50 symptoms have been documented in the context of PCS, with prevalence rates reaching up to 80% among individuals convalescing from acute infection by the virus [4].
Despite its widespread impact, the pathophysiological mechanisms underlying PCS remain in the hypothetical stage. A better understanding of these mechanisms is crucial for the optimal management of PCS patients. Several authors propose the hypothesis of persistent residual inflammation as the root cause of PCS, potentially involving induced autoimmunity [8], the presence of a viral reservoir in target tissues [6, 7], and sustained responses to mitochondrial stress [9] or endothelial dysfunction [10]. This residual inflammation, coupled with a sustained elevation of cytokines, could lead to systemic or organ-specific consequences, affecting areas such as cardiac [16, 17], neuropsychiatric [18, 19], renal [20], bone [21], and metabolic [22] functions.
Consequently, an objective assessment of cytokine levels could help identify patients with residual inflammation as a contributing factor to PCS manifestations [11]. The key findings of our study include the identification of endothelial dysfunction in a substantial majority of patients and a distinctive cytokine profile characterized by elevated pro-inflammatory cytokines and down-modulated anti-inflammatory response, particularly IL-10. Notably, our results elucidate immuno-clinical phenotypes, associating specific cytokines (IL-6, IL-27, and IL-8) with symptoms such as fatigue and dyspnea thus revealing varied pathophysiological mechanisms associated with different PCS symptoms.
Our study included 66 patients with PCS, assessed at an average of 82.8 ± 53.4 days post-SARS-CoV-2 infection. The majority of participants were women (59%), aged over 50 (67%), and had experienced a severe form of acute infection (62%). Consistent with previous findings [23–27], the most frequently reported symptoms included dyspnea in 44 patients (67%) and fatigue in 33 patients (50%).
Notably, ED was identified in 86% of the study population and was more frequent in patients who had presented a severe form of acute infection and in those who reported dyspnea. The ED prevalence in our study exceeded that which is commonly reported. For instance, the Tunisian multicenter TUN-EndCOV study, led by Charfeddine et al., investigated EF in 798 individuals recovered from SARS-CoV-2 infection using a device identical to the one employed in our study. Among the 618 patients included in this study, ED was observed in 319 patients (51.6%) [13]. Notably, our patients were recruited a large proportion of our patients were part of the TUN-EndCOV study. The higher frequency of ED observed in our study, in comparison to the study conducted by Charfeddine et al., may be attributed to a recruitment bias. Notably, a substantial proportion of our patients (62%, compared to 25.7% in the study by Charfeddine et al.) had experienced a severe respiratory form during the acute phase of SARS-CoV-2 infection. Interestingly, we observed that ED was more frequent in patients who presented a severe form of acute infection with a p value close to statistical significance, a finding consistent with some other reports in the literature [28, 29]. Moreover, ED was significantly associated with dyspnea occurrence (p = 0.031). This aligns with a case-control study by de Ambrosino et al., exclusively involving patients with a severe form of acute infection related to SARS-CoV-2, which reported a positive correlation between ED and the severity of respiratory deficiency in PCS [30]. This implies a potential heightened risk of ED during severe forms of acute infection linked to SARS-CoV-2, persisting after initial recovery, particularly in patients reporting respiratory symptoms within the context of PCS. Consequently, our results further underscore the presence of ED in the spectrum of long COVID. In line with our findings, the European Society of Cardiology recommends the evaluation of EF post-COVID-19, considering it a pertinent monitoring tool for detecting and potentially preventing the early onset of long-term complications associated with COVID-19 [31].
Significant findings emerged from the study, revealing elevated levels of two or more pro-inflammatory cytokines in all examined patients, with the majority showcasing increased levels of IP-10, IL-8, IFN-γ, MCP-1, and TNF-α. This observation strongly supports the persistence of residual inflammation during PCS. Notably, the down-modulation of IL-10, a crucial regulator of the intensity and duration of inflammatory responses, adds a critical dimension to our understanding. The negative regulation exerted by anti-inflammatory cytokines like IL-10 is pivotal in preventing excessive inflammation [32, 33]. The absence of this regulatory mechanism is particularly noteworthy and may contribute to the perpetuation of chronic inflammation, especially within the context of PCS. Similiraly, Queiroz et al. reported that low serum levels of IL-10 along with high levels of IL -17 and IL-2, may constitute a molecular signature for long COVID-19 [34].
