Abstract
Gemella morbillorum is a gram-positive coccus that is part of the normal microbiota of the human oral cavity and gastrointestinal tract. It is an opportunistic pathogen that can cause invasive infections, including septic arthritis. Septic arthritis caused by Gemella morbillorum is relatively rare, but when it occurs, it can lead to severe joint damage and other complications if not promptly diagnosed and treated. Here, we report a case of recurrent septic arthritis caused by Gemella morbillorum.
Keywords: Rare pathogen, Gemella morbillorum, Septic arthritis, MetaCAP, Case report
Clinical trial number
Not applicable.
Introduction
Gemella morbillorum is a facultative anaerobic gram-positive coccus. Discovered by R. Tunnicliff in 1917, it was first named Diplococcus morbillorum, then renamed Peptostreptococcus morbillorum, and subsequently Peptococcus morbillorum, Streptococcus morbillorum, before being officially designated as Gemella morbillorum in 1988 [1, 2]. As an opportunistic pathogen, it is generally found in the oral and gastrointestinal tracts of healthy humans and can cause conditions such as endocarditis, brain abscesses, and mediastinal abscesses in humans [3, 4]. Gemella morbillorum has been frequently reported to be resistant to penicillin [5].
In 1993, Omran et al. reported the first case of hematogenous pyogenic bone infection involving the bones and joints caused by Gemella morbillorum [6]. According to a recent systematic review, bone and joint infections caused by Gemella morbillorum have been observed in the knee joint, thoracolumbar spine, hip joint, wrist joint, and prosthetic joint [7]. We report a rare recurrent case to highlight the importance of considering Gemella morbillorum in septic arthritis, particularly in patients with underlying joint disease. These findings also underscore the importance of appropriate surgical therapy in the management of these infections.
Case report
A 65-year-old man with chronic right shoulder pain and swelling came to our clinic. He first came to our clinic and reported a 5 cm*4 cm tumor on the right shoulder. The patient presented with chronic pain and swelling but no fever or other sport symptoms. Four months before he came to our hospital, he underwent a right arm 2 cm*2 cm muscular tumor excision, and a pathology report indicated chronic suppurative inflammation. He also went to the dental clinic for cavity treatment.
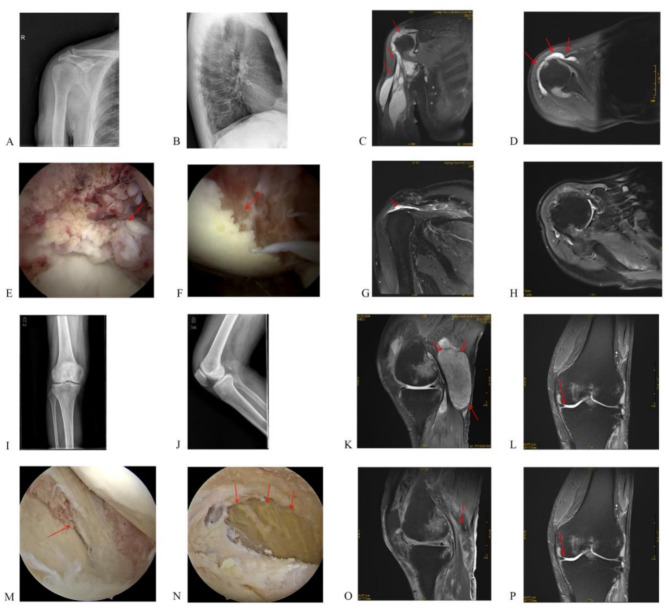
We performed an MRI examination, which revealed humeral head destruction and periarticular abscess formation (Fig. 1). Blood tests showed WBC (11.9 × 109/L), CRP (124.1 mg/L), ESR (68 mm/h), IL-6 (69.02 pg/ml) and PCT (0.04 ng/ml).The patient tested negative for rheumatoid factor (310.0 IU/ml), tuberculin, TB-Ab, and HLA-B27. The patient also had a history of smoking and drinking. Amikacin (0.8 mg/day) was used to fight infection 5 days before and 5 days after surgery. The patient then received 500 mg po bid clarithromycin (as suggested by the clinical pharmacy department) and was discharged for 10 days. On the basis of the clinical evidence, we performed arthroscopic synovectomy surgery. Synovial proliferation and humeral head necrosis were detected (Fig. 1), but the synovial fluid cultures were negative, and pathology confirmed suppurative arthritis. The pain relief and shoulder ROM improved.
Fig. 1.