Increased levels of three cytokines, namely IL-8, IP-10, and MCP-1, have been linked to age, suggesting that older adults with PCS are more prone to sustained inflammation following SARS-CoV-2 infection. This association may elucidate the high prevalence of PCS in advanced-age patients, as documented in existing literature [24].
Remarkably, severe acute COVID-19, has been correlated with elevated levels of IL-10, IL-1a, IFN-γ, MCP-1, and IL-27. A comprehensive meta-analysis including 44 studies and 7,865 patients indicated elevated levels of both pro- and anti-inflammatory cytokines—including IL-2, IL-2R, IL-4, IL-6, IL-8, IL-10, TNF-α, and IFN-γ during severe SARS-CoV-2 infection [35].
The longitudinal study conducted by Zhao et al. highlighted a correlation between elevated levels of IFN-γ, MCP-1, IL-27, and IL-10 and the severity of SARS-CoV-2 infection. Notably, patients experiencing severe disease exhibited a persistent increase in pro-inflammatory cytokines and IL-10 even four weeks post-infection [36]. This sustained elevation, particularly in pro-inflammatory cytokines, could establish an amplifying loop, creating a positive feedback mechanism that triggers a hyper-inflammatory response known as “the cytokine storm” [37]. The excessive production of cytokines may further stimulate subsequent immuno-pathological events with multivisceral involvement [38].
Schultheiß et al. introduced the concept of persistent reprogramming of specific pro-inflammatory immune cells. They proposed that self-perpetuating and uncontrolled hyper-inflammation during the severe phase of infection might lead to enduring disruptions of immune cells, resulting in residual inflammation during the PCS [39]. Consequently, it is conceivable that an increase of pro-inflammatory cytokines during PCS, coupled with an elevated level of the anti-inflammatory cytokine IL-10, may reflect increased inflammation from the acute phase that persists due to distinct pathophysiological mechanisms. This observation is particularly relevant in patients who experienced a severe form of acute SARS-CoV-2 infection and may contribute to our understanding of the underlying factors in the development of long COVID.
Our study has brought to light intriguing associations between PCS symptoms and cytokines. The reported PCS symptoms may differ in their nature, frequency and topography, suggesting the potential existence of distinct forms of long COVID with specific underlying pathophysiological mechanisms. Notably, IL-8 levels were significantly elevated in patients who reported dyspnea. This elevation of IL-8 was consistently observed in individuals with profound EF impairment, and dyspnea was also linked to endothelial dysfunction.
Studies on acute respiratory distress syndrome have demonstrated that pro-inflammatory cytokines such as IL-8 can induce airway inflammation and disrupt the alveolar-capillary barrier [40, 41]. Furthermore, research has indicated that IL-8 is involved in the pathogenesis of chronic inflammatory lung diseases [42] and plays a pivotal role in the development of pulmonary fibrosis, a condition often associated with persistent dyspnea in PCS patients [43]. The heightened levels of IL-8 may reflect persistent endotheliitis in individuals with ED who experience long COVID [44]. Notably, IL-8 is primarily secreted by endothelial cells and monocytes, playing a crucial role in endothelial inflammation and the regulation of vascular permeability [45, 46]. Exploring the role of IL-8 in the pathogenesis of dyspnea during PCS holds promise for enhancing our understanding of the underlying pathophysiological mechanisms underlying this symptom, particularly considering dyspnea is frequently reported in the literature as one of the predominant symptoms during long COVID.
In individuals reporting a cough, IL-1β levels were significantly lower hinting at potential distinct pathophysiological mechanisms compared to dyspnea such as the tissue sequelae caused during acute infection by SARS-CoV-2 [11]. Another important fact reported herein is the association between fatigue and elevated IL-27 and IL-6 levels. A study leveraging data from the UK Biobank and the Netherlands Study of Depression and Anxiety (NESDA) cohorts, based on genetic Mendelian randomization analysis, established a probable causal link between IL-6 and the onset of fatigue and sleep disorders [47]. Elevated pro-inflammatory cytokines, such as IL-6, following SARS-CoV-2 infection, have the potential to disrupt T helper (TH) 17 and regulatory T (T reg) cell responses. This disruption in the Th17/Treg balance might impact neuronal energy metabolism, manifesting clinically as fatigue and sleep disorders [48].