A, B: Preoperative X-rays for right shoulder arthroscopy surgery; C, D: Preoperative MRI scans for right shoulder arthroscopy surgery; E, F: Intraoperative photos during right shoulder arthroscopy surgery;G, H: MRI scans one month post-surgery for right shoulder arthroscopy surgery; I, J: Preoperative X-rays for left knee arthroscopy surgery; K, L: Preoperative MRI scans for left knee arthroscopy surgery; M, N: Intraoperative photos during left knee arthroscopy surgery; O, P: MRI scans one month post-surgery for left knee arthroscopy surgery
Two months after surgery patient returned with left knee and popliteal swelling. We performed MRI immediately, and the images revealed similar changes in the left knee to those in the right shoulder for the first time (Fig. 1). His blood parameters, WBC(12.10 × 109/L), CRP(14.35 mg/L), IL-6(58.60 pg/ml), and PCT(0.14 ng/ml), increased, which indicated infection in the knee. According to our previous experience, we performed knee arthroscopy surgery. Pathology revealed synovial proliferation and bone damage in the femur and tibia (Fig. 1). The patient presented with purulent knee inflammation and abscess, but cultures were still negative. After surgery, the patient’s symptoms were relieved in a short time. Although we wanted to perform further investigations to make different diagnoses, The patient insisted on follow up as an out patient.
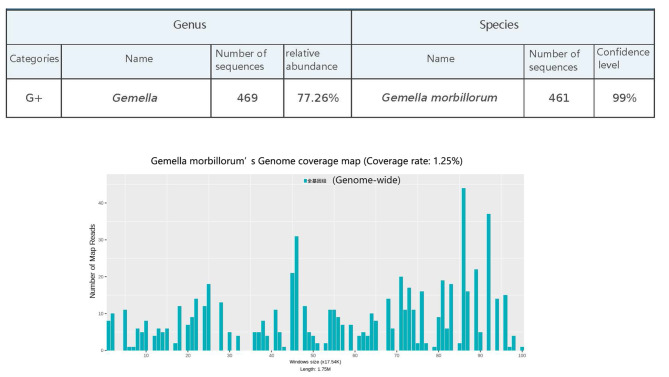
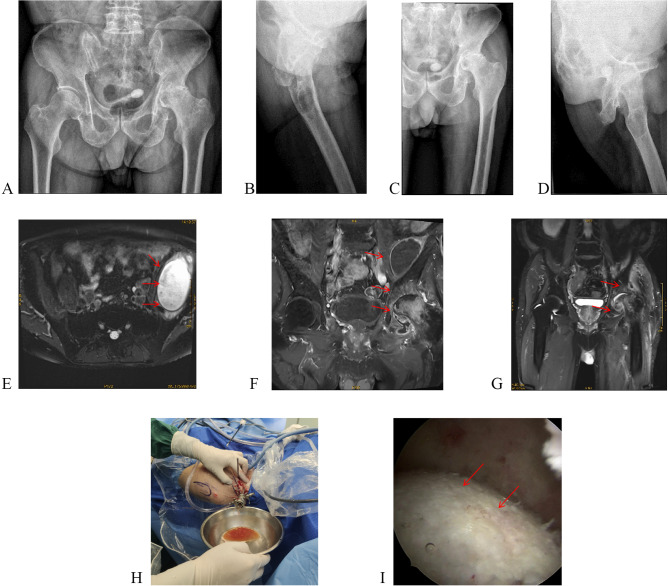
One month after left knee surgery, the patient returned with left hip pain and limited movement. This time, We performed paracentesis of synovial fluid and sent for MetaCap sequencing (Guangzhou Kingmed Diagnostics Group Co., Ltd.) (Fig. 2). The results identified Gemella morbillorum, and vancomycin (blood drug concentration: 10–20 µg/ml) for 2 weeks. However, the hip joint cavity paracentesis synovial fluid culture was negative. The final diagnosis was chronic synovitis and purulent arthritis. After antibiotic treatment, we performed arthroscopic synovectomy (Fig. 3). Two weeks of vancomycin (blood drug concentration: 10–20 µg/ml) treatment was given after the operation. One month after each of the three surgeries, MRI confirmed that the abscesses had resolved (Figs. 1, 3). This time, we followed up for 12 months, and the patient reported that no symptoms had recurred. During the 18-month follow-up, the patient’s body was checked routinely at another hospital, and the patient was diagnosed with left hip arthritis and underwent left hip arthroplasty.
Fig. 2.
MetaCap Sequencing Report
Fig. 3.