Regarding IL-27, initially identified as a pro-inflammatory cytokine acting through the expansion of Th1 cells [49], it may also exhibit immunomodulatory activity by inhibiting the expansion of Th17 cells [50]. It is plausible that elevated levels of IL-27 in patients reporting fatigue during PCS reflect its immunosuppressive activity, aiming to counteract the detrimental effects of pro-inflammatory cytokines such as IL-6 in these individuals. Enhanced comprehension, through future studies, of the roles played by IL-6 and IL-27 in the pathophysiology of fatigue— a prominent symptom in long COVID—holds promise for identifying potential diagnostic and therapeutic tools for individuals experiencing chronic fatigue during the post-acute phase of SARS-CoV-2 infection.
Finally, in subjects experiencing memory disorders, the presence of TNF-α was notably more frequent. Under normal physiological conditions, TNF-α, at low concentrations, is involved in cognitive processes including neurogenesis, neuromodulation, synaptic plasticity, and memory capacities [51]. However, during severe or chronic immune activation, elevated levels of TNF-α may lead to excitotoxicity in neuronal circuits, neurodegeneration, and subsequent disruptions in memory, neuronal plasticity, and learning [52]. Researchers have substantiated these findings by observing deleterious effects of TNF-α on memory abilities in transgenic mice [53–55].In human studies, several authors have documented an association between high TNF-α levels and memory impairment in pathological conditions marked by dysregulation of inflammatory processes, such as breast cancer [56], drug addiction [57], and type 2 diabetes [58]. Collectively, our data provide support for considering TNF-α as a potentially valuable diagnostic tool. This insight opens the possibility for targeted curative and preventive therapeutic interventions in individuals with memory disorders within the context of PCS.
Our study has some limitations. Firstly, the cross-sectional design restricted our ability to explore the dynamic changes in cytokine levels throughout the progression of long COVID. Secondly, the absence of a group comprising individuals who contracted SARS-CoV-2 but did not develop PCS represents another limitation. However, it should be noted that one of the primary objectives of our study is to compare cytokine levels in groups of PCS patients with and without endothelial dysfunction. Additionally, the relatively modest size of our study population poses a potential constraint. Technical limitations in cytokine quantification tools further constrained the size of our workforce during the study. Conversely, our study boasts several notable strengths. Given that PCS is a novel entity with yet-to-be-fully elucidated pathophysiological mechanisms, our research aimed to contribute to a comprehensive understanding for optimal patient management. Notably, our study stands as the first in Africa to investigate inflammatory cytokine levels in PCS. The prospective recruitment of patients through face-to-face medical interviews helped avoid observation bias. A distinctive feature of our study is the inclusion of unvaccinated patients against SARS-CoV-2, allowing us to eliminate potential biases associated with vaccination in cytokine profile interpretation. Lastly, our investigation delved into the cytokine profile associated with each reported symptom, an aspect rarely explored in the literature.
Conclusion
Our innovative strategy of examining the cytokine profile associated with each symptom of PCS represents an interesting and more relevant line of research. We strongly recommend the adoption of this approach in future studies, as it holds promise for refining the current definition of PCS and allowing a deeper comprehension of cytokine involvement in its pathogenesis. Cytokines, identified through this symptom-specific analysis, could potentially serve as valuable diagnostic and prognostic biomarkers during the PCS phase, opening the door to targeted therapeutic interventions such as anti-cytokine treatments. The application of these treatments, proven effective in various dysimmune pathologies, presents an attractive alternative approach in managing PCS.
Acknowledgements
The authors wish to extend their sincere gratitude to the patients who generously participated in this study. Their contribution has advanced our understanding in this field.
Abbreviations
- COVID-19
Corona Virus Disease 2019
- CT
Computed Tomography
- EF
Endothelial function
- ED
Endothelial dysfunction
- EQI
Endothelial Quality Index
- PCS
Post-COVID syndrome
Author contributions
C.T., I.Z.,Z.M, K.B. and M.B.A.: Conceptualization, Methodology, Investigation, Formal analysis, Writing - review & editingG.K. and J.B.: Formal analysis.S.C., S.A. and S.H.: Resources, Investigation.S.M., A.B.H., S.S. and Y.G.: Investigation.I.Z., Z.M., K.B. and M.B.A.: Supervision T.D.: Formal analysis, Writing - review & editing C.T., I.Z. and M.B.A.: Writing - original draft.
Funding
This work was supported by Institut Pasteur de Tunis, Tunis, Tunisia.
Data availability
All data generated or analyzed during this study are included in this published article. The data that support the findings of this study are available from the corresponding author upon reasonable req.
Declarations
Ethics approval and consent to participate
The study was approved by the ethics committee of Mongi Slim hospital (number 40/2023). All patients provided written informed consent for the collection of samples and subsequent analysis. All research was conducted according to the declaration of Helsinki principles.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Allocution liminaire du Directeur général. de l’OMS lors du point presse sur la COVID-19–5 mai 2023. [cited 2023 Sep 6]. https://www.who.int/fr/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing---5-may-2023
- 2.CDC, Post. -COVID Conditions. Centers for Disease Control and Prevention. 2022 [cited 2023 Feb 28]. https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html
- 3.COVID long. les recommandations de la Haute Autorité de santé. [cited 2022 Jan 8]. https://www.service-public.fr/particuliers/actualites/A14678
- 4.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11:16144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rights (OCR) O for C. Guidance on Long COVID as a Disability Under the ADA, Sect. 504, and Sect. 1557. HHS.gov. 2021 [cited 2022 Jan 8]. https://www.hhs.gov/civil-rights/for-providers/civil-rights-COVID19/guidance-long-COVID-disability/index.html
- 6.Patterson BK, Francisco EB, Yogendra R, Long E, Pise A, Rodrigues H, et al. Persistence of SARS CoV-2 S1 protein in CD16 + monocytes in Post-acute Sequelae of COVID-19 (PASC) up to 15 months post-infection. Front Immunol. 2022;12:746021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao XH, He ZC, Li TY, Zhang HR, Wang Y, Mou H, et al. Pathological evidence for residual SARS-CoV-2 in pulmonary tissues of a ready-for-discharge patient. Cell Res. 2020;30(6):541–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peluso MJ, Lu S, Tang AF, Durstenfeld MS, Ho H en, Goldberg SA, et al. Markers of Immune activation and inflammation in individuals with Postacute sequelae of severe Acute Respiratory Syndrome Coronavirus 2 infection. J Infect Dis. 2021;224(11):1839–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doykov I, Hällqvist J, Gilmour KC, Grandjean L, Mills K, Heywood WE. The long tail of COVID-19’ - the detection of a prolonged inflammatory response after a SARS-CoV-2 infection in asymptomatic and mildly affected patients. F1000Research. 2021;9:1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chioh FW, Fong SW, Young BE, Wu KX, Siau A, Krishnan S, et al. Convalescent COVID-19 patients are susceptible to endothelial dysfunction due to persistent immune activation. eLife. 2021;10:e64909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehandru S, Merad M. Pathological sequelae of long-haul COVID. Nat Immunol. 2022;23(2):194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Østergaard L. SARS CoV-2 related microvascular damage and symptoms during and after COVID‐19: consequences of capillary transit‐time changes, tissue hypoxia and inflammation. Physiol Rep. 2021;9(3):e14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charfeddine S, Ibn Hadj Amor H, Jdidi J, Torjmen S, Kraiem S, Hammami R, et al. Long COVID 19 syndrome: is it related to Microcirculation and endothelial dysfunction? Insights from TUN-EndCOV study. Front Cardiovasc Med. 2021;8:745758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Polymath Company. E4 - Diagnose endothelium explorer made easy for early diagnose. 2021 Feb [cited 2022 nov 20]. https://www.polymath.company/E4-Diagnose.html
- 15.Parums DV, Editorial. Long COVID, or Post-COVID Syndrome, and the global impact on Health Care. Med Sci Monit Int Med J Exp Clin Res. 2021;27:e933446–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gemayel C, Pelliccia A, Thompson PD. Arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol. 2001;38(7):1773–81. [DOI] [PubMed] [Google Scholar]
- 17.Lazzerini PE, Laghi-Pasini F, Boutjdir M, Capecchi PL. Cardioimmunology of arrhythmias: the role of autoimmune and inflammatory cardiac channelopathies. Nat Rev Immunol. 2019;19(1):63–4. [DOI] [PubMed] [Google Scholar]
- 18.Muccioli L, Pensato U, Cani I, Guarino M, Cortelli P, Bisulli F. COVID-19–Associated Encephalopathy and cytokine-mediated neuroinflammation. Ann Neurol. 2020;88(4):860–1. [DOI] [PubMed] [Google Scholar]