A, B: Preoperative X-rays for left hip arthroscopy surgery; C, D: Postoperative X-rays for left hip arthroscopy surgery; E, F: Preoperative photos for left hip arthroscopy surgery; G: MRI scans one month post-surgery for left hip arthroscopy surgery
Discussions
Gemella morbillorum(GM) is a rare pathogen. Common risk factors in previous cases include immunosuppression, the presence of prosthetics, poor dental health with dental infections, endoscopic interventions, and potential intrinsic joint diseases such as rheumatoid arthritis. However, studies on the bone and joints of the GM are limited; To the best of our knowledge, this is the first case of polyarticular infection caused by the GM.
GM is a part of oral microbiota and no matter where the infections reported are located (e.g. endocarditis [3, 8], brain abscess [9], hepatic abscess [10], aspiration pneumonia [11], mixed anaerobic lung abscess and empyema [12] etc.), the origin is from the mouth. In most infections it is part of a mixed infection except in instances such as endocarditis, where it is found as a single agent similar to S.viridans [3, 4, 7]. In early studies these infections showed an excellent response and quicker recovery with clindamycin treatment. Clindamycin has excellent tissue penetration and if the organism was susceptible should give good results [13, 14].
Septic arthritis remains a significant challenge in the field of orthopedics [15]. The symptoms of septic arthritis include persistent pain and swollen joints. If not treated promptly, irreversible damage to cartilage damage can occur, ultimately leading to permanent functional disability in patients [16]. The majority of infections are due to direct spread and some due to hematogenous spread. Septic arthritis could be from hematogenous spread or direct inoculation [7, 17–19]. The key point in the treatment of septic arthritis is early detection and antibiotic treatment [16, 20]. The outcome is better with early detection. However, in our patient, both synovial fluid and blood cultures were negative. We could not find pathogens precisely during the first and second hospitalizations. For the third examination, we chose MetaCAP examination to confirm the presence of GM infection.
Compared with traditional culture-based methods, second-generation gene sequencing technology has a higher pathogen detection rate in bone and joint infections [21]. MetaCAP is a culture-independent method capable of detecting microbiomes in various sample types. This technology, rooted in metagenomics, utilizes customized specific probes to hybridize with microbial nucleic acids in samples [22]. It can be used for the detection of samples suspected of infection in the clinic. When traditional cultures are negative, infection should be suspected based on clinical presentation.
In reviewing previous cases, the GM diagnosis was generally based on blood culture [16]. However, bone destruction is a hallmark of many inflammatory bone and joint diseases, encompassing both infectious and immune-mediated local and systemic conditions; common examples include tuberculosis infection, rheumatoid arthritis, and psoriatic arthritis [23, 24]. Most (approximately 87.5%) patients with Gemella morbillorum bone and joint infection have local symptoms, including joint pain, joint swelling, joint shrinkage and other symptoms, and a small proportion experience systemic symptoms, such as fever [7]. In this case, the patient presented with joint pain, joint swelling, and other symptoms but no systemic symptoms, such as fever. The first two arthroscopies revealed bone destruction and local abscess formation, and pathology suggested the possibility of suppuration. In addition, under standard antibiotic treatment, symptoms have decreased. Before the third surgery, to confirm the presence of the pathogen, the joint synovial fluid was sent for MetaCAP sequencing.
Thorough cleaning significantly alleviates inflammation and prevents the spread of infection, demonstrating clear benefits over open debridement [20, 25]. Thorough arthroscopic lavage and debridement are currently safe and effective methods for the treatment of septic arthritis, but these methods are characterized by rapid, minimally invasive recovery [16, 20, 25]. A review of previous cases of Gemella morbillorum-induced septic arthritis of the same type of primary joint revealed 2 cases of arthroscopic articular washout [26, 27], 3 cases of open washout [17, 19, 28], and 1 case of no irrigation [6], in which arthroscopic debridement (6 weeks) was used compared with open debridement (3 months) and antibiotics alone, resulting in a shorter treatment cycle and faster recovery. The patient’s symptoms could be relieved quickly after clearing. After infection control, patients generally recover within 6 weeks to several months [7], which is a better prognosis than other types of septic arthritis and may be attributed to its lower degree of invasiveness. Therefore, arthroscopic debridement was performed in all three instances, resulting in basic subsidence of local inflammation postsurgery, preventing further progression of conditions such as bone destruction, and improving patient prognosis. Early in purulent arthritis, cartilage destruction can occur, making the selection of appropriate antibiotics to control infection a key factor in the treatment of bone and joint infections [16, 24]. Gemella morbillorum has been shown to be susceptible to most β-lactams, particularly vancomycin. However, there are different degrees of resistance to cefoxitin, macrolides, tetracyclines and quinolones [13, 14]. Therefore, the recurrence after the first two operations in this case was possibly caused by bacterial resistance. On the basis of the patient’s condition, we chose vancomycin as the antimicrobial treatment following the patient’s third arthroscopic surgery.