- 19.Reichard RR, Kashani KB, Boire NA, Constantopoulos E, Guo Y, Lucchinetti CF. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol (Berl). 2020;140(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peleg Y, Kudose S, D’Agati V, Siddall E, Ahmad S, Nickolas T, et al. Acute kidney Injury due to collapsing Glomerulopathy following COVID-19 infection. Kidney Int Rep. 2020;5(6):940–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salvio G, Gianfelice C, Firmani F, Lunetti S, Balercia G, Giacchetti G. Bone metabolism in SARS-CoV-2 Disease: possible osteoimmunology and gender implications. Clin Rev Bone Min Metab. 2020;18(4):51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gentile S, Strollo F, Mambro A, Ceriello A. COVID-19, ketoacidosis and new‐onset diabetes: are there possible cause and effect relationships among them? Diabetes Obes Metab. 2020;22(12):2507–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernández-de-las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, Florencio LL, Cuadrado ML, Plaza-Manzano G, et al. Prevalence of post-COVID-19 symptoms in hospitalized and non-hospitalized COVID-19 survivors: a systematic review and meta-analysis. Eur J Intern Med. 2021;92:55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bai F, Tomasoni D, Falcinella C, Barbanotti D, Castoldi R, Mulè G et al. Female gender is associated with long COVID syndrome: a prospective cohort study. Clin Microbiol Infect. 2022;28(4):611.e9-611.e16. [DOI] [PMC free article] [PubMed]
- 25.Daitch V, Yelin D, Awwad M, Guaraldi G, Milić J, Mussini C, et al. Characteristics of long-COVID among older adults: a cross-sectional study. Int J Infect Dis. 2022;125:287–93. [DOI] [PubMed] [Google Scholar]
- 26.Fernández-de-las-Peñas C, Martín-Guerrero JD, Pellicer-Valero ÓJ, Navarro-Pardo E, Gómez-Mayordomo V, Cuadrado ML, et al. Female sex is a risk factor Associated with Long-Term Post-COVID related-symptoms but not with COVID-19 symptoms: the LONG-COVID-EXP-CM Multicenter Study. J Clin Med. 2022;11(2):413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munblit D, Bobkova P, Spiridonova E, Shikhaleva A, Gamirova A, Blyuss O, et al. Incidence and risk factors for persistent symptoms in adults previously hospitalized for COVID-19. Clin Exp Allergy. 2021;51(9):1107–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rovas A, Osiaevi I, Buscher K, Sackarnd J, Tepasse PR, Fobker M, et al. Microvascular dysfunction in COVID-19: the MYSTIC study. Angiogenesis. 2021;24(1):145–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tehrani S, Gille-Johnson P. Microvascular dysfunction in patients with critical COVID-19, a pilot study. Shock Augusta Ga. 2021;56(6):964–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ambrosino P, Calcaterra I, Molino A, Moretta P, Lupoli R, Spedicato GA, et al. Persistent endothelial dysfunction in Post-acute COVID-19 syndrome: a case-control study. Biomedicines. 2021;9(8):957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evans PC, Rainger GE, Mason JC, Guzik TJ, Osto E, Stamataki Z, et al. Endothelial dysfunction in COVID-19: a position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc Res. 2020;116(14):2177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kubo M, Motomura Y. Transcriptional regulation of the anti-inflammatory cytokine IL-10 in acquired immune cells. Front Immunol. 2012;3:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murakami M, Hirano T. The molecular mechanisms of chronic inflammation development. Front Immunol. 2012;3:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Queiroz MAF, Gonçalves Júnior E, da Silva Aquino M, et al. Cytokine profiles associated with acute COVID-19 and long COVID-19 syndrome. Front Cell Infect Microbiol. 2022;12:922422. 10.3389/fcimb.2022.922422. [DOI] [PMC free article] [PubMed]
- 35.Akbari H, Tabrizi R, Lankarani KB, Aria H, Vakili S, Asadian F, et al. The role of cytokine profile and lymphocyte subsets in the severity of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Life Sci. 2020;258:118167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao Y, Qin L, Zhang P, Li K, Liang L, Sun J et al. Longitudinal COVID-19 profiling associates IL-1RA and IL-10 with disease severity and RANTES with mild disease. JCI Insight. 2020; 5(13):e139834. [DOI] [PMC free article] [PubMed]
- 37.Gómez-Escobar LG, Hoffman KL, Choi JJ, Borczuk A, Salvatore S, Alvarez-Mulett SL, et al. Cytokine signatures of end organ injury in COVID-19. Sci Rep. 2021;11:12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vabret N, Britton GJ, Gruber C, Hegde S, Kim J, Kuksin M, et al. Immunology of COVID-19: current state of the Science. Immunity. 2020;52(6):910–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schultheiß C, Willscher E, Paschold L, Gottschick C, Klee B, Henkes SS, et al. The IL-1β, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep Med. 2022;3(6):100663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodman RB, Strieter RM, Martin DP, Steinberg KP, Milberg JA, Maunder RJ, et al. Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996;154(3 Pt 1):602–11. [DOI] [PubMed] [Google Scholar]
- 41.Donnelly SC, Strieter RM, Reid PT, Kunkel SL, Burdick MD, Armstrong I, et al. The Association between Mortality Rates and decreased concentrations of Interleukin-10 and Interleukin-1 receptor antagonist in the lung fluids of patients with the adult respiratory distress syndrome. Ann Intern Med. 1996;125(3):191–6. [DOI] [PubMed] [Google Scholar]
- 42.Rea IM, Gibson DS, McGilligan V, McNerlan SE, Alexander HD, Ross OA. Age and Age-Related diseases: role of inflammation triggers and cytokines. Front Immunol. 2018;9:586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crook H, Raza S, Nowell J, Young M, Edison P. Long COVID—mechanisms, risk factors, and management. BMJ. 2021;374:n1648. [DOI] [PubMed] [Google Scholar]
- 44.Haffke M, Freitag H, Rudolf G, Seifert M, Doehner W, Scherbakov N, et al. Endothelial dysfunction and altered endothelial biomarkers in patients with post-COVID-19 syndrome and chronic fatigue syndrome (ME/CFS). J Transl Med. 2022;20(1):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mirzaei H, Ferns GA, Avan A, Mobarhan MG. Cytokines and MicroRNA in coronary artery disease. Adv Clin Chem. 2017;82:47–70. [DOI] [PubMed] [Google Scholar]
- 46.Oude Nijhuis CSM, Vellenga E, Daenen SMGJ, Kamps WA, de Bont ESJM. Endothelial cells are Main Producers of Interleukin 8 through toll-like receptor 2 and 4 signaling during bacterial infection in Leukopenic Cancer patients. Clin Diagn Lab Immunol. 2003;10(4):558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Milaneschi Y, Kappelmann N, Ye Z, Lamers F, Moser S, Jones PB, et al. Association of inflammation with depression and anxiety: evidence for symptom-specificity and potential causality from UK Biobank and NESDA cohorts. Mol Psychiatry. 2021;26(12):7393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kappelmann N, Dantzer R, Khandaker GM. Interleukin-6 as potential mediator of long-term neuropsychiatric symptoms of COVID-19. Psychoneuroendocrinology. 2021;131:105295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak TW, et al. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol Baltim Md 1950. 2003;170(10):4886–90. [DOI] [PubMed] [Google Scholar]
- 50.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–42. [DOI] [PubMed] [Google Scholar]
- 51.McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev. 2009;33(3):355–66. [DOI] [PubMed] [Google Scholar]
- 52.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25(2):181–213. [DOI] [PubMed] [Google Scholar]
- 53.Fiore M, Probert L, Kollias G, Akassoglou K, Alleva E, Aloe L. Neurobehavioral alterations in developing transgenic mice expressing TNF-α in the brain. Brain Behav Immun. 1996;10(2):126–38. [DOI] [PubMed] [Google Scholar]
- 54.Bjugstad KB, Flitter WD, Garland WA, Su GC, Arendash GW. Preventive actions of a synthetic antioxidant in a novel animal model of AIDS dementia1Published on the world wide web on 24 April 1998.1. Brain Res. 1998;795(1):349–57. [DOI] [PubMed] [Google Scholar]
- 55.Matsumoto Y, Watanabe S, Suh YH, Yamamoto T. Effects of intrahippocampal CT105, a carboxyl terminal fragment of beta-amyloid precursor protein, alone/with inflammatory cytokines on working memory in rats. J Neurochem. 2002;82(2):234–9. [DOI] [PubMed] [Google Scholar]
- 56.Kesler S, Janelsins M, Koovakkattu D, Palesh O, Mustian K, Morrow G, et al. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav Immun. 2013;30(0):S109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang TY, Lee SY, Chang YH, Chen SL, Chen PS, Chu CH, et al. Correlation of cytokines, BDNF levels, and memory function in patients with opioid use disorder undergoing methadone maintenance treatment. Drug Alcohol Depend. 2018;191:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dyer AH, McKenna L, Batten I, Jones K, Widdowson M, Dunne J, et al. Peripheral inflammation and cognitive performance in Middle-aged adults with and without type 2 diabetes: results from the ENBIND Study. Front Aging Neurosci. 2020;12:605878. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article. The data that support the findings of this study are available from the corresponding author upon reasonable req.