The patient’s recurrent hospitalizations and eventual receipt of arthroplasty could be due to the following reasons. First, there was a delay in addressing Gemella morbillorum bacterial infections in the joint. Second, arthroscopic debridement should be performed early, and full-doses of antibiotics should be used to prevent bone damage. Once bone erosion and cartilage damage have started, a second-stage arthroplasty is the only solution.
Conclusion
In patients with significant bone damage and inflammation, rare infections are considered. We suggest running mNGS or a similar test when the cause of infection remains unclear. Early arthroscopic debridement and broad-spectrum antibiotics can improve outcomes by reducing inflammation and halting disease progression.
Acknowledgements
The authors acknowledged the patient in this report for permitting us to present his clinical information and relevant data.
Author contributions
Haiquan Zeng and Weijin Miao wrote the main manuscript text and Shaohua Liang prepared Figs. 1, 2 and 3. Jinli Zhang was responsible for formatting the manuscript. Wen Wang was responsible for editing and reviewing the manuscript. All authors reviewed the manuscript.
Funding
This research was supported by Guangzhou Characteristic Technology Project of Guangzhou Municipal Health Commission (fund no. 2023 C-TS11).
Data availability
All the data and materials of this case is contained within the manuscript.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
We have obtained written informed consent from the patient for the publication of the report and accompanying images. And all authors agreed to publish.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shaohua Liang, Email: shaohualeung@foxmail.com.
Wen Wang, Email: warrenwangrch@outlook.com.
References
- 1.Kilpper-Bälz R, Schleifer KH. Transfer of Streptococcus morbillorum to the Genus Gemella as Gemella morbillorum comb. nov. Int J Syst Evol Microbiol. 1988;38(4):442. 10.1099/00207713-38-4-442.
- 2.Romond C. The taxonomy of the Peptococcaceae. Infection. 1980;8(Suppl 2):S153–154. 10.1007/BF01639880. [DOI] [PubMed] [Google Scholar]
- 3.Zakir RM, Al-Dehneh A, Dabu L, Kapila R, Saric M. Mitral bioprosthetic valve endocarditis caused by an unusual microorganism, Gemella morbillorum, in an intravenous drug user. J Clin Microbiol. 2004;42:4893–6. 10.1128/JCM.42.10.4893-4896.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akiyama K, Taniyasu N, Hirota J, Iba Y, Maisawa K. Recurrent aortic valve endocarditis caused by Gemella morbillorum–report of a case and review of the literature. Jpn Circ J. 2001;65:997–1000. 10.1253/jcj.65.997. [DOI] [PubMed] [Google Scholar]
- 5.Vasishtha S, Isenberg HD, Sood SK. Gemella morbillorum as a cause of septic shock. Clin Infect Dis. 1996;22:1084–6. 10.1093/clinids/22.6.1084. [DOI] [PubMed] [Google Scholar]
- 6.Omran Y, Wood CA. Endovascular infection and septic arthritis caused by Gemella morbillorum. Diagn Microbiol Infect Dis. 1993;16:131–4. 10.1016/0732-8893(93)90007-T. [DOI] [PubMed] [Google Scholar]
- 7.Saad E, Faris ME, Abdalla MS, Prasai P, Ali E, Stake J. A Rare Pathogen of bones and joints: a systematic review of Osteoarticular infections caused by Gemella morbillorum. J Clin Med Res. 2023;15:187–99. 10.14740/jocmr4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Debast SB, Koot R, Meis JFGM, Wood C. (1993). Infections caused by gemella morbillorum. Lancet 342, 560%* https://www.elsevier.com/tdm/userlicense/561.560/ %U https://linkinghub.elsevier.com/retrieve/pii/014067369391691E [PubMed]
- 9.Thereza-Bussolaro C, Ramos EB, Yanai AL, Volpato L-E-R, Borba AM. Odontogenic (hematogenic) or sinusopathy (contiguous) brain abscess: case report. J Clin Experimental Dentistry. 2024;16:e926–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu C-Y, Su Y-C, Wang T-L, Chong C-F, Chen C-C. Gemella morbillorum liver abscess. Scand J Infect Dis. 2007;39:637–8. [DOI] [PubMed] [Google Scholar]
- 11.Hussain S, Hussain S, Ashraf M. Pneumonia and bacteraemia caused by gemella morbillorum in a previously healthy infant: first reported case in literature. BMJ case Rep. 2018;2018(bcr2018226295):bcr–2018222018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DePrez KN, Ferguson J. (2024). Endobronchial infection and bacterial lymphadenitis by gemella morbillorum leading to airway perforation and a bronchopleural fistula. Case Reports in Pulmonology 2024, 8850287. [DOI] [PMC free article] [PubMed]
- 13.Furugaito M, Arai Y, Uzawa Y, Kamisako T, Ogura K, Okamoto S, Kikuchi K. (2023). Antimicrobial Susceptibility to 27 Drugs and the Molecular Mechanisms of Macrolide, Tetracycline, and Quinolone Resistance in Gemella sp. Antibiotics (Basel) 12. 10.3390/antibiotics12101538 [DOI] [PMC free article] [PubMed]
- 14.Goldstein EJ, Merriam CV, Claros MC, Citron DM. Comparative susceptibility of Gemella morbillorum to 13 antimicrobial agents. Anaerobe. 2022;75:102573. 10.1016/j.anaerobe.2022.102573. [DOI] [PubMed] [Google Scholar]
- 15.Masters EA, Ricciardi BF, Bentley KLM, Moriarty TF, Schwarz EM, Muthukrishnan G. Skeletal infections: microbial pathogenesis, immunity and clinical management. Nat rev Microbiol. 2022;20:385–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colston J, Atkins B. Bone and joint infection. Clin Med (Lond). 2018;18:150–4. 10.7861/clinmedicine.18-2-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang FG, Yuan FL, Liu JK, Long Q. [Suppurative arthritis caused by Gemella morbillorum in a patient with rheumatoid arthritis of the knee]. Nan Fang Yi Ke Da Xue Xue Bao. 2012;32:437–8. [PubMed] [Google Scholar]
- 18.Beltramini GA, Laganà FC, Giannì AB, Baj A. Maxillary sinusitis after sinus lift due to gemella morbillorum: antibiotic and surgical treatment. J Craniofac Surg. 2013;24:e275–eU276. http://journals.lww.com/00001665-201305000-201300134. [DOI] [PubMed] [Google Scholar]
- 19.Czarnecki A, Ong GH, Pieroni P, Trepman E, Embil JM. Gemella morbillorum septic arthritis of the knee and infective endocarditis. Am J Orthop (Belle Mead NJ). 2007;36:E7–9. [PubMed] [Google Scholar]
- 20.Aim F, Delambre J, Bauer T, Hardy P. Efficacy of arthroscopic treatment for resolving infection in septic arthritis of native joints. Orthop Traumatol Surg Res. 2015;101:61–4. 10.1016/j.otsr.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Titecat M, Senneville E, Wallet F, Dezeque H, Migaud H, Courcol RJ, Loiez C. Bacterial epidemiology of osteoarticular infections in a referent center: 10-year study. Orthop Traumatol Surg Res. 2013;99:653–8. 10.1016/j.otsr.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Zhao M, Tang K, Liu F, Zhou W, Fan J, Yan G, Qin S, Pang Y. Metagenomic next-generation sequencing improves diagnosis of Osteoarticular infections from abscess specimens: a Multicenter Retrospective Study. Front Microbiol. 2020;11:2034. 10.3389/fmicb.2020.02034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DiCarlo EF, Kahn LB. Inflammatory diseases of the bones and joints. Semin Diagn Pathol. 2011;28:53–64. 10.1053/j.semdp.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Tateiwa D, Yoshikawa H, Kaito T. Cartilage and Bone Destruction in Arthritis: Pathogenesis and Treatment Strategy: A literature review. Cells. 2019;8:818. 10.3390/cells8080818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khazi ZM, Cates WT, An Q, Duchman KR, Wolf BR, Westermann RW. Arthroscopy Versus Open Arthrotomy for treatment of native hip septic arthritis: an analysis of 30-Day complications. Arthroscopy. 2020;36:1048–52. 10.1016/j.arthro.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Desmottes MC, Brehier Q, Bertolini E, Monteiro I, Terreaux W. Septic arthritis of the knee due to Gemella morbillorum. Int J Rheum Dis. 2018;21:1146–7. 10.1111/1756-185X.13293. [DOI] [PubMed] [Google Scholar]
- 27.Roche M, Smyth E. A case of septic arthritis due to infection with Gemella morbillorum. J Infect. 2005;51(e187–189). 10.1016/j.jinf.2005.01.009. [DOI] [PubMed]
- 28.van Dijk M, van Royen BJ, Wuisman PI, Hekker TA, van Guldener C. Trochanter osteomyelitis and ipsilateral arthritis due to Gemella morbillorum. Eur J Clin Microbiol Infect Dis. 1999;18:600–2. 10.1007/s100960050356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data and materials of this case is contained within the manuscript.